Abstract
The CRISP study of polycystic kidney disease (PKD) found that urinary sodium excretion associated with the rate of total kidney volume increase. Whether sodium restriction slows the progression of Autosomal Dominant PKD (ADPKD) is not known. To evaluate this we conducted a post-hoc analysis of the HALT-PKD clinical trials of renin-angiotensin blockade in patients with ADPKD. Linear mixed models examined whether dietary sodium affected rates of total kidney volume or change in estimated glomerular filtration rate (eGFR) in patients with an eGFR over 60 ml/min/1.73 m2 (Study A) or the risk for a composite endpoint of 50% reduction in eGFR, end-stage renal disease or death or the rate of eGFR decline in patients with an eGFR 25-60 ml/min/1.73 m2 (Study B) all in patients initiated on an under100 mEq sodium diet. During the trial urinary sodium excretion significantly declined by an average of 0.25 and 0.41 mEq/24 hour per month in studies A and B, respectively. In Study A, averaged and time varying urinary sodium excretions were significantly associated with kidney growth (0.43%/year and 0.09%/year, respectively, for each 18 mEq urinary sodium excretion). Averaged urinary sodium excretion was not significantly associated with faster eGFR decline (−0.07 ml/min/1.73m2/year for each 18 mEq urinary sodium excretion). In Study B, the averaged but not time-varying urinary sodium excretion significantly associated with increased risk for the composite endpoint (hazard ratio 1.08 for each 18 mEq urinary sodium excretion) and a significantly faster eGFR decline (−0.09 ml/min/1.73m2/year for each mEq 18 mEq urinary sodium excretion). Thus, sodium restriction is beneficial in the management of ADPKD.
Keywords: Autosomal dominant polycystic kidney disease, sodium, low salt diet, kidney volume, CKD progression
INTRODUCTION
Hypertension is the most common manifestation of ADPKD.1 Factors contributing to its development include activation of the intra-renal renin-angiotensin-aldosterone system (RAAS), increase in sympathetic tone and possibly a primary vascular dysfunction. It is associated with progression to ESRD and cardiovascular morbidity and mortality. Early detection, lifestyle modification and medical treatment are essential for optimal management. Angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) have become the first line therapy, based more on evidence that supports the importance of the intra-renal RAAS in the pathogenesis of hypertension in ADPKD rather than on results of randomized clinical trials.1-5 Sodium restriction may be particularly important as ADPKD patients usually have sodium-sensitive hypertension and moderation of dietary sodium has been shown to potentiate the renal and cardiovascular protective effects of RAAS blockade in other renal diseases.6,7
The importance of dietary salt restriction in ADPKD has received little attention. Nevertheless, the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) showed an association between urine sodium excretion (UNaE), a surrogate marker for dietary sodium, and the rate of increase in total kidney volume (TKV) at relatively early stages of the disease.8 Furthermore, dietary sodium has been shown to impact clinical outcomes from RAAS blockade in several randomized clinical trials for other kidney diseases. UNaE was associated with the risk for doubling the serum creatinine level or ESRD in the REIN (Ramipril Efficacy In Nephropathy9) clinical trial and with the frequency of renal and cardiovascular adverse events in RENAAL (Reduction of Endpoints in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan) and IDNT (Irbesartan Diabetic Nephropathy Trial10). On the other hand, overzealous sodium restriction in combination with ACE-I therapy may induce tubulo-interstitial damage under certain experimental conditions.11
HALT PKD was a randomized clinical trial to test whether rigorous blood pressure control slows the progression of ADPKD compared to standard blood pressure control, both with drugs blocking the renin-angiotensin system in 15 to 49 year old, healthy, hypertensive patients with good kidney function (Study A), and whether an ACEi and an ARB combination would slow the progression of the disease compared to treatment with an ACEi alone in patients with good (Study A) or moderately reduced kidney function (Study B). All participants were instructed to follow a sodium restricted diet (≤ 2.4 g/d). The goals of the present post-hoc analysis were to examine the compliance of the HALT PKD participants with the diet instructions, the effect of dietary salt on the rates of change in TKV and estimated glomerular filtration rate (eGFR), and its impact on the effects of the trial interventions on the main trial endpoints.
RESULTS
The baseline characteristics of the Study A and Study B participants are summarized in Table 1.
Table 1.
Baseline clinical and laboratory data of Study A and Study B participants
| Study A (N=558) |
Study B (N=486) |
|||
|---|---|---|---|---|
| Percent or Mean |
n | Percent or Mean |
n | |
| Male | 50.7 | 558 | 48.4 | 486 |
| Age at baseline | 36.6 ± 8.3 | 558 | 48.7 ± 8.3 | 486 |
| Height (cm) | 173.8 ± 10.2 | 547 | 173.2 ± 10.4 | 476 |
| BSA (m2) | 2.0 ± 0.2 | 546 | 2.0 ± 0.3 | 476 |
| BMI (kg/m2) | 27.2 ± 5.2 | 546 | 28.0 ± 5.2 | 476 |
| Office average systolic BP (mmHg) |
126.7 ± 13.9 | 554 | 129.1 ± 14.6 | 484 |
| Office average diastolic BP (mmHg) |
80.1 ± 11.1 | 554 | 79.4 ± 10.2 | 484 |
| Height-adjusted TKV | 692 ± 402 | 540 | -- | -- |
| Renal blood flow (mL/min/1.73 m2) |
609 ± 206 | 372 | -- | -- |
| Height-adjusted TLV | 1123 ± 460 | 539 | -- | -- |
| Liver cyst volume | 286 ± 805 | 408 | -- | -- |
| CKD EPI eGFR (mL/min/1.73 m2) |
91.5 ± 17.5 | 557 | 48.2 ± 11.8 | 486 |
| Serum sodium (mEq/L) | 139.2 ± 2.1 | 558 | 139.5 ± 2.4 | 485 |
| Serum potassium (mEq/L) | 4.1 ± 0.4 | 558 | 4.3 ± 0.5 | 486 |
| Urine volume (ml/24 hrs) | 2565 ± 1175 | 553 | 2685 ± 1072 | 475 |
| Urine sodium (mEq/24 hrs) | 178.1 ± 79.9 | 542 | 177.8 ± 81.0 | 462 |
| Urine potassium (mEq/24 hrs) | 58.3 ± 26.9 | 536 | 62.6 ± 26.5 | 462 |
| Urine creatinine (mg/24 hrs) | 1501 ± 671 | 542 | 1448 ± 618 | 462 |
| Urine aldosterone (μg/24 hrs) | 12.2 ± 9.5 | 534 | 9.7 ± 7.3 | 450 |
| Urine albumin (mg/24 hrs) | 41.5 ± 137.3 | 542 | 89.8 ± 170.2 | 462 |
Compliance with dietary instructions during HALT PKD
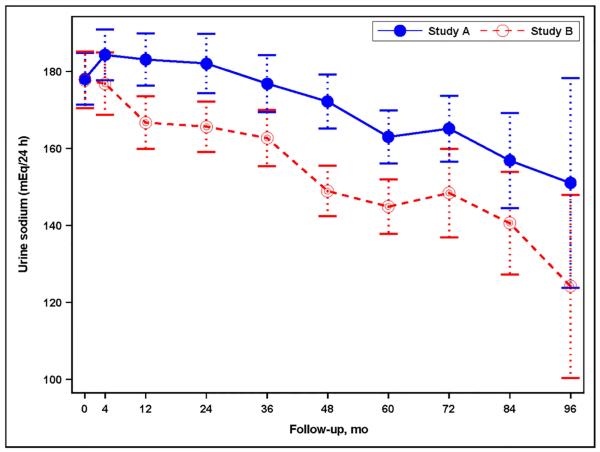
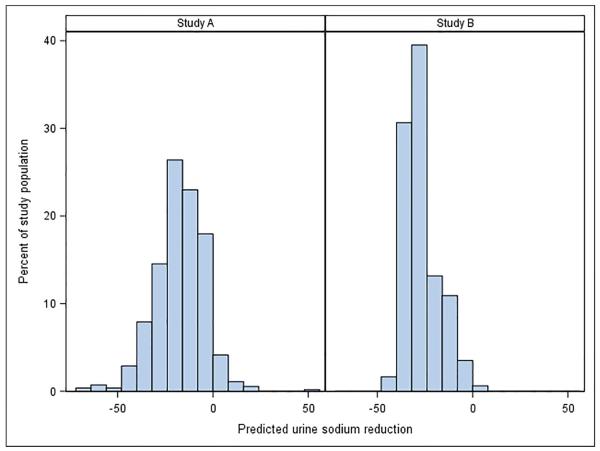
At baseline UNaE was 178.1 ± 79.9 mEq/24 hr in Study A and 177.8 ± 81.0 mEq/24 hr in Study B. During the studies UNaE declined by 0.25 ± 0.04 mEq/24 hr per month of follow-up (P<0.001) in Study A and by 0.41 ± 0.04 mEq/24 hr per month of follow-up (P<0.001) in Study B (Figure 1A). At the last follow-up, varying from 60 to 96 months, UNaE was 166.5 ± 77.5 mEq/24 hr in Study A and 152.1 ± 66.0 mEq/24 hr in Study B, and was >100 mEq/24 hr in over 80% of study participants. Therefore, reductions in UNaE were modest overall (on average, 6.5 and 14.5% reductions from baseline in Study A and Study B, respectively), but highly variable from patient to patient in Study A only (estimate of random slope SD = 0.37, p<0.001, Study A; 0.00007, P≥0.999, Study B) (Figure 1B). Changes in UNaE over time were similar regardless of assignment to BP group in Study A or to telmisartan or placebo in both studies (not shown).
Figure 1.
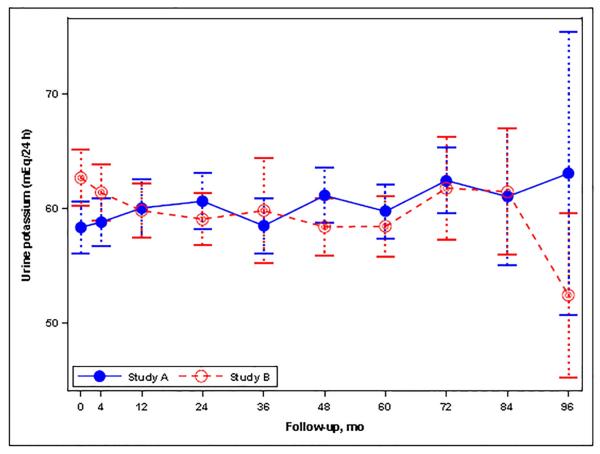
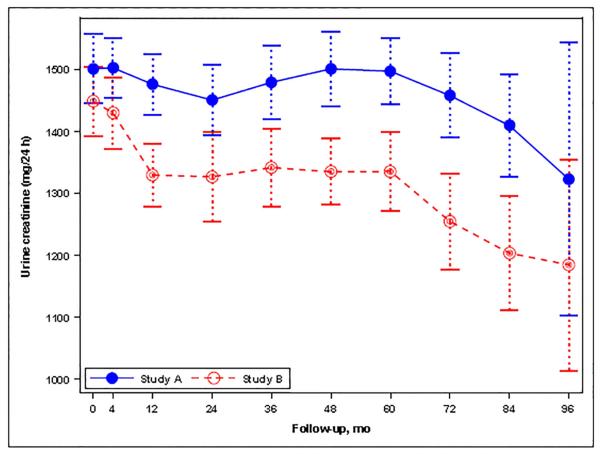
(A) Graph showing the mean urine sodium excretion in the Study A and Study B participants during the trial; I bars indicate 95% confidence intervals. (B) Histogram for the change in urine sodium excretion from the baseline to the last study visit (mEq/24 hours) in Study A and Study B patients as percent of total patients in each study. Changes were estimated from a linear mixed model with predictors for year, year-by-study drug, and year-by-blood pressure arm. (C) Graph showing the mean urine potassium excretion in the Study A and Study B participants during the trial; I bars indicate 95% confidence intervals. (D) Graph showing the mean urine creatinine excretion in the Study A and Study B participants during the trial; I bars indicate 95% confidence intervals.
Association of study averaged and time-varying UNaE with disease progression in Study A
A linear mixed model showed a significant association of averaged UNaE on the annual rate of TKV growth in Study A (0.43%/yr for each 18 mEq UNaE; P<0.001, Table 2A). A similar model showed an insignificant trend for an association between UNaE and a faster decline in eGFR (−0.067 ml/min/yr for each 18 mEq UNaE, P=0.09, Table 2B). When time-varying UNaE rather than averaged UNaE was used as a covariate, there was an association between within-person change in UNaE and the annual rate of TKV growth (0.086%/yr for each 18 mEq UNaE, P=0.005, Table 2C), but the association of UNaE with the rate of change in eGFR was insignificant (−0.004 ml/min/1.73 m2 per year for each 18 mEq increase, P=0.79) (Table 2D). Neither averaged nor time-varying UNaE differentially impacted the effect of low versus standard blood pressure control, nor the effect of ACEi/ARB combination versus ACEi monotherapy (not shown).
Table 2.
Effect of change in averaged or of time-varying urine sodium excretion (UNaE) on ADPKD progression in Study A&
| (A) Of averaged UNaE on change in annual TKV slope (%/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | 3.182 | 2.029 | 4.353 |
| Year*Low Blood Pressure | 0.001 | −1.027 | −1.642 | −0.407 |
| Averaged UNaE (per 18mEq/24hr)‡ | 0.899 | 0.126 | −1.695 | 1.972 |
| Year*Averaged UNaE (per 18mEq/24hr)‡ | <0.001 | 0.433 | 0.238 | 0.455 |
| (B) Of averaged UNaE on change in annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | −2.351 | −3.149 | −1.552 |
| Year*Low Blood Pressure | 0.428 | 0.178 | −0.261 | 0.617 |
| Averaged UNaE (per 18mEq/24hr)‡ | 0.033 | 0.586 | 0.046 | 1.125 |
| Year*Averaged UNaE (per 18mEq/24hr)‡ | 0.094 | −0.067 | −0.143 | 0.011 |
| (C) Of time-varying UNaE on change in annual TKV slope (%/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | 5.663 | 4.911 | 6.426 |
| Year*Low Blood Pressure | 0.006 | −0.908 | −1.560 | −0.252 |
| UNaE (per 18mEq/24hr) | 0.064 | −0.180 | −0.359 | 0.010 |
| Year*UNaE (per 18mEq/24hr) | 0.005 | 0.086 | 0.027 | 0.146 |
| (D) Of time-varying UNaE on change in annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | −2.750 | −3.206 | −2.294 |
| Year*Low Blood Pressure | 0.929 | −0.021 | −0.485 | 0.443 |
| UNaE (per 18mEq/24hr) | 0.009 | 0.148 | 0.038 | 0.259 |
| Year*UNaE (per 18mEq/24hr) | 0.789 | −0.004 | −0.039 | 0.030 |
All models adjusted for the following covariates: age, race, gender, and body surface area (BSA)
Averaged UNaE from 4 month visit (F5) to the end of the study (F96)
Association of study averaged UNaE with disease progression in Study B
A Cox proportional hazards model showed a significant association of the averaged UNaE with an increased risk to reach the composite end-point of 50% reduction from the baseline eGFR, ESRD or death in Study B (HR=1.083 for each 18 mEq/24hr increase in UNaE; P=0.010, Table 3A) and with a greater annual rate of decline in eGFR (−0.086 ml/min/yr for each 18 mEq/24hr increase in UNaE, P<0.001, Table 3B) using a similar linear mixed model as in Study A. When time-varying UNaE rather than averaged UNaE was used as a covariate, these associations were not statistically significant (Tables 3C and 3D). Neither averaged nor time-varying UNaE differentially impacted the effect of ACEi/ARB combination versus ACEi monotherapy.
Table 3.
Effect of change in averaged or of time-varying urine sodium excretion (UNaE) on ADPKD progression in Study B&
| (A) Of averaged UNaE on change in hazard ratio for combined endpoint of death, ESRD or 50% reduction in eGFR | ||||
| Effect | P-value | Hazard Ratio | Lower | Upper |
| ACE-I + ARB | 0.650 | 0.931 | 0.682 | 1.269 |
| Averaged UNaE (per 18mEq/24hr)‡ | 0.010 | 1.083 | 1.008 | 1.064 |
| (B) Of averaged UNaE on annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | −2.394 | −2.700 | −2.088 |
| Year*ACE-I + ARB | 0.929 | 0.007 | −0.162 | 0.177 |
| Averaged UNaE (per 18mEq/24hr)‡ | 0.405 | −0.184 | −0.614 | 0.247 |
| Year*Averaged UNaE (per 18mEq/24hr)‡ | <0.001 | −0.086 | −0.129 | −0.044 |
| (C) Of time-varying UNaE on change in hazard ratio for combined endpoint of death, ESRD or 50% reduction in eGFR | ||||
| Effect | P-value | Hazard Ratio | Lower | Upper |
| ACE-I + ARB | 0.212 | 0.746 | 0.472 | 1.181 |
| UNaE (per 18mEq/24hr) | 0.398 | 0.969 | 0.954 | 1.016 |
| (D) Of time-varying UNaE on annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | −3.097 | −3.391 | −2.803 |
| Year*ACE-I + ARB | 0.085 | 0.186 | −0.026 | 0.398 |
| UNaE (per 18mEq/24hr) | <0.001 | 0.148 | 0.063 | 0.232 |
| Year*UNaE (per 18mEq/24hr) | 0.548 | −0.013 | −0.055 | 0.029 |
All models adjusted for the following covariates: age, race, gender, and body surface area (BSA)
usodiumF596: Averaged UNaE from 4 month visit (F5) to the end of the study (F96)
Changes in urine potassium and creatinine excretions and relationship to disease progression
At baseline UKE and UCrE were 58.3 ± 26.9 mEq/24 hr and 1501 ± 671 mg/24 hr, respectively, in Study A, and 62.6 ± 26.5 mEq/24 hr and 1448 ± 618, respectively, in Study B. During Study A, UKE (0.03 mEq/24hr/month, P=0.086) and UCrE (−0.39 mg/24hr/month, P=0.192) did not change, being 59.6 ± 29.9 mEq/24 hr and 1432 ± 553 mg/24 hr, respectively, at last follow-up (Figures 1C and 1D). During Study B, however, UKE (−0.05 mEq/24hr/month, P=0.015) and UCrE (−1.58 mg/24hr/month, P<0.001) decreased slightly, being 56.8 ± 23.2 mEq/24 hr and 1290 ± 527 mg/24 hr, respectively, at last follow-up (Figures 1C and 1D). In Study A, linear mixed models showed a significant association of averaged UKE and UCrE with the rate of TKV growth (Table 4A and 4B), but the effect size was less than that of averaged UNaE (0.17, 0.04 and 0.43%/yr for each 10% increase from baseline, respectively). There was no association between averaged UKE or UCrE and the rate of change in eGFR (Table 4C and 4D). In Study B, a higher averaged UKE was associated with a reduced risk for the composite endpoint of a 50% reduction in eGFR, ESRD or death (Table 5A) and with a better preservation of eGFR (Table 5B), whereas averaged UCrE was not associated with the composite endpoint (Table 5C) and was associated with a slightly more rapid rate of decline in eGFR (Table 5D).
Table 4.
Effect of change in averaged urine potassium (UKE) and creatinine (UCreat) excretions on ADPKD progression in Study A&
| (A) Of averaged UKE on change in annual TKV slope (%/yr) | ||||
| Effect | p-value | Estimate | Lower | Upper |
| Year | <.0001 | 4.785 | 3.955 | 5.621 |
| Year*Low Blood Pressure | <.0001 | −0.896 | −1.323 | −0.467 |
| Averaged UKE (per 6mEq/24hr)‡ | 0.842 | 0.175 | −1.542 | 1.921 |
| Year*Averaged UKE (per 6mEq/24hr)‡ | <.0001 | 0.166 | 0.094 | 0.238 |
| (B) Of averaged UCreat on change in annual TKV slope (%/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | 0.157 | 0.086 | 0.228 |
| Year*Low Blood Pressure | 0.032 | −0.172 | −0.328 | −0.015 |
| Averaged UCreat (per 150mg/24hr)‡ | 0.087 | −2.225 | −4.687 | 0.331 |
| Year*Averaged UCreat (per 150mg/24hr)‡ | <0.001 | 0.038 | 0.031 | 0.044 |
| (C) Of averaged UKE on change in annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | p-value | Estimate | Lower | Upper |
| Year | <.0001 | −3.122 | −3.931 | −2.312 |
| Year*Low Blood Pressure | 0.490 | 0.155 | −0.285 | 0.595 |
| Averaged UKE (per 6mEq/24hr)‡ | 0.077 | 0.444 | −0.048 | 0.935 |
| Year*Averaged UKE (per 6mEq/24hr)‡ | 0.712 | 0.014 | −0.061 | 0.090 |
| (D) Of averaged UCreat on change in annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | −2.422 | −3.256 | −1.588 |
| Year*Low Blood Pressure | 0.467 | 0.163 | −0.276 | 0.602 |
| Averaged UCreat (per 150mg/24hr)‡ | 0.097 | −0.615 | −1.340 | 0.110 |
| Year*Averaged UCreat (per 150mg/24hr)‡ | 0.156 | −0.054 | −0.144 | 0.022 |
All models adjusted for the following covariates: age, race, gender, and body surface area (BSA)
Averaged UKE and UCreat from 4 month visit (F5) to the end of the study (F96)
Table 5.
Effect of change in averaged urine potassium (UKE) and creatinine (UCreat) excretions on ADPKD progression in Study B&
| (A) Of averaged UKE on change in hazard ratio for combined endpoint of death, ESRD or 50% reduction in eGFR | ||||
| Effect | p-value | Hazard Ratio | Lower | Upper |
| ACE-I + ARB | 0.735 | 0.947 | 0.692 | 1.296 |
| Averaged UKE (per 6mEq/24hr)‡ | 0.023 | 0.932 | 0.944 | 0.995 |
| (B) Of averaged UKE on annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | p-value | Estimate | Lower | Upper |
| Year | <0.001 | −3.303 | −3.655 | −2.950 |
| Year*ACE-I + ARB | 0.836 | 0.020 | −0.170 | 0.210 |
| Averaged UKE (per 6mEq/24hr)‡ | 0.419 | 0.013 | −0.019 | 0.045 |
| Year*Averaged UKE (per 6mEq/24hr)‡ | 0.009 | 0.588 | 0.144 | 1.031 |
| (C) Of averaged UCreat on change in hazard ratio for combined endpoint of death, ESRD or 50% reduction in eGFR | ||||
| Effect | P-value | Hazard Ratio | Lower | Upper |
| ACE-I + ARB | 0.712 | 0.943 | 0.691 | 1.288 |
| Averaged UCreat (per 150mg/24hr)‡ | 0.346 | 0.957 | 0.937 | 1.000 |
| (D) Of averaged UCreat on annual eGFR slope (ml/min/1.73 m2/yr) | ||||
| Effect | P-value | Estimate | Lower | Upper |
| Year | <0.001 | −2.774 | −3.109 | −2.438 |
| Year*ACE-I + ARB | 0.873 | 0.016 | −0.174 | 0.205 |
| Averaged UCreat (per 150mg/24hr)‡ | 0.081 | 0.570 | −0.070 | 1.211 |
| Year*Averaged UCreat (per 150mg/24hr)‡ | 0.012 | −0.044 | −0.079 | −0.010 |
All models adjusted for the following covariates: age, race, gender, and body surface area (BSA)
Averaged UKE and UCreat from 4 month visit (F5) to the end of the study (F96)
DISCUSSION
While the beneficial effects of a moderate reduction of intake of salt on blood pressure and cardiovascular and renal events in the general population are well documented,12 the optimal level of salt intake in patients with CKD is controversial6,7 with some,13-15 but not all,16 studies suggesting a U-shaped relationship between salt intake and cardiovascular and renal events risk. While dietary sodium restriction seems to potentiate the renoprotective effect of ACE inhibitors and ARBs in proteinuric renal diseases,9,10 it is uncertain whether sodium restriction modifies the effect of these drugs on the progression of diseases such as ADPKD where proteinuria is typically low grade. Therefore, this post-hoc analysis was performed to seek information on the importance of sodium restriction on the progression of this disease.
The average daily sodium intake in HALT PKD (178 mEq) was similar to those observed in other CKD and general populations.17,18 Poor adherence to sodium restriction is a common problem in clinical trials and in clinical practice.19,20 In the observational CRISP study, dietary sodium intake was found to be a relatively fixed trait.8 During HALT PKD, instructions on a sodium restricted diet at entry into the study and during the biannual study visits resulted in a modest reduction in UNaE averaging 11.6 mEq in Study A and 25.7 mEq in Study B. On the other hand, marked reductions in UNaE (range 52 to 141 mEq) have been achieved in clinical trials of short duration specifically designed to test the effect of sodium restriction on the levels of blood pressure and proteinuria in CKD patients21 and lifestyle intervention trials of hypertension prevention (TOHP I and II) have shown that it is possible to achieve sustained and substantial reductions in dietary sodium (−55.2 ± 76.9 and −42.5 ± 89.0 mEq)22 with intensive patient education. Although the averaged reductions in UNaE achieved in the HALT PKD trials were modest, they were quite variable from patient to patient and therefore potentially informative on the effect of time-varying sodium intake on the progression of ADPKD.
Averaged and time-varying UNaE in Study A, adjusted for age, gender, race, BSA, and time*blood pressure arm interaction, were significantly associated with the rate of increase in TKV. These associations suggest a causal relationship between dietary sodium and kidney growth and are consistent with the association between UNaE and rate of kidney growth observed in the CRISP Study. The level of sodium in the diet did not modify the effect of low blood pressure or the lack of effect of treatment allocation (ACE inhibitor versus ACE inhibitor ARB combination) on the rate of kidney growth.
There was only an insignificant trend for an association between UNaE and the rate of decline in eGFR, and no association between time-varying UNaE and eGFR decline in Study A. On the other hand, averaged but not time-varying UNaE was significantly associated with the rate of eGFR decline in Study B. The inability to detect an association between UNaE and eGFR decline in Study A may be due insufficient duration of the trial, the fact that effects on eGFR are more easily demonstrable at relatively advanced stages of the disease when eGFR values are consistently declining, or possibly because patients in CKD stage 3 are more salt sensitive compared to patients with normal eGFR. Neither averaged nor time-varying UNaE modified the effects of BP target or treatment assignment on the rate of decline of eGFR.
Averaged but not time-varying UNaE was also significantly associated with the risk for the composite endpoint of 50% reduction of baseline eGFR, ESRD or death. The fact that only modest reductions in UNaE were achieved during the trial may account for the lack of association between time varying UNaE and eGFR decline in both studies or between time-varying UNaE and the composite endpoint in Study B.
Strengths of this study include a clinical trial rather than an observational study setting and multiple measurements of 24 hours UNaE, the gold standard to assess dietary sodium, rather than single measurements or estimations based on morning fasting urine samples, or on dietary recall methods, food diaries or food frequency questionnaires.23 However, it was not designed to study the effect of dietary sodium in ADPKD and has the limitations inherent to a post-hoc analysis. An important confounding factor is that HALT PKD participants, by protocol, were instructed on lowering sodium in the diet to <100 mEq daily, but other dietary modifications were allowable as clinically indicated. The counterintuitive association of higher UKE with a reduced hazard ratio for the composite endpoint and better preservation of eGFR in Study B is likely due to a stricter implementation of potassium restriction in the patients with declining renal function. This may account for the small but significant decline in mean UKE noted in Study B. Aging-associated loss of lean body and muscle mass and reduction in protein intake may be responsible for the small but significant reduction in UCrE also detected in Study B.24,25 Weak but significant associations of UKE and UCrE with the rate of kidney growth were found in Study A. Therefore, we cannot exclude the possibility that diet modifications other than sodium intake could have contributed to the observed associations between UNaE and the progression of ADPKD. Unfortunately, urine urea, a better biomarker of protein intake, was not measured in HALT PKD. Associations with time-varying urine sodium should be interpreted cautiously; since urine sodium may be an endogenous covariate which could result in time-dependent confounding (i.e. eGFR at one visit could impact urine sodium at a subsequent visit). Since only a modest reduction in sodium intake was achieved in HALT PKD, the inability to demonstrate that sodium intake modifies the effects of low BP or treatment allocation on the rate of kidney growth does not rule out possible modifying effects of larger changes in sodium intake. More exhaustive initial instruction, counseling sessions and more frequent remote monitoring of food logs and feedback with additional counselling than those provided in HALT PKD would have been necessary to achieve better compliance.
In summary, this post-hoc analysis of HALT PKD points to a detrimental effect of dietary sodium on the rate of progression of ADPKD and suggests that moderate sodium restriction is beneficial in the management of ADPKD.
METHODS
This is a post-hoc analysis of the HALT-PKD studies A and B. The purpose of this post-hoc analysis was to investigate the impact of dietary salt ascertained by measurements of 24 hour UNaE on the progression of ADPKD and its response to rigorous compared to standard levels of blood pressure control and to ACEi-ARB combination therapy compared to ACEi monotherapy. The study was approved by the HALT PKD Steering Committee. The protocols and main results of the HALT PKD clinical trials have been described in detail and published previously.26-29
Design of HALT PKD
The HALT PKD trial consisted of two prospective, randomized, double-blind, multicenter trials to determine the impact of intensive blockade of the RAAS and the level of blood pressure control on progressive renal disease in individuals with early and more advanced stages of ADPKD. Study A randomized 558 patients (15-49 year-old, mean age 36 years, eGFR > 60 mL/min/1.73 m2) in a 2×2 factorial design to either low (95-110/60-75 mm Hg) or standard (120-130/70-80 mm Hg) BP goals using either the combination of lisinopril and telmisartan or lisinopril and placebo, with other medications added as needed to achieve the BP goals. Study B randomized 486 patients (18-64 year-old, mean age 48 years, eGFR 25-60 mL/min/1.73 m2) to either the lisinopril and telmisartan or lisinopril and placebo, with other medications added as needed to achieve a single BP goal of 120-130/70-80 mm Hg.
Following a formal baseline visit to confirm eligibility, a drug washout period, a baseline/randomization visit and a 4-month drug titration period, follow-up visits took place in each site’s clinical research center at 4, 7, and 12 months during the first year and subsequently every 6 months. At the baseline visit all participants were instructed to reduce their salt intake to <2.4 g (100 mEq) and Study B participants were also instructed on a moderate potassium restriction (60-80 mEq per day). Protein and phosphorus restrictions were recommended as clinically indicated. Dietary instructions were reinforced on all subsequent visits. Containers were provided to the participants for standardized 24-hour urine collections on the day preceding study visits at baseline, at the end of the 4-month drug titration, and annually thereafter. Urinary excretions of sodium, potassium, creatinine, aldosterone and albumin were performed centrally were determined at the Reference Laboratory at Cleveland Clinic Foundation, Cleveland, OH.
Clinical trial endpoints
The primary outcome for Study A was the annualized percent change in TKV measured by MRI, with several secondary outcomes including the rate of change in eGFR. The primary endpoint for Study B was the composite of time to 50% reduction in eGFR, ESRD or death, with several secondary outcomes including the rate of change in eGFR.
Measurements of TKV and eGFR
MR images of the kidneys were obtained at baseline and at 2, 4 and 5 years after the start of therapy. TKVs were measured centrally using stereology. GFR was estimated at baseline, at 4, 7 and 12 months after the start of therapy, and every 6 months thereafter using centralized measurements of serum creatinine at the Cleveland Clinic and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Statistical Analysis
Details of the analysis for the primary and secondary endpoints of the HALT PKD clinical trials are available in the protocol and have been previously published along with the main results of the trials.26-29 To ascertain the impact of dietary salt on the progression of ADPKD and its response to the interventions tested by HALT PKD, UNaE was averaged across all study visits (from month 4 through the end of study) for each participant in Study A and B. We will refer to this as “averaged UNaE”. In order to assess the relationship between averaged UNaE and outcomes in Study A (TKV and eGFR), linear mixed models were used with fixed effects for year, year-by-blood pressure arm, averaged UNaE, and year-by-averaged UNaE. Also included were baseline covariates for gender, race, age and BSA. Random effects for intercepts and slopes were included in all mixed models. A significant interaction between year and averaged UNaE indicated a meaningful association between UNaE and annual rate of change for the outcome. A similar approach was used to ascertain the association between averaged UNaE and rate of change in eGFR in Study B with the exception that shared parameter models30 were used to account for the impact of informative censoring due to reaching endpoint or study withdrawal. For the primary endpoint in Study B, Cox proportional hazards models were used to assess the relationship with averaged UNaE, adjusting for the same baseline covariates mentioned above. For both Study A and B, the effect of averaged UNaE on annual rates of change and hazard ratios was defined by a per 18 mEq/24hr increase, i.e. approximately a 10 % increase over the mean baseline UNaE. Participants were censored at the last date of follow-up. Since UNaE was collected at each study visit, it is considered to be “time-varying” or “time-dependent”. In other words, its values can change from visit to visit within the same participant as well as between participants. Therefore, we also used the same linear mixed models with time-varying UNaE, rather than the averaged UNaE described above, as a covariate to assess the relationship between within-participant changes in dietary sodium during the trial and the rate of progression of ADPKD31. The same analytical methods were used to test the associations of averaged and time varying urinary excretions of potassium (UKE) and creatinine (UCrE) with rates of change in TKV and eGFR in study A, and with time to 50% reduction in eGFR, ESRD or death and with the rate of change in eGFR in Study B. The effects of UKE and UCrE on annual rates of change and hazard ratios were defined by a per 6 mEq/24hr and 150 mg/24hr increases, i.e. approximately 10 % increases over the mean baseline values, respectively. Two observations in Study B were removed from the analyses due to extreme and implausible UNaE values that were likely due to errors in urine volume collection. All statistical analyses utilized SAS 9.3 and R 3.1.3.
ACKNOWLEDGMENTS
We thank the patients involved in the study for their participation and contribution. This work has been supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK62402 to Dr. Torres, DK082230, to Dr. Schrier, DK62411, to Dr. Perrone, DK62410, to Dr. Moore, DK62408 to Dr. Chapman, DK62401 to Washington University in St. Louis, and DK090728 to the Mayo Translational PKD Center) and the National Center for Research Resources General Clinical Research Centers (RR000585 to the Mayo Clinic, RR000039 to Emory University, RR000054 to Tufts Medical Center, RR000051 to the University of Colorado, RR023940 to the University of Kansas Medical Center, and RR001032 to Beth Israel Deaconess Medical Center), National Center for Advancing Translational Sciences Clinical and Translational Science Awards (RR024150 and TR00135 to the Mayo Clinic, RR025008 and TR000454 to Emory University, RR025752 and TR001064 to Tufts University, RR025780 and TR001082 to the University of Colorado, RR025758 and TR001102 to Beth Israel Deaconess Medical Center, RR033179 and TR000001 to the University of Kansas Medical Center, and RR024989 and TR000439 to Cleveland Clinic), by funding from the Zell Family Foundation (to the University of Colorado), and by a grant from the PKD Foundation.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schrier RW. Hypertension and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2011;57:811–813. doi: 10.1053/j.ajkd.2011.02.379. [DOI] [PubMed] [Google Scholar]
- 2.Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Eng J Med. 1990;323:1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Wilson DM, Burnett JCJ, Johnson CM, Offord KP. Effect of inhibition of converting enzyme on renal hemodynamics and sodium management in polycystic kidney disease. Mayo Clin. Proc. 1991;66:1010–1017. doi: 10.1016/s0025-6196(12)61724-8. [DOI] [PubMed] [Google Scholar]
- 4.Torres VE, et al. Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;42:364–373. doi: 10.1038/ki.1992.297. [DOI] [PubMed] [Google Scholar]
- 5.Lawson CR, Doulton TW, MacGregor GA. Autosomal dominant polycystic kidney disease: role of the renin-angiotensin system in raised blood pressure in progression of renal and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2006;7:139–145. doi: 10.3317/jraas.2006.023. [DOI] [PubMed] [Google Scholar]
- 6.Heerspink HL, Ritz E. Sodium chloride intake: is lower always better? J. Am. Soc. Nephrol. 2012;23:1136–1139. doi: 10.1681/ASN.2012010099. [DOI] [PubMed] [Google Scholar]
- 7.Humalda JK, Navis G. Dietary sodium restriction: a neglected therapeutic opportunity in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2014;23:533–540. doi: 10.1097/MNH.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VE, et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:640–647. doi: 10.2215/CJN.03250410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vegter S, et al. Sodium intake, ACE inhibition, and progression to ESRD. J. Am. Soc. Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430ASN.2011040430. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambers Heerspink HJ, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74ki201274. [pii] [DOI] [PubMed] [Google Scholar]
- 11.Hamming I, Navis G, Kocks MJ, van Goor H. ACE inhibition has adverse renal effects during dietary sodium restriction in proteinuric and healthy rats. J. Pathol. 2006;209:129–139. doi: 10.1002/path.1956. [DOI] [PubMed] [Google Scholar]
- 12.Morrison AC, Ness RB. Sodium intake and cardiovascular disease. Annu. Rev. Public Health. 2011;32:71–90. doi: 10.1146/annurev-publhealth-031210-101209. [DOI] [PubMed] [Google Scholar]
- 13.Thomas MC, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34:861–866. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekinci EI, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34:703–709. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell MJ, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 16.McQuarrie EP, et al. Association between urinary sodium, creatinine, albumin, and long-term survival in chronic kidney disease. Hypertension. 2014;64:111–117. doi: 10.1161/HYPERTENSIONAHA.113.03093. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer CM, et al. Urine sodium excretion increased slightly among U.S. adults between 1988 and 2010. J. Nutr. 2014;144:698–705. doi: 10.3945/jn.113.187914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nerbass FB, et al. Demographic associations of high estimated sodium intake and frequency of consumption of high-sodium foods in people with chronic kidney disease stage 3 in England. J. Ren. Nutr. 2014;24:236–242. doi: 10.1053/j.jrn.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 19.van Zuilen AD, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2012;82:710–717. doi: 10.1038/ki.2012.137. [DOI] [PubMed] [Google Scholar]
- 20.Welch JL, Bennett SJ, Delp RL, Agarwal R. Benefits of and barriers to dietary sodium adherence. West. J. Nurs. Res. 2006;28:162–180. doi: 10.1177/0193945905282323. discussion 181-169. [DOI] [PubMed] [Google Scholar]
- 21.McMahon EJ, Campbell KL, Bauer JD, Mudge DW. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD010070.pub2. CD010070. [DOI] [PubMed] [Google Scholar]
- 22.Cook NR, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon EJ, Campbell KL, Mudge DW, Bauer JD. Achieving salt restriction in chronic kidney disease. Int J Nephrol. 2012;2012:720429. doi: 10.1155/2012/720429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walser M. Creatinine excretion as a measure of protein nutrition in adults of varying age. JPEN. J. Parenter. Enteral Nutr. 1987;11:73S–78S. doi: 10.1177/014860718701100510. [DOI] [PubMed] [Google Scholar]
- 25.Dragsted LO. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010;84:301–307. doi: 10.1016/j.meatsci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Chapman AB, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5:102–109. doi: 10.2215/CJN.04310709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres VE, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 2012;81:577–585. doi: 10.1038/ki.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrier RW, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres VE, et al. Angiotensin Blockade in Late Autosomal Dominant Polycystic Kidney Disease. New Engl J Med. 2014;371:2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonesh EF, Greene T, Schluchter MD. Shared parameter models for the joint analysis of longitudinal data and event times. Stat. Med. 2006;25:143–163. doi: 10.1002/sim.2249. [DOI] [PubMed] [Google Scholar]
- 31.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Vol. 998. John Wiley & Sons; 2012. [Google Scholar]