1. Introduction
A central question in neurolinguistics is how semantic concepts - the generalizable knowledge of entities, people and events - are represented in the brain. Imaging studies in healthy subjects have identified broad networks involved in lexical and semantic processing, including the angular gyrus, the lateral and ventral temporal gyri, and the left inferior frontal gyrus (Binder et al., 2009; Binney et al., 2010; Davis & Gaskell, 2009; Price, 2010). Evidence suggests that semantic concepts are represented in a distributed manner, with modality-specific sensory-motor cortices, heteromodal association areas, and frontal regions being recruited partially based on modality-specific knowledge associated with the corresponding concept (Binder & Desai, 2011; Martin & Chao, 2001; Saffran, 2000). Thus, many studies have shown that different semantic and lexical categories - such as living v. manufactured, or nouns v. verbs -are supported, in part, by different combinations of these brain regions (Binder et al., 2009; Chao, Haxby & Martin, 1999; Grossman et al., 2013; Luzzatti et al., 2002). One such important categorical distinction is the representation of abstract and concrete nouns. Concrete nouns typically refer to entities that are tangible and exist in the real world, and thus have perceptible features, such as appearance, feel, or sound. These sensory features are thought to be crucial for concrete noun representations, and grounded cognition and dual coding theories propose that sensory feature knowledge - predominantly of visual features - support concrete concept processing (Paivio, 1991). Thus, activation of associated sensory knowledge during concrete word comprehension recruits corresponding visual and other sensory association cortices (Barsalou, 2008; Binder & Desai, 2011; Martin, 2007). Indeed, the importance of visual feature knowledge distinguishes concrete from abstract nouns, and the dual coding theory proposes that concrete concepts have both linguistic- and sensory-based representations, while abstract concepts have only linguistically-based representations. In support of the view that concrete nouns are supported by visual features, imaging studies in controls have shown that the visual association cortex, including the left inferior temporal lobe and the left parahippocampal gyrus, are implicated in the processing of concrete compared to abstract nouns (Bonner et al., 2015; Sabsevitz et al., 2005; Wang et al., 2010).
In contrast, abstract nouns have minimal physical or tangible qualities, and they primarily refer to entities that exist within language and thought. Whereas sensory information is thought to be more important for processing concrete concepts, the contextual and linguistic information that surrounds a word is thought to be essential for acquiring and processing abstract words (Della Rosa et al., 2010). Because they do not have tangible referents, the meanings associated with abstract nouns are more varied and less literal (Johnson & Lakoff, 1980; Lakoff & Johnson, 1999), and abstract nouns have been shown to appear in a more diverse set of contexts (Hoffman, Ralph & Rogers, 2013). Thus, the contextual availability hypothesis proposes that abstract concepts have increased contextual ambiguity compared to concrete concepts (Schwanenflugel, Harnishfeger, & Stowe, 1988; Schwanenflugel & Shoben, 1983; van Hell & de Groot, 1998). Abstract noun processing may thus require executive functioning systems for semantic selection and contextual integration, supported in part by portions of the frontal lobe (Hoffman, Jefferies & Ralph, 2010). For example, when hearing the statement, “I didn’t mean to offend, that’s just my way”, the polysemous quality of a noun like “way” requires semantic selection; to best understand this statement, the interpretation of “way” as “manner or characteristic” should be selected, and competing alternatives, such as “path or direction”, should be inhibited. A likely anatomical candidate for this role of semantic control and selection in abstract noun comprehension is the left inferior frontal gyrus (Moss et al., 2005; Thompson-Schill et al., 1997), and functional imaging studies have shown that abstract word processing activates the inferior frontal gyrus more so than concrete word processing (Hoffman, Binney & Ralph, 2015; Wang et al., 2010).
In a previous study, we examined the role of temporal and frontal regions in concrete and abstract noun comprehension using a two-alternative, forced-choice similarity task (Cousins et al., 2016). Comprehension was tested in patients with semantic variant Primary Progressive Aphasia (svPPA) and behavioral variant Frontotemporal Degeneration (bvFTD), syndromes which correspond to focal regions of cortical atrophy hypothesized to be important in concrete and abstract noun processing: the ventral temporal lobes and the inferior frontal gyrus, respectively. Our findings supported a combination of grounded cognition and contextual availability theories, and demonstrated that partially dissociable substrates support the comprehension of concrete and abstract nouns, discussed below.
1.1 Impairment of concrete noun comprehension in svPPA
Also known as Semantic Dementia, svPPA is characterized by the progressive loss of verbal and non-verbal semantic memory (i.e. single word and object comprehension). Patients with svPPA present with impaired confrontation naming and lexical retrieval (Amici et al., 2007; Hodges & Patterson, 2007; Mesulam, 2003), and some can also present with mild behavioral changes (Hodges & Patterson, 2007). Degeneration in svPPA tends to be most severe in the left anterior and ventral temporal lobes (Acosta-Cabronero et al., 2011; Mion et al., 2010; Rogalski et al., 2011). In our previous study, patients with svPPA had differentially worse knowledge for concrete nouns compared to abstract. This selective impairment in svPPA has been previously observed by several groups: while not seen by all (Jefferies et al., 2009; Hoffman, Jones, & Ralph, 2013), many studies have reported differentially worse comprehension for concrete words in svPPA (Warrington, 1975; Breedin, Saffran & Coslett, 1994; Macoir, 2009), and have implicated the anterior and ventral temporal lobes in concrete word processing (Bonner et al., 2009; Bonner et al., 2015). Similarly, we found that poor concrete noun comprehension in svPPA related to atrophy of the left anterior temporal lobe, including the left inferior temporal gyrus. The inferior temporal gyrus is part of the visual processing stream and has been shown to be involved in visual object processing (Chao, Haxby, & Martin, 1999; Martin et al., 1995; Miyashita, 1993). These findings are in agreement with the dual coding and grounded cognition perspectives that the representations of concrete nouns are supported by visual features, and that degradation of the visual association cortex in svPPA impairs concrete noun knowledge.
1.2 Impairment of abstract noun comprehension in bvFTD
Patients with bvFTD present with progressive behavioral or personality changes such as disinhibition, poor judgment, increased apathy, and loss of empathy (Piguet et al., 2011; Rascovsky et al., 2011; Wittenberg et al., 2008). The disease is associated with profound frontal lobe atrophy, including the orbitobasal and dorsolateral cortices and the anterior cingulate, as well as some modest atrophy to the anterior temporal lobe, which is commonly more prominent on the right than the left. In our previous study, we found that bvFTD patients are differentially worse at comprehending abstract compared to concrete nouns. This was a novel finding, since bvFTD is not typically associated with semantic deficits. This may be because traditional evaluations of semantic knowledge often have a highly concrete picture stimulus set (e.g. Boston Naming Test, Kaplan, Goodglass & Weintraub, 2001; Pyramids and Palm trees, Howard & Patterson, 1992) and are thus not sensitive to the deficit for abstract concepts that we observed in bvFTD. In addition, we found that poor abstract noun knowledge related to atrophy in the inferior frontal gyrus, a region shown to be important in semantic control and selection (Moss et al., 2005; Thompson-Schill et al., 1997). These results support the assertion by the contextual availability hypothesis that executive functioning and semantic selection processes in the frontal lobe are important to abstract noun processing.
These findings in svPPA and bvFTD suggest that the grounded cognition, dual coding, and context availability perspectives, while incomplete on their own, together offer some understanding of how temporal and frontal regions can differentially support the comprehension of concrete and abstract nouns. In the present study we investigate whether atrophy to these temporal and frontal regions in svPPA and bvFTD also impairs the ability to produce concrete and abstract nouns, respectively.
1.3 Noun Comprehension vs. Production
While we and others (Hoffman, Binney & Ralph, 2015; Sabsevitz et al., 2005) have found that partially dissociable regions underlie abstract and concrete noun comprehension, we do not necessarily assume that these same regions also support connected speech production for abstract and concrete nouns. The classic Broca-Wernicke-Lichtheim-Geschwind model of language processing proposes separate pathways for language comprehension and production, with Broca’s area being the anatomic center of language production and Wernicke’s area being essential to comprehension (Geschwind, 1970; Poeppel et al., 2012). Updated models suggest that, while comprehension and production operations are distinct, there may be portions of these lexical processing networks that are common to both comprehension and production (Indefrey, 2011; Price, 2010; Price, 2012; Shalom & Poeppel, 2007). Thus, structures involved in the comprehension of concrete and abstract nouns might also be recruited to support retrieval during production.
This study aims to expand our previous work and examine the underlying structures and processes that support retrieval of abstract and concrete nouns during connected speech. To elicit semi-structured speech samples, svPPA and bvFTD patients participated in the Cookie Theft picture description task (Goodglass & Kaplan, 1983). By using the Cookie Theft picture, we were able to prompt connected speech concerning a known target, which was more natural than confrontation naming of a picture, while also modestly constraining the concreteness of the content and its complexity. This enabled comparisons of performance across patient groups while still requiring self-directed lexical retrieval. If there is some overlap between brain regions supporting the comprehension of concrete and abstract nouns and the regions supporting their production, then we expect patients with svPPA to produce nouns that are less concrete, and for this decreased concreteness to be related to visual association cortex, such as left anterior and ventral temporal regions. Similarly, we expect that bvFTD patients will produce less abstract nouns during the picture description task and that this decreased abstractness will be related to regions implicated in semantic selection and control, such as the inferior frontal gyrus.
2. Materials and Methods
2.1 Subjects
Forty-two bvFTD and 20 svPPA patients participated in the current study. Subjects were native English speakers and diagnoses were made by consensus based on criteria outlined by Rascovsky et al. (2011) and Gorno-Tempini et al. (2011), and independently from the neuropsychological features reported below. Typical of the svPPA spectrum, 15 cases had mild behavioral symptoms that presented following their difficulties with word comprehension and retrieval (Hodges & Patterson, 2007). None of the bvFTD cases were found to have semantic deficits (i.e. single-word and object knowledge loss) on bed-side clinical examination. We further evaluated patient groups’ performance on standard language and neuropsychological assessments. All patients were evaluated on global cognition, as measured by the Mini Mental State Examination (MMSE), and subsets participated in supplementary tasks to evaluate semantic knowledge and executive functioning. Semantic knowledge was assessed using the Boston Naming Test (Howard & Patterson, 1992) Pyramids and Palm Trees Test (Kaplan, Goodglass & Weintraub, 2001) and Animal and Tool Fluency (Lezak, Howieson & Loring, 1983). Executive functioning and control were assessed using the digit-symbol substitution test (Libon et al., 2011) and the Philadelphia Brief Assessment of Cognition (PBAC) disinhibition severity measure (McLeod et al., 1982). Performance was compared to 32 controls. Table 1 lists the demographic information for all groups. Patient groups were matched for age, education, disease duration, and gender distribution (all p>0.05). Control patients were matched for education and gender distribution, but were significantly older. However age within and across groups did not correlate with any of the neuropsychological or linguistic measures, or concreteness of nouns produced (see below).
Table 1.
Demographics for all groups
| Control | svPPA | bvFTD | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Disease Duration (months) | - | - | 46.95 | 24.12 | 48.76 | 34.88 |
| Education (years) | 15.88 | 2.52 | 14.95 | 2.95 | 16.17 | 3.15 |
| Age (years) | 68.4 | 8.21 | 63.4 | 7.70 | 62.3 | 8.35 |
| Control | svPPA | bvFTD | ||||
| Female | Male | Female | Male | Female | Male | |
| Sex (count) | 21 | 11 | 9 | 11 | 18 | 24 |
2.2 Cookie Theft Procedure and Analysis
Subjects were asked to verbally describe the Cookie Theft picture (Goodglass & Kaplan, 1983) and descriptions were digitally recorded, transcribed and coded. The total number of words produced and the number of nouns produced was tallied for each subject. Words were identified as nouns based on dominant form (Brysbaert, New & Keuleers, 2012) and sentential context, and the number of nouns per 100 words was calculated. Repeated words and dysfluencies were not included. Nouns were rated for concreteness/abstractness on a scale from 1–5 using published norms, with 1 being the least concrete/most abstract and 5 being the most concrete/least abstract (Brysbaert, Warriner & Kuperman, 2014). Nouns were also rated on frequency of occurrence (Brysbaert, New & Keuleers, 2012) and semantic diversity (Hoffman, Ralph & Rogers, 2013). Ratings were not available for a small number of nouns produced (e.g. Mary Janes, countertop, the fifties, flats), and colloquialisms were also not rated (e.g. boo-boos). Thus 13 nouns were not rated: 9 nouns for controls, 4 nouns for bvFTD patients, and 0 nouns for svPPA. The number of unrated nouns was not significantly different between groups (chi-square: p>0.05). To compare the performance of svPPA and bvFTD patients, z-scores were calculated for all measures relative to control subject performance. We further calculated how traditional measures of semantic knowledge related to the concreteness of nouns produced for both patient groups using the Pyramids and Palm Trees Test (Howard & Patterson, 1992), Boston Naming Test (Kaplan, Goodglass & Weintraub, 2001), and Animal and Tool Fluency (Lezak, Howieson & Loring, 1983). A composite semantic score was obtained by calculating z-scores and averaging performance across all measures.
2.3 Imaging Methods
Twenty-nine of the bvFTD patients and 14 of the svPPA patients agreed to undergo magnetic resonance imaging (MRI). Subjects were right-handed, except for 2 left-handed bvFTD patients, and 1 left-handed and 2 ambidextrous svPPA patients. These patient subgroups were not significantly different from the full patient groups on either Cookie Theft performance or on neuropsychological evaluations (all p-values > 0.1). T1-weighted three-dimensional spoiled gradient-echo images were collected on a Siemens 3.0T Trio scanner using an 8-channel head coil with repetition time=1620ms, echo time=3ms, flip angle=15°, matrix=192 × 256, slice thickness=1 mm, and in-plane resolution=0.9 × 0.9 mm. Images were acquired within an average of 96 days of the task (SD=107). T1 images were also collected on an independent group of 18 healthy seniors, who were age- and education-matched to both patient groups.
Images were normalized to a standard space and segmented using the PipeDream interface (http://sourceforge.net/projects/neuropipedream/) and the Advanced Normalization Tools kit (http://www.picsl.upenn.edu/ANTS/). See Tustison et al. (2014) for additional details. Grey matter probability (GMP) images were calculated, transformed into Montreal Neurological Institute (MNI) space, down-sampled to 2mm3 resolution, and smoothed using a 2-mm full-width half-maximum (FWHM) Gaussian kernel. The preprocessed images were unadjusted and were compared and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). To outline regions of atrophy in bvFTD and svPPA, we compared patient GMP to controls using a two-sample t-test with a threshold of p<0.05 (controlled for multiple comparisons with family-wise error), and a minimum cluster size of 50 voxels. To examine how atrophy affected the ability to produce concrete nouns in svPPA and abstract nouns in bvFTD, we related grey matter atrophy for each patient group to the average concreteness rating of nouns produced. To do so, we performed voxelwise multiple regressions constrained to regions of grey matter atrophy for both patient groups, using a height threshold of p<0.02 (uncorrected) and a minimum cluster size of 50 voxels.
3. Results
Neuropsychological evaluations demonstrated different patterns of impairment for bvFTD and svPPA patients (Table 2). While bvFTD and svPPA patients were similarly impaired on global cognition (MMSE: p>0.1), svPPA patients were significantly more impaired on measures of semantic knowledge than bvFTD patients, as assessed by the Boston Naming Test (t(33)=7.61, p<0.001), Pyramids and Palm Trees Test (t(25)=2.08, p<0.05), Animal (t(36)=3.14, p<0.001) and Tool (t(34)=3.39, p<0.01) Fluency, as well as the composite semantic score (t(39)=5.01, p<0.001). Conversely, bvFTD patients were significantly more impaired on measures of executive functioning, as assessed by the digit-symbol substitution test (t(16)=2.27, p<0.05) and PBAC disinhibition severity (t(46)=2.20, p<0.05).
Table 2.
Neuropsychological and linguistic measures
| svPPA | bvFTD | t-test | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | |
| Mini Mental State Exam (out of 30) | 21.7 | 6.36 | 24.3 | 4.25 | n.s. | |
| Boston Naming Test (30) | 7.1 | 6.32 | 23.4 | 5.70 | 7.61 | <0.001 |
| Pyramids & Palm Trees (104) | 75.8 | 16.06 | 89.1 | 16.12 | 2.08 | <0.05 |
| Animals (timed) | 4.4 | 4.44 | 10.5 | 5.97 | 3.14 | <0.01 |
| Tools (timed) | 3.9 | 1.99 | 5.9 | 1.83 | 3.39 | <0.01 |
| Composite Semantic Score (z-score) | −3.54 | 1.48 | −1.29 | 1.27 | 5.01 | <0.001 |
| Digit Symbol (timed) | 46.4 | 10.01 | 35.7 | 7.98 | 2.41 | <0.05 |
| Disinhibition (3) | 2.7 | 1.05 | 1.9 | 1.19 | 2.20 | <0.05 |
3.1 Cookie Theft Behavioral Results
The total number of words and nouns produced during the Cookie Theft picture description task was tallied for each group (Table 3). Both svPPA and bvFTD patients produced significantly shorter descriptions than controls (t(50)=2.89, p<0.01; t(72)=4.26, p<0.001). To compare patient groups, we converted patient performance to z-scores relative to control performance. Between-group comparisons revealed that svPPA and bvFTD patients were not significantly different from each other, neither in the length of their descriptions nor in the number of nouns per 100 words produced (Table 3).
Table 3.
Cookie Theft Performance
| Control | svPPA | bvFTD | t-test between svPPA & bvFTD |
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | t | p | |
| Words Produced | ||||||||
| Average | 172 | 57.46 | 124 | 61.82 | 109 | 67.49 | - | > 0.1 |
| z-score | - | - | −0.87 | 1.11 | −1.11 | 1.19 | - | > 0.1 |
| Nouns per 100 Words | ||||||||
| Average | 19.3 | 4.02 | 15.7 | 7.29 | 18.4 | 7.43 | - | > 0.1 |
| z-score | - | - | −0.90 | 1.814 | −0.23 | 1.88 | - | > 0.1 |
| Concreteness | ||||||||
| Average | 4.59 | 0.19 | 4.53 | 0.21 | 4.70 | 0.14 | 3.99 | <0.001 |
| z-score | - | - | −0.33 | 1.12 | 0.62 | 0.75 | 3.95 | <0.001 |
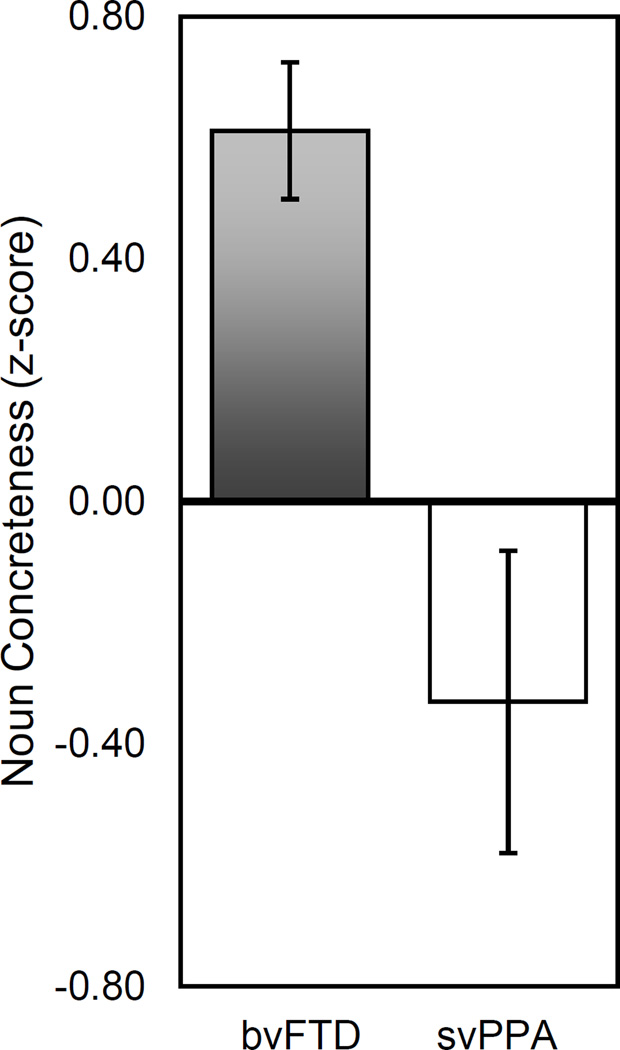
We next scored the concreteness of all nouns produced using ratings published by Brysbaert, Warriner, & Kuperman, (2014). Subjects produced nouns across the concreteness spectrum (Control: min=1.66, max=5; svPPA: min=1.61, max=5; bvFTD min=1.61, max=5), though all groups were most likely to produce very concrete nouns when describing the scene (mode=5). When comparing patients (Table 4; Figure 1), we found that svPPA patients tended to produce nouns that were more abstract and bvFTD patients tended to produce nouns that were more concrete, and that these were significantly different from each other (t(60)=3.96, p<0.001). Furthermore, concreteness of nouns was positively correlated with semantic knowledge scores for svPPA patients, and was negatively correlated with semantic knowledge scores for bvFTD patients (Table 4). In other words, svPPA patients who were more impaired on traditional measures of semantic knowledge produced less concrete speech, while bvFTD patients who were more impaired on independent measures of semantic knowledge produced less abstract speech. In addition, nouns produced by bvFTD patients had significantly lower semantic diversity and frequency ratings than those produced by svPPA patients (Semantic Diversity: t(60)=2.26, p<0.05; Frequency: t(60)=3.78, p<0.001).
Table 4.
Correlations of Concreteness with Semantic Knowledge.
| Boston Naming Test |
Pyramids & Palm Trees |
Animals | Tools | Composite Semantic Score |
||
|---|---|---|---|---|---|---|
|
Concreteness in svPPA |
rho | 0.689 | 0.427 | 0.452 | 0.628 | 0.631 |
| p | 0.019 | 0.219 | 0.140 | 0.029 | 0.021 | |
| N | 11 | 10 | 12 | 12 | 13 | |
|
Concreteness in bvFTD |
rho | −0.507 | −0.790 | −0.451 | −0.422 | −0.523 |
| p | 0.011 | 0.000 | 0.021 | 0.040 | 0.004 | |
| N | 24 | 17 | 26 | 24 | 28 |
Figure 1. Average Noun Concreteness ratings for bvFTD and svPPA patients (z-scores).
The average noun concreteness rating for bvFTD and svPPA patients is plotted, z-score converted. bvFTD patients produce nouns that are more concrete than elderly control patients (grey bar), while svPPA patients produce nouns that are more abstract (white bar).
3.2 Imaging Results
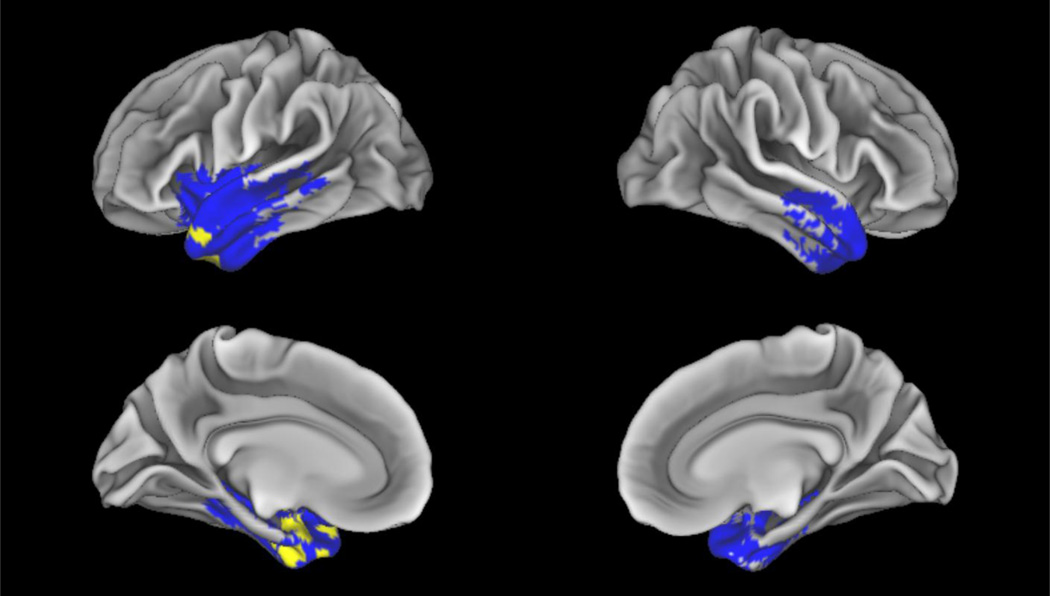
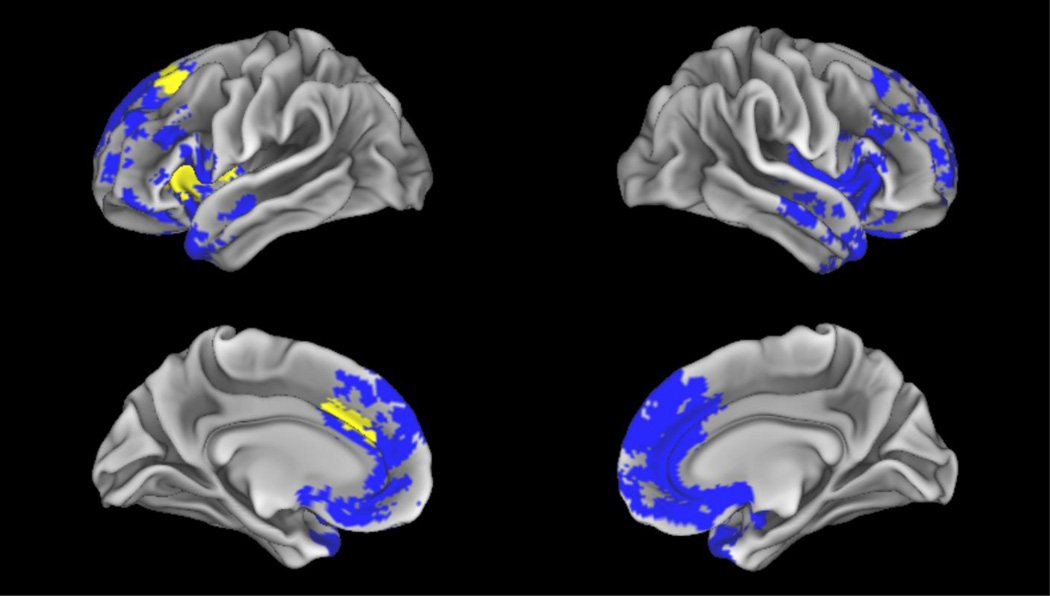
We calculated GMP and compared patients to age- and education-matched controls to determine regions of atrophy for svPPA and bvFTD groups (Figure 2 and Figure 3). Analyses revealed that svPPA patients have significant grey matter atrophy in the left anterior inferior temporal gyrus, left fusiform gyrus, and right inferior temporal gyrus (Table 5). By comparison, bvFTD patients had broad bilateral atrophy in frontal lobe regions, which also extended into temporal lobes. Areas of significant grey matter atrophy in bvFTD include the middle and inferior frontal gyri, the orbitofrontal cortex, and the superior temporal gyri (Table 6). These findings are consistent with previously published structural imaging studies of bvFTD and svPPA (Hodges & Patterson, 2007; Pereira et al., 2009; Rascovsky et al., 2011) and thus help confirm our diagnostic accuracy of these focal cortical syndromes.
Figure 2.
Regions of significantly decreased grey matter in svPPA compared to controls are in blue (FWE, p<0.05, k=50). Regression of decreased average concreteness in svPPA with decreased grey matter is in yellow (p<0.02, k=50).
Figure 3.
Regions of significantly decreased grey matter in bvFTD compared to controls are in blue (FWE, p<0.05, k=50). Regression of decreased average abstractness in bvFTD with decreased grey matter is in yellow (p<0.02, k=50).
Table 5.
Regressions of task performance with grey matter atrophy in svPPA
| MNI Coordinates |
||||||
|---|---|---|---|---|---|---|
| svPPA | BA | x | y | z | z score | Cluster size (voxels) |
| Grey Matter Atrophy, svPPA<Control | ||||||
| Left Anterior Temporal Cortex | 38 | −32 | 12 | −30 | Inf | 51870 |
| Left Fusiform Gyrus | 37 | −48 | −54 | −20 | 7.01 | 51 |
| Right Inferior Temporal Gyrus | 20 | 42 | −12 | −36 | Inf | 19250 |
| Regression of Decreased Concreteness with Grey Matter Atrophy | ||||||
| Left Parahippocampal Gyrus | −26 | 2 | −24 | 3.89 | 58 | |
| Left Anterior Inferior Temporal Cortex | 38 | −46 | 20 | −22 | 3.24 | 51 |
Table 6.
Regressions of task performance with grey matter atrophy in bvFTD
| MNI Coordinates |
||||||
|---|---|---|---|---|---|---|
| bvFTD | BA | x | y | z | z score | Cluster size (voxels) |
| Grey Matter Atrophy, bvFTD<Control | ||||||
| Left Middle Frontal Gyrus | 46 | −40 | 26 | 24 | 6.82 | 284 |
| Left Inferior Frontal Gyrus | 47 | −38 | 26 | −4 | 7.21 | 856 |
| Left Inferior Frontal Gyrus | 47 | −26 | 30 | −18 | 7.02 | 249 |
| Left Orbitofrontal Cortex | 11 | −10 | 30 | −22 | 7.25 | 177 |
| Left Putamen | 49 | −20 | 14 | −4 | 7.03 | 310 |
| Left Anterior Superior Temporal Gyrus | 38 | −34 | 14 | −40 | 6.67 | 224 |
| Left Superior Temporal Gyrus | 21 | −52 | −4 | −18 | 5.97 | 55 |
| Right Superior Frontal Gyrus | 9 | 24 | 56 | 32 | 6.15 | 79 |
| Right Middle Frontal Gyrus | 8 | 26 | 20 | 44 | 6.42 | 76 |
| Right Anterior Inferior Frontal Gyrus | 46 | 46 | 26 | 20 | 6.53 | 248 |
| Right Inferior Frontal Gyrus | 47 | 28 | 34 | −16 | 7.36 | 156 |
| Right Orbitofrontal Cortex | 11 | 8 | 30 | −26 | 7.20 | 178 |
| Right Anterior Cingulate | 32 | 10 | 40 | 16 | Inf | 3551 |
| Right Insula | 13 | 40 | 20 | −4 | 7.13 | 1019 |
| Right Anterior Superior Temporal Gyrus | 38 | 34 | 14 | −40 | 6.45 | 260 |
| Right Anterior Middle Temporal Gyrus | 38 | 56 | 8 | −24 | 6.44 | 236 |
| Regression of Decreased Abstractness with Grey Matter Atrophy | ||||||
| Left Inferior Frontal Gyrus | 47 | −34 | 28 | 0 | 4.68 | 391 |
| Left Superior Frontal Gyrus | 8/6 | −14 | 28 | 58 | 3.47 | 59 |
| Left Cingulate Gyrus | 32 | −10 | 24 | 32 | 3.27 | 85 |
| Left Caudate | 48 | −18 | 16 | 8 | 3.25 | 129 |
| Right Caudate | 48 | 16 | 10 | 12 | 3.35 | 204 |
Finally, we sought to identify the neuroanatomy involved in the production of concrete and abstract nouns in svPPA and bvFTD, and to assess if these processing regions were shared or were partially distinct. To determine how atrophy in svPPA and bvFTD patients affected the production of concrete and abstract nouns, a regression analysis related GMP to noun concreteness (Figures 2 and 3). Decreased concreteness in svPPA was related to grey matter atrophy of the left parahippocampal gyrus and anterior portions of the left temporal lobe (Table 5). No clusters were significant in regions of atrophy associated with bvFTD, which confirmed that decreased concreteness in svPPA was not associated with the frontal lobe. In bvFTD, the left inferior frontal gyrus, left superior frontal gyrus, left anterior cingulate, and bilateral caudate were implicated in decreased abstract noun production (Table 6). No clusters were significant in regions of atrophy associated with svPPA, which confirmed that decreased abstractness in bvFTD was not associated with atrophy in the temporal lobe.
4. Discussion
Here we examined concrete and abstract noun production in svPPA and bvFTD patients during the Cookie Theft description task. While svPPA and bvFTD patients produced a statistically equivalent number of words and nouns, patients differed in the concreteness of their speech, with svPPA patients producing nouns that were less concrete and bvFTD patients producing nouns that were less abstract. This dissociation was related to the degree of impairment: svPPA patients with decreased semantic knowledge produced less concrete nouns, while bvFTD patients with decreased semantic knowledge produced less abstract nouns. Regression analyses revealed that atrophy of the left anterior inferior temporal gyrus and left parahippocampal gyrus in svPPA was related to decreased concreteness, while atrophy of the left inferior frontal gyrus and bilateral caudate in bvFTD was related to decreased abstractness.
The anatomical regions implicated here overlap with results from our previous study, which examined comprehension of concrete and abstract nouns in svPPA and bvFTD (Cousins et al., 2016): GMP in the left inferior temporal gyrus and the left inferior frontal gyrus was related to the comprehension of concrete and abstract nouns, respectively. It must be noted that we do not conclude that concrete and abstract representations are entirely dissociated, nor that the regions revealed by our analyses are comprehensive of semantic processing. However, results do indicate that aspects of concrete and abstract noun processing are partially dissociable, and that the concreteness/abstractness of the stimuli can somewhat determine the extent to which these supporting regions are recruited. Below we discuss the significance of our results, the roles of the left ventral and inferior temporal cortices and left frontal areas in concrete and abstract noun processing, and our interpretations as they relate to speech production.
4.1 Speech production and concreteness
To examine the regions involved in the production of concrete and abstract nouns, we used the Cookie Theft picture as a semi-constraining stimulus probe. Speech samples produced by a less constraining probe, such as an Autobiographical Memory Interview (Kopelman, Wilson & Baddeley, 1990), can vary in complexity across individuals and the intended target is not equivalent across individuals or groups. By using the Cookie Theft picture, the intended target of the semistructured speech sample was kept consistent, and speech samples could be compared across patient groups. A limitation of a visual stimulus probe is that it elicits highly concrete speech. In this study, a majority of all nouns produced by all groups were very concrete, though speech samples from all groups also included moderately abstract nouns, and some very abstract nouns. It should also be noted that we did not eliminate misidentified nouns or substitution errors from our analysis. During description of the Cookie Theft picture, some svPPA patients produced a small number of concrete nouns that were not present in the scene. Such substitution errors are consistent with their disease, and these speech irregularities in svPPA have been previously observed (Meteyard & Patterson, 2009). This choice was conservative, as the most detectable errors are for highly concrete nouns. Thus, elimination of substitution errors from analyses would have made the average concreteness of speech in svPPA lower than it already was. Despite this conservative choice, we were still able to detect differences between our patient groups. In addition, we focused on the production of nouns, a choice which allows us to consider these results in relation to previous findings of concrete and abstract noun comprehension (Cousins et al., 2016; Sabsevitz et al., 2005). While this limits the scope of this study, feature characteristics vary considerably between different parts of speech, and this can make direct comparisons difficult. In particular, verbs tend to be more frequent and more semantically diverse than nouns, and these features covary with concreteness (Brysbaert, New & Keuleers, 2012). Most importantly, the perceived concreteness of a verb can change depending on the concreteness of the subject noun it is modifying, while the perceived concreteness of a noun is more stable (Scorolli et al., 2011). In this study we focus on the differences in concreteness between nouns because the concreteness differences of verbs is harder to interpret - though previous studies have demonstrated that svPPA patients also tend to have more difficulty with concrete than abstract verbs (Bonner et al., 2009; Yi et al., 2007). Understanding how concreteness interacts with parts of speech is an important avenue for future study.
4.2 The left ventral and inferior temporal cortices and concreteness
Our findings that svPPA patients produce less concrete and more frequent nouns are similar to those reported in a previous behavioral study by Bird and colleagues (2000), who also examined speech production during the Cookie Theft picture description in 3 semantic dementia patients. The authors observed that the svPPA patients produce less imageable but more frequent words than controls. They demonstrated that the lowest frequency nouns produced were also highly imageable and concluded that the tendency to produce low imageability words in svPPA patients is the consequence of poor knowledge for low frequency items. Another behavioral study examined speech samples from Autobiographical Memory Interviews in 7 svPPA patients (Hoffman, Meteyard & Patterson, 2014). Again, it was observed that svPPA patients tended to produce low imageability, high frequency words. However, this group suggested that impairments in svPPA cause a shift towards less specific, more generic vocabulary. Therefore they argued that semantic diversity, not word frequency, is the feature which best characterizes the speech patterns in svPPA.
We agree that disease in the temporal lobes in svPPA can result in a vocabulary composed of more semantically diverse and frequent words. However, our results do not indicate that these features fully explain the deficit we see for concrete nouns in svPPA; we show that diminished concrete noun production is related to reduced grey matter in the anterior inferior temporal and parahippocampal gyri. We therefore believe our findings suggest that degraded visual feature knowledge in svPPA plays an important role in the pattern to produce speech that is less concrete. Moreover, in the context of our assessment of bvFTD (see section 4.3), we believe that the increased abstractness and semantic diversity of svPPA vocabulary are in part related to relatively preserved frontal lobe regions that support semantic selection. One limitation of this study is that our imaging analyses included a smaller number of svPPA patients compared to bvFTD patients: Although our cohorts are relatively large for these uncommon conditions, we were able to assess only 14 svPPA patients, compared to 29 bvFTD patients. The discrepancy in sample size reflects the fact that svPPA is a rare neurodegenerative disorder (Hodges & Patterson, 2007). Because of this, our regression analyses used a slightly more lenient threshold (p<0.02) so that we could accurately detect the significant association between grey matter atrophy in svPPA and decreased production of concrete nouns. Our findings here, which indicate a link between concrete noun processing and the ventral temporal lobe, are corroborated by previous studies in svPPA and control subjects. Converging evidence from fMRI, lesion and PET studies indicate that the inferior and ventral temporal lobes are important to visual feature and object processing (Carlson et al., 2014; Chao, Haxby, & Martin, 1999; Miyashita, 1993). Ventral temporal regions are part of the visual processing stream, where visual input is progressively transformed and increasingly conceptualized as it travels anteriorly towards the temporal pole (Bonner & Price, 2013; Visser et al., 2012). Specifically, the left parahippocampal gyrus has been shown to be involved during semantic processing (Binney et al., 2010), and has been demonstrated to have a clear role during processing of visual feature knowledge associated with concrete nouns (Bonner et al., 2015; Sabsevitz et al., 2005; Wang et al., 2010).
4.3 The frontal lobes and abstractness
We are not aware of other studies which have examined abstract noun production in bvFTD patients. Compared to the temporal lobe predominant svPPA, bvFTD patients demonstrate relatively preserved comprehension and production on classic language measures. However, some studies have observed mild deficits on language measures and sentence comprehension in bvFTD patients which may relate to their executive functioning deficits (Ash et al., 2006; Cooke et al., 2003; Gunawardena et al., 2010). In this study, we found that bvFTD patients show reduced abstract noun production. This is a novel finding, since bvFTD patients are typically characterized as having preserved semantic memory. Indeed, in this study bvFTD patients performed significantly better on all measures of semantic knowledge than svPPA patients. However, these traditional assessments of semantic memory - such as the Boston Naming Test - often focus on confrontation naming or comprehension with picture stimuli that elicit highly imageable words. Because of the concrete nature of the stimuli used, these measures may not be sensitive to the deficit for abstract noun processing that we observed. Thus, our methods using a semi-structured speech sample provide higher sensitivity to detect these changes.
Our results show that bvFTD patients had extensive frontal lobe atrophy and that decreased abstract noun production in bvFTD is related to the left inferior frontal gyrus, dorsolateral prefrontal cortex, anterior cingulate, and bilateral caudate. While canonical semantic regions are located in the temporal and parietal lobes, frontal regions have been consistently implicated during semantic tasks which require selection or control. The left inferior frontal gyrus is part of so-called Broca’s area and was originally suggested to be the center of language production (Broca, 1865; Geschwind, 1970). However, it a heterogeneous region which has been shown to support a variety of language processes, including phonemic, syntactic, and semantic processing (Brodmann, 1994; Shalom & Poeppel, 2007). Evidence suggests that the left inferior frontal gyrus plays a role in controlled retrieval of semantic information (Fiez, 1997), selection among competing semantic alternatives (Moss et al., 2005; Thompson-Schill et al., 1997) and contextual information processing (Hoffman, Binney & Ralph, 2015). We hypothesize that these selection and integration processes are particularly important for abstract noun processing; the polysemous and contextually diverse characteristics of abstract nouns may require executive functions to select the correct word or meaning from a set of competing alternatives. While it could be argued that the left inferior frontal gyrus was implicated because of its role in speech production, we found that only abstract noun production was related to the left inferior frontal gyrus, and not concrete noun production. Our previous study also found that the inferior frontal gyrus was related to abstract noun comprehension (Cousins et al., 2016). In addition, other regions important for executive functioning and control were implicated in our analyses, and we found that decreased GMP in the left anterior cingulate and left dorsolateral prefrontal cortex was related to decreased abstract noun production in bvFTD (Barch et al., 1997; Botvinick et al., 2001; Braver et al., 2001). Therefore, these results suggest that frontal regions support the production of abstract nouns, and that this may be due to the semantic and contextual ambiguity of abstract nouns, which increase demands on executive control and semantic selection.
In addition to left frontal cortical regions, decreased GMP of the bilateral caudate was also related to decreased abstract noun production. While the role of the caudate during semantic processing is still being studied, there is evidence that the basal ganglia are important to language selection processes. Studies of language and semantic processing in polyglots have demonstrated involvement of the basal ganglia during tasks which require language switching (Crinion et al., 2006; Klein et al., 1995; Price et al., 1999). It has been hypothesized that the basal ganglia are necessary for recruitment of cortical regions involved in linguistic selection and control (Friederici, 2006). It may be that atrophy in the caudate limits recruitment of frontal regions involved in semantic selection processes during abstract noun processing.
4.4 Conclusions
In this study, the production of abstract and concrete nouns in svPPA and bvFTD patients was assessed using semistructured speech samples elicited from an oral description of the Cookie Theft picture. Behavioral findings demonstrated that svPPA patients’ speech was less concrete and that bvFTD patients’ speech was less abstract. Regression analyses in these patient groups identified neural regions that are critical for effective abstract and concrete noun production. We found that decreased concrete noun production in svPPA is related to atrophy in the visual association cortex, including the left anterior and ventral temporal lobes. This relationship suggests that visual feature representations are important to supporting concrete noun production, as proposed by the grounded cognition perspective. Yet, in line with the context availability hypothesis, we found that decreased abstract noun production in bvFTD is related to atrophy in the left inferior frontal gyrus and other frontal regions, suggesting that the production of abstract nouns depends in part on semantic selection and control. Taken together, our findings indicate that partially dissociable neuroanatomical regions underlie the production of concrete and abstract nouns, and they support a model of lexical representation which integrates premises from both grounded cognition and context availability perspectives.
Highlights.
Investigation of anatomical regions underlying concrete and abstract noun production
Examined semistructured speech samples in svPPA and bvFTD compared to controls
Decreased concrete noun production in svPPA related to left ventral temporal lobe
Decreased abstract noun production in bvFTD related to left inferior frontal gyrus
Behavioral and anatomic double-dissociation between abstract and concrete nouns
Acknowledgments
This work was supported in part by NIH (AG017586, AG038490, AG053488) and the Wyncote Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- Acosta-Cabronero J, Patterson K, Fryer TD, Hodges JR, Pengas G, Williams GB, Nestor PJ. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2011;134(7):2025–2035. doi: 10.1093/brain/awr119. [DOI] [PubMed] [Google Scholar]
- Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, Gorno-Tempini ML. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cognitive and Behavioral Neurology. 2007;20(4):203–211. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66(9):1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35(10):1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, Ralph MAL. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cerebral Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Bird H, Ralph MAL, Patterson K, Hodges JR. The rise and fall of frequency and imageability: Noun and verb production in semantic dementia. Brain and language. 2000;73(1):17–49. doi: 10.1006/brln.2000.2293. [DOI] [PubMed] [Google Scholar]
- Bonner MF, Vesely L, Price C, Anderson C, Richmond L, Farag C, Grossman M. Reversal of the concreteness effect in semantic dementia. Cognitive Neuropsychology. 2009;26(6):568–579. doi: 10.1080/02643290903512305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Price AR. Where is the anterior temporal lobe and what does it do? The Journal of Neuroscience. 2013;33(10):4213–4215. doi: 10.1523/JNEUROSCI.0041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Price AR, Peelle JE, Grossman M. Semantics of the Visual Environment Encoded in Parahippocampal Cortex. Journal of cognitive neuroscience. 2015 doi: 10.1162/jocn_a_00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological review. 2001;108(3):624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of the concreteness effect in a patient with semantic dementia. Cognitive Neuropsychology. 1994;11(6):617–660. [Google Scholar]
- Broca P. On the seat of the faculty of articulate language. Bull Soc Anthropol Paris. 1865;6:337–393. [Google Scholar]
- Brodmann K. Localisation in the cerebral cortex. London: Smith-Gordon (original work published 1909); 1994. [Google Scholar]
- Brysbaert M, New B, Keuleers E. Adding part-of-speech information to the SUBTLEX-US word frequencies. Behavior Research Methods. 2012;44(4):991–997. doi: 10.3758/s13428-012-0190-4. [DOI] [PubMed] [Google Scholar]
- Brysbaert M, Warriner AB, Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Behavior research methods. 2014;46(3):904–911. doi: 10.3758/s13428-013-0403-5. [DOI] [PubMed] [Google Scholar]
- Carlson TA, Simmons RA, Kriegeskorte N, Slevc LR. The emergence of semantic meaning in the ventral temporal pathway. Journal of cognitive neuroscience. 2014;26(1):120–131. doi: 10.1162/jocn_a_00458. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature neuroscience. 1999;2(10):913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cooke A, DeVita C, Gee J, Alsop D, Detre J, Chen W, Grossman M. Neural basis for sentence comprehension deficits in frontotemporal dementia. Brain and language. 2003;85(2):211–221. doi: 10.1016/s0093-934x(02)00562-x. [DOI] [PubMed] [Google Scholar]
- Cousins KAQ, York C, Bauer L, Grossman M. Cognitive and anatomic double dissociation in the representation of concrete and abstract words in semantic variant and behavioral variant frontotemporal degeneration. Neuropsychologia. 2016;84:244–251. doi: 10.1016/j.neuropsychologia.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, Devlin JT, Usui K. Language control in the bilingual brain. Science. 2006;312(5779):1537–1540. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364(1536):3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rosa PA, Catricalà E, Vigliocco G, Cappa SF. Beyond the abstract— concrete dichotomy: Mode of acquisition, concreteness, imageability, familiarity, age of acquisition, context availability, and abstractness norms for a set of 417 Italian words. Behavior Research Methods. 2010;42(4):1042–1048. doi: 10.3758/BRM.42.4.1042. [DOI] [PubMed] [Google Scholar]
- Friederici AD. What’s in control of language? Nature neuroscience. 2006;9(8):991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970 doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2nd. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Peelle JE, Smith EE, McMillan CT, Cook P, Powers J, Camp E. Category-specific semantic memory: converging evidence from bold fMRI and Alzheimer’s disease. Neuroimage. 2013;68:263–274. doi: 10.1016/j.neuroimage.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena D, Ash S, McMillan C, Avants B, Gee J, Grossman M. Why are patients with progressive nonfluent aphasia nonfluent? Neurology. 2010;75(7):588–594. doi: 10.1212/WNL.0b013e3181ed9c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. The Lancet Neurology. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jefferies E, Ralph MAL. Ventrolateral prefrontal cortex plays an executive regulation role in comprehension of abstract words: convergent neuropsychological and repetitive TMS evidence. The Journal of Neuroscience. 2010;30(46):15450–15456. doi: 10.1523/JNEUROSCI.3783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, Ralph MAL. The degraded concept representation system in semantic dementia: damage to pan-modal hub, then visual spoke. Brain. 2012;135(12):3770–3780. doi: 10.1093/brain/aws282. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, Ralph MAL. Be concrete to be comprehended: consistent imageability effects in semantic dementia for nouns, verbs, synonyms and associates. Cortex. 2013;49(5):1206–1218. doi: 10.1016/j.cortex.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Ralph MAL, Rogers TT. Semantic diversity: a measure of semantic ambiguity based on variability in the contextual usage of words. Behavior Research Methods. 2013;45(3):718–730. doi: 10.3758/s13428-012-0278-x. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Meteyard L, Patterson K. Broadly speaking: vocabulary in semantic dementia shifts towards general, semantically diverse words. Cortex. 2014;55:30–42. doi: 10.1016/j.cortex.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Binney RJ, Ralph MAL. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex. 2015;63:250–266. doi: 10.1016/j.cortex.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson KE. The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company. 1992 [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Frontiers in psychology. 2011;2(255):1–16. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Jones RW, Lambon Ralph MA. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23(4):492. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Lakoff G. Metaphors we live by. Chicago: U of Chicago P; 1980. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. Pro-ed. 2001 [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: a bilingual functional-imaging study. Proceedings of the National Academy of Sciences. 1995;92(7):2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview (manual) England: Thames Valley Test Company, Bury St. Edmunds; 1990. [Google Scholar]
- Lakoff G, Johnson M. Philosophy in the flesh: The embodied mind and its challenge to western thought. Basic books; 1999. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychology assessment. Vol. 28. New York: 1983. [Google Scholar]
- Libon DJ, Rascovsky K, Gross RG, White MT, Xie SX, Dreyfuss M, Coslett HB. The Philadelphia Brief Assessment of Cognition (PBAC): a validated screening measure for dementia. The Clinical Neuropsychologist. 2011;25(8):1314–1330. doi: 10.1080/13854046.2011.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatti C, Raggi R, Zonca G, Pistarini C, Contardi A, Pinna GD. Verb- noun double dissociation in aphasic lexical impairments: The role of word frequency and imageability. Brain and language. 2002;81(1):432–444. doi: 10.1006/brln.2001.2536. [DOI] [PubMed] [Google Scholar]
- Macoir J. Is a plum a memory problem?: Longitudinal study of the reversal of concreteness effect in a patient with semantic dementia. Neuropsychologia. 2009;47(2):518–535. doi: 10.1016/j.neuropsychologia.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Current opinion in neurobiology. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu. Rev. Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavior Research Methods & Instrumentation. 1982;14(5):463–466. [Google Scholar]
- Mesulam M, Grossman M, Hillis A, Kertesz A, Weintraub S. The core and halo of primary progressive aphasia and semantic dementia. Annals of Neurology. 2003;54(S5):S11–S14. doi: 10.1002/ana.10569. [DOI] [PubMed] [Google Scholar]
- Meteyard L, Patterson K. The relation between content and structure in language production: An analysis of speech errors in semantic dementia. Brain and language. 2009;110(3):121–134. doi: 10.1016/j.bandl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, Nestor PJ. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, Tyler LK. Selecting among competing alternatives: selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex. 2005;15(11):1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Annual review of neuroscience. 1993;16(1):245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Paivio A. Dual coding theory: Retrospect and current status. Canadian Journal of Psychology. 1991;45(3):255. [Google Scholar]
- Peelle JE, Troiani V, Grossman M. Interaction between process and content in semantic memory: An fMRI study of noun feature knowledge. Neuropsychologia. 2009;47(4):995–1003. doi: 10.1016/j.neuropsychologia.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JMS, Williams GB, Acosta-Cabronero J, Pengas G, Spillantini MG, Xuereb JH, Nestor PJ. Atrophy patterns in histologic vs clinical groupings of frontotemporal lobar degeneration. Neurology. 2009;72(19):1653–1660. doi: 10.1212/WNL.0b013e3181a55fa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. The Lancet Neurology. 2011;10(2):162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Hickok G. Towards a new functional anatomy of language. Cognition. 2004;92(1):1–12. doi: 10.1016/j.cognition.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Emmorey K, Hickok G, Pylkkänen L. Towards a new neurobiology of language. The Journal of Neuroscience. 2012;32(41):14125–14131. doi: 10.1523/JNEUROSCI.3244-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Green DW, Von Studnitz R. A functional imaging study of translation and language switching. Brain. 1999;122(12):2221–2235. doi: 10.1093/brain/122.12.2221. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Hillis AE. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Grossman M, McCawley G. Concreteness effects in lexical processing of semantic dementia. Brain and Language. 2006;99(1):157–158. [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam MM. Anatomy of language impairments in primary progressive aphasia. The Journal of Neuroscience. 2011;31(9):3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27(1):188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Schwanenflugel PJ, Harnishfeger KK, Stowe RW. Context availability and lexical decisions for abstract and concrete words. Journal of Memory and Language. 1988;27(5):499–520. [Google Scholar]
- Schwanenflugel PJ, Shoben EJ. Differential context effects in the comprehension of abstract and concrete verbal materials. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1983;9(1):82. [Google Scholar]
- Scorolli C, Binkofski F, Buccino G, Nicoletti R, Riggio L, Borghi AM. Abstract and concrete sentences, embodiment, and languages. Front. Psychol. 2011;2(227):10–3389. doi: 10.3389/fpsyg.2011.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom DB, Poeppel D. Functional anatomic models of language: assembling the pieces. The Neuroscientist. 2007 doi: 10.1177/1073858407305726. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, Avants BB. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- van Hell JG, De Groot AM. Disentangling Context Availability and Concreteness in Lexical Decision and Word Translation. The Quarterly Journal of Experimental Psychology: Section A. 1998;51(1):41–63. [Google Scholar]
- Visser M, Jefferies E, Embleton KV, Ralph MAL. Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. Journal of Cognitive Neuroscience. 2012;24(8):1766–1778. doi: 10.1162/jocn_a_00244. [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: A meta-analysis of neuroimaging studies. Human Brain Mapping. 2010;31(10):1459–1468. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg D, Possin KL, Rascovsky K, Rankin KP, Miller BL, Kramer JH. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychology review. 2008;18(1):91–102. doi: 10.1007/s11065-008-9056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in patients with semantic dementia. Neuropsychology. 2007;21(1):9. doi: 10.1037/0894-4105.21.1.9. [DOI] [PubMed] [Google Scholar]