Abstract
Repeated exposure to psychosocial stress is a robust sympathomimetic stressor and as such has adverse effects on cardiovascular health. While the neurocircuitry involved remains unclear, the physiological and anatomical characteristics of the locus coeruleus (LC)-norepinephrine (NE) system suggest that it is poised to contribute to stress-induced cardiovascular vulnerability. A major theme throughout is to review studies that shed light on the role that the LC may play in individual differences in vulnerability to social stress-induced cardiovascular dysfunction. Recent findings are discussed that support a unique plasticity in afferent regulation of the LC, resulting in either excitatory or inhibitory input to the LC during establishment of different stress coping strategies. This contrasting regulation of the LC by either afferent regulation, or distinct differences in stress-induced neuroinflammation would translate to differences in cardiovascular regulation and may serve as the basis for individual differences in the cardiopathological consequences of social stress. The goal of this review is to highlight recent developments in the interplay between the LC-NE and cardiovascular systems during repeated stress in an effort to advance therapeutic treatments for the development of stress-induced cardiovascular vulnerability.
Keywords: locus coeruleus, individual differences, social stress, neuroinflammation, opioid, corticotropin-releasing factor
Introduction
Acute episodes of psychosocial stress robustly activate the sympathetic nervous system and impact cardiovascular function, increasing blood pressure and heart rate, which serve a functional role in an organism’s fight or flight mechanism. Although beneficial during short-term stress responses, chronic activation of the sympathetic nervous system can have deleterious effects on cardiovascular health. Epidemiological studies examining the effects of chronic stress such as “permanent stress” at work or marital stress indicate a direct link to increased risk of cardiovascular disease (Rosengren et al., 2004)(Orth-Gomer et al., 2000). In addition, it is clear from animal studies that there is a direct relationship between chronic stress and the development of cardiovascular disease, but the exact neurological pathways that are affected remain to be determined.
During stress, norepinephrine (NE) is released in both the brain and the periphery to affect cardiovascular responses. The majority of the NE in the central nervous system is released from neurons of the locus coeruleus (LC), and in this review we will discuss the key anatomical and physiological features of the LC-NE system that make it ideal to function as a major hub of the stress response system. Furthermore, we discuss evidence supporting a role for the LC in cardiovascular regulation. The resident-intruder model of social stress in rodents is a well-characterized model producing consequences that parallel psychosocial stress in humans. As a result, this model is ideal to study the negative cardiovascular effects that occur in response to coping with social stress and its link to the LC-NE system. An important factor when faced with a social stress is the strategy used to cope with the stressor. Therefore, a major theme of this review will focus on how the individual differences in coping strategy during social stress can lead to differences in vulnerability to cardiovascular insults. Moreover, the recent discovery that coping responses and cardiovascular vulnerability during repeated stress are paralleled by a distinct plasticity in LC afferent regulation and distinct neuroinflammatory profiles is discussed. The goal of this review is to highlight recent developments in the interplay between the LC/NE and cardiovascular systems during repeated stress in an effort to advance therapeutic treatments for the development of stress-induced cardiovascular vulnerability.
The locus coeruleus-norepinephrine stress response system
Under most circumstances stress activates the hypothalamic-pituitary-adrenal (HPA) axis and leads to increased levels of circulating glucocorticoids. In addition, sustained activation of the brain NE system parallels that of the HPA axis during a chronic stress response and may also be considered a hallmark of stress. Although activation of the LC-NE system is thought to function as an arousal and cognitive measure of the stress response, recent evidence suggests that the brain norepinephrine system also plays an important role in the cardiovascular consequences of stress.
Anatomy of the LC-NE system
The LC is located in the posterior portion of the rostral pons and is a primary source for the synthesis of NE in the brain (Grzanna and Molliver, 1980; Swanson and Hartman, 1976). More recent studies using novel tract tracing tools have advanced our knowledge of the LC’s numerous excitatory afferent inputs as well as widespread efferent projections to many brain regions (Robertson et al., 2013; Schwarz and Luo, 2015). The LC is characterized by a homogenous population of NE neurons and its widespread efferent projections to the entire neuraxis (Aston-Jones et al., 1995; Swanson and Hartman, 1976). Of importance to the focus of this review, the LC can regulate cardiovascular function both indirectly through the nucleus ambiguous (Amb)-rostroventrolateral medulla (RVLM) circuit (Jones and Yang, 1985; McKitrick and Calaresu, 1996) as well as directly through projections to the preganglionic sympathetic neurons of the spinal cord (Jones and Yang, 1985; Spyer, 1992). Stress-sensitive brain regions such as the central nucleus of the amygdala (CNA) receive LC projections and represent another projection region with cardiovascular impact (Kravets et al., 2015; Mason and Fibiger, 1979). Furthermore, tract tracing studies identified that the LC projects to the region of preganglionic parasympathetic cardiac neurons within the dorsal motor vagal nucleus (DMV) (Ter Horst et al., 1991). In support of a role for LC projections regulating cardiovascular function, LC neurons are also labeled transsynaptically from the heart (Standish et al., 1995). Herein, we discuss evidence that supports the hypothesis that the LC has inhibitory effects on parasympathetic neurons of the heart as well as excitatory effects on preganglionic neurons.
Data that identify sources of LC afferents are a point of contention due to differences in retrograde tracing reagents and whether the injection was confined to the neuronal cell bodies (Aston-Jones et al., 1991). The expansive dendrites of LC neurons can reach several hundred microns into the pericoerulear zone, therefore axons terminating within this region can contact LC dendrites, thus having an impact on LC activity even though they are not labeled by retrograde tracers (Shipley et al., 1996). This leads to an underestimation of the number of LC afferents, including the dorsal cap of the paraventricular hypothalamic nucleus, the nucleus prepositus hypoglossi and nucleus paragigantocellularis (PGi) in the ventrolateral medulla (Aston-Jones et al., 1990). The PGi coordinates central noradrenergic activity with peripheral sympathetic activity through inputs from preganglionic sympathetic neurons (Van Bockstaele and Aston-Jones, 1995), and is a major source of enkephalinergic innervation into the LC (Drolet et al., 1990). The importance of this input in stress coping strategy and vulnerability to cardiovascular dysfunction will be discussed below. Interestingly, when retrograde tracers are injected into the pericoerulear regions near LC dendrites, combined with anterograde labeling from putative LC afferents, electron microscopy has verified synaptic connections and more LC afferents have been identified (Luppi et al., 1995; Van Bockstaele et al., 2001; Van Bockstaele et al., 1998; Van Bockstaele et al., 1999). One of these regions is the central nucleus of the amygdala, which transmits information about the cardiovascular system to the LC (Curtis et al., 2002). Through afferents to the LC this region also activates the LC-NE system by releasing the stress neuropeptide corticotropin-releasing factor (CRF) in response to stress (Curtis et al., 2002; Van Bockstaele et al., 1998).
Physiological characteristics of LC neurons
The spontaneous firing rate of LC neurons correlates positively with the state of arousal, which implicates the LC-NE system in the regulation of arousal and attention (Aston-Jones and Bloom, 1981a, b; Aston-Jones and Cohen, 2005; Foote et al., 1980; Williams and Marshall, 1987). In fact, this relationship has been determined to be more than correlative since chemically-induced LC activation or inhibition can affect cortical and hippocampal activity. These data suggest a direct relationship between rate of neuronal discharge in the LC and forebrain indices of arousal (Berridge and Foote, 1991; Berridge et al., 1993). Importantly, activation of LC neurons has been shown to be required for hypotensive stress-induced cortical activation (Lechner et al., 1997; Page et al., 1993) (see below). Thus, the LC may be a critical route by which autonomic changes can affect cortical function.
In addition to the average firing rate, LC neurons fire in both tonic and phasic synchronous bursting patterns, and the latter has been linked to the state of arousal. When a salient sensory stimuli is encountered, a burst of LC neuronal discharge precedes the orientation to the stimuli (Aston-Jones and Bloom, 1981b; Foote et al., 1980), suggesting that the LC-NE system is responsible for the initial redirection of attention to the salient stimuli.
Both the phasic and tonic firing of LC neurons are thought to lead to different behavioral consequences with regards to focus and attention. High tonic activity is associated with lack of focus and going off task, while phasic LC discharge is associated with a more focused attention and an ability to stay on task (see for reviews (Aston-Jones and Cohen, 2005; Bouret and Sara, 2005)) (Rajkowski et al., 1994; Usher et al., 1999). On the other hand, a high rate of tonic LC discharge is thought to disengage animals from focused attention, allowing the animal to scan for threats or other alternative behaviors that might produce a more favorable outcome (Aston-Jones and Cohen, 2005). Shifting between tonic and phasic firing patterns would allow the animal to dynamically change its behavior in a complex environment.
A stressor that activates the LC-NE system initiates the cognitive limb of the stress response, and correspondingly activates the endocrine stress response that is initiated by the HPA axis. Various endpoints of LC activity have been used to verify its activation in response to stressors such as auditory stress, shock, immune system challenges, restraint and social stress and include expression of c-fos and tyrosine hydroxylase, neuronal activity of the LC, as well as NE turnover and release (Beck and Fibiger, 1995; Bonaz and Tache, 1994; Britton et al., 1992; Campeau and Watson, 1997; Cassens et al., 1981; Cassens et al., 1980; Chan and Sawchenko, 1995; Chang et al., 2000; Curtis et al., 2012; Dun et al., 1995; Duncan et al., 1993; Funk and Amir, 2000; Graham et al., 1995; Ishida et al., 2002; Kollack-Walker et al., 1997; Korf et al., 1973; Lacosta et al., 2000b; Makino et al., 2002; Rusnak et al., 2001; Sabban and Kvetnansky, 2001; Smagin et al., 1994; Smith et al., 1992; Smith et al., 1991; Thierry et al., 1968; Valentino et al., 1991b). Examining LC firing rate in both anesthetized and awake rats using electrophysiology has indicated that acute stress (including hypotensive stress, see below) and exposure to CRF can shift the LC firing rate from phasic to high tonic state, favoring cognitive flexibility and heightened arousal (Curtis et al., 2012; Valentino and Foote, 1987; Valentino and Foote, 1988; Valentino and Wehby, 1988; Zitnik et al., 2015). In agreement with these results, intra-LC CRF microinfusion during an attentional set-shifting task facilitated cognitive flexibility suggesting an adaptive mechanism in this dynamic environment (Snyder et al., 2012). On the other hand, if the same hyperactivity of the LC-NE system occurs under resting, non-stress conditions this would translate to impaired concentration and hyperarousal, key features of stress-related psychiatric disorders such as post-traumatic stress disorder and major depression (Southwick et al., 1999; Wong et al., 2000). Furthermore, as outlined below, pathological activation on the LC-NE system is also poised to promote hyperactivation of the sympathetic nervous system, thereby promoting cardiovascular dysfunction. Taken together, like many other neurotransmitter and neuropeptide systems, CRF can promote both healthy adaptation to stress as well as maladaptive consequences.
Cross-talk between the LC and cardiovascular system
LC responses to cardiovascular stimuli
As discussed above, the anatomical features of the LC-NE system favor bidirectional communication with brain regions involved in control of the cardiovascular system. The most clearly understood intercommunication between these centers is the activation of LC neurons by hypotensive stress. During a hemorrhage, hypovolemia was shown to cause an increase in firing rates of LC neurons in rats similar to the hypotensive agent sodium nitroprusside (Elam et al., 1985; Svensson, 1987). Acute cardiovascular challenges such as hypotensive stress, cause the pattern of LC discharge to shift towards a high tonic state that facilitates increased arousal and behavioral flexibility (Valentino and Wehby, 1988). A hypotensive challenge leads to LC neuronal responses that cause arousal-like behaviors such as increased forebrain NE release and cortical electroencephalogram (EEG) activation. This adaptive response by the LC-NE system during hypotension is an example of cognitive flexibility that increases arousal if an animal is severely injured and hemorrhaging.
During a hypotensive episode, endogenous CRF serves to activate LC neurons. These data are supported by initial experiments whereby microinfusion of CRF antagonists into the LC during hypotension inhibited the activation of LC neurons (Valentino et al., 1991b). Furthermore, the finding that intra-LC CRF antagonists also inhibited cortical activation during hypotension indicates that CRF-induced activation of LC neurons during a hypotensive challenge functions to increase arousal (Page et al., 1993). Importantly, the source of CRF that activates the LC during an episode of hypotensive stress was found to be primarily from the central nucleus of the amygdala (CNA), as determined by using lesion studies and functional anatomy experiments (Curtis et al., 2002).
Following LC activation, another important feature of the LC response to stress is inhibition of LC-NE activity allowing recovery once the stress has ended (Curtis et al., 2001; Valentino et al., 1991b). When a CRF antagonist is administered, blocking the excitatory input to the LC, this inhibition becomes exaggerated and with more potent antagonists LC inhibition can even occur during the hypotensive response itself (Curtis et al., 2001; Valentino et al., 1991a). When the opioid antagonist naloxone is administered directly into the LC this inhibition is prevented and slows recovery of LC neuronal activity back to pre-stress levels indicating that this process is mediated by endogenous opioids (Curtis et al., 2001). Taken together, these data suggest that hypotensive stress engages central nucleus of the amygdala-corticotropin releasing factor (CNA-CRF) afferents shifting neuronal activity of the LC to a high tonic state while simultaneously engaging endogenous opioid afferents which function to dampen LC neuronal excitation, thus promoting recovery of LC activation to pre-stress levels. Importantly, the opposing actions of CRF/opioid LC afferents that fine tune LC activity during hypotension are not unique to hypotensive stress. Other more recent studies have shown a similar dual CRF/opioid effect on LC neuron activation during predator stress (Curtis et al., 2012). An opioid input to the LC that my be responsible for this inhibition could be a PGI-enkephalin (ENK), since it densely innervates the LC (Drolet et al., 1992). Furthermore, there is a high expression of µ-opioid receptors (MOR) in the LC and these opioid receptors respond to ENK innervation (Mansour et al., 1994; Tempel and Zukin, 1987).
LC-elicited cardiovascular responses
In addition to activation of the LC by cardiovascular stimuli, a bidirectional relationship exists between the autonomic nervous system and the LC-NE system such that LC activation also initiates a cardiovascular response. It has been shown that when a harmful sensory stimulus is encountered both the LC-NE system and the peripheral sympathetic system are activated in parallel, thus suggesting a relationship between LC-induced arousal and the peripheral sympathetic nervous system (Elam et al., 1986). Although data strongly suggest that LC activation is capable of eliciting cardiovascular responses, the functional effect of LC activation on the cardiovascular system remains a point of debate. For example, some studies report that electrical stimulation of LC neurons can produce noradrenergic and cardiovascular changes, inline with activation of the sympathetic nervous system (Crawley et al., 1980). In addition, a pressor response occurs upon peptidergic activation of the LC by CRF or thyrotropin releasing hormone (Brown, 1986; Paakkari et al., 1987). On the other hand, when the excitatory neurotransmitter L-glutamate is used to activate LC neurons some studies have reported a depressor response and bradycardia (Sved and Felsten, 1987), while others cite findings of increased blood pressure (Chen and Huang, 1997). These conflicting results have led to the hypothesis that data collected from electrical stimulation was the result of activating non-noradrenergic cells or fibers of passage within the LC (Crawley et al., 1980). It is clear now that the contrasting results of LC activation on sympathetic output were likely due to differences in factors such as anesthetized versus awake animal recordings, different anesthetics used, or variations in strains of animals (Chen and Huang, 1997; Kawamura et al., 1978; Sved and Felsten, 1987).
Optogenetic stimulation of LC neurons has begun to clarify the role of the LC in cardiovascular function by revealing a direct influence on cardioinhibitory vagal neurons (Wang et al., 2014). During this study, an increase in the frequency of inhibitory postsynaptic currents was seen in cardiac vagal neurons following photostimulation of LC neurons. The nucleus ambiguous (Amb) is also capable of decreasing heart rate and blood pressure by suppressing the sympathoexcitatory rostroventrolateral medulla (RVLM) (McKitrick and Calaresu, 1996). Comparable to the DMV, the LC also exhibits inhibitory control over the Amb (Jones and Yang, 1985; Samuels and Szabadi, 2008a, b) thereby releasing the Amb-induced brake on this sympathoexcitatory region. Recent advances have also shed light on LC-induced cardiovascular responses using optogenetics. These studies revealed that if LC-NE neurons were activated via astrocytic release of L-lactate, an increase in heart rate and blood pressure ensued (Tang et al., 2014). Moreover, LC stimulation has been shown to result in peripheral catecholamine release, increased heart rate and blood pressure, providing physiological evidence of the combined effects of the LC’s excitatory projections to sympathetic centers and inhibitory tone over parasympathetic neurons (Drolet and Gauthier, 1985; Gurtu et al., 1984; Kawamura et al., 1978).
Individual differences in stress vulnerability
Social stress is a common stressor during daily life. One common laboratory paradigm that mimics aspects of human social stress is the resident-intruder model. This stress paradigm first described by Miczek (Miczek, 1979) has been modified by many labs and typically involves placing an “intruder” rodent into the cage of a more aggressive “resident” rodent’s cage, resulting in aggressive attacks towards the intruder (Koolhaas et al., 1997). Recurrent exposure to this social stress causes a myriad of pathologies including decreased social interaction, anxiety-like behaviors, HPA dysfunction, anhedonia, decreased heart rate variability and self-administration of drugs of abuse (Miczek et al., 2004; Rygula et al., 2005; Tornatzky and Miczek, 1994; Wood et al., 2012b; Wood et al., 2010). Results from the resident-intruder paradigm reveal considerable individual differences in vulnerability to these stress-related consequences. For example, in a mouse model of social stress mice that are vulnerable to the negative consequences of social stress exhibit low levels of social interaction following stress, while the mice that are resilient display social interaction levels comparable to controls (Krishnan et al., 2007). When rats are subjected to social defeat stress, differences in stress susceptibility as measured by HPA function, anhedonia and heart rate variability all correlate with individual differences in coping strategy (Chaijale et al., 2013a; Wood et al., 2012b; Wood et al., 2010; Wood et al., 2015). Daily exposure to the resident-intruder paradigm results in the occurrence of two distinct phenotypes that can be separated based on the rat’s latency to assume the supine defeat posture. Analysis of these two populations reveals that they occur in a bimodal distribution (Wood et al., 2010) reinforcing the notion that these are likely two distinct populations. Over the course of the resident-intruder paradigm, initial defeat latencies are short but after repeated exposures the resistance or vulnerability to the stress becomes established. Interestingly, over the course of subsequent exposures to the stress paradigm, a population of intruder rats develops an active upright coping posture that co-occurs with the increase in defeat latency. The other subset of rats exhibit short defeat latencies (SL rats) and are characterized by an anhedonic response in the sucrose preference test, a decrease in heart variability, immobility in the forced swim test and neural changes related to a propensity for self-administration of drugs of abuse. Furthermore, when compared to animals that have a long latency to defeat (LL rats), the SL rats have changes in HPA function similar to that reported in human depression (Wood et al., 2012b; Wood et al., 2010; Wood et al., 2015).
Individual differences in social stress-induced LC regulation
Recent functional neuroanatomy studies have shed some light on the neurobiological mechanisms that lead to the development of either the SL (passive) or LL (active) coping strategy during social stress, and suggest that a unique plasticity of LC afferent regulation is established during the onset of these coping strategies (Reyes et al., 2015b). During the initial exposure to social stress it is common for all rats to exhibit passive coping strategies, resulting in a short latency to defeat. C-fos expression analysis in this study determined that during the first stress exposure LC neurons and CRF-CNA afferents are activated along with ENK-PGi afferents to the LC. As discussed earlier, these data are in line with electrophysiological studies that show that LC discharge rates are increased during acute stress and both opioid and CRF afferents to the LC are engaged. Interestingly, by the fifth day of the resident-intruder paradigm, the SL and LL phenotypes have developed and only the SL rats have increased cfos expression in the LC and CRF-CNA afferents. With repeated exposure to this stressor, the SL rats lose the activation of ENK-PGi neurons in the LC, consistent with the increase in cfos expression over that of control rats. Unlike passive coping SL rats, LL rats have an activation of ENK-PGi LC afferents while the CRF-CNA activation is lost. As a result, by the fifth stress exposure there is a decrease in LC neuronal activation comparable to control animal levels (as determined by c-fos expression). Consistent with these data, LL rats have higher levels of postsynaptic MOR and lower postsynaptic CRF1 receptor levels in LC neurons compared to SL or control rats (Chaijale et al., 2013b). These data suggest that the emergence of the LL phenotype is associated with a change in the equilibrium of LC afferent control toward inhibition by the ENK-MOR pathway, while the SL phenotype is associated with a loss of this inhibition. One point that requires further characterization is whether or not these changes in afferent control in the LC are causal to the development of the distinct coping strategies. In support of this hypothesis, our work has shown that administration of CRF antagonists prior to the resident-intruder paradigm shifts the population towards the LL phenotype and reduces some of the negative cardiovascular effects seen in the SL phenotype (see below) (Wood et al., 2012b).
Distinct differences in the cardiovascular response to social defeat
It is evident that the resident-intruder paradigm can be used as an ethologically relevant model system to study the ongoing effects of social stress. In particular, social stress has a dramatic impact on sympathetic activation, specifically the cardiovascular system. For example, it has been shown that social defeat results in intense sympathetic activation that elicits 30 times the number of arrhythmias (ventricular premature beats) during social defeat, as compared to other non-social stressors (i.e. restraint or foot shock) (Sgoifo et al., 1999). Indeed, the ability of social defeat to elicit long-term consequences is clearly defined by its negative effects on the cardiovascular system. Examples include disruption of the circadian rhythm of heart rate (Carnevali et al., 2013a; Sgoifo et al., 2002), promotion of atherosclerosis (Black and Garbutt, 2002) and decreased resting heart rate variability (HRV) (Wood et al., 2012b). This shift in HRV indicates a decrease in parasympathetic and an increase in the sympathetic nervous system, which are suggestive of enhanced cardiovascular disease risk (Novak et al., 1997; Tsuji et al., 1996). One major pathological consequence arising from the autonomic imbalance caused by social defeat is maladaptive cardiac hypertrophy (Carnevali et al., 2013a; Gelsema et al., 1994). In fact, a recent study conducted a comprehensive analysis of cardiac electrophysiological variables where high density epicardial mapping analysis revealed increased ventricular myocardial excitability, a shortening of the effective refractory period, and decreased transversal conduction velocity of the wavefront in socially defeated rats compared with controls (Carnevali et al., 2013b). These cardiac electrophysiological variables were attributed to an increased sympathetic response, and are important determinants of arrhythmias (Carnevali et al., 2013b). In addition, social defeat is also capable of producing fibrosis within cardiac tissue, another precipitating factor to cardiac arrhythmias (Costoli et al., 2004). One important question to answer is whether rodents are uniformly sensitive to the cardiovascular consequences of stress or, like in humans, is susceptibility related to the coping strategy adopted during stress exposure?
Studies taking into account individual differences confirmed that cardiovascular susceptibility is related to coping strategy. Specifically, when rats adopted a passive coping response their reduction in resting HRV (increased LF/HF ratio) was exaggerated 24–48 hours after social defeat (Wood et al., 2012b). More recently, continuous elevations in resting systolic blood pressure recordings were observed following defeat only in passive coping rats (Lombard et al., unpublished manuscript). Another related study determined active coping as the number of counter attacks by the intruder. This study found an association between increased counter attacks and reduced/shorter lasting disruption of heart rate circadian rhythms, when compared to passive coping rats (Meerlo et al., 1999). Furthermore, in a separate study, passive coping was associated with hypertension when coping style was determined prior to repeated intermittent stress (Hawley et al., 2010). Moreover, rats that exhibit either low or high aggression also display distinctly different cardiovascular responsivity; with high aggression rats displaying increased tachyarrhythmias and decreased vagal antagonism compared to non-aggressive rats (Carnevali et al., 2013a). All together, these data suggest that behavioral coping responses are linked with stress-induced cardiovascular susceptibility.
LC regulation of individual differences in cardiovascular vulnerability
Since the LC plays a critical role in the behavioral stress response and an organism’s cardiovascular function, it seems plausible that insight into the susceptibility to stress-related cardiac pathology should begin with a better understanding of LC afferent and efferent signals. As mentioned earlier, various cardiovascular centers of the brain are targeted by LC efferents, namely DMV and Amb parasympathetic centers are inhibited while preganglionic sympathetic neurons (PSN) are activated. In active coping rats, the inhibitory PGi-ENK afferent regulation of the LC is enhanced and would translate to decreased activity of the LC’s cardiovascular targets (Figure 1). This change in activity would promote resilience to stress-related cardiovascular pathology through blunted inhibition of the parasympathetic DMV and the cardioinhibitory Amb as well as dampened excitation of the PSN. In addition to social defeat, restraint stress has also been shown to increase ENK expression in the ventrolateral medulla (the region containing PGi neurons) (Boone and McMillen, 1994). The role of ENK in this region is to inhibit sympathetic nerve activity, thereby causing a depressor response and bradycardia (Punnen et al., 1984). In addition to the cardioprotective effects of ENK within the LC, increases in ENK within the PGi/ventrolateral medulla would also exert a cardioprotective influence in active coping rats by decreasing sympathetic activity. In contrast, exaggerated CNA-CRF afferent activation and resulting hyperactivity of LC neurons are a characteristic of the passive coping phenotype, and would promote cardiovascular vulnerability by exaggerating the inhibition of parasympathetic centers and enhancing sympathetic drive (Figure 1). Consistent with this supposition, when a daily CRF antagonist was administered prior to social defeat the population of rats shifted their coping strategy towards the active phenotype. Furthermore, the cardiovascular consequences associated with the SL, passive coping strategy, were also blocked as evidenced by normalization of the HRV (Wood et al., 2012b). While there is clear evidence demonstrating that CNA afferents are distinct regulators of the LC in passive coping rats (Reyes et al., 2015a), it has also been shown that the LC projects to the CNA and contributes to the cardiovascular response to stress (Kravets et al., 2015; Mason and Fibiger, 1979). As an example of this, stimulation of the CNA in awake rats provokes an increase in blood pressure (Chiou et al., 2009). It is also clear that the CNA plays a role in stress-induced blood pressure as lesions of the CNA attenuate the pressor response to acute stress (Sanders et al., 1994). These data suggest that the exaggerated sympathetic activation in the passive coping phenotype may arise from the anticipated increase in excitatory drive from the LC to the CNA. Together, this work suggests that a feed-forward relationship between excitatory afferents to the LC, and the resulting increase in LC efferent activity to sympathoexcitatory regions (and blockade at sympathoinhibitory brain regions) may be partially responsible for the enhanced cardiovascular vulnerability seen in passive coping animals. Recent findings from our group support this hypothesis. Using intracerebroventricular administration of the noradrenergic neurotoxin DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride) to partially lesion LC-NE projections prior to social defeat, we identified that an intact LC is necessary for the chronic increase in resting blood pressure to develop in passive coping SL rats (Lombard et al., unpublished manuscript). Overall, the work outlined in this review support the hypothesis that exaggerated LC activation is central to cardiovascular vulnerability during social stress.
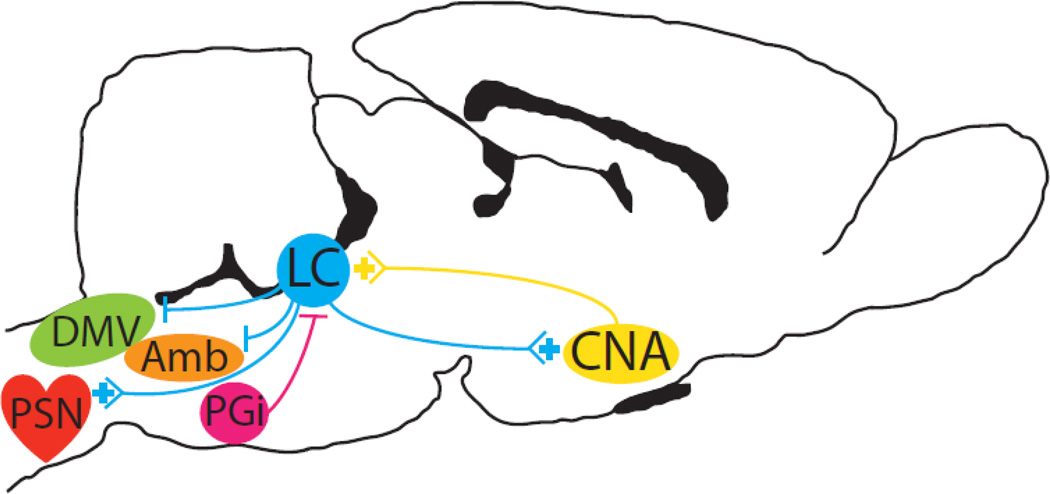
Figure 1.
Schematic depicting afferent and efferent communication of the LC with brain regions involved in the regulation of the cardiovascular system.
Opposing CRF excitatory afferents from the CNA and ENK inhibitory afferents from the PGi regulate the function of the LC. In addition, LC efferents inhibit parasympathetic/cardioinhibitory centers (DMV and Amb) and excite the sympathoexcitatory preganglionic sympathetic neurons (PSN) and CNA. Recent findings indicate that during social defeat stress, adopting an active coping strategy (resulting in a long latency to defeat, LL) biases the LC towards PGi-ENK inhibitory afferents and a decrease in CRF afferent activation of the LC from the CNA. On the other hand, when a passive coping response (short latency to be defeated, SL) is adopted this afferent regulation of the LC is shifted towards the CNA-CRF excitatory influence and reduced PGi-ENK afferents. These data lead us to hypothesize that a bias towards either an excitatory or inhibitory input to the LC may in part underlie the basis for the individual differences in cardiovascular susceptibility seen during social defeat stress. Excitatory and inhibitory projections are denoted by a (+) or a solid line perpendicular to the projection (−).
Beyond CRF: Neuroinflammation in the LC as a vehicle for promoting stress-induced cardiovascular vulnerability
This review focuses predominately on the roles of CRF and opioids in the LC-NE system, however many neurotransmitters, neuropeptides and neurochemicals are capable of modulating the activity of the LC. One such example is proinflammatory cytokines. Social stress is well recognized as promoting the release of proinflammatory cytokines and therefore may promote cardiovascular disease under conditions of chronic or repeated stress exposure. For example, psychosocial stress exposure is recognized as promoting atherosclerosis (Black and Garbutt, 2002), which may be mediated in part by inflammatory leukocytes (Heidt et al., 2014). The link between cardiovascular disease and inflammation in the peripheral tissues or plasma is not a novel concept and has been reviewed extensively. Therefore in this review we have focused on how inflammation has the potential to function through the LC-NE system to mediate neurogenic mechanisms promoting cardiovascular vulnerability.
Animal studies indicate a direct effect of cytokines on NE expression within discrete brain regions. While proinflammatory cytokines have an inhibitory effect on NE in reproductive-related regions, several reports demonstrate that cytokines facilitate NE levels in stress-related brain regions. For example, interleukin (IL)-1β or IL-2 increased NE levels in stress-related brain regions including the paraventricular nucleus, central nucleus of the amygdala, and prefrontal cortex (Lacosta et al., 2000a; Sirivelu et al., 2012). Direct excitatory effects on LC-NE neurons is one manner by which cytokines could stimulate NE levels in the brain. Intra-LC microinjection of IL-1β or lipopolysaccharide (LPS) increases the activity of these noradrenergic neurons (Borsody and Weiss, 2002, 2004), and a single LPS injection increased LC neuronal activity for at least 7 days (Borsody and Weiss, 2004). Furthermore, IL-1 receptor antagonist treatment reversed the LPS-induced increase in spontaneous discharge rate (Borsody and Weiss, 2002, 2004). These cytokine-induced increases in LC activity likely result in concomitant release of NE to the expansive network of LC targets within the brain. In sum, cytokines have discrete and dynamic effects on NE synthesis and release, which may drive various aspects of stress-related cardiovascular vulnerability.
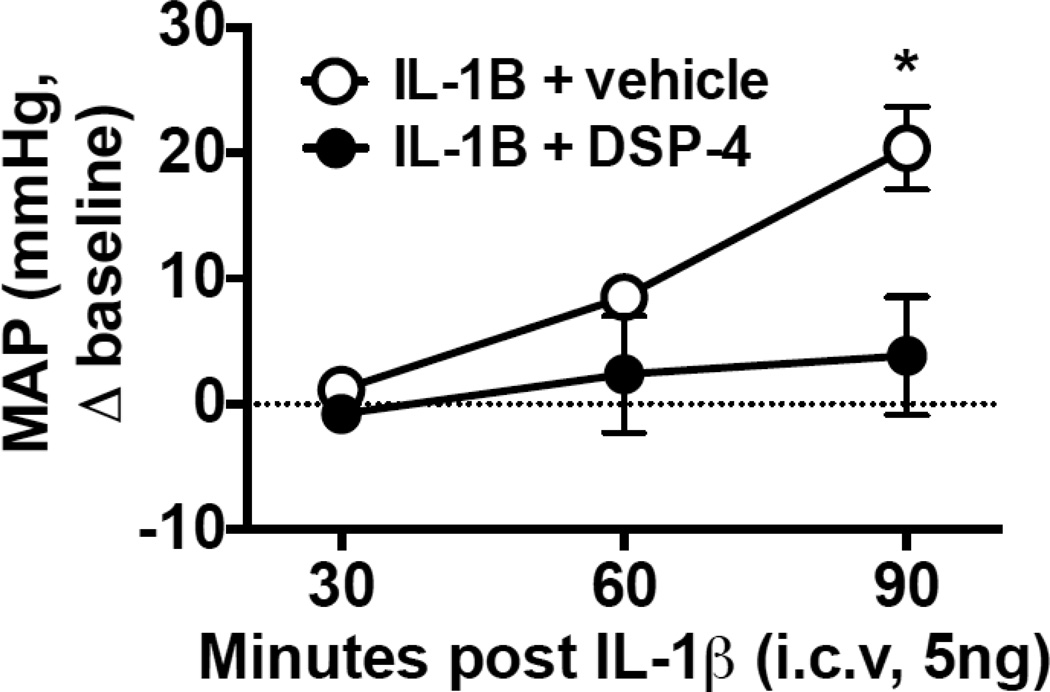
Evidence of a role for central inflammation in hypertension is supported by studies demonstrating a pressor response following an intracerebroventricular injection of IL-1β (Ye et al., 2000). Furthermore, IL-1β is suggested to mediate stress-induced cardiovascular responses because centrally administered IL-1 receptor antagonist (IL-1ra) blunts the pressor response to air jet stress and footshock. In addition, this study confirmed the role of brain versus peripheral IL-1β in this stress-induced pressor response through findings that intravenous IL-1ra was ineffective (Ufnal et al., 2008; Zou et al., 2001). In support of the role of endogenous IL-1β in stress-related cardiovascular vulnerability, the SL susceptible (passive coping) phenotype exhibited increased IL-1β in the LC 24 hours after the final stress exposure, while the LL phenotype and controls did not (Wood et al., 2015). Importantly, this is the same time point that the SL group displayed decreases in resting heart rate variability, indicative of increased sympathetic activity and decreased parasympathetic tone (Wood et al., 2012a), also an independent risk factor for cardiac mortality (Carney et al., 1995). Interestingly, these studies are consistent with clinical findings indicating that passive stress coping is associated with an increased vulnerability to hypertension (Harburg et al., 1964). While the role of central IL-1β in stress-induced hypertension and the specific brain regions it impacts is unclear, our group recently conducted studies that suggest a critical role for the LC in IL-1β-induced pressor responses. In a subset of rats, LC-NE projections were either left intact or lesioned using DSP-4 (400 ug/rat, intracerebroventricular (icv); see figure 2), a dose that produces a 70% reduction in NE levels in LC target regions such as the hippocampus (Sziray et al., 2010). One week later all rats were challenged with an icv infusion of IL-1β, and only rats with intact LC-NE projections exhibited the typical IL-1β-induced pressor response (Figure 2). Taken together, persistent elevations in central IL-1β, especially in the context of repeated stress, could promote cardiovascular vulnerability through hyperactivity of the LC-NE system.
Figure 2.
Effect of LC-NE lesion on IL-1β induced pressor response. Rats were implanted with cardiovascular transmitters (HD-S11, Data Sciences Intl.) and an intracerebroventricular (icv) cannula and injected with N-[2-chloroethyl]-N-ethyl-2-bromobenzylamine hydrochloride (400 ug/rat/4min, icv, DSP-4; R & D Systems) or vehicle 30 mins after citalopram hydrobromide (10mg/kg, IP; AK Scientific). One-week later rats were injected with IL-1β (5ng, icv) and the change in mean arterial pressure (MAP, mmHg) from the pre-IL-1β injection baseline was quantified. Rats with a DSP-4 induced LC-NE lesion lacked the IL-1β-induced pressor response. 2-way ANOVA significant interaction: F(2,4)=7.9; p=0.04; Bonferroni post-hoc *p<0.05 vs. DSP-4 treated rat at 90 mins post injection.
Conclusion
Gaining a better understanding of adaptations within the brain that are related to coping strategy will be critical in identifying the etiology of susceptibility to stress-induced cardiovascular disease. As outlined in this review, evidence of distinct regulation of LC afferents in active versus passive coping rats (Reyes et al., 2015a) as well as distinct differences in neuroinflammation (Wood et al., 2015), may provide a window into the neurobiological changes promoting vulnerability to stress-related cardiopathology. Consistent with this perspective, a study in humans combined functional neuroimaging to measure brain activity with measurements of HRV and demonstrated that the high frequency index of HRV, reflecting cardiovagal output (Novak et al., 1997), was negatively correlated with LC activation (Napadow et al., 2008), underscoring the inhibitory influence that the LC has on parasympathetic tone. LC hyperactivity has also been implicated in depression and post-traumatic stress disorder (PTSD)(Southwick et al., 1997; Wong et al., 2000), psychiatric disorders that both result in a striking increase in cardiovascular disease risk that is proportional to severity of symptoms (Halaris, 2013; Kemp et al., 2010)(Agorastos et al., 2013; Shah et al., 2013)(Wood, 2014; Wood and Bhatnagar, 2015). Interestingly, a recent study showed that marines are significantly more likely to develop PTSD if they start off with lower HRV before deployment (Minassian et al., 2014). This suggests that if mechanisms capable of reducing HRV are engaged before stress, for example exaggerated LC activation, this may increase susceptibility to stress-related disorders including cardiovascular dysfunction. We put forth the hypothesis that individual differences in afferent regulation of the LC, as well as a unique milieu of neuroinflammation in the context of repeated stress exposure plays a significant role in how one individual demonstrates susceptibility in the face of stress while another remains resilient.
Highlights.
The LC may play a role in individual differences in vulnerability to social stress-induced cardiovascular dysfunction.
Recent findings support a unique plasticity in afferent regulation of the LC, resulting in either excitatory or inhibitory input to the LC during establishment of different stress coping strategies.
Findings of individual differences in neuroinflammation within the LC are also related to stress susceptibility.
A better understanding of the interplay between the LC-NE and cardiovascular systems during chronic stress may reveal therapeutic targets for stress-induced cardiovascular disease.
Acknowledgments
Efforts of SKW and CSW were supported by a Scientist Development Grant from the American Heart Association 15SDG22430017 and the National Institute of Health (NIGMS) grant 5P20GM103641. Efforts of RJV were supported by 093981 and DA009082
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, Haji U, Motazedi A, Yanagi MA, Baker DG, Stiedl O. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress. 2013;16:300–310. doi: 10.3109/10253890.2012.751369. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, Van Bockstaele EJ, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, Charlety P, Valentino R, Williams JT. Afferent regulation of locus coeruleus neurons : Anatomy, physiology and pharmacology. Prog. Brain Res. 1991;85:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Ennis M, Williams JT, Pieribone VA. Restricted afferent control of locus coeruleus neurons revealed by anatomical, physiological and pharmacological studies. In: Marsden CA, Heal DJ, editors. The Pharmacology of Noradrenaline in the Central Nervous System. Oxford, Great Britain: Oxford University Press; 1990. pp. 187–247. [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Brain. Academic Press; 1995. pp. 183–213. [Google Scholar]
- Beck CHM, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J. Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. neuroscience. 1993;55:381–383. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002;52:1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- Boone JB, Jr, McMillen D. Differential effects of prolonged restraint stress on proenkephalin gene expression in the brainstem. Brain Res Mol Brain Res. 1994;27:290–298. doi: 10.1016/0169-328x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. Alteration of locus coeruleus neuronal activity by interleukin-1 and the involvement of endogenous corticotropin-releasing hormone. Neuroimmunomodulation. 2002;10:101–121. doi: 10.1159/000065186. [DOI] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. The effects of endogenous interleukin-1 bioactivity on locus coeruleus neurons in response to bacterial and viral substances. Brain Res. 2004;1007:39–56. doi: 10.1016/j.brainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Brown M. Corticotropin releasing factor: central nervous system sites of action. Brain Res. 1986;399:10–14. doi: 10.1016/0006-8993(86)90595-0. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral response and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Trombini M, Porta A, Montano N, de Boer SF, Sgoifo A. Vagal withdrawal and susceptibility to cardiac arrhythmias in rats with high trait aggressiveness. PLoS One. 2013a;8:e68316. doi: 10.1371/journal.pone.0068316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L, Trombini M, Rossi S, Graiani G, Manghi M, Koolhaas JM, Quaini F, Macchi E, Nalivaiko E, Sgoifo A. Structural and electrical myocardial remodeling in a rodent model of depression. Psychosom Med. 2013b;75:42–51. doi: 10.1097/PSY.0b013e318276cb0d. [DOI] [PubMed] [Google Scholar]
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. The American journal of cardiology. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- Cassens G, Kuruc A, Roffman M, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism and behavior induced by environmental stimuli previously paired with inescapable shock. Brain Res. 1981;2:387–407. doi: 10.1016/0166-4328(81)90020-6. [DOI] [PubMed] [Google Scholar]
- Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science. 1980;209:1138–1139. doi: 10.1126/science.7403874. [DOI] [PubMed] [Google Scholar]
- Chaijale NN, Curtis AL, Wood SK, Zhang XY, Bhatnagar S, Reyes BA, Van Bockstaele EJ, Valentino RJ. Social Stress Engages Opioid Regulation of Locus Coeruleus Norepinephrine Neurons and Induces a State of Cellular and Physical Opiate Dependence. Neuropsychopharmacology. 2013a doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijale NN, Curtis AL, Wood SK, Zhang XY, Bhatnagar S, Reyes BA, Van Bockstaele EJ, Valentino RJ. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013b;38:1833–1843. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Hemodynamic regulation of tyrosine hydroxylase messenger RNA in medullary catecholamine neurons: a c-fos-guided hybridization histochemical study. neuroscience. 1995;66:377–390. doi: 10.1016/0306-4522(94)00600-a. [DOI] [PubMed] [Google Scholar]
- Chang MS, Sved AF, Zigmond MJ, Austin MC. Increased transcription of the tyrosine hydroxylase gene in individual locus coeruleus neurons following footshock stress. neuroscience. 2000;101:131–139. doi: 10.1016/s0306-4522(00)00352-3. [DOI] [PubMed] [Google Scholar]
- Chen YY, Huang ZS. Stimulation of locus coeruleus increases arterial pressure in rabbits. Zhongguo yao li xue bao = Acta pharmacologica Sinica. 1997;18:437–440. [PubMed] [Google Scholar]
- Chiou RJ, Kuo CC, Liang KC, Yen CT. State-dependent amygdala stimulation-induced cardiovascular effects in rats. The Chinese journal of physiology. 2009;52:432–440. doi: 10.4077/cjp.2009.amh046. [DOI] [PubMed] [Google Scholar]
- Costoli T, Bartolomucci A, Graiani G, Stilli D, Laviola G, Sgoifo A. Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. Am J Physiol Heart Circ Physiol. 2004;286:H2133–H2140. doi: 10.1152/ajpheart.00869.2003. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Maas JW, Roth RH. Evidence against specificity of electrical stimulation of the nucleus locus coeruleus in activating the sympathetic nervous system in the rat. Brain Res. 1980;183:301–311. doi: 10.1016/0006-8993(80)90466-7. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connally KR, Valentino RJ. Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J. Neuroendocrinol. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ. Endogenous opioids in the locus coeruleus function to limit the noradrenergic response to stress. J. Neurosci. 2001;21:RC152. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Leiser SC, Snyder K, Valentino RJ. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology. 2012;62:1737–1745. doi: 10.1016/j.neuropharm.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet G, Akaoka H, Van Bockstaele E, Aston-Jones G, Shipley MT. Opioid afferents to the locus coeruleus from the rostral medulla as detective by retrograde transport combined with immunohistochemistry. Soc. Neurosci. 1990;16:1027. Abstract. [Google Scholar]
- Drolet G, Gauthier P. Peripheral and central mechanisms of the pressor response elicited by stimulation of the locus coeruleus in the rat. Can J Physiol Pharmacol. 1985;63:599–605. doi: 10.1139/y85-100. [DOI] [PubMed] [Google Scholar]
- Drolet G, Van Bockstaele EB, Akaoka H, et al. Robust enkephalin innervation of the locus coeruleus from the rostral medulla. J. Neurosci. 1992;12:3162–3174. doi: 10.1523/JNEUROSCI.12-08-03162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Shen E, Tang H, Huang R, Chiu TH. c-fos expression as a marker of central cardiovascular neurons. Biol. Signals. 1995;4:117–123. doi: 10.1159/000109431. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GR. Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of fos-like immunoreactivity. J. Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam M, Svensson TH, Thoren P. Differentiated cardiovascular afferent regulation of locus coeruleus neurons and sympathetic nerves. Brain Res. 1985;358:77–84. doi: 10.1016/0006-8993(85)90950-3. [DOI] [PubMed] [Google Scholar]
- Elam M, Thoren P, Svensson TH. Locus coeruleus neurons and sympathetic nerves: activation by visceral afferents. Brain Res. 1986;375:117–125. doi: 10.1016/0006-8993(86)90964-9. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos resonses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Gelsema AJ, Schoemaker RG, Ruzicka M, Copeland NE. Cardiovascular effects of social stress in borderline hypertensive rats. J Hypertens. 1994;12:1019–1028. [PubMed] [Google Scholar]
- Graham JC, Hoffman GE, Sved AF. c-fos expression in brain in response to hypotension and hypertension in conscious rats. J. Auton. Nerv. Sys. 1995;55:92–104. doi: 10.1016/0165-1838(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- Gurtu S, Pant KK, Sinha JN, Bhargava KP. An investigation into the mechanism of cardiovascular responses elicited by electrical stimulation of locus coeruleus and subcoeruleus in the cat. Brain Res. 1984;301:59–64. doi: 10.1016/0006-8993(84)90402-5. [DOI] [PubMed] [Google Scholar]
- Halaris A. Inflammation, heart disease, and depression. Curr Psychiatry Rep. 2013;15:400. doi: 10.1007/s11920-013-0400-5. [DOI] [PubMed] [Google Scholar]
- Harburg E, Julius S, McGinn NF, McLeod J, Hoobler SW. Personality Traits and Behavioral Patterns Associated with Systolic Blood Pressure Levels in College Males. J Chronic Dis. 1964;17:405–414. doi: 10.1016/0021-9681(64)90101-8. [DOI] [PubMed] [Google Scholar]
- Hawley DF, Bardi M, Everette AM, Higgins TJ, Tu KM, Kinsley CH, Lambert KG. Neurobiological constituents of active, passive, and variable coping strategies in rats: integration of regional brain neuropeptide Y levels and cardiovascular responses. Stress. 2010;13:172–183. doi: 10.3109/10253890903144621. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nature medicine. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Hashiguchi H, Takeda R, Ishizuka Y, Mitsuyama Y, Kannan H, Nishimori T, Nakahara D. Conditioned-fear stress increases Fos expression in monoaminergic and GABAergic neurons of the locus coeruleus and dorsal raphe nuclei. Synapse. 2002;45:46–51. doi: 10.1002/syn.10086. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Gunn CG, Frohlich ED. Cardiovascular alteration by nucleus locus coeruleus in spontaneously hypertensive rat. Brain Res. 1978;140:137–147. doi: 10.1016/0006-8993(78)90243-3. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J. Neurosci. 1997;17:8842–8865. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Korf J, Aghajanian GK, Roth RH. Increased turnover of norepinephrine in the rat cerebral cortex during stress: role of the locus coeruleus. Neuropharmacology. 1973;12:933–938. doi: 10.1016/0028-3908(73)90024-5. [DOI] [PubMed] [Google Scholar]
- Kravets JL, Reyes BA, Unterwald EM, Van Bockstaele EJ. Direct targeting of peptidergic amygdalar neurons by noradrenergic afferents: linking stress-integrative circuitry. Brain Struct Funct. 2015;220:541–558. doi: 10.1007/s00429-013-0674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Central monoamine activity following acute and repeated systemic interleukin-2 administration. Neuroimmunomodulation. 2000a;8:83–90. doi: 10.1159/000026457. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Central monoamine activity following acute and repeated systemic interleukin-2 administration. Neuroimmunomodulation. 2000b;8:83–90. doi: 10.1159/000026457. [DOI] [PubMed] [Google Scholar]
- Lechner S, Curtis A, Brons R, Valentino R. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Lombard CM, Melson MN, Muniz BL, Sanner C, Fadel JR, Wood CS, Wilson LB, Wood SK. The role of the locus coeruleus in the cardiovascular response to social stress is associated with coping strategy. unpublished manuscript. [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and phaseolus vulgaris leucoagglutinin. neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res. 2002;943:216–223. doi: 10.1016/s0006-8993(02)02647-1. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain research. 1994;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;187:703–724. doi: 10.1002/cne.901870405. [DOI] [PubMed] [Google Scholar]
- McKitrick DJ, Calaresu FR. Nucleus ambiguus inhibits activity of cardiovascular units in RVLM. Brain Res. 1996;742:203–210. doi: 10.1016/s0006-8993(96)00971-7. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 1999;113:1283–1290. doi: 10.1037//0735-7044.113.6.1283. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neuroscience and biobehavioral reviews. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Minassian A, Geyer MA, Baker DG, Nievergelt CM, O’Connor DT, Risbrough VB Marine Resiliency Study, T. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med. 2014;76:292–301. doi: 10.1097/PSY.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. NeuroImage. 2008;42:169–177. doi: 10.1016/j.neuroimage.2008.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V, Saul JP, Eckberg DL. Task Force report on heart rate variability. Circulation. 1997;96:1056–1057. [PubMed] [Google Scholar]
- Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA. 2000;284:3008–3014. doi: 10.1001/jama.284.23.3008. [DOI] [PubMed] [Google Scholar]
- Paakkari I, Siren AL, Nurminen ML, Svartstrom-Fraser M. Injection of thyrotropin releasing hormone into the locus coeruleus increases blood pressure. Eur Heart J. 1987;(8 Suppl B):147–151. doi: 10.1093/eurheartj/8.suppl_b.147. [DOI] [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci. Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- Punnen S, Willette R, Krieger AJ, Sapru HN. Cardiovascular response to injections of enkephalin in the pressor area of the ventrolateral medulla. Neuropharmacology. 1984;23:939–946. doi: 10.1016/0028-3908(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Activity of locus coeruleus neurons in monkey: phasic and tonic changes correspond to altered vigilance. Brain Res. Bull. 1994;35:607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Zitnik G, Foster C, Van Bockstaele EJ, Valentino RJ. Social Stress Engages Neurochemically-Distinct Afferents to the Rat Locus Coeruleus Depending on Coping Strategy(1,2,3) eNeuro. 2015a:2. doi: 10.1523/ENEURO.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Zitnik GA, Foster C, Van Bockstaele EJ, Valentino RJ. Social Stress Engages Neurochemically-Distinct Afferents to the Rat Locus Coeruleus Depending on Coping Strategy. [accepted October 20, 2015];eNeuro. 2015b doi: 10.1523/ENEURO.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–1023. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S, investigators I. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Kvetnansky R, Jelokova J, Palkovits M. Effect of novel stressors on gene expression of tyrosine hydroxylase and monoamine transporters in brainstem noradrenergic neurons of long-term repeatedly immobilized rats. Brain Res. 2001;899:20–35. doi: 10.1016/s0006-8993(01)02126-6. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91–98. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008a;6:235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part II: Physiological and Pharmacological Manipulations and Pathological Alterations of Locus Coeruleus Activity in Humans. Curr Neuropharmacol. 2008b;6:254–285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders BJ, Wirtz-Nole C, DeFord SM, Erling BF. Central amygdaloid lesions attenuate cardiovascular responses to acute stress in rats with borderline hypertension. Physiol Behav. 1994;56:709–713. doi: 10.1016/0031-9384(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Current biology : CB. 2015;25:R1051–R1056. doi: 10.1016/j.cub.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Koolhaas JM, Musso E, De Boer SF. Different sympathovagal modulation of heart rate during social and nonsocial stress episodes in wild-type rats. Physiol Behav. 1999;67:733–738. doi: 10.1016/s0031-9384(99)00134-1. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Meerlo P, Costoli T, Manghi M, Stilli D, Olivetti G, Musso E. Intermittent exposure to social defeat and open-field test in rats: acute and long-term effects on ECG, body temperature and physical activity. Stress. 2002;5:23–35. doi: 10.1080/102538902900012387. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry. 2013;73:1103–1110. doi: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Fu L, Ennis M, Liu W, Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J. Comp. Neurol. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Sirivelu MP, MohanKumar PS, MohanKumar SM. Interleukin-1 beta simultaneously affects the stress and reproductive axes by modulating norepinephrine levels in different brain areas. Life Sci. 2012;91:878–884. doi: 10.1016/j.lfs.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Swiergiel AH, Dunn AJ. Sodium nitroprusside infusions activate cortical and hypothalamic noradrenergic systems in rats. Neurosci. Res. Comm. 1994;14:85–91. [Google Scholar]
- Smith MA, Banerjee S, Gold PW, Glowa J. Induction of c-fos mRNA in rat brain by conditioned and unconditioned stressors. Brain Res. 1992;578:135–141. doi: 10.1016/0006-8993(92)90240-a. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brady LS, Glowa J, Gold PW, Herkenham M. Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in the locus ceruleus by in situ hybridization. Brain Res. 1991;544:26–32. doi: 10.1016/0006-8993(91)90881-u. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, 3rd, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, Charney DS. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- Spyer KM. Central nervous control of the cardiovascular system. In: Bannister R, Mathias CJ, editors. Autonomic Failure. 3rd. Oxford: Oxford University Press; 1992. pp. 54–77. [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. The Journal of neuroscience : the official journal of the Society for neuroscience. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF, Felsten G. Stimulation of the locus coeruleus decreases arterial pressure. Brain Res. 1987;414:119–132. doi: 10.1016/0006-8993(87)91332-1. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: Putative implications for psychiatry and psychopharmacology. Psychopharmacology. 1987;92:1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat using dopamine-B-hydroxylase as a marker. J. Comp. Neurol. 1976;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Sziray N, Kuki Z, Nagy KM, Marko B, Kompagne H, Levay G. Effects of single and simultaneous lesions of serotonergic and noradrenergic pathways on open-space and bright-space anxiety-like behavior in two animal models. Behav Brain Res. 2010;209:93–98. doi: 10.1016/j.bbr.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nature communications. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the µ, d and k opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc. Natl. Acad. Sci. U S A. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst GJ, Toes GJ, Van Willigen JD. Locus coeruleus projections to the dorsal motor vagus nucleus in the rat. neuroscience. 1991;45:153–160. doi: 10.1016/0306-4522(91)90111-z. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Javoy F, Glowinski J, Kety SS. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat: modification of norpinephrine turnover. J. Pharmacol. Exp. Ther. 1968;163:163–171. [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology (Berl) 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Ufnal M, Sikora M, Szczepanska-Sadowska E. Interleukin-1 receptor antagonist reduces the magnitude of the pressor response to acute stress. Neurosci Lett. 2008;448:47–51. doi: 10.1016/j.neulet.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J. Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain research. 1991a;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991b;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: Evidence for a neurotransmitter role in the locus coeruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Aston-Jones G. Integration in the ventral medulla and coordination of sympathetic, pain and arousal functions. Clin Exp Hypertens. 1995;17:153–165. doi: 10.3109/10641969509087062. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Valentino RJ. Differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol. Psych. 1999;46:1352–1363. doi: 10.1016/s0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Pinol RA, Byrne P, Mendelowitz D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. J Neurosci. 2014;34:6182–6189. doi: 10.1523/JNEUROSCI.5093-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Marshall KC. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. The Journal of neuroscience : the official journal of the Society for neuroscience. 1987;7:3687–3694. doi: 10.1523/JNEUROSCI.07-11-03687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK. Cardiac autonomic imbalance by social stress in rodents: understanding putative biomarkers. Frontiers in psychology. 2014;5:950. doi: 10.3389/fpsyg.2014.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Bhatnagar S. Resilience to the effects of social stress: evidence from clinical and preclinical studies on the role of coping strategies. Neurobiology of Stress. 2015;1:164–173. doi: 10.1016/j.ynstr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology (Berl) 2012a;222:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology. 2012b;222:325–336. doi: 10.1007/s00213-012-2648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, Valentino RJ. Inflammatory Factors Mediate Vulnerability to a Social Stress-Induced Depressive-like Phenotype in Passive Coping Rats. Biol Psychiatry. 2015;78:38–48. doi: 10.1016/j.biopsych.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Mozayeni P, Gamburd M, Zhong H, Campese VM. Interleukin-1beta and neurogenic control of blood pressure in normal rats and rats with chronic renal failure. American journal of physiology. Heart and circulatory physiology. 2000;279:H2786–H2796. doi: 10.1152/ajpheart.2000.279.6.H2786. [DOI] [PubMed] [Google Scholar]
- Zitnik GA, Curtis AL, Wood SK, Arner J, Valentino RJ. Adolescent Social Stress Produces an Enduring Activation of the Rat Locus Coeruleus and Alters its Coherence with the Prefrontal Cortex. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou CJ, Liu JD, Zhou YC. Roles of central interleukin-1 on stress-induced-hypertension and footshock-induced-analgesia in rats. Neurosci Lett. 2001;311:41–44. doi: 10.1016/s0304-3940(01)02140-1. [DOI] [PubMed] [Google Scholar]