Abstract
The essential oil was obtained by hydrodistillation and the identification and quantification of components were achieved with the use of GC-MS analysis. The antioxidant activity was evaluated by the method of sequestration of DPPH. Essential oils were used for study the cytotoxic front larvae of Artemia salina. In the evaluation of the antimicrobial activity of essential oils, we employed the disk-diffusion method. The potential larvicide in mosquito larvae of the third stage of development of Aedes aegypti to different concentrations of essential oils was evaluated. The major compounds found in the essential oils of M. piperita were linalool (51.8%) and epoxyocimene (19.3%). The percentage of antioxidant activity was 79.9 ± 1.6%. The essential oil showed LC50 = 414.6 μg/mL front of A. saline and is considered highly toxic. It shows sensitivity and halos significant inhibition against E. coli. The essential possessed partial larvicidal efficiency against A. aegypti.
1. Introduction
The International Standard Organization (ISO) defines the essential oils (EO) as volatile products extracted from plants by steam distillation, in most cases. They are usually complex mixtures of volatile, lipophilic, liquid, and odoriferous substances and associated with various functions necessary for the survival of the plant in its ecosystem, playing a key role in the defense against microorganisms and predators and in attracting insects and other fertilizing agents. In traditional medicine, essential oils have a long tradition of use [1, 2].
The Lamiaceae family has potential in obtaining EO and it plays and important role in various sectors of the economy, for example, the pharmaceutical industry, perfumery, cosmetics, and food. Several biological functions are associated with species by popular medicine, used for the treatment of burns, headache, colic, fever, reports of antiflu activity, antiemetic, carminative, insecticide, repellent, antibacterial, and combat intestinal parasites [3–5].
The genus Mentha, popularly known in Brazil as “hortelãs, hortelã-pimenta, menta, menta-inglesa, hortelã-apimentada, hortelã das cozinhas and sândalo,” includes 25–30 species that are known mainly due to the characteristic and refreshing taste. It is native to Europe and was brought by settlers to Brazil, where it is cultivated as medicinal plant in gardens [6, 7].
The literature logs its spasmolytic activities: antivomitive, carminative, stomachic and anthelmintic, topical antibacterial, antifungal, and antipruritic. In the preliminary phytochemical analysis of the essential oil, there was the presence of the main constituents: the majority were menthol, menthone, and menthofuran compounds which were responsible for pleasant odor [8–11].
The Amazon region with its immense biodiversity offers a great potential for the discovery of new flavors and products. However, there is great scarcity of chemical and biological activities information associated with plant species due to deficient support in research work in biotechnology. The aim of this study is to evaluate the chemical composition and in vitro antioxidant, cytotoxic, antimicrobial, and larvicidal activities of the essential oil of Mentha piperita L. (Lamiaceae).
2. Material and Methods
2.1. Collection of Plant Material
The species were collected in the city of Macapá, AP, under the coordinates 00°02′23′′S and 51°06′29′′W and, later, sent to the Herbarium of the Institute of Scientific and Technological Research of the State Amapá, IEPA, for taxonomic identification procedures and preparation of voucher specimen, under the registration number RRS 001.
2.2. Obtaining Essential Oil
The extraction of essential oils was held in Pharmacognosy and Phytochemistry Laboratory of the Federal University of Amapá (UNIFAP). The leaves were treated (washed and dried) for extracting their essential oils. The essential oil was obtained by hydrodistillation (temperature 100°C) in a Clevenger-type apparatus for 4 h [12].
2.3. Chromatographic Analysis (GC-MS)
The essential oil analysis was performed by gas chromatography coupled to mass spectrometry (GC-MS) at the Federal University of São Carlos (UFSCar). We used Shimadzu equipment, GCMS Shimadzu QP 5000, and employed a capillary column of fused silica OPTIMA® 5–0.25 μm, 30 m in length, and 0.25 mm internal diameter and nitrogen as a carrier gas. The gas chromatograph operating conditions were as follows: internal column pressure of 67.5 kPa, split ratio 1 : 20, the gas flow in column 1.2 mL/min. (210°C), injector temperature 260°C, and the temperature detector or the interface (GC-MS) of 280°C. The initial column temperature was 50°C followed by an increase of 6°C/min. up to 260°C and maintained constant for 30 min. The mass spectrometer was programmed to perform readings in a range 29–400 Da, 0.5 s intervals with ionization energy of 70 eV.
2.4. Component Identification
The identification of the constituents of essential oils was performed in comparison with the Kovats Index (KI) of the homologous series of n-alkanes (C8–C26 + C28) and literature [13]. Further identification was made by combining their mass spectra with the registered and stored equipment in the Wiley version 2.5/MBP GC-MS system library.
2.5. Analysis of Antioxidant Activity
Evaluation of antioxidant activity was held in Pharmacognosy and Phytochemistry Laboratory of the Federal University of Amapá (UNIFAP). Based on the methodology proposed, we used 2,2-diphenyl-1-picrylhydrazyl (DPPH) with some modifications [14–16].
A methanol solution of DPPH at the concentration of 40 μg·mL−1 was prepared. The essential oils were diluted in methanol concentrations (5, 1, 0.75, 0.50, and 0.25 mg·mL−1). For the evaluation, they were added into a test tube of 2.7 mL of the stock solution of DPPH, followed by addition of 0.3 mL of the essential oil solution. Meanwhile, the negative control was prepared, this being a mixture of 2.7 mL of methanol and the methanolic solution of evaluated compounds. After 30 minutes, the readings were taken in a spectrophotometer (Biospectro SP-22) at a wavelength of 517 nm. For comparison, ascorbic acid was evaluated.
2.6. Toxicity Test
The toxicity test against A. salina Leach was held in Pharmacognosy and Phytochemistry Laboratory of the Federal University of Amapá (UNIFAP). Initially, 250 mL of synthetic sea salt solution (35.5 g/L) was prepared for incubation of 25 mg of A. salina eggs, which were exposed to artificial light in 24-hour period for onset of lava (metanauplius); then, the metanauplii were separated and placed in the dark for 24 h period to reach nauplii stage [17–19].
The solution was prepared to contain 62.5 mg of essential oil, 28 mL of synthetic sea salt solution, and 2 mL of dimethylsulfoxide (DMSO) to facilitate its solubilization. Subsequently, at the end of the period in the dark, they were selected and divided into 7 groups of 10 subjects in each test tube. Each group has added a solution of the rate (3125, 2500, 1250, 625, 250, 25, and 2.5 μL). The volume of 5 mL with synthetic sea salt solution was reached, to yield final solutions with the following concentrations of 1250, 1000, 500, 250, 100, 10, and 1 μg/mL. Thereby, the groups were designated according to their respective concentration and all tests were performed in triplicate. At the end, the number of nonsurvivors were counted, to determine LC50 through probit analysis of the SPSS® software.
2.7. Antimicrobial Activity
The microbiological activity of essential oils was studied in the Microbiology Laboratory of the Faculty Estacio SEAMA. It used two strains of bacteria, one Gram-positive (Staphylococcus aureus, ATCC 25923) and another,Gram-negative (Escherichia coli, ATCC 25922), being performed according to the rules and procedures of Clinical and Laboratory Standards Institute (CLSI) [20]. The test for evaluation of the antibacterial activity was performed in triplicate.
2.7.1. Culture Media
The medium Mueller-Hinton (MH) agar was used for antimicrobial activity assays and prepared according to manufacturer's instructions, followed by release of 25 mL of agar medium per Petri dish of 90 × 15 mm.
2.7.2. Bacterial Suspension and Inoculum
The growth of Mueller-Hinton agar, after incubation for 24 hours at 37°C, peaked up from 2 to 4 colonies in 1 mL of sterile 0.85% saline solution, until a turbidity similar to the 0.5 McFarland scale obtained a final concentration of 1,5 × 108 UFC/mL.
2.7.3. Evaluation of Antimicrobial Activity
To evaluate the antimicrobial activity of essential oils, we used the disk agar diffusion method. Each bacterial suspension was plated (in triplicate) with the aid of a stereodisposable swab across the surface of Mueller-Hinton agar. A solution was prepared with a solubilized concentration of 250 mg/mL with Tween 80 and then, dilutions were held to obtain concentrations of 100, 50, and 10 mg/mL. After that, it was soaked on filter paper discs (Whatman, type 3) of 6 mm diameter each 10 L concentrations, respectively. After incubation of the plates at 35°C for 24 hours, a reading of the results by measuring the halo formed around disks containing the oil was performed. The result of each dilution, the average of the three measurements, and susceptible halo of an equal size or greater than 8 mm diameter were considered [21, 22].
The disk-diffusion experiment was controlled by using disks containing the antibiotics reference: cefoxitin, oxacillin, and gentamicin to verify the sensitivity of the test microorganism and negative controls were impregnated with discs containing water and Tween 80.
2.8. Larvicidal Activity
2.8.1. Larvae
The larvae of A. aegypti used in the bioassays were from the colony maintained at the Insectarium of the Arthropoda Laboratory at the Federal University of Amapá, all of F6 generation, in the third young stadium, Macapá, Amapá lineage.
2.8.2. Bioassays
The biological tests were conducted in a room (3 m × 4 m) with controlled climatic conditions: temperature 25 ± 2°C, relative humidity of 75 ± 5%, and photoperiod of 12 hours, located at Arthropoda Laboratory of the Federal University of Amapá, Macapá.
The methodology followed the standard WHO protocol with modifications to the test container [23, 24]. After analyzing preliminary test series, the concentrations were selected: 500, 400, 300, 200, and 130 ppm.
A stock solution was prepared with essential oil, presolubilized in Tween 80, and dissolved in 93 mL of water to obtain a concentration of 1500 ppm. From this solution, a dilution series was prepared to obtain the concentrations of solutions 500, 400, 300, 200, and 130 ppm. For each replicate of a treatment, 10 larvae were used, pipetted into a beaker containing 100 mL of distilled water. Then, the larvae were removed from the beaker into the test container, thus minimizing the time between the preparation of the first and last sample. The safety of the solvent was observed in the concentration used, with the same present also in control of the replicas. During the experiment, the average water temperature was 25°C. After 24 and 48 hours, dead larvae were counted, being considered as all those unable to reach the surface. The data obtained from the mortality (%) × concentration (ppm) were analyzed by SPSS in probit graph to determine the lethal concentration that causes 50% mortality of the population (LC50).
2.9. Statistical Analysis
Statistical analysis was performed by analysis of variance (ANOVA). The significant differences between averages were determined by Tukey' test.
3. Results and Discussion
The yield of essential oil obtained from the hydrodistillation process of the species M. piperita was 0.08% (m/m). In total, 15 components were identified, representing 94.9% of the total amount. Compounds identified in the essential oil of the species were as follows: thuja-2,4(10)-diene (0.3%), verbenene (2.6%), β-pinene (3.8%), mentha-2,8-diene (0.4%), β-ocimene (0.4%), linalool (51.8%), epizonarene (0.6%), epoxyocimene (19.3%), sesquiphellandrene (9.4%), cadinene (4.0%), and germacrene B (2.3%).
To the best of our knowledge, this is the first record of the chemical composition of the M. piperita essential oil collected in the city of Macapá, Brazil. The chemical composition of the essential oil (Table 1) shows a high percentage of oxygenated monoterpenes (71.1%).
Table 1.
Major constituents of the essential oils of M. piperita.
| Number | tR (min) | KI | Components | Peak Area (%) |
|---|---|---|---|---|
| 1 | 8.88 | 960 | Thuja-2,4(10)-diene | 0.3 |
| 2 | 9.08 | 967 | Verbenene | 2.6 |
| 3 | 9.96 | 979 | β-Pinene | 3.8 |
| 4 | 10.30 | 983 | Mentha-2,8-diene | 0.4 |
| 5 | 13.20 | 1177 | β-Ocimene | 0.4 |
| 6 | 16.12 | 1096 | Linalool | 51.8 |
| 7 | 18.31 | 1501 | Epizonarene | 0.6 |
| 8 | 18.50 | 1142 | Epoxyocimene | 19.3 |
| 9 | 19.07 | 1522 | Sesquiphellandrene | 9.4 |
| 10 | 19.30 | 1538 | Cadinene | 4.0 |
| 11 | 19.85 | 1561 | Germacrene B | 2.3 |
|
| ||||
| Monoterpene hydrocarbons | 7.5 | |||
| Oxygenated monoterpenes | 71.1 | |||
| Sesquiterpene hydrocarbons | 16.3 | |||
|
| ||||
| Total | 94.9 | |||
tR: retention time; KI: Kovats Index [13].
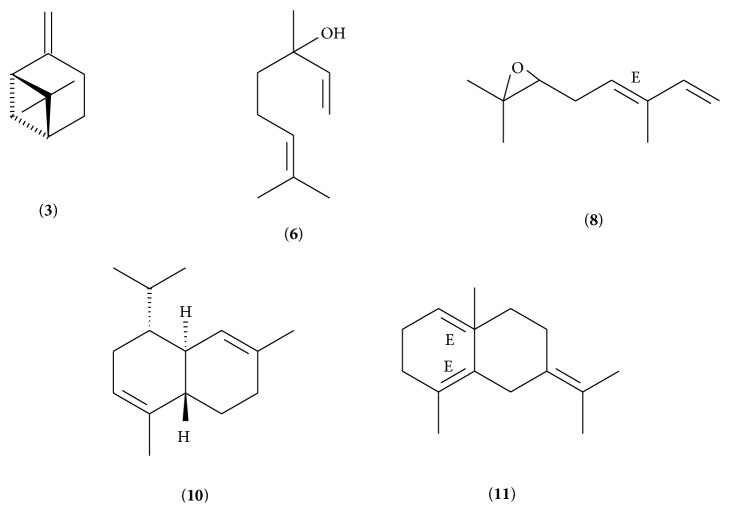
The essential oil of M. piperita contains compounds with properties of biological interest. Some authors believe that the linalool has antifungal, antimicrobial [25, 26], antitumor [27], antimutagenic [28], analgesic, antispasmodic [29], anti-inflammatory [30], antiparasitic [31], antiplatelet, and antioxidant activity [32]. The chemical structure of major compounds identified in the essential oil of the species is shown in Figure 1.
Figure 1.
Chemical structure of major compounds identified in the essential oil.
Other studies of Sartoratto et al. [33] showed that the essential oils obtained from the dried leaves of the species M. piperita have the major compound linalool (51.0%) in its chemical constitution. The results are in agreement with previous studies of M. piperita essential oil collected in Brazil, characterizing linalool (51.8%) as the most important major component. However, several studies of the chemical composition found that, in other countries, menthol is the major component. The diversity of chemical compounds in the essential oil of the species is attributed to soil factors, biosynthetic, and collection time [34–38].
3.1. Antioxidant Activity
The antioxidant activity (ability or antioxidant potential) is a widely used parameter to characterize different biological materials. This activity is related to compounds capable of protecting a biological system against the harmful effects of processes or reactions that cause excessive oxidation involving reactive oxygen species [39, 40].
The vast majority of compounds with antioxidant properties have a molecular structure having at least one aromatic ring and a hydroxyl group, including phenols, flavonoids and isoflavones, esters, lignin, coumarins, flavones, and oligomeric proantocianidinas. In mixtures of these compounds, an antioxidant arrangement is produced that may act by different mechanisms to give an effective defense system against free radicals. Alcohols are the second class of oxygenated monoterpenes, which comprise the essential oil, and more active in oxidizing activities [41].
The mean values of percentage of antioxidant activity (%AA) of essential oils are shown in Table 2.
Table 2.
Percentages results of antioxidant activity (% AA) of essential oil.
| Species | Concentration (mg·mL−1) | |||||
|---|---|---|---|---|---|---|
| 5 | 1 | 0.75 | 0.5 | 0.25 | IC50 | |
| M. piperita | 79.9 ± 1.7a | 54.9 ± 1.8bf | 53.0 ± 0.3cf | 47.2 ± 0.5dg | 47.6 ± 1.04eg | 0.54 |
Values (% AA) that followed the same characters do not show statistically significant differences for ANOVA (p < 0.05).
The species M. piperita has significant antioxidant activity and p < 0.0001 in the concentration of 100 mg·mL−1 with % AA 79.9 ± 1.6. The antioxidant activity is associated with the presence of linalool major compound (51.80%). The inhibitory concentration (IC50) by linear regression presented a value of 0.54 mg·mL−1, showing the strong coefficient correlation (R 2) of 0.9770. On the other hand, if polar compounds, such as ascorbic acid (IC50 0.12 mg·mL−1) compared to the essential oil, were only tested by it, they would be considered weak antioxidants.
The results of antioxidant activity are similar to previous studies of Derwich et al. [35] % AA values ≥ 81.09 in the concentration of 150 μg·mL−1 and IC50 ≥ 53.67 μg·mL−1. However, previous studies of essential oil species showed menthol as the major compound, in which the radical scavenging activity is associated with the presence of compounds menthone and menthol, both compounds with the presence of the hydroxyl radical (-OH). The species M. piperita is described by several authors as a potential antioxidant, and there are also reports of cytotoxic activity. Some authors describe the possible elimination of monoterpene ketones (menthone and isomenthone) and 1,8-cineole in changing the chemical composition of the species [35].
3.2. Toxicity Test
Table 3 refers to the average mortality readings performed within 48 h, in the cytotoxic activity of the essential oil of M. piperita. The results are significant and expressed in percentage of mortality (%).
Table 3.
Results of the mortality of M. piperita front toxicity test against larvae of A. salina.
| Species | Concentration (μg·mL−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1250 | 1000 | 500 | 250 | 100 | 10 | 1 | LC50 | |
| M. piperita | 95.4a | 80.7b | 60.3c | 45d | 20e | 12e | 0f | 414.6 |
Different lowercase characters represent significant differences in mortality between the concentrations of the oils.
The essential oil of M. piperita showed LC50 414.6 μg·mL−1 and correlation coefficient of 0.7443, confirming its moderate toxicity front of A. salina. The oil of Mentha spp. has a calming pain action and is clinically used as an ingredient in many analgesics creams and in the treatment of arthritis and other musculoskeletal conditions. The chemical compound menthol relieves discomfort by afferent modulator, causing pain impulses, as part of an astringent effect, and exciting the nerves that recognize the sensation of cold.
Essential oils were characterized as good bioactive agents, because of oils and extracts from plants presenting LC50 values below 1000 μg·mL−1 and previous studies by Andrade et al. [41], Meyer et al. [42], and Nascimento et al. [43] considered them as bioactive. Specifically, oils and plant extracts have degrees of toxicity against larvae of A. salina. According to the intervals, they demonstrated activity at or below 100 μg·mL−1 and were considered as having a strong cytotoxic activity. LC50 values between 100 and 500 μg·mL−1 were considered to be moderately toxic. LC50 values between 500 and 1000 μg·mL−1 were considered as weak cytotoxic activity, while LC50 values greater than 1000 μg·mL−1 were categorized as nontoxic [44].
Several dangerous and even fatal side effects have been reported about herbal products. These side effects may occur by several different mechanisms, including direct toxicity, contamination, and interactions with drugs and other herbs. The essential oil of M. piperita is associated with side effects such as heartburn, nausea, vomiting, allergic reactions, flushing, and headaches.
3.3. Antimicrobial Activity
Natural products have become an excellent therapeutic alternative, where the research for plant compounds that have some pharmacological property has intensified significantly in recent years. That has awakened new looks to the Amazon region, because its flora is estimated at more than 55,000 species and less than 1% of it has been scientifically investigated [45, 46].
A Gram-positive bacterium protects its cytoplasmic membrane with a thick cell wall. The layers of peptidoglycan prevent the passage of hydrophilic compounds due to the presence of sugars and amino acids. The outer lipoprotein membrane presents characteristics and the bacteria need to have a mechanism enabling the entry of hydrophilic compounds like sugars, amino acids, and ions. Therefore, the outer membrane has special channels called porins, which allow the passive diffusion of hydrophilic compounds [47].
The oils mechanism of action about antimicrobial effect is acting directly on the bacterial cell wall structure, denaturing and coagulating proteins. Specifically, they act by altering the permeability of the cytoplasmic membrane by hydrogen ions (H+) and potassium (K+). The amendment of the ion concentration gradients leads to deterioration of the essential processes of cell, such as transport of electrons, translocation proteins, stages of oxidative phosphorylation, and other reactions dependent enzymes, resulting in loss of chemiosmotic control of the affected cell, leading to cell death [37].
Tables 4 and 5 refer to the halo measurement results of inhibition of essential oil M. piperita (expressed in mm) against strains of Staphylococcus aureus and Escherichia coli.
Table 4.
Measurement results of the inhibition halos (mm) of the essential oil of M. piperita against strains of Staphylococcus aureus.
| Species | Concentration | |||
|---|---|---|---|---|
| 100 mg·mL−1 | 50 mg·mL−1 | 10 mg·mL−1 | 1 mg·mL−1 | |
| M. piperita | 7.6 ± 0.57 | NA | NA | NA |
NA: did not present an inhibition halo.
Table 5.
Measurement results of the inhibition halos (mm) of the M. piperita essential oil against Escherichia coli strains.
| Species | Concentration | |||
|---|---|---|---|---|
| 100 mg·mL−1 | 50 mg·mL−1 | 10 mg·mL−1 | 1 mg·mL−1 | |
| M. piperita | 7.6 ± 0.57 | 7 ± 0.0 | 9 ± 1.0 | 7.3 ± 0.57 |
The essential oil of M. piperita has submitted inhibition zone in 100 mg·mL−1 concentrations at 7.6 ± 0,57 mm and 10 mg·mL−1 with 9 ± 1.0 mm for S. aureus and E. coli, respectively. Compared with positive control and concentrations tested, there was significant difference between the inhibition zones, in which the halo in the positive control showed halo of 15 mm. The nonoccurrence of menthol may have influence on the occurrence of only one strain of bacteria, whereas linalool is also described such as potential agent in the control of bacteria.
The extract of M. piperita being either an essential oil or an alcoholic extract has antimicrobial properties [48]. The linalool is a major component and it contributes to the antimicrobial potential of this plant. This potential essential oil of M. piperita has proven in this study that the results show antimicrobial activity only for the bacteria E. coli.
3.4. Larvicidal Activity
Several studies demonstrate the activity of essential oils and plant extracts against different species of mosquitoes including A. aegypti [49–51]. The study of potential larvicide, insecticide, and repellent of essential oils is an alternative technology to synthetic products destined to vector control, since, to date, there is no vaccine ready for use against the four serotypes of dengue. Active agents at potential larvicide essential oils and plant extracts at LC50 values of <100 ppm are considereed.
The researches for vector control come showing the efficiency of the larvicidal effect of essential oils. Some studies detect the effects of the chemical composition of monoterpenes, as well as, some sesquiterpenes, which serve as repellents at toxicity significant to insects but negligible toxicity to mammals. Mixtures of these volatile low molecular weight compounds provide plants, such as Mentha piperita, Citrus limon, Ocimum basilicum, and Salvia officinalis, with their distinctive odor, which are commercially available, produced, and important ingredients for fragrance and flavor creation because of their specific sensory characteristics [52].
Table 6 refers to the average mortality readings performed in the period of 48 h of the larvicidal activity of essential oils from M. piperita. The results are significant and expressed in percentage of mortality (%).
Table 6.
Mortality percentage and lethal concentration (LC50) of M. piperita essential oil front Aedes aegypti larvae.
| Species | Concentration (ppm) | |||||
|---|---|---|---|---|---|---|
| 500 | 400 | 300 | 200 | 130 | LC50 | |
| M. piperita | 86.6a | 66.8b | 40.8c | 17d | 3.4e | 367.6 |
Different lowercase characters represent significant differences in mortality between the concentrations of the oils.
According to the bioassay, the species M. piperita has a low potential larvicide at LC50 367.6 ppm, p (value) < 0.01, and coefficient of determination (R 2) of 0.9980; these results are statistically significant. The study is similar to research by Kumar et al. [52]. Reduction of LC50 in periods of 24 or 48 hours (111.9 and 98.66 ppm) in the potential larvicide of the species was observed. The bioactivity is ineffective for the larvae, because they develop from pupas into adults in the 60–72 h period.
The essential oils extracted from various plants have been reported to have larvicidal and repellent properties against A. aegypti [53, 54]. Although some of the reports are available in relation to repellent potential of the M. piperita essential oil against insects, the research is of fundamental importance to respect the larvicidal effect evaluation of the mosquito in the preparation of a bioproduct that does not harm the environment.
The evaluation of larvicidal activity is in agreement with studies made by Pavela et al. [54]. That determined the value LC50 > 100 mg·L−1 against larvae of Culex quinquefasciatus, having as a major component the monoterpene which is L-(−)-menthol, in which the participation in the EO differs depending on the genotype of 20.5% to 57.3%, together with carvone (0.0 to 56.8%) and piperitenone oxide (0.0 to 31.8%).
4. Conclusions
The results found in this study show a great potential for use/application of the essential oil of M. Piperita species because it has a diversified class of chemical compounds at antioxidant, microbial, and cytotoxic activities. The information obtained in this research corroborates the use of species reported by traditional communities and literature.
Additionally, the use of these plants for the prevention and treatment of various human diseases seems reasonable and useful. The research contributes to enhancement and aggregation of the products of the Brazilian biodiversity, in the field of utilization of the species and insertion in the treatment of underlying disease in isolated regions where drugs in primary care health centers are sometimes not provided.
Acknowledgments
The authors acknowledge SUS Research Program (PPSUS), Federal University of Amapá (UNIFAP/PROPESPG), and Prof. M.S. Rosangela Sarquis of the Microbiology Laboratory of the Faculty Estacio SEAMA.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Raimundo Nonato Picanço Souto contributed equally to this work. João Batista Fernandes and Lourivaldo da Silva Santos also contributed equally to this work.
References
- 1.Radaelli M., da Silva B. P., Weidlich L., et al. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens . Brazilian Journal of Microbiology. 2016;47(2):424–430. doi: 10.1016/j.bjm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenzi H. Plantas Medicinais no Brasil: Nativas e Exóticas. 2nd. Nova Odessa, Brazil: Instituto Plantarum; 2008. [Google Scholar]
- 3.Kruppa P., Russomanno O. Ocorrência de fungos em sementes de plantas medicinais, aromáticas e condimentares da família Lamiaceae. Tropical Plant Pathology. 2008;33(1) doi: 10.1590/S1982-56762008000100013. [DOI] [Google Scholar]
- 4.Almeida A. C., Oliveira L. d., Paulo P. D., et al. Potencial antimicrobiano dos óleos essenciais de cravo-da-índia (Syzygium aromaticum L.) e alfavacão (Ocimum gratissimum L.) em carne moída de ovinos contaminada experimentalmente com Staphylococcus aureus . Revista Brasileira de Ciência Veterinária. 2013;20(4):248–251. doi: 10.4322/rbcv.2014.010. [DOI] [Google Scholar]
- 5.Ramos R., Rodrigues A., Almeida S. S. M. S. Preliminary study of the extract of the barks of Licania macrophylla benth: phytochemicals and toxicological aspects. Biota Amazônia. 2014;4(1):94–99. doi: 10.18561/2179-5746/biotaamazonia.v4n1p94-99. [DOI] [Google Scholar]
- 6.Mimica-Dukic N., Bozin B., Sokovic M., Mihajlovic B., Matavuj M. Antimicrobial and antoxidant activities of three Mentha species essential oil. Plant Med. 2003;(63):413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 7.Costa J., Rodrigues F., Angélico E., et al. Estudo químico-biológico dos óleos essenciais de Hyptis martiusii, Lippia sidoides e Syzigium aromaticum frente às larvas do Aedes aegypti . Revista Brasileira de Farmacognosia. 2005;15(4):304–309. doi: 10.1590/s0102-695x2005000400008. [DOI] [Google Scholar]
- 8.Koz£owska M., Laudy A., Przyby£ J., Ziarno M., Majewska E. Chemical composition and antibacterial acrivity of some medicinal plants from lamiaceae family. Acta Poloniae Pharmaceutica-Drug Research. 2015;72(4):757–767. [PubMed] [Google Scholar]
- 9.McKay D. L., Blumberg J. B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytotherapy Research. 2006;20(8):619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 10.Ansari M. A., Vasudevan P., Tandon M., Razdan R. K. Larvicidal and mosquito repellent action of peppermint (Mentha piperita) oil. Bioresource Technology. 2000;71(3):267–271. doi: 10.1016/s0960-8524(99)00079-6. [DOI] [Google Scholar]
- 11.Búfalo J., Rodrigues T. M., de Almeida L. F., Tozin L. R., Marques M. O., Boaro C. S. PEG-induced osmotic stress in Mentha x piperita L.: structural features and metabolic responses. Plant Physiology and Biochemistry. 2016;105:174–184. doi: 10.1016/j.plaphy.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Farmacopéia Brasileira. 5th. Brasília, Brazil: ANVISA; 2010. [Google Scholar]
- 13.Adams R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corporation; 2012. [Google Scholar]
- 14.Souza T. M., Moreira R. R., Pietro R. C., Isaac V. L. Avaliação da atividade anti-séptica de extrato seco de Stryphnodendron adstringens (Mart.) Coville e de preparação cosmética contendo este extrato. Revista Brasileira de Farmacognosia. 2007;17(1):71–75. doi: 10.1590/s0102-695x2007000100015. [DOI] [Google Scholar]
- 15.Lopes-Lutz D., Alviano D. S., Alviano C. S., Kolodziejczyk P. P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69(8):1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Andrade M. A., Das Graças Cardoso M., Batista L. R., Mallet A. C. T., Machado S. M. F. Óleos essenciais de Cymbopogon nardus, Cinnamomum zeylanicum e Zingiber officinale: composição, atividades antioxidante e Antibacteriana. Revista Ciência Agronômica. 2012;43(2):399–408. doi: 10.1590/s1806-66902012000200025. [DOI] [Google Scholar]
- 17.Cansian R. L., Vanin A. B., Orlando T., et al. Toxicity of clove essential oil and its ester eugenyl acetate against Artemia salina . Brazilian Journal of Biology. 2016 doi: 10.1590/1519-6984.12215. [DOI] [PubMed] [Google Scholar]
- 18.Araújo M., Cunha W., Veneziani R. Estudo fitoquímico preliminar e bioensaio toxicológico frente a larvas de Artemia salina Leach. de extrato obtido de frutos de Solanum lycocarpum A. St.-Hill (Solanaceae) Revista de Ciências Farmacêuticas Básica e Aplicada. 2010;31:205–209. [Google Scholar]
- 19.Lôbo K., Athayde A., Silva A., et al. Avaliação da atividade antibacteriana e prospecção fitoquímica de Solanum paniculatum Lam. e Operculina hamiltonii (G. Don) D. F. Austin & Staples, do semi-árido paraibano. Revista Brasileira de Plantas Medicinais. 2010;12(2):227–235. doi: 10.1590/s1516-05722010000200016. [DOI] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 3rd. Wayne, Pa, USA: CLSI; 2009. (M27-A3). [Google Scholar]
- 21.Garcia C., Ueda S., Mimica L. Avaliação da atividade antibacteriana in vitro de extratos hidroetanólicos de plantas sobre Staphylococcus aureus MRSA e MSSA. Revista do Instituto Adolfo Lutz. 2011;70(4):589–598. [Google Scholar]
- 22.Mendes L., Maciel K., Vieira A., et al. Atividade antimicrobiana de extratos etanólicos de Peperomia pellucida e Portulaca pilosa . Revista de Ciências Farmacêuticas Básica e Aplicada. 2011;32:121–125. [Google Scholar]
- 23.World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicidas. World Health Organization Communicable Diseasecontrol, Prevention and Eradication WHO Pesticide Evaluation Scheme. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 24.WHO. Dengue and Severe Dengue. Geneva, Switzerland: WHO; 2014. (Factsheetno. 117). [Google Scholar]
- 25.Rao A., Zhang Y., Muend S., Rao R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrobial Agents and Chemotherapy. 2010;54(12):5062–5069. doi: 10.1128/AAC.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khadir A., Sobeh M., Gad H. A., et al. Chemical composition and biological activity of the essential oil from Thymus lanceolatus . Zeitschrift für Naturforschung C. 2016;71(5-6):155–163. doi: 10.1515/znc-2016-0005. [DOI] [PubMed] [Google Scholar]
- 27.Jaafari A., Mouse H. A., Rakib E. M., et al. Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Revista Brasileira de Farmacognosia. 2007;17(4):477–491. [Google Scholar]
- 28.Yin Q.-H., Yan F.-X., Zu X.-Y., et al. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology. 2012;64(1):43–51. doi: 10.1007/s10616-011-9389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavares A. C., Gonçalves M. J., Cavaleiro C., et al. Essential oil of Daucus carota subsp. halophilus: composition, antifungal activity and cytotoxicity. Journal of Ethnopharmacology. 2008;119(1):129–134. doi: 10.1016/j.jep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues V., Cabral C., Évora L., et al. Chemical composition, anti-inflammatory activity and cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. essential oil and its main compounds. Arabian Journal of Chemistry. 2015 doi: 10.1016/j.arabjc.2015.08.026. [DOI] [Google Scholar]
- 31.Sobral-Souza C. E., Leite N. F., Brito D. I. V., et al. Avaliação da atividade citotóxica e potencial antiparasitário in vitro do a-pineno e carvacrol. Acta Toxicológica Argentina. 2014;22(2) [Google Scholar]
- 32.Dastgerdi G. F., Goli S. A. H., Kadivar M. A new antioxidant active film based on HDPE and peppermint essential oil for packaging soybean oil. Journal of the American Oil Chemists' Society. 2016;93(5):657–664. doi: 10.1007/s11746-016-2806-9. [DOI] [Google Scholar]
- 33.Sartoratto A., Machado A. L. M., Delarmelina C., Figueira G. M., Duarte M. C. T., Rehder V. L. G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazilian Journal of Microbiology. 2004;35(4):275–280. doi: 10.1590/S1517-83822004000300001. [DOI] [Google Scholar]
- 34.Işcan G., Kirimer N., Kurkcuoglu M., Başer K. H. C., Demirci F. Antimicrobial screening of Mentha piperita essential oils. Journal of Agricultural and Food Chemistry. 2002;50(14):3943–3946. doi: 10.1021/jf011476k. [DOI] [PubMed] [Google Scholar]
- 35.Derwich E., Chabir R., Taouil R., Senhaji O. In-vitro antioxidant activity and GC/MS studies on the leaves of Mentha piperita (Lamiaceae) from Morocco. International Journal of Pharmaceutical Sciences and Drug Research. 2011;3:130–136. [Google Scholar]
- 36.Singh R., Shushni M. A. M., Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arabian Journal of Chemistry. 2015;8(3):322–328. doi: 10.1016/j.arabjc.2011.01.019. [DOI] [Google Scholar]
- 37.Smaoui S., Hsouna A. B., Lahmar A., et al. Bio-preservative effect of the essential oil of the endemic Mentha piperita used alone and in combination with BacTN635 in stored minced beef meat. Meat Science. 2016;117:196–204. doi: 10.1016/j.meatsci.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Ghabraie M., Vu K. D., Tata L., Salmieri S., Lacroix M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT—Food Science and Technology. 2016;66:332–339. doi: 10.1016/j.lwt.2015.10.055. [DOI] [Google Scholar]
- 39.Alam M. N., Bristi N. J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Alarcón C., Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Analytica Chimica Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 41.Andrade M. A., Das Graças Cardoso M., Batista L. R., Mallet A. C. T., Machado S. M. F. Óleos essenciais de Cymbopogon nardus, Cinnamomum zeylanicum e Zingiber officinale: composição, atividades antioxidante e Antibacteriana. Revista Ciencia Agronomica. 2012;43(2):399–408. doi: 10.1590/s1806-66902012000200025. [DOI] [Google Scholar]
- 42.Meyer B. N., Ferrigni N. R., Putnam J. E., Jacobsen L. B., Nichols D. E., McLaughlin J. L. Brine shrimp: a convenient general bioassay for active plant constituents. Journal of Medicinal Plants Research. 1982;45:31–34. [PubMed] [Google Scholar]
- 43.Nascimento J. E., Melo A. F. M., Lima e Silva T. C., et al. Estudo fitoquímico e bioensaio toxicológico frente a larvas de Artemia salina Leach. de três espécies medicinais do gênero Phyllanthus (Phyllanthaceae) Revista de Ciências Farmacêuticas Básica e Aplicada. 2008;29:145–150. [Google Scholar]
- 44.Rebelo M. M., Da Silva J. K. R., Andrade E. H. A., Maia J. G. S. Antioxidant capacity and biological activity of essential oil and methanol extract of Hyptis crenata Pohl ex Benth. Revista Brasileira de Farmacognosia. 2009;19(1):230–235. doi: 10.1590/s0102-695x2009000200009. [DOI] [Google Scholar]
- 45.Lorenzi H. `. Plantas Medicinais no Brasil: Nativas e Exóticas. 2nd. Nova Odessa, Brazil: Instituto Plantarum; 2008. [Google Scholar]
- 46.Simões C. M. O. Farmacognosia: da Planta ao Medicamento. 6th. Florianópolis, Brazil. Editora da UFRGS, Porto Alegre, Brazil; Editora da UFSC: 2010. [Google Scholar]
- 47.Tepe B., Daferera D., Sokmen A., Sokmen M., Polissiou M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chemistry. 2005;90(3):333–340. doi: 10.1016/j.foodchem.2003.09.013. [DOI] [Google Scholar]
- 48.Nguta J. M., Mbaria J. M., Gakuya D. W., Gathumbi P. K., Kabasa J. D., Kiama S. G. Biological screeing of Kenya medicinal plants using Artemia salina L. (Artemiidae) Pharmacologyonline. 2011;2:458–478. [Google Scholar]
- 49.Dorman H. J. D., Deans S. G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 50.Furtado R. F., Lima M. G. A., Neto M. A., Bezerra J. N. S., Silva E. M. G. V. Atividade larvicida de óleos essenciais contra Aedes aegypti L. (Diptera: Culicidae) Neotropical Entomology. 2005;34(5):843–847. [Google Scholar]
- 51.Porto K. R. A., Roel A. R., Silva M. M., Coelho M. M., Scheleder E. J. D., Jeller A. H. Atividade larvicida do óleo de Anacardium humile Saint Hill sobre Aedes aegypti (Linnaeus, 1762) (Diptera, Culicidae) Revista da Sociedade Brasileira de Medicina Tropical. 2008;41(6):586–589. doi: 10.1590/s0037-86822008000600008. [DOI] [PubMed] [Google Scholar]
- 52.Skalicka-Woźniak K., Walasek M. Preparative separation of menthol and pulegone from peppermint oil (Mentha piperita L.) by high-performance counter-current chromatography. Phytochemistry Letters. 2014;10:94–98. doi: 10.1016/j.phytol.2014.06.007. [DOI] [Google Scholar]
- 53.Kumar S., Wahab N., Warikoo R. Bioefficacy of Mentha piperita essential oil against dengue fever mosquito Aedes aegypti L. Asian Pacific Journal of Tropical Biomedicine. 2011;1(2):85–88. doi: 10.1016/s2221-1691(11)60001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavela R., Kaffková K., Kumšta M. Chemical composition and larvicidal activity of essential oils from different Mentha L. and Pulegium species against Culex quinquefasciatus say (Diptera: Culicidae) Plant Protection Science. 2014;50(1):36–42. [Google Scholar]