Abstract
Objectives
This study sought to perform a systematic review and meta-analysis to understand the prognostic value of myocardial scarring as evidenced by late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR) in patients with known or suspected cardiac sarcoidosis.
Background
Although CMR is increasingly used for the diagnosis of cardiac sarcoidosis, the prognostic value of CMR has been less well described in this population.
Methods
PubMed, Cochrane CENTRAL, and meta-Register of Controlled Trials were searched for CMR studies with ≥ 1 year of prognostic data. Primary end-points were all-cause mortality and a composite outcome of arrhythmogenic events (ventricular arrhythmia, ICD shock, sudden cardiac death) plus all-cause mortality during follow-up. Summary effect estimates were generated with random-effects modeling.
Results
Ten studies were included, involving a total of 760 patients with a mean follow up of 3.0 ± 1.1 years. Patients had a mean age of 53 years, 41% were male, 95.3% had known extra-cardiac sarcoidosis, and 21.6% had known cardiac sarcoidosis. The average ejection fraction was 57.8 ± 9.1%. Patients with late gadolinium enhancement had higher odds for all-cause mortality (odds ratio [OR]: 3.06, p < 0.03) and higher odds of the composite outcome (OR: 10.74, p< .00001) compared to those without LGE. Patients with LGE had an increased annualized event rate of the composite outcome (11.9% v. 1.1%; p<0.0001)
Conclusions
In patients with known or suspected cardiac sarcoidosis, the presence of late gadolinium enhancement on cardiac MRI is associated with increased odds of both all-cause mortality and arrhythmogenic events.
Keywords: Cardiac MR, late gadolinium enhancement, cardiac sarcoid, cardiovascular outcomes
Introduction
Sarcoidosis is an inflammatory granulomatous disease of unknown origin, characterized histologically by non-caseating granulomas in multiple organs, including the lungs, skin, lymphatics, and central nervous system (1). Cardiac involvement is associated with ventricular arrhythmias, sudden cardiac death, and congestive heart failure. It is thought that two-thirds of sarcoid-related deaths are attributable to involvement of the myocardium (2-4). Thus, clinical diagnosis of cardiac sarcoidosis (CS) is crucial for timely therapeutic management and consideration of immunosuppressive therapies. Furthermore, a better understanding of cardiovascular risk factors in this population could have implications for device therapy for the prevention of sudden cardiac death.
Cardiac magnetic resonance imaging (CMR) has been shown to have excellent diagnostic accuracy for detection of CS and is becoming the gold standard for its diagnosis (5, 6). CMR may detect myocardial edema and inflammation using T2 weighted imaging as well as detect myocardial scarring and fibrosis using late gadolinium enhancement (LGE) (7). Multiple recent studies have been published regarding CMR assessment of prognosis in CS, in particular examining the presence of LGE and its association with adverse outcomes (8-10). However, the broad applicability of many of these studies is limited because they are small and single-centered. Prognostic validation of CMR is crucial, as the presence of LGE is thought to confer a higher risk of major adverse cardiac events such as new or worsening heart failure, life-threatening arrhythmias, and sudden cardiac death resulting from myocardial scarring and fibrosis as has been demonstrated in other cardiac pathologies (11-13).
In the current environment of escalating medical costs, the prognostic utility of CMR may help justify its use and guide therapies in patients with sarcoidosis. Prognostic CMR data might provide valuable information for risk stratification and resource allocation, such as clarifying which patients benefit from ICD placement or when immunosuppressive medications, which have significant patient side-effects, are indicated.
Given the multiple small and single-centered studies, we performed a systematic review and meta-analysis of studies reporting prognostic data from patients undergoing CMR for evaluation of known or suspected cardiac sarcoidosis.
Methods
Search strategy
To identify eligible studies for inclusion in the current systematic review and meta-analysis, three independent reviewers (GCC, PS, and PB) systematically searched (July 2015) PubMed, Cochrane, and meta-Register of Controlled Trials for studies assessing prognosis in patients undergoing CMR with known or suspected cardiac sarcoidosis. Keywords used were “sarcoid late gadolinium enhancement”, “sarcoid delayed enhancement”, and “cardiac MRI and sarcoid.” Since the initial search, no further articles have been identified as of December 2015. Studies were considered eligible for inclusion if CMR was used (alone or in addition to other imaging modalities) to assess for myocardial scarring from biopsy-proven or clinically suspected sarcoidosis; in cohorts of ≥ 5 patients; with ≥ 1 year of prognostic follow-up data, including event data for ventricular arrhythmia, sudden cardiac death, aborted cardiac death and/or appropriate ICD discharge, hospital admission for congestive heart failure, cardiac mortality, and all-cause mortality. Studies with populations known to have coronary artery disease or cardiomyopathies of non-sarcoid etiology were excluded.
In addition, we consulted experts, reviewed citations from eligible studies, and contacted some authors for additional unpublished data. The search was limited to studies published in peer-reviewed journals, therefore excluded trials presented in abstract form only. We restricted the review to studies that enrolled adults only with no language restriction. The current systematic review and meta-analysis was performed in accordance with guidelines of the MOOSE (Meta-analysis of Observational Studies in Epidemiology) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) groups (14, 15).
Study selection
Three investigators (GCC, PS, and PB) independently and in duplicate scanned all abstracts and obtained full-text reports of articles that indicated or suggested eligibility. After obtaining full reports, the same reviewers independently assessed eligibility from the full-text articles, with divergences resolved after consensus.
The quality of included studies was assessed by two investigators (JAG, GCC) using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (16), in which the quality of the selected trials was determined on the basis of selection of the study groups (0 to 4 points), comparability of the study groups (0 to 2 points), and ascertainment of the outcome of interest (0 to 3 points).
Data collection
Data abstraction and study appraisal were performed by the same aforementioned investigators. Clinical outcomes of interest were cardiovascular death, all-cause mortality, and a composite of arrhythmogenic events defined as ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation), sudden cardiac death, or aborted sudden cardiac death (appropriate ICD discharge) during follow-up. Clinical outcomes data was directly abstracted. Annualized event rates were calculated for studies by dividing the number of events by the follow-up duration.
Data analysis
Dichotomous variables are reported as proportions (percentages); continuous variables are reported as mean ± SD or median (range). Binary outcomes from individual studies were combined with a random-effects model, leading to computations of odds ratios (ORs) and 95% confidence intervals (CIs). I2 was calculated as a measure of statistical heterogeneity, with values of 25%, 50%, and 75% representing mild, moderate, and severe inconsistency, respectively. Small study or publication bias was explored with funnel plots and Egger's test. Finally, meta-regression and sensitivity analyses (including exclusion of one study at a time) were conducted to explore heterogeneity.
Statistical analysis was performed using Review Manager (RevMan) version 5.3.5 freeware package (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and R version 3.2.2, with statistical significance for hypothesis testing set at the 0.05 two-tailed level. Meta-regression analysis was performed using the package “metafor” in R. For studies with zero events in a group, the convention of adding 0.5 events to all cells was adopted (17).
Results
Results of the literature search
The literature search identified 519 relevant abstracts of full-text articles: 58 unique articles were abstracted for review, 27 of these warranted full-text review, 17 articles were excluded for various reasons including cohort overlap with other articles, lack of specified outcomes, or incomplete CMR data (18-33). Ten articles remained for detailed study (6, 8-10, 34-39). Details of the search strategy are outlined in the QUORUM diagram in Figure 1.
Figure 1. QUORUM Diagrama.
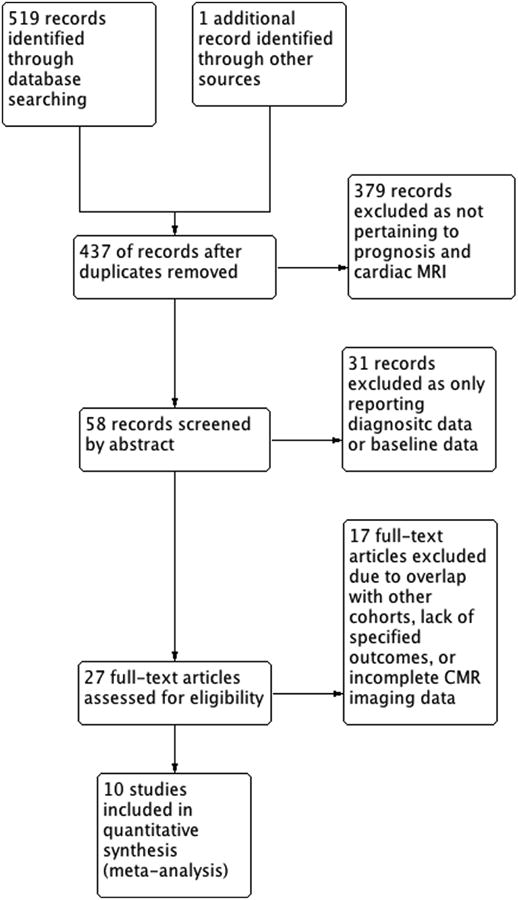
Overview of the Study Review Process
Study Characteristics
Study characteristics are presented in Table 1. The 10 studies included a total of 760 patients with known or suspected cardiac sarcoidosis undergoing CMR. Four studies were prospective and three studies were multi-center. The follow up duration ranged from 1.5 years to 4.9 years with a weighted mean follow-up duration of 3.0 ± 1.1 years. Baseline patient characteristics are shown in Table 2. Patients had a weighted mean age of 53 ± 10.0 years and 41% were male. The weighted average ejection fraction was 57.8 ± 9.1%. 95.3% of patients had known extra-cardiac sarcoidosis and 21.6% had known cardiac sarcoidosis. The prevalence of LGE ranged from 13% to 89% with a weighted mean prevalence of LGE of 33%. The prevalence of LGE in each study had a strong negative correlation with the mean LVEF (R=0.95, p<0.001) (Figure 2). Eight of the studies included patients undergoing CMR at 1.5 T; the remaining two studies do not report MRI field strength. Data for immunosuppressive therapy including corticosteroid use was inconsistently reported.
Table 1. Study Characteristics.
| Study | Year | No. of Pts included herein | Average Follow-up (yrs) | Outcome measure | Study design | Quality Assessment Score | Population |
|---|---|---|---|---|---|---|---|
| Blankstein et al (34) | 2014 | 39 | 1.5 | death from any cause or sustained VT | prospective, single-center | 4,2,3 | known or suspected cardiac sarcoidosis referred for PET, no hx of CAD or MI |
| Crawford et al (35) | 2014 | 51 | 4.0 ± 1.7 | VT/VF free survival | retrospective, multi-center | 4,2,3 | biopsy-proven extra cardiac sarcoid, LVEF > 35%, and diagnosis of cardiac involvement |
| Greulich et al (8) | 2013 | 153 | 2.6 | death, aborted SCD, appropriate ICD shock, VT, VF | prospective, multi-center | 4,1,3 | biopsy-proven or clinical systemic sarcoidosis with suspected cardiac involvement, no hx of CAD or MI |
| Murtagh et al (36) | 2015 | 205 | 3.0 ± 1.5 | death, VT | retrospective, single-center | 4,1,3 | biopsy-proven extracardiac sarcoid, LVEF > 50% |
| Nadel et al (9) | 2015 | 106 | 3.1 ± 1.7 | composite (SCD, VT, VF), all-cause death, SCD/aborted SCD | retrospective, single-center | 4,2,3 | biopsy-proven extracardiac and/or presumed cardiac sarcoidosis |
| Nagai et al (10) | 2014 | 61 | 4.2 ± 1.0 | composite (all-cause death, HF admission, ventricular arrhythmia, bradyarrhythmia requiring pacemaker) | prospective, single-center | 4,2,3 | histological and/or clinically diagnosed extracardiac sarcoid, no cardiac symptoms, and LVEF > 50% |
| Patel et al (6) | 2009 | 81 | 1.8 ± 0.7 | death, ICD shock, pacemaker requirement | prospective, single-center | 4,2,3 | biopsy-proven extracardiac sarcoidosis without known cardiac involvement, no hx of CAD or MI |
| Poyhonen et al (37) | 2014 | 8* | ≥ 2 | VT, VF | retrospective, single-center | 4,2,3 | suspected non-ischemic cardiomyopathy, no hx of CAD or MI. (*86 total patients; 8 diagnosed with cardiac sarcoidosis) |
| Shafee et al (38) | 2012 | 37 | 3.8 ± 2.6 | VT, VF and composite (VA, HF admission, cardiovascular mortality) | retrospective, single-center | 4,2,3 | diagnosed cardiac sarcoidosis (revised JMHW criteria) |
| Watanabe et al (39) | 2013 | 19 | 4.9 ± 1.4 | death, VT, VF | retrospective, multi-center | 4,1,3 | diagnosed cardiac sarcoidosis (revised JMHW criteria) |
Table 2. Baseline Patient Characteristics.
| Study | Total n | Included n | LG E+ (%) |
Age (yrs) |
Mal e (%) |
White (%) | African American (%) |
LV EF (%) |
LV EDV (mL) | Know n extra- cardia c sarcoi d (%) |
Know n cardia c sarcoi d (%) |
Any steroid use (%) |
Syncope (%) | Palpitations (%) | CHF NYHA I-II (%) |
CHF NYHA III (%) |
CHF NYHA IV (%) |
ICD/pacemaker at any point (%) |
Baseline EKG - any BBB (%) |
Baseline EKG - any AV block (%) |
Prior VT (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blankstein et al (34) | 118 | 39 | 67 | 51.5±11.2 | 57 | 77 | 16 | 47±16 | 154±90 | 18 | 32 | 26 | 17 | 6 | 14 | NR | NR | 54 | 24 | 37 | 20 |
| Crawford et al (35) | 51 | 51 | 63 | 51.1±10.3 | 16 | 45 | 47 | 52±9 | 175±55 | 100 | 8 | 47 | 2 | 37 | 94 | 6 | 0 | 61 | 27 | 6 | 22 |
| Greulich et al (8) | 155 | 153 | 25 | 49.7±13 | 59 | NR | NR | 63 | 126 | 98 | NR | NR | 6 | 30 | NR | NR | NR | 8 | NR | NR | NR |
| Murtagh et al (36) | 205 | 205 | 20 | 56±7 | 31 | NR | 59 | 61±5.6 | 72.9±14.9 | 100 | 0 | 47 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Nadel et al (9) | 106 | 10 6 | 30 | 51±12.2 | 57 | NR | NR | 56.6±10.5 | NR | 75 | 30 | NR | NR | NR | NR | NR | NR | 22 | NR | NR | NR |
| Nagai et al (10) | 61 | 61 | 13 | 57±15 | 34 | NR | NR | 63.1±7.1 | 104.6±23.9 | 100 | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 2 | 7 | 7 | 0 |
| Patel et al (6) | 81 | 81 | 26 | 46±11 | 38 | 26 | 73 | 56 | 101 | 100 | 12 | 91 | 2 | 7 | 4 | 1 | 0 | 11 | 6 | 4 | NR |
| Poyhonen et al (37) | 86 | 8* | 88 | 51 | 6 | NR | NR | 43 | 77 | 1 | 0 | NR | 3 | 9 | NR | NR | NR | NR | 1 | 10 | 8 |
| Shafee et al (38) | 61 | 37 | 70 | 57±12 | 30 | NR | NR | 50±16 | 52±8 | 88 | 100 | NR | NR | NR | 82 | 15 | 3 | NR | NR | NR | NR |
| Watanabe et al (49) | 19 | 19 | 89 | 58.2±10.9 | 11 | NR | NR | 37.5±19.8 | 175.2±76.4 | 89 | 100 | NR | 5 | 32 | NR | NR | NR | NR | 5 | 37 | NR |
| TOTALS | 943 | 760 | 95.3** | 21.6** | |||||||||||||||||
| Weighted Mean | 33 | 52.5±10.0 | 48 | 57.8±9.1 | 91.6±16.6 | **% calculated from number of patients | 27.9 | 2.7 | 10.5 | 11.4 | 1.4 | 0.2 | 12.9 | 4.4 | 4.3 | 2.6 | |||||
NR: Not Reported
8 diagnosed with cardiac sarcoidosis; others with non-sarcoid cardiomyopathies
Figure 2. Correlation of LGE and LVEF.
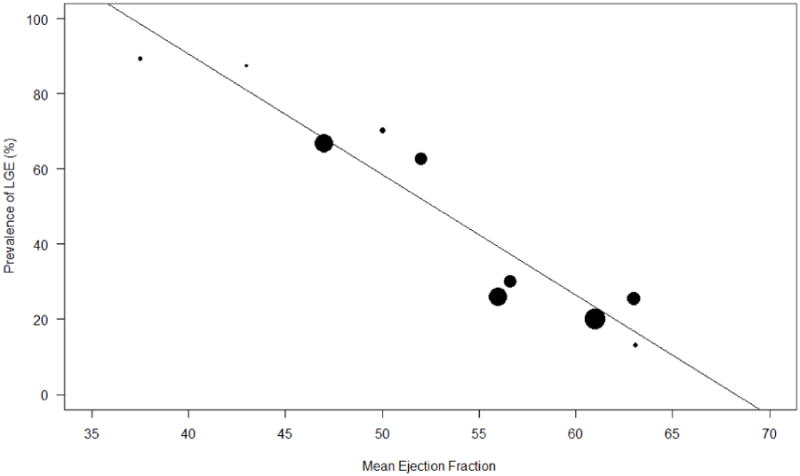
Relationship between LGE prevalence and mean LVEF in each study population (R=0.9526, p<0.001)
Study Quality
Overall, the included studies were of high quality, with all 10 studies receiving maximal scores on the Newcastle-Ottawa Quality Assessment Scale in the areas of study group selection and ascertainment of the desired outcome (Table 1). Seven of ten studies also received maximal scores in the third domain of comparability of study groups. Thus, the pooled data from these high quality studies is collectively robust.
Late Gadolinium Enhancement and Cardiovascular Outcomes
Composite Endpoint
Of the 10 studies reporting outcome data for ventricular arrhythmias, sudden cardiac death, appropriate ICD discharge/aborted SCD, and all-cause mortality, patients with LGE had greater odds of having the combined outcome of arrhythmogenic events plus all-cause mortality compared to those without LGE (overall OR: 10.74 [95% CI: 4.12 to 27.90]; p < 0.00001, I2 = 45%) (Figure 3). When comparing annualized event rates for the composite endpoint, patients with LGE had significantly higher rates of events than patients without LGE (11.9% vs. 1.1%; p<0.001) (Figure 4).
Figure 3. Forrest Plot for Composite Outcome.
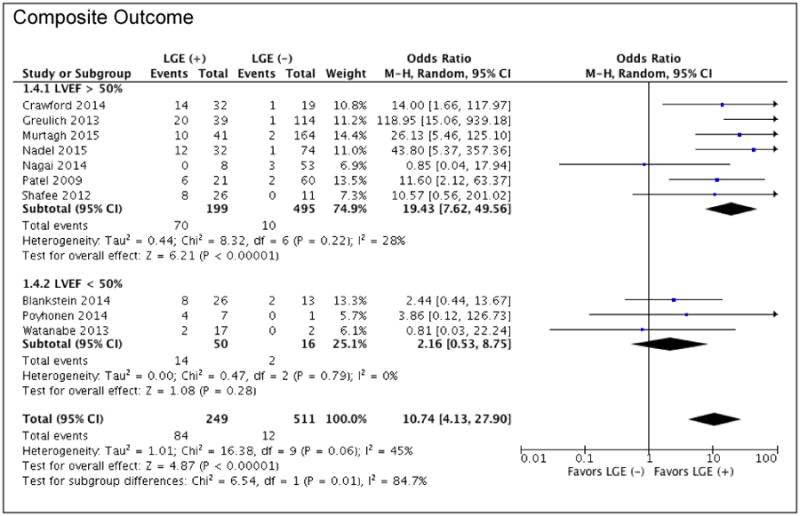
Clinical outcomes of patients with known or suspected cardiac sarcoid with the presence or absence of LGE on CMR: Composite outcome of all-cause mortality plus arrhythmogenic events stratified by LVEF; arrhythmogenic events defined as ventricular arrhythmias (VT/VF), sudden cardiac death, and appropriate ICD discharge / aborted SCD.
Figure 4. Annualized Event Rates of Cardiovascular Outcomes for CMR.
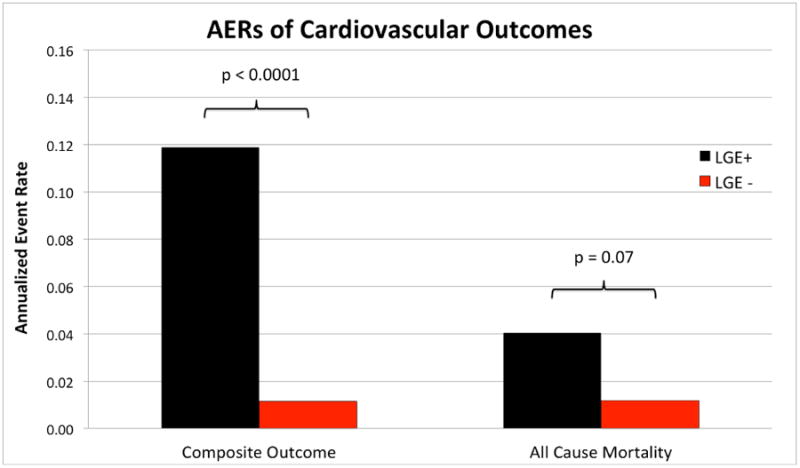
Weighted mean annualized event rates for the composite outcome of mortality plus arrhythmogenic events and all-cause mortality comparing patients with myocardial scarring as evidenced by the presence of LGE (black bars) with patients without LGE (red bars).
Moderate heterogeneity (I2=45%) was noted in the meta-analysis. To investigate this heterogeneity, we performed meta-regression to determine whether any clinical variables were associated with the composite cardiovascular outcome. There was adequate data to explore the effects of gender, age, LVEF, percentage of patients with known extra-cardiac sarcoidosis, and duration of follow-up using a mixed-model approach. LVEF was the only significant covariate and inclusion of LVEF in the meta-regression model accounted for all remaining heterogeneity (I2=0%).
The OR for the association of LGE with adverse events was higher in studies with greater mean LVEF. However, the total prevalence of events was higher in studies with a mean LVEF<50% (24%) than those with mean LVEF≥50% (11%). Two of the larger studies (10, 36) had a pre-specified LVEF cutoff of ≥50%, and to explore this association further we performed a stratified analysis based on this LVEF cutoff (Figure 3). Among studies with a mean LVEF≥50%, the presence of LGE was associated with greater odds of the combined endpoint (OR 19.43 [95% CI: 7.62 to 49.56], p<0.00001), with only mild-moderate residual heterogeneity (I2=28%). In this population, the AER for the composite outcome was significantly greater for those with LGE compared to those without LGE (11.59% vs. 0.69%, p=0.0011). In contrast, among studies with mean LVEF<50%, where there was a very high prevalence of LGE positivity, patients with LGE were not at increased odds of having the composite endpoint.
Mortality Endpoints
From seven studies reporting all-cause mortality, patients with LGE had significantly greater odds of death from any cause compared to patients without LGE (OR: 3.06 [1.14 to 8.20]; p =0.03, I2 = 37%) (Figure 5). Of these studies, only one (Watanabe et al.) had a mean LVEF<50%. A trend towards a higher AER for all-cause mortality was also noted in patients with LGE compared to those without LGE (4.0% vs. 1.2%; p=0.07) (Figure 4).
Figure 5. Forrest Plot for All-Cause Mortality.
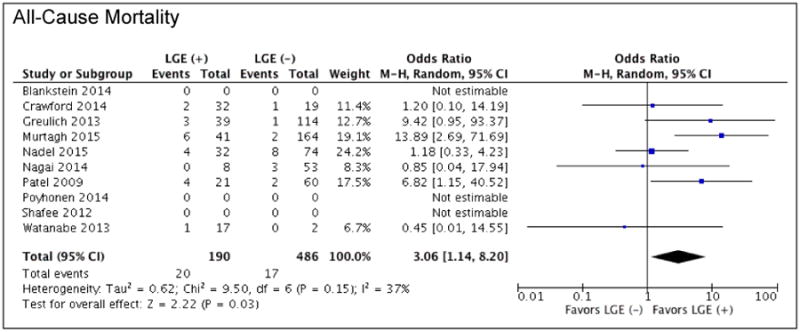
Clinical outcomes of patients with known or suspected cardiac sarcoid with the presence or absence of LGE on CMR: all-cause mortality.
Only three studies provided specific data for cardiovascular mortality including 151 patients. No significant association between the presence of LGE and increased odds of cardiovascular death (OR: 3.24 [0.43 to 24.63]; p=0.26, I2 = 31%) was seen.
Study Variability
Two studies included in the analysis (10, 39) individually showed near-neutral odds ratios for the composite outcome. This discordance may be explained on the basis of individual study characteristics. Watanabe et al. retrospectively studied 19 subjects with cardiac sarcoid; 17 of the 19 patients (89%) demonstrated LGE and the mean EF was 37.5%. Only two events among all 19 subjects were noted, both in LGE(+) patients. The neutral OR from this study is likely due to the small sample size and biased distribution. Nagai et al. prospectively studied 61 patients with known extra-cardiac sarcoid, no evidence of cardiac involvement, and LVEF>50%. In this cohort, only 13% of patients had LGE and the overall event rate was low in both groups (there were three total events, all non-cardiac in nature among patients without LGE), which likely lead to the neutral OR for the composite endpoint. These two studies only contributed 16% weight to the overall meta-analysis.
Assessment of bias
Visual inspection of funnel plots and Egger's test for funnel plot asymmetry did not demonstrate significant asymmetry. Sensitivity analysis, which was performed by excluding one study at a time from the outcomes analysis, demonstrated that the measured effect for the composite cardiovascular outcome was not sensitive to any individual studies. However, sensitivity analysis in the model for all-cause mortality did demonstrate sensitivity to three included studies (6, 8, 36), as exclusion of one of these studies at a time no longer rendered the model significant. Notably, these are three of the larger studies and therefore had the largest effect sizes.
Discussion
The findings of this systematic review and meta-analysis show that the presence of myocardial scarring as evidenced by the presence of LGE in CMR provides meaningful prognostic information in patients with known or suspected cardiac sarcoidosis. The data demonstrates that patients with LGE have increased likelihood of death from any cause as well as increased odds of future arrhythmogenic events. The correlation of LGE and adverse outcomes seen in this meta-analysis supports the role of cardiac MRI for detection of cardiac involvement in patients with sarcoidosis when cardiac involvement may not be evident clinically. Our findings also support prior work advocating cardiac MRI in patients with suspected cardiac sarcoidosis and normal LVEF (18).
Multiple prior studies have shown equivocal or insignificant associations between LGE and future risk of death or ventricular arrhythmias (25, 40, 41) which may be due to population differences or differences in MRI techniques as pointed out in the 2014 Heart Rhythm Society (HRS) Expert Consensus Statement (42). The studies that do show an association between myocardial scarring and worse prognosis are small (8, 9, 38). Despite this limited data, the Heart Rhythm Society reached a consensus that CMR imaging for the purpose of sudden death risk stratification was reasonable in patients with cardiac sarcoidosis, even in those with LVEF >35%. This meta-analysis helps to validate the HRS position statement by bolstering the growing body of evidence showing an association between LGE and adverse outcomes and justifying the role for CMR in patients with known or suspected cardiac sarcoidosis, including those with near-normal LVEF. The current analysis shows that the presence of LGE in sarcoid patients with normal or near-normal LVEF is prognostically significant and greatly increases the likelihood of adverse events.
Implications for ICD and Future Directions
The 2014 HRS guidelines indicate that sarcoid patients with LGE on CMR and normal LVEF should have an EP study; if the EP study is positive, then an ICD may be indicated (Class IIa recommendation). The results of this meta-analysis may justify consideration of device therapy without further EP testing. Further prospective studies are needed to clarify the role of both CMR and EP testing with regard to ICD implantation in patients with cardiac sarcoidosis. Although key concerns regarding inappropriate shocks and adverse events related to device therapy remain (43), this new data should be considered when deciding on ICD implantation given the adverse prognosis associated with myocardial scarring in patients with cardiac sarcoid. As the optimal management of CS patients continues to evolve, there is a need for prospective studies enrolling patients with normal EF and reduced EF to further evaluate the interaction between LVEF and myocardial scarring on cardiovascular outcomes. Outcomes analysis adjusted for LVEF was only available for two of the included studies and was inadequate for pooled analysis. In the study by Nadel et al. (N=106), adjusted Cox analysis including LVEF and the presence of LGE demonstrated that the presence of LGE was the only independent variable that was predictive of the composite cardiovascular outcome (Hazard ratio 12.52, 95% C.I. 1.35–116.18, P < 0.03). Multivariate Cox regression analysis by Greulich et al. (N=155) including the presence of LGE and the initial LVEF demonstrated that LGE presence was the best independent predictor of the composite endpoint (Hazard ratio 31.6, p=0.0014). Patient-level data was available for the cohort in Murtagh et al. (N=205) and we performed a multivariate analysis including LVEF and LGE and found that LGE was an independent predictor of adverse outcomes (hazard ratio 29.79, 95% CI 6.05-146.76, p<0.0001). In the current analysis, the majority of adverse cardiovascular events (73%) were in LGE+ patients with a mean LVEF ≥50%, suggesting that LGE provides risk stratification for adverse events in patients with CS beyond LVEF assessment alone.
Future prospective studies using quantitative assessment of LGE may provide a more nuanced risk stratification model. Furthermore, as the inflammation and fibrosis may be more diffuse in sarcoid, there may be a role for parametric mapping techniques such as T1 or T2 mapping (44, 45).
Study limitations
Certain limitations inherent to systematic reviews are pertinent to the current analysis, including non-uniform reporting of data from included studies and variable duration of follow-up. Additional limitations include heterogeneity of methods for quantifying EF, lack of LGE quantification or pattern data, and variable inclusion criteria, such as a pre-specified LVEF cutoff ≥50% in some studies. As only study-level covariates were available for analysis, the relationship between LVEF and LGE could not be assessed on a per patient basis; future prospective studies may help mitigate selection bias and provide patient-level insights. Despite these differences among studies, we demonstrate that meta-regression analysis showed no residual heterogeneity when LVEF was accounted for (I2 = 0%).
Finally, we were only able to include a composite of all-cause mortality and arrhythmogenic events due to insufficient breakdown of events in some of the studies. However, the direction of effect was similar to that of all-cause mortality. In the studies separately reporting arrhythmogenic events, the effect size for the OR was similar to the composite endpoint.
Conclusion
Cardiac MRI with late gadolinium enhancement provides important prognostic risk stratification for patients with known or suspected cardiac sarcoidosis. Patients with the presence of LGE are at increased risk of death from any cause and arrhythmogenic events, even if their cardiac function is normal or near normal. This study illustrates how the presence or absence of LGE likely has important implications for optimizing therapy in patients with known or suspected cardiac sarcoidosis.
Perspectives.
Competency in Medical Knowledge 1: Cardiac MR imaging is excellent in the diagnosis of cardiac sarcoidosis and the presence of late gadolinium enhancement provides prognostic risk stratification.
Competency in Medical Knowledge 2: The presence of late gadolinium enhancement confers an increased risk of death by any cause and arrhythmogenic events in patients with known or suspected cardiac sarcoidosis.
Competency in Patient Care and Procedural Skills: With the ability to provide both diagnostic and prognostic information, cardiac MR imaging should be strongly considered in the management of patients with suspected cardiac sarcoidosis.
Competency in Interpersonal and Communication Skills: Cardiac MR imaging provides important prognostic information to providers which can help direct patient management.
Translational Outlook 1: A prospective registry with strict entry criteria for patients with cardiac sarcoidosis would be helpful to better define the association between LGE and other adverse prognostic factors.
Translational Outlook 2: Additional research in the quantification of LGE in patients with cardiac sarcoidosis may provide more nuanced risk stratification.
Acknowledgments
Funding Sources: Drs. Peter Shaw, Pelbreton Balfour, and Jorge Gonzalez receive grant support from the NIH T32 5T32EB003841.
Dr. Michael Salerno acknowledges grant support from NIH K23 HL112910.
Disclosures: Dr. Salerno receives research support from Siemens Healthcare. Dr. Patel receives research support from Philips Healthcare and Astellas Pharma.
Abbreviations
- AER
Annualized Event Rate
- CS
Cardiac sarcoidosis
- CMR
Cardiac Magnetic Resonance Imaging
- ICD
Implantable Cardioverter-Defibrillator
- LGE
Late Gadolinium Enhancement
- LVEF
Left Ventricular Ejection Fraction
- OR
Odds Ratio
- SCD
Sudden Cardiac Death
- VF
Ventricular Fibrillation
- VT
Ventricular Tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Statement on sarcoidosis. joint statement of the american thoracic society (ATS), the european respiratory society (ERS) and the world association of sarcoidosis and other granulomatous disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee, february 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–10. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 3.Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J. 2009;157:9–21. doi: 10.1016/j.ahj.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) Am J Med. 1977;63:86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 5.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–90. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Patton DJ, Friedrich MG. The emerging clinical role of cardiovascular magnetic resonance imaging. Can J Cardiol. 2010;26:313–22. doi: 10.1016/s0828-282x(10)70396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–11. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Nadel J, Lancefield T, Voskoboinik A, Taylor AJ. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging. 2015;16:634–41. doi: 10.1093/ehjci/jeu294. [DOI] [PubMed] [Google Scholar]
- 10.Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014;146:1064–72. doi: 10.1378/chest.14-0139. [DOI] [PubMed] [Google Scholar]
- 11.Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–7. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: A systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250–8. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: A systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:940–52. doi: 10.1016/j.jcmg.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O'Connell D, et al. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2015;2015 [Google Scholar]
- 17.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. Studies with zero-cell counts (section 16.9.2) [updated March 2011] ed. [Google Scholar]
- 18.Patel AR, Klein MR, Chandra S, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur J Heart Fail. 2011;13:1231–7. doi: 10.1093/eurjhf/hfr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mc Ardle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by (1)(8)F-fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6:617–26. doi: 10.1161/CIRCIMAGING.112.000289. [DOI] [PubMed] [Google Scholar]
- 20.Panda S, Kaur D, Lalukota K, Sundar G, Pavri BB, Narasimhan C. Pleomorphism during ventricular tachycardia: A distinguishing feature between cardiac sarcoidosis and idiopathic VT. Pacing Clin Electrophysiol. 2015;38:694–9. doi: 10.1111/pace.12626. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21:925–39. doi: 10.1007/s12350-014-9901-9. [DOI] [PubMed] [Google Scholar]
- 22.Cheong BY, Muthupillai R, Nemeth M, et al. The utility of delayed-enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy-proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:39–46. [PubMed] [Google Scholar]
- 23.Kron J, Sauer W, Mueller G, et al. Outcomes of patients with definite and suspected isolated cardiac sarcoidosis treated with an implantable cardiac defibrillator. J Interv Card Electrophysiol. 2015;43:55–64. doi: 10.1007/s10840-015-9978-3. [DOI] [PubMed] [Google Scholar]
- 24.Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: Role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2011;4:43–8. doi: 10.1161/CIRCEP.110.958322. [DOI] [PubMed] [Google Scholar]
- 25.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: Diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–35. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 26.Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–81. doi: 10.1111/jce.12401. [DOI] [PubMed] [Google Scholar]
- 27.Orii M, Hirata K, Tanimoto T, et al. Comparison of cardiac MRI and (18)F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complete heart block. Heart Rhythm. 2015;12:2477–85. doi: 10.1016/j.hrthm.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Paz YE, Bokhari S. The role of F18-fluorodeoxyglucose positron emission tomography in identifying patients at high risk for lethal arrhythmias from cardiac sarcoidosis and the use of serial scanning to guide therapy. Int J Cardiovasc Imaging. 2014;30:431–8. doi: 10.1007/s10554-013-0339-y. [DOI] [PubMed] [Google Scholar]
- 29.Smedema JP, Snoep G, van Kroonenburgh MP, et al. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128:1629–37. doi: 10.1378/chest.128.3.1629. [DOI] [PubMed] [Google Scholar]
- 30.Tezuka D, Terashima M, Kato Y, et al. Clinical characteristics of definite or suspected isolated cardiac sarcoidosis: Application of cardiac magnetic resonance imaging and 18F-fluoro-2-deoxyglucose positron-emission tomography/computerized tomography. J Card Fail. 2015;21:313–22. doi: 10.1016/j.cardfail.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama R, Miyagawa M, Okayama H, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015;195:180–7. doi: 10.1016/j.ijcard.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 32.Ise T, Hasegawa T, Morita Y, et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165–72. doi: 10.1136/heartjnl-2013-305187. [DOI] [PubMed] [Google Scholar]
- 33.Betensky BP, Tschabrunn CM, Zado ES, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:884–91. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawford T, Mueller G, Sarsam S, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–15. doi: 10.1161/CIRCEP.113.000156. [DOI] [PubMed] [Google Scholar]
- 36.Murtagh G, Laffin LJ, Beshai JF, et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: Risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9:e003738. doi: 10.1161/CIRCIMAGING.115.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poyhonen P, Holmstrom M, Kivisto S, Hanninen H. Late gadolinium enhancement on CMR and sustained ventricular tachycardia predict severe cardiac inflammation. Acta Cardiol. 2014;69:637–47. doi: 10.1080/ac.69.6.1000006. [DOI] [PubMed] [Google Scholar]
- 38.Shafee MA, Fukuda K, Wakayama Y, et al. Delayed enhancement on cardiac magnetic resonance imaging is a poor prognostic factor in patients with cardiac sarcoidosis. J Cardiol. 2012;60:448–53. doi: 10.1016/j.jjcc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe E, Kimura F, Nakajima T, et al. Late gadolinium enhancement in cardiac sarcoidosis: Characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging. 2013;28:60–6. doi: 10.1097/RTI.0b013e3182761830. [DOI] [PubMed] [Google Scholar]
- 40.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the netherlands. Chest. 2005;128:30–5. doi: 10.1378/chest.128.1.30. [DOI] [PubMed] [Google Scholar]
- 41.Vignaux O, Dhote R, Duboc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: Initial results of a prospective study. J Comput Assist Tomogr. 2002;26:762–7. doi: 10.1097/00004728-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 43.Kron J, Sauer W, Schuller J, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–54. doi: 10.1093/europace/eus316. [DOI] [PubMed] [Google Scholar]
- 44.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–22. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189:109–12. doi: 10.1164/rccm.201309-1668LE. [DOI] [PMC free article] [PubMed] [Google Scholar]


