Abstract
Nanotechnology is increasingly playing important roles in various fields including virology. The emerging use of metal or metal oxide nanoparticles in virus targeting formulations shows the promise of improved diagnostic or therapeutic ability of the agents while uniquely enhancing the prospects of targeted drug delivery. Although a number of nanoparticles varying in composition, size, shape, and surface properties have been approved for human use, the candidates being tested or approved for clinical diagnosis and treatment of viral infections are relatively less in number. Challenges remain in this domain due to a lack of essential knowledge regarding the in vivo comportment of nanoparticles during viral infections. This review provides a broad overview of recent advances in diagnostic, prophylactic and therapeutic applications of metal and metal oxide nanoparticles in Human Immunodeficiency Virus, Hepatitis virus, influenza virus and Herpes virus infections. Types of nanoparticles commonly used and their broad applications have been explained in this review.
Introduction
Nanomaterials have attracted enormous interest in field of targeted therapeutics and diagnostics in the past decade [1–2]. As the nanoparticles typically span in the size range of 10–500 nm, their interactions with mammalian cells can be programmed based on the particle size and functional requirement. In addition to more conventional polymeric nanoparticles, metal and metal oxide nanoparticles can also play a major role in detection [2], and external control over drug delivery [3]. Over the past two decades, various nanoparticles and drug delivery models have been designed and implemented to treat cancers [3–4], offset diabetes [5], control bacterial infections via antimicrobial coatings [6], cross the blood brain barrier [7], and form vaccines [8].
Nanomaterials offer a plethora of opportunities in the field of medicine owing to the unique quantum mechanical properties exhibited in their size range. The market share of these medical innovations based on nanotechnology has grown approximately 20 times in the past decade. The emergence of commercially available quantum nanodots, superparamagnetic nanoparticles and nanopolymeric suspensions has given previously unavailable tools to scientists who did not have sophisticated labs to produce the same. This has not only increased the use of nanomaterials in biological sciences, but has seen the birth of new fields of study such as nanovaccinology and nanobiotechnology.
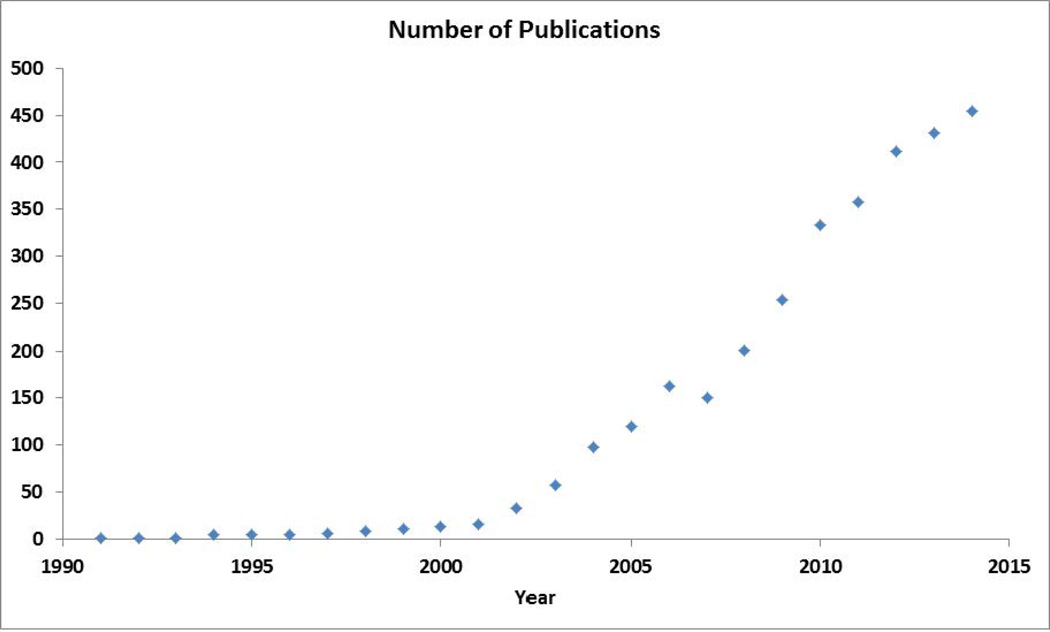
The role of nanotechnology in virology, in particular, has been increasing exponentially in the past decade (figure 1). In diagnostic, prophylactic and therapeutic approaches, nanoparticles have been used for imaging purposes [9], and/or as drug carrying adjuvant to enhance virucidal properties [10]. Virucidal nanoparticles and drug delivery models have been used mainly for the fighting Human immunodeficiency virus (HIV), hepatitis (type A, B, C and E) and herpes simplex virus (HSV-1&2). The use of nanoparticles for virucidal outcomes is still under investigation and has not been approved for clinical or pre-clinical trials.
Figure 1.
Number of publications returned using the search terms “nanoparticle* and virus*” from Scopus (http://www.scopus.com/; results for a search conducted on 19 October 2015)
In this review, we provide an overview of recent advances made in the broad area of virology with specific focus on the use of metal and metal oxide nanoparticles. We first survey the advances made in the general field of nanomaterials for virology followed by their specific use in fighting HIV, hepatitis and herpesvirus infections. Each of them would be further sub-divided to highlight their usage in detection, and treatment of viral diseases.
1. Use of Nanoparticles in Virology
A broad survey of literature revealed that majority of the published studies which use nanomaterials were focused on detecting/and/or treating HIV, followed by hepatitis (A, B and C) virus, influenza virus and herpes simplex virus (1&2). A few other studies have also been conducted in the areas of detection and treatment of human papilloma virus, human rotavirus and Japanese encephalitis virus. Further, it is interesting to note that the majority of studies use polymeric systems such as polyethylene glycol (PEG) [11], poly-Lactic-co-glycolic acid (PLGA) [12], and liposomes [8] for the drug delivery systems. While the use of metal nanoparticles have been strictly restricted to gold and silver, metal oxide nanoparticles such as iron oxide (magnetite), zinc oxide (ZnO), and titanium dioxide (TiO2) have been used in the past studies as well. Although many reports relating to the use of virus like particles (VLPs) [13] and nano-DNA vaccines [8] have been discussed, this review will limit to the study of metal and metal oxide nanoparticles used in virological studies. A broad scopus search for the terms ‘Virus’ and ‘nanoparticles’ showed numerous studies published since 1990s’ (Fig. 1).
2.1 Studies on HIV
2.1.1 Detection
Traditionally HIV has been detected using enzyme linked immunosorbent assay (ELISA). This remains to be the gold standard for the detection of HIV in a clinical scenario. Over the past decade, various enhancements have been made to this process in order to increase the efficiency several fold. The more prominent detection process involves the use of polymerase chain reaction (PCR) for enhancing viral DNA in a given sample and hence pertaining to the detection of the same.
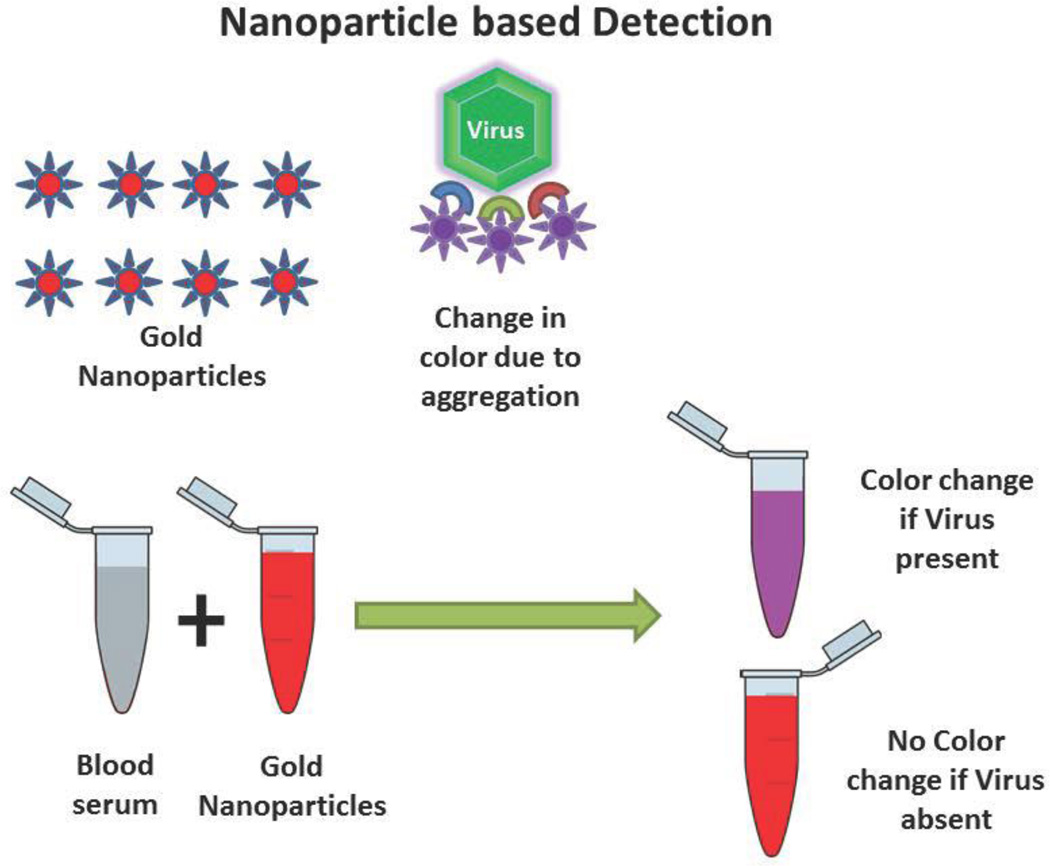
With the advent of nanotechnology, detection mechanism of HIV has been modified using various nanomaterials. One of the simplest methods for detection of viruses involves the measurement of aggregation (and resultant color change) in gold nanoparticles attached to viruses (figure 2). Xiansong et al. [14] used gold magnetic particles functionalized with antibodies against the HIV capsid protein p24 and gold nanoparticles functionalized with both oligonucleotide barcodes and antibodies against a non-overlapping region of the cognate p24. Using this, they improved the real time immuno-PCR amplification process for the detection of HIV. Limit of detection of this model was as low as 100 copies of p24 antigens which show that real-time immune-PCR through nanoparticle based barcode amplification offers an disruptive approach to p24 detection and quantification, thus may potentially shorten the window of HIV-I diagnosis. Using a similar bio-barcode based method. Kim et al. [15] evaluated the efficiency of this technique at the Chicago component of the multicenter AIDS cohort study. The results indicated that the bio-barcode-amplification method was superior to the conventional ELISA assay for the detection of HIV-1 p24 Gag protein in plasma with a breadth of coverage for diverse HIV-1 subtypes. Although bio-barcode based methods have further been used by many other groups with greater efficiency, the use of gold nanoparticles remain to be the common link in these processes [16–18].
Figure 2.
Nanoparticle based detection methods. The nanoparticle based detection methods in general explore a chromatic or electrochemical change due to the presence of target virus antigen or virus particles itself. Here we represent a mechanism by which aggregation of nanoparticles cause the shift in solution color from red to purple due to the presence of virus particles. This method has been followed by many scientists and in detail by Sajjanar et al [87]
In a novel study, Mahmoud et al. [19–20], exploited the chemical interaction between HIV-1 protease and pepstatin for the detection and subsequent evaluation of its inhibitors. A ferrocene tagged, gold nanoparticle coated single walled nanotube (SWNT/AuNP) was used as the electrode in an electrochemical impedance spectroscopy technique for this purpose. Later the same group reported the detection of HIV-1 protease in the patient sera using the aforementioned setup [21]. Detection efficiency of 10 picomolar was reported using this method. The most interesting part of this study was the use of disposable screen printing process for the development of the setup. In a different study, Lee at al [22], discussed the detection of HIV-1 and virus like particles in the range of 600 fg/mL to 375 pg/mL using gold nanoparticle coated indium tin oxide substrates (ITO). Although an electrochemical detection mechanism was used, the efficiency of the method because of the material used largely improved by the direct electron transfer technique used in this study.
Europium nanoparticle immunoassay technique reported by Liu et al. [23] does not use any catalytic enzymes and signal amplification, while has a sub-pg/mL detection resolution with significantly high signal to noise ratio. This microchip based platform has promising qualities for the development of a simple and cheap bedside HIV detection system.
2.1.2 Treatment
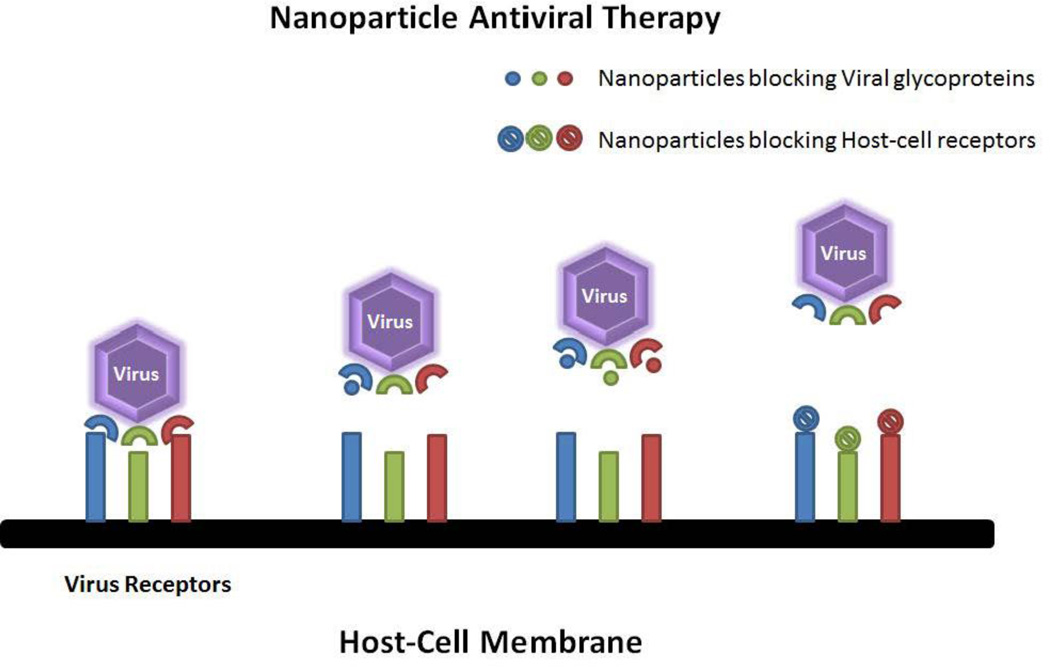
Similar to the reports seen in HIV detection, prophylactic/therapeutic models for HIV have also been dominated by gold and silver nanoparticles followed closely by superparamagnetic iron oxide nanoparticles. Although it does not fall under the scope of this review article, it is interesting to note that, a majority of the studies conducted using metal and metal oxide nanoparticles use the transactivator of transcription (TAT) protein as an adjuvant to target cells of interest for intracellular delivery of nanoparticles [24–31]. Most treatment strategies discussed in this paper involve the inhibition of viral glycoproteins by nanoparticles (figure 3). Thus constrained viruses would not be able to attach to the host cells and in turn inhibiting infection.
Figure 3.
Nanoparticle based antiviral therapy. The common site exploited for antiviral therapy is the entry mechanism of the virus in to the host cell. By electrostatically blocking the viral receptors (on virus or on host cell), one can significantly reduce virus entry into a cell, thereby inhibiting viral infection.
Gold nanoparticles present active surface and bind to sulphated biological ligands with ease. This along with excellent conductivity, biocompatibility and facile preparation methods make them a promising material for antiviral applications. In this regard, Martinez et al. [32] developed prophylactic glyconanoparticle antivirals using gold nanoparticles tagged with truncated (oligo) mannosides of the high-mannose undecasaccharide Man(9)GlcNAc(2). Here, gold nanoparticles provide a surface for the attachment and aggregation of sugars in a small volume. Further, the anti-gp120 activity of gold nanoparticles allows for a synergistic affect when used in conjunction with glyco-conjugates. The authors further reported the use of other highly efficient antiviral glycol-conjugates tagged to gold nanoparticles [33–34] and N-linked high-mannose glycans [34–36] which raise new possibilities in HIV treatment. Later, they developed a highly active antiretroviral therapy (HAART) which used carbohydrate-coated gold nanoparticles loaded with anti-HIV prodrug candidates’ abacavir and lamivudine in a pH-mediated release model [37]. In summary, gold nanoparticles facilitate great adhesion sites for the immobilization of various ligands.
Although silver nanoparticles were proven to have anti-HIV properties [38] at non-cytotoxic concentrations, the mechanism behind the same was discussed later by Lara et al. [39]. The study suggested that silver nanoparticles exert anti-HIV activity at an early stage of viral replication, most likely as a virucidal agent or as an inhibitor of viral entry by binding to viral envelope protein gp120, thereby preventing CD4-dependent virion binding, fusion, and infectivity. Post this study, further evaluation was conducted by preparing a spermicidal gel consisting of polyvinylpyrrolidone coated silver nanoparticles (PVP-AgNPs) as a topical vaginal microbicide to prevent HIV transmission [40]. Silver nanoparticles along with neutralizing antibodies (NABs) have shown to have additive effect when combined against cell-associated HIV-1 infection in vitro [41]. Further studies on incorporation of silver nanoparticles into condoms [42] and enhanced inhibition of HIV protease by silver nanoparticles [43] elucidates the importance of silver in HIV therapy.
HAART for HIV has proven to be a very efficient model for removing active HIV from the body by limiting HIV replication. However, latent forms of HIV pose threat of remission of the disease preventing complete eradication. Thus, novel strategies for the treatment of these forms are being designed using superparamagnetic iron oxide nanoparticles (SPIONs). Mononuclear phagocyte ghost cells of bone marrow and blood monocytes, tissue macrophages, microglia, and dendritic cells loaded with anti-retroviral drugs and SPIONs are being used as undercover carrier vehicles to deliver drugs to latent forms of HIV. In a study conducted by Gorantla et al. [44], bone marrow macrophages loaded with indinavir and SPIONs studied for their distribution throughout the body by magnetic resonance imaging (MRI). Owing to their contrasting property, SPIONs were able successfully report the location of the drugs in a mice model. The results indicated an elevated drug levels (exceeding 200–350 fold therapeutic concentration) through day 1 to day 14.
Magnetic hyperthermia, which uses SPIONs and alternating magnetic field for the generation of heat exceeding 45°C has been used for anti HIV treatments. In a first of kind proof-of-concept study conducted by Williams et al. [45], latent reservoirs of HIV were targeted by SPIONs and thermo-ablated to enhance cytotoxic T-lymphocyte targeting of HIV-infected cells. In another novel approach [46], an anti-HIV drug (tenofovir) and a latency-breaking agent (vorinostat) were co-encapsulated using magnetically guided layer-by-layer (LbL) assembled nanocarriers for the treatment of neuroAIDS. SPIONs coated with the bilayer resulted in an increased drug loading capacity (2.8 fold for tenofovir) and drug release (30 fold with 100% release) over a period of 5 days. Use of SPIONs to cross the blood brain barrier (BBB) has been shown by Fiandra et al. [47]. A structurally complex drug enfuvitride, which normally is unable to cross the BBB, was able to cross the same when tagged onto SPIONs and coated with PMA amphiphilic polymer. The study proved that to completely eradicate HIV, novel studies that clear the BBB have to be taken into consideration and SPIONs play a major role in the same.
2.2 Studies on Hepatitis
Viral Hepatitis is a major health concern all over the world. Although vaccines against the same have been developed in the past decade, many developing and war-torn countries still lack the basic functionalities to deliver the same. In this mayhem, it is important to have fast and simple detection methods to determine the disease status of a person without enduring costly analytical methods or avoiding misuse of the vaccine. Over the past decade, many improvements have been made in detection technologies with the advent of nanotechnology. Here we discuss the role of metal and metal oxide nanoparticles in the detection and treatment of hepatitis A, B, C and E virus.
2.2.1 Detection
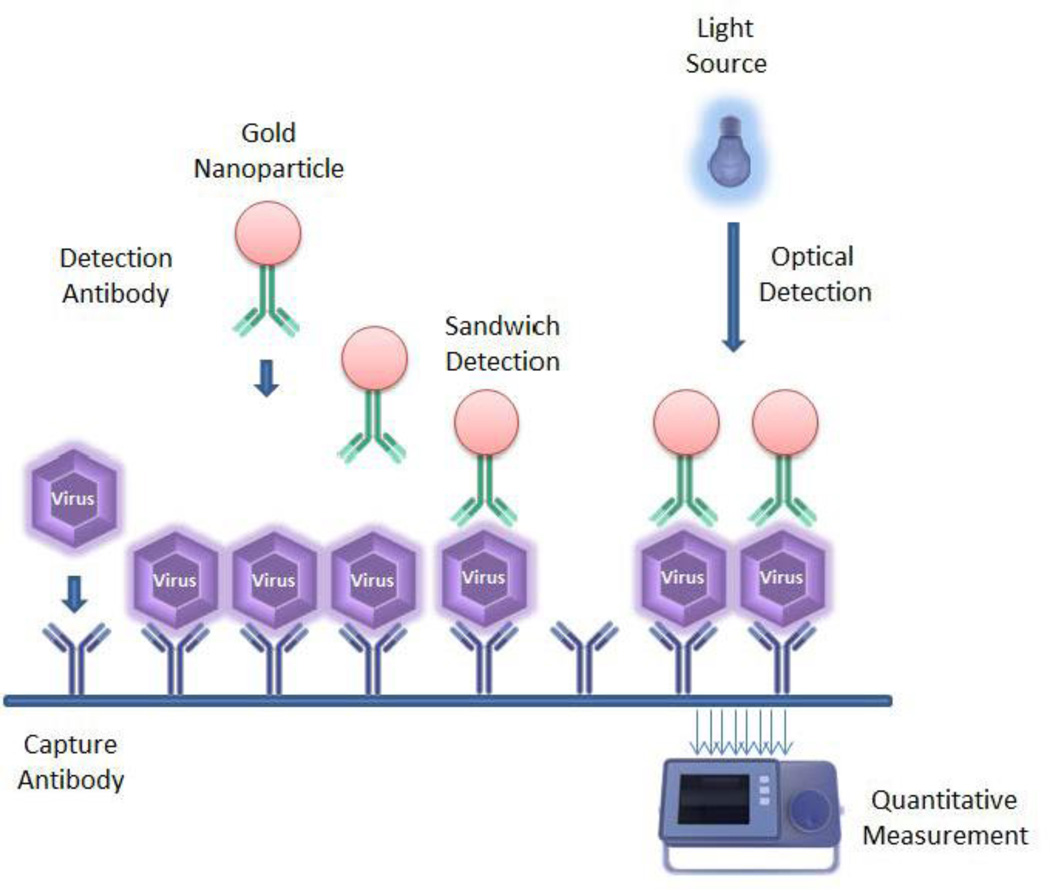
One of the first studies in this century regarding nanoparticle based detection of hepatitis B and C virus was conducted at the University of Wuhan, China by Pang et al. [48]. Their study used a gold nanoparticle based sandwich hybridization model for the detection of target DNA in patient sera using “gene chips”. The same model was tested using silver nanoparticles owing to their surface plasmon resonant state similar to gold nanoparticles. The advantage of this method was direct visual detection, high sensitivity and rapid results compared to traditional PCR based or ELISA based methods (figure 4). In a slightly modified model, the same group reported gold nanoparticle/DNA conjugate (biotin/streptavidin conjugate) based detection probe for voltametric detection of virus DNA [49]. This paved a path for a more quantitative detection model as opposed to the aforementioned qualitative model. The study reported a detection limit of 2 pM viral DNA using this model. Anchoring on these studies, further research to improve the analytical sensitivity of detection has been done by Wang et al. [50] (from the same university). Furthermore, protein analysis chips for the simultaneous detection of hepatitis B and C were developed by Duan et al. [51]. These studies progressed to form a highly sensitive bio-sensor based clinical microarray system for the detection of hepatitis B [52–54] and hepatitis E virus [53] in patient sera. A recent study by Liu et al. [55–56] shows simultaneous colorimetric detection and quantification of two molecules using gold nanoparticles and magnetic beads as two separating agents and boasts the advantages of high sensitivity and low sample consumption. Evidently, the use of gold nanoparticles for detection based assays has not been limited to HIV. Hepatitis detection using gold nanoparticles tagged to a variety of molecules has been reported. Gold nanoparticles attached to magnetic micro-particles and functionalized with multiple restriction enzyme targets (BamHI for HAV target, SpeI for HBV target, and EcoRV for HIV target) have been used for femto molar detection of hepatitis viral DNA [57]. Multiple studies based on antibody coated gold nanoparticles for potentiometric [58], amperometric [59], impedance [60], and electrochemical [61–63] immuno-sensors have also been developed for the detection hepatitis antigens in human serum.
Figure 4.
Sandwich based assay using gold nanoparticles for the optical detection of virus/viral proteins. The process uses a capture antibody for the immobilization of the desired target followed by the attachment of a detection antibody tagged to gold nanoparticles which can then be quantified using an optical sensor.
Silica nanoparticles, owing to their biocompatible nature and reactive surface qualities, have been used in a variety of immune-linked assays to detect hepatitis virus. Yang et al. [64] reported one of the first uses of silica nanoparticles as ultrasensitive fluorescent dyes for the detection of hepatitis B antigens in the ng/mL range. Their usage was supported based on the photo-stability and simple preparation techniques compared to conventional quantum dots and existing dye molecules. Further these fluorescent silica nanoparticles were modified with lanthanide chelates for developing highly sensitive time resolved immunofluorometric assays [65]. Luminescent lanthanide (Eu and Tb) chelate coated silica nanoparticles were tagged with detection antibodies/bridging proteins for the detection of Hepatitis B surface antigen and Hepatitis B e antigen. The notable quality of these nanoparticles was their uniform size (55 nm) and their long shelf life (> 2 years). These nanoparticles were further used in a lateral flow immunoassay [66] to overcome the poor quantitative discrimination and low analytical sensitivity problems faced in conventional assays. The results indicated 100 fold higher sensitivity when compared to gold based lateral flow assays. These particles were later also used ELISA [67] and PCR [68] based methods for the detection of viral DNA from human serum samples. In a novel study, the detection of Hepatitis B Virus DNA at femtomolar concentrations was done using a silica nanoparticle-enhanced microcantilever sensor [69]. Although microcanilever assays use highly sophisticated instrumentation and technologically advanced methodologies for analysis, the qualitative and quantitative data generated from the say are accurate and rapid. Low target antibody usage and concise detection stand to be the most promising qualities of these assays. Similar studies using quartz nano-pillars attached to virus nanoparticles [70] have also been developed which exploit the piezo-electric property of these materials for the detection of virus DNA. Furthermore, fluorescent dye tagged silica nanoparticles [71] have also been used for a cheaper more rapid detection of hepatitis in the human serum samples.
In other interesting studies, magnetic iron oxide nanoparticles were used as single materials for immune-chromatographic detection of hepatitis B virus surface antigens [71]. Nano-graphene tagged with DNAzyme was used as simultaneous monitoring and silencing mechanism of hepatitis C virus in infected cells in-vitro.
2.2.2 Treatment
Although many scientific articles exist on how nanoparticles have been/are being used for the delivery of therapeutic agents to infectious sites, very few publications demonstrate the actual delivery process for the treatment of hepatitis in human or animal models. A single study on this topic was conducted by Lee et al. [72], who reported the treatment of hepatitis virus through a HA-AuNP/IFNα complex (Hyaluronic acid-Gold nanoparticle/Interferon alpha). The treatment was compared against standard PEG (polyethylene glycol) tagged IFNα system and found the novel nanoparticles had comparable biological activity with enhanced stability in human serum. Another study revealed that the use of gold nanorods in hepatitis infected patients can lead to exacerbated liver damage and aggravate hepatitis severity [73]. The study also noted that these results are evidenced only via flow-cytometry and gene expression studies and that gold nanorods cause significant polarization of liver macrophages even at low concentrations. Another 2014 study revealed that gold nanoparticles do not interact in anyway with hepatitis C viral when incubated for a certain period [74]. A 30 sample test revealed no significant difference between the activity of naïve viral load and gold nanoparticle treated viral load and that gold nanoparticles may not be used directly as anti-infective agents for hepatitis C virus.
Similar to the method discussed earlier regarding the use of DNAzyme for the effective killing of hepatitis virus, SPIONs tagged DNAzymes have been used to kill hepatitis C virus [75]. SPIONs not only help provide a magnetic anchor towards desired molecules, but also function as in-vivo magnetic resonance imaging (MRI) contrast agents. Furthermore, SPIONs coated and with lipids and loaded with antivirals and vaccines are able to deliver their carrier loads to selective targets [76].
In other recent study, the use of silver and gold nanoclusters coated with polyvinyl pyrrolidone [77] were able to carry higher payloads such as DNAzyme to target site compared to hollow nanoshells developed earlier for drug delivery purposes. This meagre number of reports, suggest that studies on the role of nanoparticles in therapeutic aspects of virology are still in their nascent stage and is a potential area for future research.
2.3 Studies on Influenza Virus
The most common seasonal disease caused by viruses is undoubtedly flu. Although many developed countries are able to provide substantial protection to their citizens by annual vaccination campaigns, several developing and under-developed countries suffer from flu epidemics every year with very little medical interventions available. Novel treatments and detection strategies, involving metal and metal oxide nanoparticles, have emerged in the past decade which has the potential to accurately diagnose and treat patients suffering from frequent episodes of disease and are likely to be cost effective for use in the developing world.
2.3.1 Detection
In a recent 2015 review article, Shojaei et al. [78] have reported the various detection strategies that emerged for avian flu in the past decade. Their comprehensive study also encompassed the role of metal and metal oxide nanoparticles in the detection process. As evident from previous sections, gold, silica and magnetic SPIONs form the majority of the reports for detection of this disease.
In their own words, they describe that nanoparticle-based techniques have been introduced in biological and medical sciences in a broad variety of applications, such as drug and gene delivery [79], separation or purification of biological molecules, cells and viruses [80] as well as detection of pathogens [81]. Moreover, nanoparticle technology includes usage of a broad variety of materials, such as gold nanoparticles (AuNPs) [82], magnetic nanoparticles [86], carbon-nanotubes [84], and mesoporous silica nanoparticles [85].
Gold nanoparticle modified electrodes for the amperometric detection of H5N1 virus through a gold nanoparticle/DNA-aptamer/H5N1/antiH5N1-Alkaline-phosphatase sandwich model has been able to detect H5N1 concentration in the femto molar range (figure 5). This study conducted by Diba et al. [86] has obtained lowest detectable concentration within the 100 fM to 10 pM range using differential pulse voltammetry. Further, visual detection by the aggregation of citrate stabilized gold nanoparticles attached to virus particles reveals a much simpler qualitative method for the detection of viruses. Sajjanar et al. [87] suggest that gold nanoparticles in the presence of virus particles aggregate and change color from bright red to purple. This method not only enables for the visual detection of the virus, but also quantitatively asses the viral load through simple UV-Visual spectroscopy. In another novel study [88], a bio-nanogate comprised of nanoporous (~20 nm) goldfilm attached to H5N1 specific aptamers, was capable of controlling enzymatic reaction for avian influenza virus H5N1 sensing within 1 hour with a detection limit of 2–9HAU (hemagglutination units). It is also interesting to note that no interference was observed from non-target avian influenza virus subtypes such as H1N1, H2N2, H4N8 and H7N2. The authors propose that the developed approach could be adopted for sensing of other viruses.
Figure 5.
Amperometric detection of H5N1 virus through a gold nanoparticle/DNA-aptamer/H5N1/antiH5N1-Alkaline-phosphatase sandwich model. The model showed a detection limit in the femto molar range.
In other interesting studies, a review on the role of magnetic nanoparticles in cheap and rapid detection of infectious diseases including influenza virus have been discussed by Carinelli et al. [89]. In their enthralling review, they address the promising features of the magnetic particles for the detection of biomarkers in emerging technologies related with infectious diseases affecting global health, such as malaria, influenza, dengue, tuberculosis or HIV. Further, the role of graphene oxide and platinum functionalized cerium oxide nanoparticles for the detection of influenza virus was discussed by Yang et al. [90]. In their study, they report an amplified electrochemical immuno-sensor based on 1-napthol (electroactive substance) and platinum/graphene-oxide/cerium-oxide (catalytic amplifier) for the detection of influenza virus. The proposed immune-sensor was able to achieve a detection limit of 0.43 pg/mL. A fluorescent aptamer based sensor tagged on silica core and silver shell nanoparticle was reported by Pang et al. [91]. The metal enhanced fluorescent sensing platform developed by this group used thiazole orange as the fluorescent tag which detected the hemagglutinin protein of the H5N1 virus. The group also reported that the sensor could be used in a clinical setting, where human serum would be used as the input sample with a detection limit of 3.5 ng/mL, in under 30 minutes.
2.3.2 Treatment
In a review by Adriar B.M. [92], the state of the art nanoparticle based therapeutic techniques against various respiratory viruses (influenza, RSV,or PI-3V) was discussed in extensive detail. While the paper outlines some of the most significant research done in the field of therapeutic medicine, most of the details limit to the use of polymeric nanoparticles for the same. Although it is true that polymeric nanoparticles play a major role in the field of therapeutic nanotechnology, many reports, especially for the inhibition of influenza virus, have been reported using metal and metal oxide nanoparticles. While gold nanoparticles have not shown any direct involvement in the inhibition of influenza virus, their usage has been significant in determining the efficacy of certain vaccines. Gold nanoparticles conjugated to the highly conserved extracellular region of the matrix protein 2 of influenza A virus were used to test the efficacy of the vaccine in a mouse influenza challenge model [93]. However, surface-activated anionic gold nanoparticles have shown remarkable success against influenza virus. While vaccines are very specific to their viral counterparts, gold nanoparticles with activated surfaces showed inactivation of many viruses by occupying active sites on the virus [94].
Silver nanoparticles stand tall when it comes to the inhibition of influenza viruses. Due to their success in inhibiting influenza viruses, their use has been fore-shadowed on other viruses. The use of silver nanoparticles for the inhibition of influenza virus has been extensively studied by Xiang et al. [95] in their comprehensive study over the years. In their first study, they reported the treatment of silver nanoparticles with H3N2 virus. The study revealed that the silver nanoparticles selectively destruct the morphological viral structure in a matter of 30 minutes to 2 hours. In-vitro studies proved that while silver nanoparticles did not have any direct cytotoxic effect on the cell lines, they significantly improved cell viability when treated with H3N2 virus. In a follow up study by Miao et al. [96], it was shown that silver nanoparticles had an atoxic concentration of 25 µg/mL, while TCID50 of influenza virus H3N2 on MDCK cell line was 10 - 3.5/0. 1 µg/mL. Also, the survival rate of MDCK cells was shown to be ~ 98% after the 50 µg/mL silver nanoparticle solution mixed with 40 TCID50 of influenza virus H3N2 in 2 hours, and the survival rate of MDCK cells was ~ 35% in the influenza virus H3N2 control group with 20 TCID. Silver nanoparticles (25 µg/mL) were able to effectively inhibit the apoptosis of MDCK cells induced by 20 TCID50 influenza virus H3N2. Further studies also showed similar activity against the influenza virus H1N1 by the same group [97]. In a recent study, montmorillonite clay based nano silicate platelets surface-modified by silver nanoparticles were reported to have anti-viral activity against influenza A virus [98]. The group also claimed a broad spectrum anti-viral usage of this nanoparticle, given the stability and biocompatibility of the material.
In a study conducted by Cui et al. [99], titanium dioxide nanoparticles in their anatase phase were shown to inhibit H9N2 avian influenza virus. It is interesting note that the nanoparticles activated in the presence of UV light showed greater inhibitory activity compared to those not exposed. In a follow up study by Jiang et al. [100], Copper doped titanium dioxide (Cu+2/TiO2) nanoparticles with greater photocatalytic activity were tested for their antiviral activity. The results indicated a greater antiviral activity compared to their titanium dioxide counterparts. The inactivating rate on H9N2 viruses was shown to reach up to 100%, when the UV intensity is 0.5 mW/cm2, the quantity of H9N2 is 0.1 ml, and the UV illumination time is 2.5 h. In another study [101], titanium dioxide nanoparticles electrostatically bound to DNA (v3’) targeted to the 3’ end of the non-encoding region of the viral (H3N2) DNA was able to efficiently inhibit virus reproduction. Further follow up studies showed a similar activity of the DNA tagged titanium dioxide nanoparticles against H5N1 and H1N1 viruses [102].
Other interesting studies include the use of calcium compounds for the inactivation of influenza virus. Scallop shell powder ground to nanometer range showed exceptional inactivation property against avian influenza virus (also Newcastle disease virus and goose parvovirus) within 5 seconds of incubation. The study [103] showed that calcium oxide, which does not show any activity at 2 µm size, elicit inhibitory behavior at 550 nm size. In another study [104], the ability of vaccines to induce a T-cell response was mimicked by calcium phosphate nanoparticles. Functionalized (with viral antigen) nanoparticles were able to selectively target viruses and illicit an immune response resulting in the clearance of infected cells from the system. These biodegradable nanoparticles have a great potential as a novel vaccination tool with substantial flexibility and wide applicability [105]. In a thought-provoking article [106], the role of zinc oxide nanoparticles in influenza infection has been discussed. It was shown that zinc oxide nanoparticles impair the host pulmonary immune response and attenuate macrophage response to infections and hence may not be used during flu infections.
Recent studies have shown that magnetic nanoparticles tagged with specific viral binding agents can be used in high gradient magnetic separation for therapeutic hemofiltration [107]. This state of the art, emerging process has great promise in the field of therapeutic and diagnostic virology where human blood can be filtered by magnetic materials tagged to target agents.
2.4 Studies on Herpes simplex virus
Herpes simplex virus (HSV) is a double-stranded DNA virus, belonging to Alphaherpesvirinae family, a subfamily of the Herpesviridae family. There are two highly related serotypes of HSV: HSV-1 and HSV-2. Ocular HSV-1 infection may manifest in different clinical situations, including as conjunctivitis, iridocyclitis, acute retinal necrosis, or keratitis. HSV-2, on the other hand, predominantly causes genital herpes. The development of novel strategies to eradicate HSV is a global public health priority and yet, despite this fact, there have been very few major breakthroughs in the detection, treatment or prevention of the virus.
2.4.1 Detection
Although, upto 80% of the industrialized population is affected by HSV (either type 1 or 2), the number of studies conducted on nanoparticle improvised detection procedures have been low. Some of the most significant reports on nanoparticle based detection technologies are mentioned below.
In line with previous sections, magnetic nanoparticles and colloidal gold nanoparticles play a major role in the detection of HSV. Laderman et al. [108] studied a lateral flow immune-chromatographic assay based on colloidal gold nanoparticles. In this report, they described the development and evaluation of an HSV-2 immunoglobulin G (IgG)-specific antibody with sensitivity (100%) and specificity (97%) equivalent to commercially available detection kits (HerpeSelect ELISA). Both whole blood samples and serum samples were evaluated with similar results. Further Tan et al. [109] described the visual colorimetric detection of HSV by isothermal DNA amplification using gold nanoparticles by a novel technique called EXPAR (Exponential amplification reaction). The study reported the detection of HSV -1 with limitation in its detection limit due to non-specific background amplification.
Magnetic particle pull-down assay using a DNA sandwich model for the detection of HSV-1 in swine cerebrospinal fluid samples was reported by Thomson et al. [110]. The technique used both magnetic nanoparticles and fluorescent polystyrene for the pull down assay by hybridizing either end of the target DNA to the nanoparticles. The study reported a detection limit of 0.8 pM was achieved in a total sample volume of 0.09 mL. Further Ran et al. [111] reported a rapid and sensitive, label free magnetic bead aggregation assay for the capture and detection of model proteins of HSV. The biotin streptavidin based magnetic bead assay had the sensitivity in the femtomolar range with an analysis time less than 10 minutes. The study also reported the detection of HSV-1 virus under circumstances where only 200 viruses/mL were present in a sample. The authors of the paper suggest that this magnetic bead assay could be the next forward for resource-limited point of care settings.
2.4.2 Treatment
In a previous review report [112], our group reported the benefits and recent treatment trends of HSV using nanoparticles. While discussing the benefits of targeting the initial interaction between the virus and host cells, we reported that targeting cellular heparan sulfate (entry receptor for numerous viruses) pave a pathway for the preparation of broad-spectrum antiviral drugs. Recent advances in nanotechnology have spurred the development of metal oxide nanostructured compounds that have binding affinity to viral glycoproteins. Several nanostructures from metal-based materials have shown antiviral properties such as zinc oxide (ZnO) [113], tin oxide (SnO) [114], and gold nanoparticles capped with mercaptoethance sulfonate (Au-MES) [115]. The use of zinc oxide tetrapods with prophylactic, therapeutic and virostatic potential has been shown by our group against both HSV-1 [116] and HSV-2 [117]. In this study, it was conferred that ZnO tetrapod type structures counteract HSV infection at different levels. Their ability to control and trap viruses also makes them of specific interest in the development of low cost prophylactics having the potential to treat an existing infection as well. The antiviral property is the result of the attraction of negatively charged nanoparticles (to viruses), which are similar to the natural target receptors on the cell membrane. It might also be possible to present these compounds as virus trappers that stimulate immune response while providing protection from virus infection as microbicides [114]. The combined effect would lead to improved viral clearance and overall antiviral effectiveness. Reduced entry also translates to decreased replication and spread to other cells. These new drugs that target other critical steps in viral lifecycle, such as entry and cell-to-cell spread, can help reduce the likelihood of viral resistance to therapeutic agents.
In a much recent trend, the use of silver nanoparticles as inhibiting agents for HSV has been studied. With increasing number of studies regarding the interaction between silver nanoparticles and viruses (discussed in previous sections), the role of silver nanoparticles as potential anti-HSV agents were studied. A size dependent study regarding the role of silver nanoparticles produced by fungi, in inhibiting HSV-1&2 were studied by Gaikwad et al. [118]. The study concluded that non-toxic silver nanoparticles in the size range of 4–23 nm (and produced by F. oxysporum and Curvularia species) had better anti-viral activity. The authors proposed that the antiviral activity could be attributed to the interaction of silver nanoparticles with the surface of the virus inhibiting them from infecting the host cell. Similar studies conducted by Hu et al. [119] revealed that silver nanoparticles at concentrations 100 µg/mL were able to completely inhibit the HSV-2 infection for 24 hours in Vero cell lines. Tannic acid modified silver nanoparticles of various sizes (sized 13 nm, 33 nm and 46 nm) used by Orlowski et al. [120], showed potency in inhibiting HSV-2 virus both in-vitro and in-vivo. They showed that the antiviral activity was size related and that smaller nanoparticles induced chemokine and cytokine production responsible for antiviral response.
2. Discussion
Study of viruses and their structure has seen dramatic progress in the past decade with advancement in biomedical and instrumentation technology. We know more about the intricacies of virus entry and egress together with proteomics and molecular biology of infection and modes of inhibition. With advancement in informatics, we now know a great deal regarding the structure of proteins and polysaccharides (both viral and host cell) responsible for viral entry and egress. Advancements in nanotechnology have given rise to highly sensitive detection systems which are accurate at detecting molecules in the pico molar range. Although these advancements were not primarily aimed at detection of viral components, their applications towards virus detection has seen an upsurge in the past decade. Femto molar detection of avian influenza viral proteins by amperometric sensors [86] is a good example of cutting edge, state of the art detection system that is available through nanotechnological tools. Metal and metal oxide nanoparticles in these detection systems play an important role by providing stable, conductive and suitable attachment surfaces for detection ligands.
In the past decade, nanoparticles have not only been used as biocompatible drug carriers for anti-viral therapy, but also as anti-virals themselves. The use of nanoparticles as anti-virals reduces the risk of developing drug resistance which is widely seen in molecular anti-virals. Commonly silver nanoparticles have been used for anti-viral therapy than any other nanoparticles. However, this trend is changing with the advent of zinc oxide nanoparticles showing new promise as effective adjuvants and antigen-presenting platforms [121]. These specifically designed nanoparticles have been shown to trap herpes virus particles, rendering them unable to infect cells and subsequently generating an immunogenic via antigen presenting cells. This new model of treatment, called microbivac, could pave the way for longterm immunotherapy of persistent viral infection by nanoparticles. In summary, there is hope for the development of resistance free, biocompatible metal and metal oxide nanoparticle based HSV antivirals that could be used in the clinical scenario in the near future.
3. Conclusions
The advent of multifunctional materials that simplify diagnosis and prophylaxis/therapy, using metal and metal oxide nanoparticles into clinical development has established nanotherapeutics as a credible option for future drug development. Although nanochemistry can produce a large number of metals or metal oxides in the nano-scale, the real challenge is to engineer their effective medical presentation by shape, size and surface modification, while keeping them biocompatible and non-toxic. Growing capability of this multi-disciplinary field is facilitating the design of more refined secondary technologies with bio-mimetic elements, and enhanced targeting of ligands and multi-dimensional applications. There is real hope that one day, these biomimetic nano-sized metal and metal oxide nanoparticles will provide the improved diagnostic abilities and antiviral treatments to address ever increasing global requirement.
Acknowledgments
This work was supported by National Institutes of Health grant AI103754 to D.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(d,l-lactide-co-glycolide) and its derivatives. Journal of Controlled Release. 2008;125:3. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Lee WG, Kim Y-G, Chung BG, Demirci U, Khademhosseini A. Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Advanced Drug Delivery Reviews. 2010;62:1201. doi: 10.1016/j.addr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadavalli T, Ramasamy S, Chandrasekaran G, Michael I, Therese HA, Chennakesavulu R. Dual responsive PNIPAM-chitosan targeted magnetic nanopolymers for targeted drug delivery. Journal of Magnetism and Magnetic Materials. 2015;380:180. [Google Scholar]
- 4.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:1. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 5.Jeevanandam J, Danquah MK, Debnath S, Meka VS, Chan YS. Opportunities for nano-formulations in type 2 diabetes mellitus treatments. Current Pharmaceutical Biotechnology. 2015;16:6. doi: 10.2174/1389201016666150727120618. [DOI] [PubMed] [Google Scholar]
- 6.Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7:1. doi: 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Mailänder V, Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10:15. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 8.Ghaffar KA, Giddam AK, Zaman M, Skwarczynski M, Toth I. Liposomes as nanovaccine delivery systems. Current Topics in Medicinal Chemistry. 2014;14:38. doi: 10.2174/1568026614666140329232757. [DOI] [PubMed] [Google Scholar]
- 9.Cruz LJ, Tacken PJ, Bonetto F, Buschow SI, Croes HJ, Wijers M, De Vries IJ, Figdor CG. Multimodal imaging of nanovaccine carriers targeted to human dendritic cells. Molecular Pharmaceutics. 2011;8:53. doi: 10.1021/mp100356k. [DOI] [PubMed] [Google Scholar]
- 10.Nangmenyi G, Li X, Mehrabi S, Mintz E, Economy J. Silver-modified iron oxide nanoparticle impregnated fiberglass for disinfection of bacteria and viruses in water. Materials Letters. 2011;65:8. [Google Scholar]
- 11.Bailon P, Won C-Y. PEG-modified biopharmaceuticals. Expert Opinion on Drug Delivery. 2009;6:1. doi: 10.1517/17425240802650568. [DOI] [PubMed] [Google Scholar]
- 12.Herrero-Vanrell R, Ramirez L. Biodegradable PLGA microspheres loaded with ganciclovir for intraocular administration. Enapsulation technique in vitro release profiles, and sterilization process. Pharmaceutical Research. 2000;17:1323. doi: 10.1023/a:1026464124412. [DOI] [PubMed] [Google Scholar]
- 13.Unzueta U, Saccardo P, Domingo-Espín J, Cedano J, Conchillo-Solé O, García-Fruitós E, Céspedes MV, Corchero JL, Daura X, Mangues R, Ferrer-Miralles N, Villaverde A, Vázquez E. Sheltering DNA in self-organizing, protein-only nano-shells as artificial viruses for gene delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:535. doi: 10.1016/j.nano.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Xiansong W, Yi S, Shan J, Xuemet M, Yi Z. Combining gold nanoparticles with real-time immuno-PCR for analysis of HIV p24 antigens. 2007 1st International Conference on Bioinformatics and Biomedical Engineering. 2007;1198:1201. [Google Scholar]
- 15.Kim E-Y, Stanton J, Korber BTM, Krebs K, Bogdan D, Kunstman K, Wu S, Phair JP, Mirkin CA, Wolinsky SM. Detection of HIV-1 p24 Gag in plasma by a nanoparticle-based bio-barcode-amplification method. Nanomedicine. 2008;3:293. doi: 10.2217/17435889.3.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang S, Hewlett I. Nanoparticle-based immunoassays for sensitive and early detection of HIV-1 capsid (p24) antigen. The Journal of infectious diseases. 2010;201(Suppl 1):64. doi: 10.1086/650386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan N, Hou J, Hu F, Zheng L, Ni M, Cao Y. An amperometric immunosensor based on a polyelectrolyte/gold magnetic nanoparticle supramolecular assembly- modified electrode for the determination of hiv P24 in serum. Molecules. 2010;15:5053. doi: 10.3390/molecules15075053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong H, Liu J, Zhu H, Ou C-Y, Xing W, Qiu M, Zhang G, Xiao Y, Yao J, Pan P, Jiang Y. Two types of nanoparticle-based bio-barcode amplification assays to detect HIV-1 p24 antigen. Virology Journal. 2012;9:180. doi: 10.1186/1743-422X-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoud KA, Luong JHT. Impedance method for detecting HIV-1 protease and screening for its inhibitors using ferrocene-peptide conjugate/Au nanoparticle/single-walled carbon nanotube modified electrode. Analytical Chemistry. 2008;80:7056. doi: 10.1021/ac801174r. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud KA, Luong JHT. A sensitive electrochemical assay for early detection of HIv-1 protease using ferrocenepeptide conjugate/AU nanoparticle/single walled carbon nanotube modified electrode. Analytical Letters. 2010;43:1680. [Google Scholar]
- 21.Choi J-H, Kang S-R, Kim H, Um SH, Shin K, Choi J-W, Oh B-K. Dye-doped silica nanoparticle with HIV-1 TAT peptide for bioimaging. Journal of Biomedical Nanotechnology. 2013;9:291. doi: 10.1166/jbn.2013.1542. [DOI] [PubMed] [Google Scholar]
- 22.Lee J-H, Oh B-K, Choi J-W. Electrochemical sensor based on direct electron transfer of HIV-1 Virus at Au nanoparticle modified ITO electrode. Biosensors and Bioelectronics. 2013;49:535. doi: 10.1016/j.bios.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Du B, Zhang P, Haleyurgirisetty M, Zhao J, Ragupathy V, Lee S, DeVoe DL, Hewlett IK. Development of a microchip europium nanoparticle immunoassay for sensitive point-of-care HIV detection. Biosensors and Bioelectronics. 2014;61:183. doi: 10.1016/j.bios.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 24.Lewin M, Carlesso N, Tung C-H, Tang X-W, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature Biotechnology. 2000;18:410. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 25.Tkachenko AG, Xie H, Liu Y, Coleman D, Ryan J, Glomm WR, Shipton MK, Franzen S, Feldheim DL. Cellular trajectories of peptide-modified gold particle complexes: Comparison of nuclear localization signals and peptide transduction domains. Bioconjugate Chemistry. 2004;15:482. doi: 10.1021/bc034189q. [DOI] [PubMed] [Google Scholar]
- 26.Console S, Marty C, García-Echeverría C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) "protein transduction domains" promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. Journal of Biological Chemistry. 2003;278:35109. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 27.Sun RW-Y, Chen R, Chung NP-Y, Ho C-M, Lin C-LS, Che C-M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chemical Communications. 2005;40:5059. doi: 10.1039/b510984a. [DOI] [PubMed] [Google Scholar]
- 28.Dodd CH, Hsu H-C, Chu W-J, Yang P, Zhang H-G, Mountz JD, Jr, Zinn K, Forder J, Josephson L, Weissleder R, Mountz JM, Mountz JD. Normal T-cell response and in vivo magnetic resonance imaging of T cells loaded with HIV transactivator-peptide-derived superparamagnetic nanoparticles. Journal of Immunological Methods. 2001;256:89. doi: 10.1016/s0022-1759(01)00433-1. [DOI] [PubMed] [Google Scholar]
- 29.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. Journal of Nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowman M-C, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. Journal of the American Chemical Society. 2008;130:6896. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Avila O, Hijazi K, Marradi M, Clavel C, Campion C, Kelly C, Penadés S. Gold manno-glyconanoparticles: multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chemistry (Weinheim an der Bergstrasse, Germany. 2009;15:9874. doi: 10.1002/chem.200900923. [DOI] [PubMed] [Google Scholar]
- 33.Di Gianvincenzo P, Marradi M, Martínez-ávila OM, Bedoya LM, Alcamí J, Penadés S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorganic and Medicinal Chemistry Letters. 2010;20:2718. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 34.Arnáiz B, Martínez-ávila O, Falcon-Perez JM, Penadés S. Cellular uptake of gold nanoparticles bearing HIV gp120 oligomannosides. Bioconjugate Chemistry. 2012;23:814. doi: 10.1021/bc200663r. [DOI] [PubMed] [Google Scholar]
- 35.Marradi M, Di Gianvincenzo P, Enríquez-Navas PM, Martínez-ávila OM, Chiodo F, Yuste E, Angulo J, Penadés S. Gold nanoparticles coated with oligomannosides of HIV-1 glycoprotein gp120 mimic the carbohydrate epitope of antibody 2G12. Journal of Molecular Biology. 2011;410:798. doi: 10.1016/j.jmb.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 36.Di Gianvincenzo P, Chiodo F, Marradi M, Penadés S. Gold manno-Glyconanoparticles for Intervening in HIV gp120 Carbohydrate-Mediated Processes. Methods in Enzymology. 2012;509:21. doi: 10.1016/B978-0-12-391858-1.00002-2. [DOI] [PubMed] [Google Scholar]
- 37.Chiodo F, Marradi M, Calvo J, Yuste E, Penadés S. Glycosystems in nanotechnology: Gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein Journal of Organic Chemistry. 2014;10:1339. doi: 10.3762/bjoc.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. Journal of Nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. Journal of Nanobiotechnology. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lara HH, Ixtepan-Turrent L, Garza Treviño EN, Singh DK. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. Journal of Nanobiotechnology. 2011;9:38. doi: 10.1186/1477-3155-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shing C-Y, Whiteley CG, Lee D-J. HIV protease: Multiple fold inhibition by silver nanoparticles-Spectrofluorimetric, thermodynamic and kinetic analysis. Journal of the Taiwan Institute of Chemical Engineers. 2014;45:1140. [Google Scholar]
- 43.Mohammed Fayaz A, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan PT, Yao X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: A new approach to inhibit HIV- and HSV-transmitted infection. International Journal of Nanomedicine. 2012;7:5007. doi: 10.2147/IJN.S34973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorantla S, Dou H, Boska M, Destache CJ, Nelson J, Poluektova L, Rabinow BE, Gendelman HE, Mosley RL. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. Journal of Leukocyte Biology. 2006;80:1165. doi: 10.1189/jlb.0206110. [DOI] [PubMed] [Google Scholar]
- 45.Williams JP, Southern P, Lissina A, Christian HC, Sewell AK, Phillips R, Pankhurst Q, Frater J. Application of magnetic field hyperthermia and superparamagnetic iron oxide nanoparticles to HIV-1-specific T-cell cytotoxicity. International journal of nanomedicine. 2013;8:2543. doi: 10.2147/IJN.S44013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayant RD, Atluri VSR, Agudelo M, Sagar V, Kaushik A, Nair M. Sustained-release nanoART formulation for the treatment of neuroAIDS. International Journal of Nanomedicine. 2015;10:1077. doi: 10.2147/IJN.S76517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiandra L, Colombo M, Mazzucchelli S, Truffi M, Santini B, Allevi R, Nebuloni M, Capetti A, Rizzardini G, Prosperi D, Corsi F. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11:1387. doi: 10.1016/j.nano.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y-F, Pang D-W, Zhang Z-L, Zheng H-Z, Cao J-P, Shen J-T. Visual gene diagnosis of HBV and HCV based on nanoparticle probe amplification and silver staining enhancement. Journal of Medical Virology. 2003;70:205. doi: 10.1002/jmv.10379. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Li J, Baca AJ, Hu J, Zhou F, Yan W, Pang D-W. Amplified voltammetric detection of DNA hybridization via oxidation of ferrocene caps on gold nanoparticle/streptavidin conjugates. Analytical Chemistry. 2003;75:3941. doi: 10.1021/ac0344079. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y-F, Shen J-T, Liu H-H. Analytical performance of and real sample analysis with an HBV gene visual detection chip. Journal of Virological Methods. 2004;121:79. doi: 10.1016/j.jviromet.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Duan L, Wang Y, Li SS-C, Wan Z, Zhai J. Rapid and simultaneous detection of human hepatitis B virus and hepatitis C virus antibodies based on a protein chip assay using nano-gold immunological amplification and silver staining method. BMC Infectious Diseases. 2005;5:53. doi: 10.1186/1471-2334-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan Z, Wang Y, Shawn S-CL, Duan L, Zhai J. Development of array-based technology for detection of HAV using gold-DNA probes. Journal of Biochemistry and Molecular Biology. 2005;38:399. doi: 10.5483/bmbrep.2005.38.4.399. [DOI] [PubMed] [Google Scholar]
- 53.Liu H-H, Cao X, Yang Y, Liu M-G, Wang Y-F. Array-based nano-amplification technique was applied in detection of hepatitis E virus. Journal of Biochemistry and Molecular Biology. 2006;39:247. doi: 10.5483/bmbrep.2006.39.3.247. [DOI] [PubMed] [Google Scholar]
- 54.Song J-W, Xin Z, Yao L, Li X-F, Tang J-X, Zhou X-J, Wu B, Sun A-J, Wu Z-Q. Development of clinical highly sensitive biosensor-based microarray system. World Chinese Journal of Digestology. 2008;16:1628. [Google Scholar]
- 55.Xiao Y, Wu Z, Wong K-Y, Liu Z. Hairpin DNA probes based on target-induced in situ generation of luminescent silver nanoclusters. Chemical Communications. 2014;50:4849. doi: 10.1039/c4cc01154f. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Fang W, Wu Z, Zhou G, Yi W, Zhou X, Shen A, Hu J. A one-tube multiplexed colorimetric strategy based on plasmonic nanoparticles combined with non-negative matrix factorization. Talanta. 2014;128:305. doi: 10.1016/j.talanta.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 57.Jang K-J, Lee H, Hua-Lan Jin, Park Y, Nam J-M. Restriction-enzyme-coded gold-nanoparticle probes for multiplexed DNA detection. Small. 2009;5:2665. doi: 10.1002/smll.200901105. [DOI] [PubMed] [Google Scholar]
- 58.Tang DP, Yuan R, Chai YQ, Zhong X, Liu Y, Dai JY, Zhang LY. Novel potentiometric immunosensor for hepatitis B surface antigen using a gold nanoparticle-based biomolecular immobilization method. Analytical Biochemistry. 2004;333:345. doi: 10.1016/j.ab.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 59.Zhuo Y, Yuan R, Chai Y, Zhang Y, Li XL, Zhu Q, Wang N. An amperometric immunosensor based on immobilization of hepatitis B surface antibody on gold electrode modified gold nanoparticles and horseradish peroxidase. Analytica Chimica Acta. 2005;548:205. [Google Scholar]
- 60.Fu Y-Z, Yuan R, Chai Y-Q. Reagentless immunosensing assay via electrochemical impedance for hepatitis B surface antigen monitoring based on polypyrrole and gold nanoparticles as matrices. Chinese Journal of Chemistry. 2006;24:59. [Google Scholar]
- 61.Tang D, Yuan R, Chai Y, Zhong X, Liu Y, Dai J. Electrochemical detection of hepatitis B surface antigen using colloidal gold nanoparticles modified by a sol-gel network interface. Clinical Biochemistry. 2006;39:309. doi: 10.1016/j.clinbiochem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 62.He X, Yuan R, Chai Y, Zhang Y, Shi Y. A new antibody immobilization strategy based on electro-deposition of gold nanoparticles and Prussian Blue for label-free amperometric immunosensor. Biotechnology Letters. 2007;29:149. doi: 10.1007/s10529-006-9211-7. [DOI] [PubMed] [Google Scholar]
- 63.Tang D, Li H, Liao J. Ionic liquid and nanogold-modified immunosensing interface for electrochemical immunoassay of hepatitis B surface antigen in human serum. Microfluidics and Nanofluidics. 2009;6:403. [Google Scholar]
- 64.Yang W, Zhang CG, Qu HY, Yang HH, Xu JG. Novel fluorescent silica nanoparticle probe for ultrasensitive immunoassays. Analytica Chimica Acta. 2004;503:163. [Google Scholar]
- 65.Xu Y, Li Q. Multiple fluorescent labeling of silica nanoparticles with lanthanide chelates for highly sensitive time-resolved immunofluorometric assays. Clinical Chemistry. 2007;53:1503. doi: 10.1373/clinchem.2006.078485. [DOI] [PubMed] [Google Scholar]
- 66.Xia X, Xu Y, Zhao X, Li Q. Lateral flow immunoassay using europium chelate-loaded silica nanoparticles as labels. Clinical Chemistry. 2009;55:179. doi: 10.1373/clinchem.2008.114561. [DOI] [PubMed] [Google Scholar]
- 67.Ke R, Yang W, Xia X, Xu Y, Li Q. Tandem conjugation of enzyme and antibody on silica nanoparticle for enzyme immunoassay. Analytical Biochemistry. 2010;406:42229. doi: 10.1016/j.ab.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H, Xu Y, Huang Q, Yi C, Xiao T, Li Q. Natural phage nanoparticle-mediated real-time immuno-PCR for ultrasensitive detection of protein marker. Chemical Communications. 2013;49:3778. doi: 10.1039/c3cc40688a. [DOI] [PubMed] [Google Scholar]
- 69.Lee J-H, Kim JS, Park J-S, Lee W, Lee KE, Han S-S, Lee KB, Lee J. A three-dimensional and sensitive bioassay based on nanostructured quartz combined with viral nanoparticles. Advanced Functional Materials. 2010;20:2004. [Google Scholar]
- 70.Cha BH, Lee S-M, Park JC, Hwang KS, Kim SK, Lee Y-S, Ju B-K, Kim TS. Detection of Hepatitis B Virus (HBV) DNA at femtomolar concentrations using a silica nanoparticle-enhanced microcantilever sensor. Biosensors and Bioelectronics. 2009;25:130. doi: 10.1016/j.bios.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Bae SW, Tan W, Hong J-I. Fluorescent dye-doped silica nanoparticles: New tools for bioapplications. Chemical Communications. 2012;48:2270. doi: 10.1039/c2cc16306c. [DOI] [PubMed] [Google Scholar]
- 72.Lee M-Y, Yang J-A, Jung HS, Beack S, Choi JE, Hur W, Koo H, Kim K, Yoon SK, Hahn SK. Hyaluronic acid-gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano. 2012;6:9522. doi: 10.1021/nn302538y. [DOI] [PubMed] [Google Scholar]
- 73.Bartneck M, Ritz T, Keul HA, Wambach M, Bornemann J, Gbureck U, Ehling J, Lammers T, Heymann F, Gassler N, Lüdde T, Trautwein C, Groll J, Tacke F. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano. 2012;6:8767. doi: 10.1021/nn302502u. [DOI] [PubMed] [Google Scholar]
- 74.Sora Yasri, Viroj Wiwanitkit. Effect of gold nanoparticle on viral load of hepatitis C virus. Journal of Coastal Life Medicine. 2014;2:500. [Google Scholar]
- 75.Ryoo S-Rd, Jang Hd, Kim K-Sb, Lee Bb, Kim KBb, Kim Y-Kd, Yeo W-Sc, Lee Yd, Kim D-Eb, Min D-Ha. Functional delivery of DNAzyme with iron oxide nanoparticles for hepatitis C virus gene knockdown. Biomaterials. 2012;33:2754. doi: 10.1016/j.biomaterials.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 76.Al-Jamal WT, Kostarelos K. Liposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine. 2007;2:85. doi: 10.2217/17435889.2.1.85. [DOI] [PubMed] [Google Scholar]
- 77.Jang H, Min D-H. Spherically-clustered porous Au-Ag alloy nanoparticle prepared by partial inhibition of galvanic replacement and its application for efficient multimodal therapy. ACS Nano. 2015;9:2696. doi: 10.1021/nn506492s. [DOI] [PubMed] [Google Scholar]
- 78.Shojaei TR, Tabatabaei M, Shawky S, Salleh MAM, Bald D. A review on emerging diagnostic assay for viral detection: the case of avian influenza virus. Molecular Biology Reports. 2015;42:187. doi: 10.1007/s11033-014-3758-5. [DOI] [PubMed] [Google Scholar]
- 79.Pantarotto D, Prtidos CD, Hoebeke J, Brown F, Kramer E, Briand JP, Muller S, Prato M, Bianco A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem Biol. 2003;10:961. doi: 10.1016/j.chembiol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 80.Ma J, Wong H, Kong LB, Peng KW. Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology. 2003;14:619. [Google Scholar]
- 81.Nam JM, Thaxton CC, Mirkin CA. Nanoparticles-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;30:1884. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 82.Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 2008;1:17. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhumpa R, Bu M, Handberg KJ, Wolff A, Bang DD. Rapid sample preparation for detection and identification of avian influenza virus from chicken faecal samples using magnetic bead microsystem. J Virol Methods. 2010;16:228. doi: 10.1016/j.jviromet.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 84.Santra S, Xu J, Wang K, Tan W. Luminescent nanoparticle probes for bioimaging. J Nanosci Nanotechnol. 2004;4:590. doi: 10.1166/jnn.2004.017. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Y, Trewyn BG, Slowing II, Lin VSY. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 86.Diba FS, Kim S, Lee HJ. Amperometric bioaffinity sensing platform for avian influenza virus proteins with aptamer modified gold nanoparticles on carbon chips. Biosensors and Bioelectronics. 2015;72:355. doi: 10.1016/j.bios.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 87.Sajjanar B, Kakodia B, Bisht D, Saxena S, Singh AK, Joshi V, Tiwari AK, Kumar S. Peptide-activated gold nanoparticles for selective visual sensing of virus. Journal of Nanoparticle Research. 2015;17:234. [Google Scholar]
- 88.Wang R, Xu L, Li Y. Bio-nanogate controlled enzymatic reaction for virus sensing. Biosensors and Bioelectronics. 2015;67:400. doi: 10.1016/j.bios.2014.08.071. [DOI] [PubMed] [Google Scholar]
- 89.Carinelli S, Martí M, Alegret S, Pividori MI. Biomarker detection of global infectious diseases based on magnetic particles. New Biotechnology. 2015;32:521. doi: 10.1016/j.nbt.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Yang Z-H, Zhuo Y, Yuan R, Chai Y-Q. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosensors and Bioelectronics. 2015;69:321. doi: 10.1016/j.bios.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 91.Pang Y, Rong Z, Wang J, Xiao R, Wang S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF) Biosensors and Bioelectronics. 2015;66:527. doi: 10.1016/j.bios.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 92.Adair BM. Nanoparticle vaccines against respiratory viruses. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1:405. doi: 10.1002/wnan.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tao W, Ziemer KS, Gill HS. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine. 2014;9:237. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sametband M, Shukla S, Meningher T, Hirsh S, Mendelson E, Sarid R, Gedanken A, Mandelboim M. Effective multi-strain inhibition of influenza virus by anionic gold nanoparticles. MedChemComm. 2011;2:421. [Google Scholar]
- 95.Xiang D, Zheng Y, Duan W, Li X, Yin J, Shigdar S, O'Connor ML, Marappan M, Zhao X, Miao Y, Xiang B, Zheng C. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. International Journal of Nanomedicine. 2013;8:4103. doi: 10.2147/IJN.S53622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miao Y-Q, Zheng C-L. Inactivation of nanosilver on influenza virus H3N2. Journal of Dalian Medical University. 2010;32:119. [Google Scholar]
- 97.Miao Y-Q, Zheng C-L. Inhibiting effect of silver nanoparticles on influenza virus H1N1. Chinese Journal of New Drugs. 2010;19:605. [Google Scholar]
- 98.Liang J-J, Wei J-C, Lee Y-L, Hsu S-H, Lin J-J, Lin Y-L. Surfactant-Modified nanoclay exhibits an antiviral activity with high potency and broad spectrum. Journal of Virology. 2014;88:4218. doi: 10.1128/JVI.03256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang J-F, Cui H-X, Yang T, Cai H-C, Wu D-L. Inactivation efficiency of nano-Cu2+/TiO2 on avian influenza (H9N2) Gongneng Cailiao/Journal of Functional Materials. 2009;40:1403. [Google Scholar]
- 100.Cui H, Jiang J, Gu W, Sun C, Wu D, Yang T, Yang G. Photocatalytic inactivation efficiency of anatase nano-TiO2 sol on the H9N2 avian influenza virus. Photochemistry and Photobiology. 2010;86:1135. doi: 10.1111/j.1751-1097.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 101.Levina AS, Repkova MN, Ismagilov ZR, Shikina NV, Mazurkova NA, Zarytova VF. Eficient inhibition of human influenza A virus by oligonucleotides electrostatically fixed on polylysine-containing TiO2 nanoparticles. Bioorganicheskaia khimiia. 2014;40:196. doi: 10.1134/s1068162014020095. [DOI] [PubMed] [Google Scholar]
- 102.Levina AS, Repkova MN, Mazurkova NA, Makarevich EV, Ismagilov ZR, Zarytova VF. Knockdown of different influenza A virus subtypes in cell culture by a single antisense oligodeoxyribonucleotide. International Journal of Antimicrobial Agents. 2015;46:125. doi: 10.1016/j.ijantimicag.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Thammakarn C, Satoh K, Suguro A, Hakim H, Ruenphet S, Takehara K. Inactivation of avian influenza virus, Newcastle disease virus and goose parvovirus using solution of nano-sized scallop shell powder. Journal of Veterinary Medical Science. 2014;76:1277. doi: 10.1292/jvms.14-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Knuschke T, Sokolova V, Rotan O, Wadwa M, Tenbusch M, Hansen W, Staeheli P, Epple M, Buer J, Westendorf AM. Immunization with biodegradable nanoparticles efficiently induces cellular immunity and protects against influenza virus infection. Journal of Immunology. 2013;190:6221. doi: 10.4049/jimmunol.1202654. [DOI] [PubMed] [Google Scholar]
- 105.Zhou W, Moguche AO, Chiu D, Murali-Krishna K, Baneyx F. Just-in-time vaccines: Biomineralized calcium phosphate core-immunogen shell nanoparticles induce long-lasting CD8+ T cell responses in mice. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:571. doi: 10.1016/j.nano.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin C-D, Kou Y-Y, Liao C-Y, Li C-H, Huang S-P, Cheng Y-W, Liao W-C, Chen H-X, Wu P-L, Kang J-J, Lee C-C, Lai C-H. Zinc oxide nanoparticles impair bacterial clearance by macrophages. Nanomedicine. 2014;9:1327. doi: 10.2217/nnm.14.48. [DOI] [PubMed] [Google Scholar]
- 107.Frodsham G, Pankhurst QA. Biomedical applications of high gradient magnetic separation: Progress towards therapeutic haeomofiltration. Biomedizinische Technik. 2015;60:393. doi: 10.1515/bmt-2015-0056. [DOI] [PubMed] [Google Scholar]
- 108.Laderman EI, Whitworth E, Dumaual E, Jones M, Hudak A, Hogrefe W, Carney J, Groen J. Rapid, sensitive, and specific lateral-flow immunochromatographic point-of-care device for detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in serum and whole blood. Clinical and Vaccine Immunology. 2008;15:159. doi: 10.1128/CVI.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan E, Erwin B, Dames S, Voelkerding K, Niemz A. Isothermal DNA amplification with gold nanosphere-based visual colorimetric readout for herpes simplex virus detection. Clinical Chemistry. 2007;53:2017. doi: 10.1373/clinchem.2007.091116. [DOI] [PubMed] [Google Scholar]
- 110.Thomson DAC, Dimitrov K, Cooper MA. Amplification free detection of Herpes Simplex Virus DNA. Analyst. 2011;136:1599. doi: 10.1039/c0an01021a. [DOI] [PubMed] [Google Scholar]
- 111.Ran Y-F, Fields C, Muzard J, Liauchuk V, Carr M, Hall W, Lee GU. Rapid, highly sensitive detection of herpes simplex virus-1 using multiple antigenic peptide-coated superparamagnetic beads. Analyst. 2014;139:6126. doi: 10.1039/c4an00774c. [DOI] [PubMed] [Google Scholar]
- 112.Hadigal S, Shukla D. Exploiting herpes simplex virus entry for novel therapeutics. Viruses. 2013;5:1447. doi: 10.3390/v5061447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Antoine TE, Mishra YK, Trigilio J, Tiwari V, Adelung R, Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir. Res. 2012;96:363. doi: 10.1016/j.antiviral.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trigilio J, Antoine TE, Paulowicz I, Mishra YK, Adelung R, Shukla D. Tin oxide nanowires suppress herpes simplex virus-1 entry and cell-to-cell membrane fusion. PLoS One. 2012;7:e48147. doi: 10.1371/journal.pone.0048147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baram-Pinto D, Shukla S, Gedanken A, Sarid R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small. 2010;6:1044. doi: 10.1002/smll.200902384. [DOI] [PubMed] [Google Scholar]
- 116.Antoine TE, Mishra YK, Trigilio J, Tiwari V, Adelung R, Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antiviral Research. 2012;96:363. doi: 10.1016/j.antiviral.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mishra YK, Adelung R, Röhl C, Shukla D, Spors F, Tiwari V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Research. 2011;92:305. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, Russo L, Galdiero S, Galdiero M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. International Journal of Nanomedicine. 2013;8:4303. doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu RL, Li SR, Kong FJ, Hou RJ, Guan XL, Guo F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genetics and Molecular Research. 2014;13:7022. doi: 10.4238/2014.March.19.2. [DOI] [PubMed] [Google Scholar]
- 120.Orlowski P, Tomaszewska E, Gniadek M, Baska P, Nowakowska J, Sokolowska J, Nowak Z, Donten M, Celichowski G, Grobelny J, Krzyzowska M. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE. 2014;9:8. doi: 10.1371/journal.pone.0104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Antoine Thessicar E, Hadigal Satvik R, Yakoub Abraam M, Mishra Yogendra Kumar, Bhattacharya Palash, Haddad Christine, Valyi-Nagy Tibor, Adelung Rainer, Prabhakar Bellur S, Shukla Deepak. Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital Herpes. The Journal of Immunology. 2016 doi: 10.4049/jimmunol.1502373. [DOI] [PMC free article] [PubMed] [Google Scholar]