Abstract
Dietary restriction is known to extend lifespan in many species. It has now been shown to reduce DNA damage and extend lifespan in mice modelling human DNA-repair disorders.
The accumulation of DNA damage is an inevitable side effect of living, and is one of the main causes of cellular and organismal ageing. Compromised DNA repair leads to persistent DNA damage, causing age-related disorders and shortening lifespans. In humans, this can manifest as progeroid syndromes, in which children or adults age at a greatly accelerated rate. On page 427, Vermeij et al.1 demonstrate that a relatively modest degree of dietary restriction can greatly increase the lifespans of two mouse models of these human syndromes.
The authors’ mouse strains harbour mutations in genes involved in a DNA-repair process called nucleotide excision repair (NER). In one strain, a mutation reduces production of the protein ERCC1, which normally forms a complex with a DNA endonuclease enzyme to create DNA breaks and excise damaged sequences. ERCC1 mutations can cause three diseases in humans2 — the accelerated-ageing disorders Cockayne syndrome and XFE progeroid syndrome, and xeroderma pigmentosum, in which people are extremely sensitive to DNA damage by sunlight. In the other mouse strain, a mutation inhibits production of another DNA endonuclease, called XPG. Human XPG mutations can present as xeroderma pigmentosum and Cockayne syndrome3.
It has previously been shown4 that mice harbouring mutations in Ercc1 exhibit many of the metabolic responses to stress that are seen in healthy mice subjected to dietary restriction — in both, biological pathways involved in physiological maintenance are enhanced at the expense of pathways involved in growth. This is thought to be a survival response that helps to protect NER-deficient mice. Vermeij et al. therefore investigated whether dietary restriction could enhance these protective responses in their animal models. Indeed, a 30% restriction led to a substantial increase in lifespan in both strains of mouse, as compared with siblings given unlimited access to food (those fed ad libitum).
A weakness of many investigations into dietary restriction is their failure to carefully investigate physiological and structural traits of the organism under study, which together can be used to gauge healthy lifespan. Vermeij and colleagues’ study is a welcome exception, because it investigates a wide range of relevant traits — including those involving the brain and neuromuscular systems, which are particularly vulnerable to damage in human DNA-repair disorders5. A striking finding was that mutant mice subjected to dietary restriction retained 50% more neurons than did siblings fed ad libitum. Moreover, markers of DNA damage were reduced in the diet-restricted animals (Fig. 1), and transcriptional profiles were better preserved.
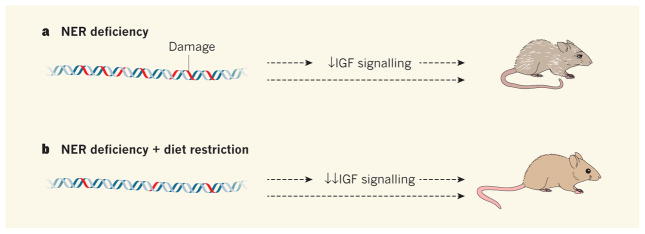
Figure 1. Living better for longer.
a, Nucleotide excision repair (NER) is a process by which DNA damage is repaired. Mice that harbour genetic mutations in the genes Ercc1 or Xpg are NER deficient. DNA damage accumulates, signalling through the protein IGF1 is suppressed, and mice age at an accelerated rate. The pathways by which these processes influence ageing are unclear (dashed arrows). b, Vermeij et al.1 report that restricting the diets of NER-deficient mice reduces DNA damage and further suppresses IGF signalling. The average lifespan of the mice significantly increases compared with counterparts that eat freely, and they remain healthy for much longer.
There were several other interesting findings. For instance, the authors showed that, in ERCC1-deficient mice fed ad libitum, genes that encode large proteins were more damaged than those that encode small ones. This makes sense, because DNA damage occurs randomly. As such, long genes suffer disproportionate amounts of stochastic damage. As another example, the weights of NER-deficient mice fed ad libitum gradually decreased over time, and Vermeij et al. found that these animals died when they reached around the same weight as diet-restricted mutants, which initially lost weight rapidly but then maintained a constant weight. Again, this makes sense — weight loss in mutants fed ad libitum reflects physiological decline, whereas initial weight loss related to scheduled dietary restriction actually enhances physiology.
Dietary restriction has long been known to extend healthy lifespan in many animal species5. In usual ageing, its effects are modulated mainly through inhibition of the IGF1 and mTOR molecular signalling pathways6, which have roles in nutrient sensing. IGF signalling is already suppressed in NER-deficient mice2, so it comes as something of a surprise that the defects seen in these animals can be partially rescued by dietary restriction. Nonetheless, the authors confirmed that the IGF1 and mTOR pathways are further suppressed in the dietary-restricted mutants, indicating that the pathways’ repression modulates lifespan extension, at least in part.
But how does dietary restriction reduce the accumulation of DNA damage? Although Vermeij et al. say it is inconceivable that there is a role for compensatory pathways that enhance DNA repair, it is a speculation that, in our opinion, deserves further research. The authors also speculate that there is an exaggerated response to DNA damage in NER-deficient mice, perhaps as part of an increase in the organism’s response to various stress signals. Concomitant adjustments in metabolic regulation, together with alterations in the function of energy-producing organelles called mitochondria, may also shift cellular metabolism towards roles that protect the genome from damage.
Another observation by Vermeij et al. that might point towards a mechanism for dietary-restriction-dependent reductions in DNA damage is that molecular stress responses are increased in ERCC1-deficient animals. Such stress responses are modulated, in part, by mTOR signalling6. Long-term treatment with rapamycin, a molecule that inhibits mTOR signalling, reduces the accumulation of DNA damage in another genomic-instability disorder, Werner syndrome7. There have been other examples of daily rapamycin treatments causing substantial extensions in lifespan — for instance, rapamycin approximately triples the lifespan of mice that lack a mitochondrial protein called Ndufs4, which is involved in energy production8.
Vermeij and colleagues’ study greatly strengthens the evidence supporting the idea that genomic instability is a major mechanism underlying human progeroid syndromes9. Moreover, modest dietary restriction could be rapidly and cheaply tested in patients with these conditions. There is little doubt that the authors’ findings will lead to peer-reviewed clinical trials of modest dietary restriction, and also probably of mTOR inhibitors, in patients with progeroid syndromes that involve defective DNA repair.
Finally, the study should provide much-needed momentum for efforts to discover pharmacological mimetics of dietary restriction that can be used in humans. But given the enormous genetic and environmental diversity between humans, and the remarkably varied responses of different strains of mice to dietary restriction10, the responses of individuals to such drugs will probably vary greatly. Large-scale clinical trials will be required before dietary restriction can be recommended as a general treatment for protecting genes during usual ageing.
References
- 1.Vermeij WP, et al. Nature. 2016;537:427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manandhar M, Boulware KS, Wood RD. Gene. 2015;569:153–161. doi: 10.1016/j.gene.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulen W, Jaeken J, Jaspers NG, Bootsma D, Hoeijmakers JH. Am J Hum Genet. 1993;53:185–192. [PMC free article] [PubMed] [Google Scholar]
- 4.Niedernhofer LJ, et al. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 5.Laugel V. Mech Ageing Dev. 2013;134:161–170. doi: 10.1016/j.mad.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Partridge L, Longo VD. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha B, Cypro A, Martin GM, Oshima J. Aging Cell. 2014;13:573–575. doi: 10.1111/acel.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SC, et al. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisama FM, Oshima J, Martin GM. Cold Spring Harb Perspect Med. 2016;6:a025882. doi: 10.1101/cshperspect.a025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao CY, Johnson TE, Nelson JF. Exp Gerontol. 2013;48:1025–1029. doi: 10.1016/j.exger.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]