Abstract
Background
Prisoners are at high risk of blood borne and sexually transmitted infections due to their high involvement in risky behaviors. In this descriptive/cross-sectional study, the prevalence, sero-prevalence, and risk factors for bloodborne tumor viruses including HTLV-I, HBV, HCV, and KSHV were evaluated among inmates of two central prisons in the northeast of Iran.
Methods
Blood samples of 1114 inmates were analyzed for the presence of anti HTLV-I, KSHV, and HCV antibodies and HBsAg by ELISA. PCR tests were performed to confirm the presence of these viruses in plasma and identify the current infections.
Results
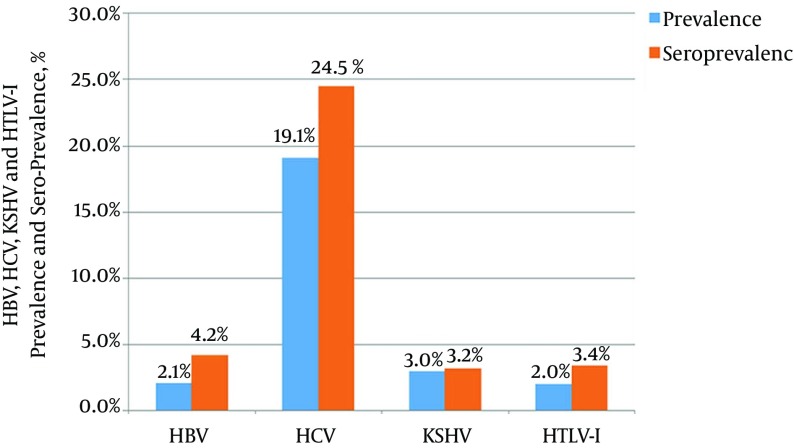
The sero-prevalence of HCV, HBV, HTLV-I, and KSHV was 24.5%, 4.2%, 3.4%, and 3.2% and the prevalence of HCV, HBV, HTLV-I, and KSHV was 19.1%, 2.1%, 2%, and 3%, respectively. HCV infection was significantly associated with history of imprisonment, tobacco consumption, alcohol consumption, intravenous drug use, length of imprisonment, and type of crime committed. Thirty one (2.8%) prisoners had HCV-KSHV co-infection, 16 (1.5%) had HCV-HTLV-I co-infection, and 14 (1.3%) had HBV-HCV co-infection. Triple co-infection was observed in seven cases and one case had four infections concomitantly.
Conclusions
This epidemiological study indicated different rates and transmission risks for these viruses. HCV was the most contagious viral infection and HTLV-I was the weakest in the prisoners. Apart from KSHV infection which its prevalence was as twice as in the general population, the prevalence of HBV and HTLV-I in prisoners was nearly in ranges of the general population.
Keywords: Epidemiology, Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), Human T-Cell Leukemia Virus Type I (HTLV-I), Kaposi’s Sarcoma-Associated Herpes Virus (KSHV), Prison
1. Background
Human T-cell leukemia virus type I (HTLV-I), hepatitis B virus (HBV), hepatitis C virus (HCV), and Kaposi’s sarcoma-associated herpes virus (KSHV) are four bloodborne, sexually transmissible viruses that can cause human cancers such as adult T-cell leukemia/lymphoma (ATL), hepatocellular carcinoma (HCC), and kaposi sarcoma (1, 2). These viruses usually are transmitted via sexual contact or exposure to the infected blood, through either blood transfusion or sharing contaminated needles or transmission from mother to child (3-7). HTLV-I belongs to Retroviridae family and is associated with adult T-cell leukemia (ATL) and the inflammatory condition of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). This virus infects 15 - 20 million people worldwide. Razavi Khorasan province (with 6.9 million populations) located in the northeast of Iran is an endemic region for this virus (8). Over two percent (2.12%) of residents in Mashhad, the capital of Razavi Khorasan province, are HTLV-I carriers (9, 10). According to recent studies, 5 provinces in Iran are endemic for this virus (9, 11-13). HBV infection is among the top ten causes of death worldwide due to chronic liver disease and accounts for an estimated 370 million chronic infections (14, 15). HCV is a positive-stranded RNA virus, which belongs to the Flaviviridae family. More than 180 million people are infected with HCV worldwide (2, 16). KSHV has a large double-stranded DNA genome. This tumor virus is identified as the cause of Kaposi’s sarcoma (KS) and uncommon primary-effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (7). The prevalence of the virus in the northeast of Iran is around 1.7% (17). The routine screening tests for most of the viruses usually are based on antibody detection (serology) tests such as ELISA and molecular detection methods such as PCR. After viral encounter, 90% of the patients show detectable specific anti-viral antibodies in three months and the remaining may take longer, despite the presence of viremia. Therefore, the chances of false negativity and false positivity are common. In addition, in most of viral infections, around 10% - 15% of infected subjects can clear virus and remain serologically positive for lifelong. Therefore, the positive tests do not show the presence of infection (18). The PCR analysis makes possible the diagnosis of viral infection through the sensitive detection of specific viral nucleic acids and represents the presence of viral infection in the body (19). Therefore, to evaluate the presence of viral infection (prevalence) or the possibility of exposure to the virus (sero-prevalence), it is necessary to choose appropriate methods (20). Taken together, our epidemiological knowledge of bloodborne infections is based on serological tests which demonstrate the exposure to these viruses (sero-prevalence); although the method is useful for epidemiological studies, it is not a perfect method to have a precise estimation of the current infection. Therefore, the PCR test is applied to identify the current virus infection (prevalence) (21). The abovementioned tumor virus infections are more common among prisoners. About thirty million inmates usually go back to their respective community each year and this could potentially threaten the community health (22, 23). Most of the prisoners originate from marginal socio-economic rural areas and are subject to extreme poverty, pervasive social health problems, limited educational opportunities, and illegal behaviors, such as drug injection and unsafe sexual activities. Up to 35% of the prisoners are addicted to drugs and they may transmit these infections to others while they return home (24). The prevalence of HCV infection in prisoners in Lebanon, Hungary, Spain, Indonesia, and Croatia was reported about 3.4%, 4.9%, 22.7%, 18.6%, and 8.3%, respectively (25-29). Furthermore, the prevalence of HBV infection in the same countries were reported to be about 2.4%, 1.5%, 2.6%, 5.8%, and 11.3%, respectively (25-29). The prevalence of HCV among prisoners in Tehran, Iran, was 34.5% in injection drug users (IDUs), which was strongly associated with female gender, length of imprisonment, and use of shared needles, drug injection frequency of more than once per day, drug usage for more than 10 years, and unmarried status (30). Based on other Iranian studies conducted in Nahavand and Birjand, the histories of surgery, imprisonment, tattooing, extramarital sexual intercourses, and drug addiction were the main risk factors for HBV and HCV infections (31). The significant growth rates in prison coupled with related risky behaviors among prisoners and the high-rate of returning to communities emphasize the necessity of detection and treatment of these tumor virus infections. The study was carried out in Razavi Khorasan province (northeast of Iran) with a population around 6.9 million according to the 2006 Census. Mashhad, the capital of Razavi Khorasan province, is the second largest city in Iran, a holly pilgrimage city with a population about 2.2 million and a mobile population of visitors around 25 million a year (Census, 2006). The motives of mobility, the process of the international movement, particularly those from countries at war such as Iraq and Afghanistan and heterogeneity of domestic population render this region in need of management of life threatening viruses. Furthermore, the most populated prisons in this region are located in the holly city of Mashhad
2. Objectives
Therefore, the study was conducted on demand of prison administrators. They assumed that cancers were more prevalent among the prisoners in recent years and granted the study. The aim of the present study was to evaluate the prevalence, sero-prevalence, and risk factors for bloodborne tumor viruses including HTLV-I, HBV, HCV, and KSHV among inmates of two central prisons in the northeast of Iran.
3. Methods
3.1. Study Population and Sample Size
The study was performed according to the world medical association declaration of Helsinki. All prisoners had full rights to decide whether they wanted to participate in the study. An informed consent was taken from each subject. Cases having risk factors or symptoms of diseases were referred to the prison health center for medical attention. This descriptive/cross-sectional study was conducted between April and September 2008 upon 1114 prison inmates from 11170 prisoners in two central prisons, Mashhad, Iran. Prisoners were selected randomly according to stratified sampling method and divided into two strata (female and male). The total sample size, by assuming a less than 5% refusal rate amongst the prisoners, amounted to 1175 that was chosen based on statistical test of population proportion, frequencies of risk factors among the population and previous published studies. The research ethics committee of Mashhad University of Medical Sciences (MUMS) approved this study (Number: 86675).
3.2. Data Collection Procedures
After taking permissions from the prison administration and Biomedical Ethics Committee, a random sampling was carried out. The questioners and physicians were trained in data collection and physical examination techniques. All participants were interviewed using a standard questionnaire that included demographic information and risk factors e.g., addiction to drugs, various ways of drugs usage, alcohol consumption, tobacco consumption, and infection to HTLV-I, HBV, HCV, and KSHV. The physicians then examined all inmates. Medical history and related signs and symptoms were meticulously recorded.
3.3. Sample Collection and Serological Assay
After obtaining informed consent, 5 mL blood sample was collected from each of the participants in EDTA- containing sterile tubes. Serum and PBMCs samples were kept at minus 20°C until analysis. Serum samples were tested for the presence of anti-HTLV-I (Dia.pro, Italy), anti-HCV (Dia.pro, Italy), anti-KSHV (Biotrin, Ireland), and the surface antigen of hepatitis B virus (Radim, Italy), by enzyme-linked immunosorbent assay (ELISA) according to manufacturers’ instructions. All serological and molecular tests were performed in inflammation and inflammatory diseases research center laboratory, MUMS, Iran. The positive specimens were further confirmed by polymerase chain reaction (PCR) using specific primers for HCV, HBV, HTLV-I (Tax and LTR), and KSHV. DNA and RNA were extracted using available commercial kits (DNA blood mini kit and viral RNA mini kit, Qiagen, Germany). Then, DNA samples were kept at -20°C until use. The HCV RNA was reverse transcribed using a Revert Aid TM H minus First Strand cDNA Synthesis kit (Fermentas, Germany). The reverse transcription was carried out at 42°C for 60 minutes followed by RT inactivation at 70°C for 5 minutes. The cDNA was kept at -20°C until use and was used as a template in the nested PCR method for amplification of a gene fragment of HCV with the following protocol: 25 µL reaction of 10x buffer, MgCl2 (1.5 mM), dNTPs (200 µM), 10 pm of each primer, 0.5-U of Taq DNA polymerase, and 3 µL cDNA. The HBV PCR amplification was carried out in a tube containing 25 µL reaction of 10x buffer, MgCl2 (2 mM), d NTPs (200 µM), 10 pm of each primer, 0.5-U of Taq DNA polymerase, and 100 ng DNA. To confirm HTLV-I infection, a conventional PCR was also carried out for Tax and LTR region on PBMCs-extracted DNA. The PCR amplification was carried out in a tube containing 25 µL reaction of 10x buffer, MgCl2 (2mM), d NTPs (200 µM), 10 pm of each primer, 0.5-U of Taq DNA polymerase, and 100 ng DNA. Table 1 shows the sequence of primers and PCR conditions for these viruses.
Table 1. Primers Sequences and PCR Conditions for the Virus Detection, HTLV-I, HBV.
| Nucleotide Position | Sequence (5′ - 3′) | PCR Condition |
|---|---|---|
| HTLV-Taxf | AGGGTTTGGACAGAGTCTT | 94°C for 3 min and 35 cycles at 94°C for 45 s, 60°C for 45 s, 72°C for 45 s, final extension at 72°C for 7 min |
| HTLV-Taxr | AAGGACCTTGAGGGTCTTA | |
| HTLV-LTRf | CATAAGCTCAGACCTCCGGG | |
| HTLV-LTRr | GGATGGCGGCCTCAGGTAGG | |
| (256-278) HBVf | GTGGTGGACTTCTCAATTTTC | 94°C for 3 min and 40 cycles at 94°C for 45 s, 56°C for 45 s, 72°C for 1 min, final extension at 72°C for 7 min |
| (776-796) HBVr | CGGTAAAAAGGGACTCAAGAT | |
| HCVF1 | CAT AgA TCA CTC CCC TgT gAg g | 94°C for 3 min and 20 cycles for the first run, 30 cycles for the second run at 94°C for 40 s, 58°C for 40 s, 72°C for 40 s, final extension at 72°C for 5 min |
| HCVR1 | ggC ggT Tgg TgT TAC gTT Tgg T | |
| HCVF2 | CCC TgT gAg gAA CTA CTg TCT TC | |
| HCVR2 | ggT gCA Cgg TgT ACg AgA CCT C | |
| KSHV f | AGCCGAAAGGATTCCACCAT | 94°C for 3 min and 35 cycles at 94°C for 45 s, 60°C for 45 s, 72°C for 45 s, final extension at 72°C for 7 min |
| KSHV R | TCCGTGTTGTCTACGTCCAC 3 |
3.4. Statistical Analysis
Data on demographic characteristics, medical history, and physical examination, related to risk factors, were analyzed by SPSS software version 11.5. Descriptive data were summarized as mean, standard deviation and/or percentages. Chi-Square test was used to examine the association of demographical and behavioral variables with HCV, HBV, HTLV-I, and KSHV infections. Mann-Whitney test was performed to analyze quantitative variables with abnormal distribution. Odd ratios (ORs) and their 95% confidence interval (95% CI) were calculated for each risk factor in univariate analysis. Logistic regression analysis was applied to identify independent risk factors for virus infections. P values < 0.05 were considered statistically significant.
4. Results
4.1. Demographic Data
This study was conducted on 1114 inmates in Razavi Khorasan prisons in Iran. The majority of participants were imprisoned for drug-related crimes (50%) and robbery (30%). The mean length of imprisonment was 53.37 ± 54.9 months (median: 33, 25 percentile: 12 and 75 percentile: 81). 615 individuals (58.2%) were reported as drug users (data not shown). The study population consisted of 992 (89%) males and 122 (11%) females. The mean age of males and females was 34.42 ± 10.95 years and 40.67 ± 14.2 years, respectively. This difference was significant (P < 0.001). The data showed that 72.6% of males and 100% of females were married.
4.2. Prevalence and Sero-Prevalence of Viruses
According to the serology tests, sero-prevalence of HCV, HBV, HTLV-I and KSHV was 24.5%, 4.2%, 3.4%, and 3.2%, respectively. According to the results of PCR, the prevalence of HCV, HBV, HTLV-I, and KSHV was 19.1%, 2.1%, 2%, and 3% respectively (Figure 1). The sero-prevalence of HCV, HBV, HTLV-I, and KSHV among females was 19.5% (22), 3.5% (4), 4.4% (5), and 1.8% (2), respectively, while in males was 25.1% (247), 4.3% (42), 3.2% (32), and 3.2% (33), respectively. No significant differences were existed in sero-prevalence of these viruses between females and males (P > 0.05).
Figure 1. Prevalence and Sero-Prevalence of HBV, HCV, KSHV, and HTLV-I among Razavi Khorasan Prisoners in Iran.
4.3. Risk Factors for Virus Infections
As shown in Table 2, there was a significant association between HCV, HTLV-I, and KSHV infections and history of imprisonment. HBV infection was associated just with the length of imprisonment and HCV infection was significantly associated with the length of imprisonment, number of prisoners inside the cell, and number of prisoners inside in the wing. Table 3 shows the univariate analysis of behavioral risk factors for HCV, HBV, HTLV-I, and KSHV infections among prisoners. There were statistically significant differences between HCV, HTLV-I, and KSHV infections among addict and non-addict, drug injecting, and shoplifting in this study (Table 3). Moreover, a statistically significant difference was observed among prisoners with HCV and HTLV-I infections who consumed tobacco versus who did not, and also those who committed robbery (Table 3). Table 4 shows odd ratios (OR) and corresponding 95% confidence intervals (CI) for HCV, HBV, HTLV-I, and KSHV positive cases who were drug users.
Table 2. Associations of HBV, HCV, HTLV-I and KSHV Infections With Socio-Demographic Variables among Razavi Khorasan Prisoners in Irana.
| Infection | Socio-Demographic Variables | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Length of Imprisonment, mo | Number of Prisoner Inside the Cell | Number of Inmates in the Wing | History of Imprisonment | |||||||||||
| Mean ± SD | No | P Value | Mean ± SD | No | P Value | Mean ± SD | No | P Value | Mean ± SD | No | P Value | Mean ± SD | No | P Value | |
| HBV | |||||||||||||||
| + | 37.35 ± 12.7 | 45 | 0.22 | 80.74 ± 70.48 | 45 | 0.004 | 37.93 ± 52.14 | 46 | 0.34 | 429.84 ± 309.36 | 44 | 0.41 | 4.10 ± 5.76 | 46 | 0.69 |
| - | 34.82 ± 11.41 | 1035 | 52.05 ± 53.85 | 1047 | 28.37 ± 18.48 | 1046 | 444.16 ± 267.22 | 981 | 3.06 ± 4.03 | 1050 | |||||
| HCV | |||||||||||||||
| + | 34.91 ± 10.29 | 266 | 0.43 | 66.58 ± 62.31 | 267 | 0.001 | 24.62 ± 10.28 | 268 | 0.002 | 521.67 ± 280.99 | 249 | 0.001 | 5.25 ± 6.04 | 268 | 0.001 |
| - | 34.94 ± 11.85 | 813 | 48.96 ± 51.58 | 824 | 28.86 ± 23.45 | 823 | 418 ± 260.41 | 775 | 2.40 ± 2.94 | 827 | |||||
| HTLV-I | |||||||||||||||
| + | 36.19 ± 11.41 | 36 | 0.43 | 68.70 ± 76.89 | 37 | 0.33 | 26.45 ± 11.64 | 37 | 0.71 | 573.88 ± 291.23 | 34 | 0.003 | 5.10 ± 6.31 | 37 | 0.001 |
| - | 34.88 ± 11.48 | 1044 | 52.69 ± 53.93 | 1055 | 27.86 ± 21.32 | 1055 | 439.08 ± 267.25 | 991 | 3.03 ± 4 | 1059 | |||||
| KSHV | |||||||||||||||
| + | 34.22 ± 10.93 | 35 | 0.81 | 56.51 ± 57.41 | 35 | 0.89 | 26.91 ± 10.89 | 35 | 0.98 | 505.43 ± 259.90 | 32 | 0.09 | 5.82 ± 6.54 | 35 | 0.02 |
| - | 34.95 ± 11.5 | 1045 | 53.12 ± 54.83 | 1057 | 27.84 ± 21.32 | 1057 | 441.55 ± 269.19 | 993 | 3.01 ± 3.98 | 1061 | |||||
aα = 0.0.
Table 3. Univariate Analysis of Behavioral Risk Factors for HCV, HBV, HTLV-I, and KSHV Infections among Razavi Khorasan Prisoners in Iran.
| Behavioral Variables | Inmates | HCV Sero-Positive | HBV Sero-Positive | HTLV-I Sero-Positive | KSHV Sero-Positive | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CL) | No. (%) | OR (95% CL) | No. (%) | OR (95% CL) | No. (%) | OR (95% CL) | ||
| Addition to drugs | |||||||||
| Yes | 606 (58.3) | 210 (78.1) | 2.54 (1.96 - 3.31) | 22 (51.2) | 0.75 (0.41 - 1.35) | 30 (81.1) | 3.07 (1.36 - 6.93) | 27 (77.1) | 2.42 (1.11 - 5.28) |
| No | 403 (41.7) | 59 (21.9) | 21 (48.4) | 7 (18.9) | 8 (22.9) | ||||
| Alcohol consumption | |||||||||
| Yes | 363 (35.1) | 124 (46.3) | 1.58 (1.29 - 1.94) | 14 (32.6) | 0.89 (0.47 - 1.66) | 16 (44.4) | 1.47 (0.77 - 2.81) | 15 (42.9) | 1.38 (0.71 - 2.67) |
| No | 670 (64.9) | 144 (53.7) | 29 (67.4) | 20 (55.6) | 20 (57.1) | ||||
| Tobacco consumption | |||||||||
| Yes | 729 (70.2) | 225 (83.6) | 2.17 (1.62 - 2.92) | 29 (67.4) | 0.88 (0.47 - 1.64) | 33 (89.2) | 3.5 (1.25 - 9.81) | 28 (80) | 1.70 (0.75 - 3.85) |
| No | 310 (29.9) | 44 (16.4) | 14 (32.6) | 4 (10.8) | 7 (20) | ||||
| Injection drug use | |||||||||
| Yes | 111 (18.3) | 79 (37.6) | 2.68 (2.22 - 3.24) | 6 (27.3) | 1.67 (0.67 - 4.17) | 60 (20) | 1.11 (0.46 - 2.66) | 8 (29.6) | 1.87 (0.84 - 4.17) |
| No | 495 (81.7) | 131 (62.4) | 16 (72.7) | 24 (80) | 19 (70.4) | ||||
| Type of crime committed | |||||||||
| Financial crime | 50 (4.7) | 9 (3.5) | 0.98 (0.96-1.01) | 1 (2.2) | 0.97 (0.93-1.02) | 0 | - | 1 (2.9) | 0.98 (0.92-1.04) |
| Rape | 18 (1.7) | 2 (8) | 0.99 (0.97-1) | 0 | - | 0 | - | 0 | - |
| Accidental killing | 19 (1.8) | 7 (2.7) | 1.01 (0.99-1.04) | 1 (2.2) | 1.01 (0.9-1.05) | 1 (2.7) | 1.01 (0.96-1.07) | 0 | - |
| Intentional homicide | 85 (8) | 13 (5) | 0.96 (0.93-0.99) | 2 (4.4) | 0.96 (0.90-1.03) | 1 (2.7) | 0.94 (0.89-0.99) | 3 (8.6) | 1.01 (0.91-1.16) |
| Shoplifting | 253 (23.9) | 92 (35.7) | 1.24 (1.13-1.37) | 9 (20) | 0.95 (0.82-1.1) | 13 (35.1) | 1.18 (0.93-1.49) | 18 (51.4) | 1.59 (1.13-2.24) |
| Drug -related crimes | 587 (54.6) | 123 (47.7) | 0.83 (0.72-0.95) | 30 (66.7) | 1.38 (0.91-2.11) | 19 (51.4) | 0.93 (0.66-1.31) | 11 (31.4) | 0.65 (0.51-0.82) |
| Other | 56 (5.3) | 12 (4.7) | 0.99 (0.96-1.02) | 2 (4.4) | 0.99 (0.93-1.06) | 3 (8.1) | 1.03 (0.94-1.14) | 2 (5.7) | 1.01 (0.92-1.09) |
Table 4. Odd Ratios (ORs) and Corresponding 95% Confidence Intervals (CIs) for HCV, HBV, HTLV-I, and KSHV Sero-Positivity Based on Methods of Using Drug Among Razavi Khorasan Prisoners in Iran.
| Methods of Using Drugs | Inmates | HCV Sero-Positive | HBV Sero-Positive | HTLV-I Sero-Positive | KSHV Sero-Positive | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CL) | No. (%) | OR (95% CL) | No. (%) | OR (95% CL) | No. (%) | OR (95% CL) | ||
| Injection | 19 (3.2) | 13 (6.3) | 1.05 (1.01-1.09) | 0 | - | 0 | - | 0 | - |
| Oral | 35 (5.8) | 8 (3.8) | 0.97 (0.93 - 1.01) | 0 | - | 0 | - | 1 (3.7) | 0.98 (0.91 - 1.06) |
| Inhalation | 377 (62.6) | 95 (45.7) | 1.05 (1.01 - 1.09) | 13 (59.1) | 0.97 (0.95 - 0.98) | 20 (66.7) | 0.97 (0.95 - 0.98) | 14 (51.9) | 0.97 (0.95 - 0.98) |
| Both inhalation and oral | 78 (13) | 25 (12) | 0.98 (0.92 - 1.05) | 3 (13.6) | 1.01 (0.85 - 1.19) | 4 (13.3) | 1.01 (0.87 - 1.16) | 4 (14.8) | 1.02 (0.87 - 1.2) |
| Both injection and oral | 3 (5) | 3 (1.4) | 1.02 (0.99 - 1.03) | 0 | - | 0 | - | 1 (3.7) | 1.04 (0.96 - 1.11) |
| Both inhalation and injection | 42 (7) | 32 (15.4) | 1.15 (1.08 - 1.22) | 3 (13.6) | 1.08 (0.91 - 1.27) | 2 (6.7) | 0.99 (0.90 - 1.10) | 5 (18.5) | 1.15 (0.96 - 1.38) |
| Injection, oral and inhalation together | 47 (7.8) | 31 (14.9) | 1.13 (1.06 - 1.2) | 3 (13.6) | 1.07 (0.91 - 1.26) | 4 (13.3) | 1.07 (0.93 - 1.23) | 2 (3) | 0.99 (0.89 - 1.11) |
4.4. Co-infections
HBV, HCV, HTLV-I, and KSHV co-infections among prisoners are shown in Table 5. The results showed that HCV-KSHV co-infection was the most common co-infection among prisoners (31, 2.8%). HCV-HTLV-I co-infection and HBV-HCV co-infection were observed in 16 (1.5%) and 14 (1.3%) of prisoners, respectively. HCV-KSHV and HBV-HCV co-infections were more common among males than females. Triple co-infection was also observed in seven cases and one case had all of four infections.
Table 5. HBV, HCV, HTLV-I, and KSHV Co-Infections among Razavi Khorasan Prisoners in Irana.
| Infection | Inmates, No. (%) | Gender | No. (%) | Risk Estimate OR (95% CI) | P Value |
|---|---|---|---|---|---|
| HBV, HCV | 14 (1.3) | Female | 1 (0.1) | 1.49 (0.19 - 1.29) | 0.69 |
| Male | 13 (1.2) | ||||
| HBV.HTLV-I | 1 (0.1) | Female | 0 | - | 0.73 |
| Male | 1 (0.1) | ||||
| HBV, KSHV | 4 (0.4) | Female | 0 | - | 0.5 |
| Male | 4 (0.4) | ||||
| HCV, HTLV-I | 16 (1.5) | Female | 3 (0.3) | 0.5 (0.14 - 1.71) | 0.26 |
| Male | 13 (1.2) | ||||
| HCV, KSHV | 31 (2.8) | Female | 2 (0.2) | 1.66 (0.40 - 6.88) | 0.47 |
| Male | 29 (2.6) | ||||
| HTLV-I, KSHV | 2 (0.2) | Female | 0 | - | 0.63 |
| Male | 2 (0.2) | ||||
| HBV, HCV, HTLV-I | 1 (0.1) | Female | 0 | - | 0.73 |
| Male | 1 (0.1) | ||||
| HBV, HCV, KSHV | 3 (0.3) | Female | 0 | - | 0.5 |
| Male | 3 (0.3) | ||||
| HCV, HTLV-I, KSHV | 2 (0.2) | Female | 0 | - | 0.63 |
| Male | 2 (0.2) | ||||
| KSHV, HTLV-I, HBV | 1 (0.1) | Female | 0 | - | 0.73 |
| Male | 1 (0.1) | ||||
| HBV,HCV, KSHV, HTLV-I | 1 (0.1) | Female | 0 | - | 0.73 |
| Male | 1 (0.1) |
aα = 0.05.
5. Discussion
Cancer is the leading cause of death in developed countries and the second most cause of death in developing countries. According to report of cancer incidence and mortality, 14.1 million new cancer cases and 8.2 million cancer deaths occurred in 2012 worldwide (32). Human tumor viruses are associated with a variety of human malignancies; and around 15% of human cancers are virus-related (33). Low socioeconomic level, high risk behaviors like tattooing, using drugs, needle sharing, and sexual activity of prisoners put them at a high risk of infection with tumor viruses such as HBV, HCV, HTLV-I, and KSHV. Beside these risk factors, the inadequate living conditions such as overcrowding along with the absence of early diagnosis and treatment in the prisons may be involved in the spread of infection among inmates at a faster pace. Subsequently, when prisoners are released, they may transfer these infections to the general population (1, 26, 34, 35). In this study, we evaluated the sero-prevalence, prevalence, and risk factors of HBV, HCV, HTLV-I, and KSHV infections among prisoners in Mashhad, Iran. This study showed higher overall prevalence of HBV, HCV, HTLV-I, and KSHV among prisoners compared to the general population of Iran (9, 36-38). It is possibly because of their behavioral patterns and frequent use of risky behaviors (such as drug abuse, alcohol consumption, and sexual practices). However, the sero-prevalence of HCV and KSHV was dramatically higher compared to the general population in Khorasan provinces. The results of the present study showed that the sero-prevalence of tumor viruses for HCV, HBV and KSHV was two times more in prisoners than the general population; however, for HTLV-I it was nearly same as the general population. These differences between the spread ratio of HTLV-I and that of HBV, HCV, and KSHV in prisoners may be due to the differences in infectivity and amount of these viruses which are necessary factors for the mounting an infection. In addition, HTLV-I transmits via viable HTLV-I infected cells through the specialized sites of cell-to-cell contact called “virological synapses (39)”. Apart from HTLV-I and KSHV, for HBV and HCV, other studies around the world have demonstrated similarly a higher prevalence of these viral infections in prisoners than the general population (1, 26, 34, 40, 41). Furthermore, the prevalence of HBV (4.2%), HCV (19.1%), and HTLV-I (3%) among prisoners in the present study was higher than the ratios reported by a similar study conducted in Brazil (2.4%, 6.4%, and 1.09%, respectively) (42). These higher rates of prevalence might be due to the larger sample size in the present study, different methodologies, and the effect of infection accumulation progressing with age. Moreover, the Brazilian study was carried out on a teenage population (11 - 18 years old).
The sero-prevalence of HBV (8.1%) and HCV (31.1%) infections among the inmates of a prison in Bologna, Italy, was higher compared to the present study. The higher rates might be due to that the majority of the prisoners in Italy were addicted to drugs and 33.9% of them were IDUs (43). The present study also demonstrated that HCV infection was significantly associated with the number of prisoners inside the cell, history of imprisonment, number of inmates in the wing, length of imprisonment, tobacco consumption, and methods of using drugs. HBV infection was significantly related to the length of imprisonment while KSHV infection showed a statistical association with history of imprisonment. Furthermore, there was a significant association among HTLV-I with history of imprisonment, number of inmates in the wing, and tobacco consumption. Our findings indicated that, the majority of HCV and HTLV-I and KSHV positive prisoners were addicted to drugs. Additionally, 29.6% of KSHV positive prisoners and 37.6% of HCV positive prisoners were injection drug user which can be the cause of higher prevalence of HCV among inmates. Moreover, HCV and KSHV infections were statistically correlated with the type of crime committed. Among HCV positive inmates, drug-related crimes and robbery and among KSHV positive inmates, robbery and drug-related crimes were more frequent.
In a study conducted on Ghanaian prison inmates aged 17 - 46 years who had HCV, HBV, HIV and syphilis infections, the main risk factors were unmarried status, illiteracy, female gender, being incarcerated for longer than median time served of 36 months, history of homosexuality, history of intravenous drug use, history of sharing syringes, drug paraphernalia, history of participation in paid sexual activity, and history of sexually transmitted diseases (1).
Also, in a study conducted to evaluate the sero-prevalence, co-infection, and risk factors of HIV, HBV, and HCV among inmates in Nasarawa State, Nigeria, the length of incarceration, previous incarceration (for HIV, HBV, and HCV), intra-prison anal sex, multiple sex partners (for HIV and HBV), and ignorance of transmission modes, blood transfusion, and alcohol consumption (for HBV and HCV) were risk factors among the population of study (44).
In our study, HCV co-infection was more common than other co-infections amongst the prisoners. We observed HCV-KSHV co-infection in 2.8%, HCV-HTLV-I co-infection in 1.5%, and HCV-HBV co-infection in 1.3% of prisoners. Among prisoners who were addicted to drugs, 2.8% had HCV-KSHV co-infection and 1.5% had HCV-HTLV-I co-infection.
Furthermore, approximately all prisoners with HBV-HCV and HCV-KSHV co-infections were injection drug users. These results emphasis the transmission mode of these co-infections (possibly through infected needles and syringes shared) among prisoners. Co-infection results found in this study are different from those of other studies. For example, HCV-HBV co-infection with the rate of 3.83% (30 cases) among prisoners in Lahore was more prevalent compared to our study (45).
In contrast, HCV-HBV co-infection of prisoners (the prevalence of 0.7%) in Nasarawa State, Nigeria, was lower than the rate found in our study (44). This lower rate of co-infection was expected because none of the inmates in the mentioned study were exposed to drug use. In the present study, the co-infected prisoners had no symptoms; however, they were referred to Qaem Hospital, affiliated to MUMS, for monitoring.
In summary, this study demonstrated that tumor virus infections (particularly HCV infection) are highly prevalent in the prison population. Furthermore, the potential transmission rates for these viruses are different. HCV was the most contagious viral infection and HTLV-I was the weakest one in prisoners. Apart from KSHV infection which its prevalence in prisoners was as twice as the prevalence in the general population, the prevalence of HBV and HTLV-I was nearly in ranges of the general population. Rubbery and drug injection were the main risk factors for HCV, KSHV, and HTLV-I infections. The high rate of HCV infection (and KSHV infection in a lower extent) among inmates is an important health concern. Effective programs must be undertaken to mitigate these risks. Health education, regular testing, routine screening of infected prisoners with high-risk behaviors particularly among injection drug users and facilitating access to treatment are essential to mitigate the associated risks and curb its spread within the infected population.
The study faced some limitations and the authors tried to minimize their effects. Prisoners are special population to study, and it needs much coordination with different organizations to obtain their informed consent. Any transportation was prohibited; the authorities did not allow our team of researchers to enter the prison, except the main researcher. Therefore, we had to educate their physicians and health center employees to take part. Due to loss of samples, the researchers had to consider 20 percent attrition rate to calculate the sample size.
Acknowledgments
We do appreciate the prison organization of Razavi Khorasan province and the vice chancellor for research of MUMS for funding and their kind cooperation and assistance. Many thanks to the vice chancellor for research deputy of Mashhad University of Medical Sciences (MUMS) for ethics approval of the study and a great thank to Antonio Giovanni for proof reading of the article. The authors appreciate all inmates who participated in this study.
Footnotes
Funding/Support:We thank the research deputy of Mashhad University of Medical Sciences (MUMS) and Razavi Khorasan prison organization for their kind cooperation and assistance.
References
- 1.Adjei AA, Armah HB, Gbagbo F, Ampofo WK, Boamah I, Adu-Gyamfi C, et al. Correlates of HIV, HBV, HCV and syphilis infections among prison inmates and officers in Ghana: A national multicenter study. BMC Infect Dis. 2008;8:33. doi: 10.1186/1471-2334-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha A, Kaul R, Murakami M, Robertson ES. Tumor viruses and cancer biology: Modulating signaling pathways for therapeutic intervention. Cancer Biol Ther. 2010;10(10):961–78. doi: 10.4161/cbt.10.10.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arjmand B, Aghayan SH, Goodarzi P, Farzanehkhah M, Mortazavi SM, Niknam MH, et al. Seroprevalence of human T lymphtropic virus (HTLV) among tissue donors in Iranian tissue bank. Cell Tissue Bank. 2009;10(3):247–52. doi: 10.1007/s10561-008-9117-9. [DOI] [PubMed] [Google Scholar]
- 4.Lam NC, Gotsch PB, Langan RC. Caring for pregnant women and newborns with hepatitis B or C. Am Fam Physician. 2010;82(10):1225–9. [PubMed] [Google Scholar]
- 5.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–25. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 6.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52(4):1497–505. doi: 10.1002/hep.23808. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Shao X, Chen Y, Zhang T, Minhas V, Wood C, et al. Human herpesvirus 8 seroprevalence, China. Emerg Infect Dis. 2012;18(1):150–2. doi: 10.3201/eid1801.102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24(39):6058–68. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 9.Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, et al. High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. J Clin Virol. 2011;52(3):172–6. doi: 10.1016/j.jcv.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Statistical Centre of Iran. Pebuara 2006. 2010. [Google Scholar]
- 11.Rafatpanah H, Rezaee A, Etemadi MM, Hosseini RF, Khorram B, Afsahr L, et al. The impact of interferon-alpha treatment on clinical and immunovirological aspects of HTLV-1-associated myelopathy in northeast of Iran. J Neuroimmunol. 2012;250(1-2):87–93. doi: 10.1016/j.jneuroim.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Shoeibi A, Etemadi M, Moghaddam Ahmadi A, Amini M, Boostani R. "HTLV-I Infection" Twenty-Year Research in Neurology Department of Mashhad University of Medical Sciences. Iran J Basic Med Sci. 2013;16(3):202–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Kalavi K, Moradi A, Tabarraei A. Population-based Seroprevalence of HTLV-I Infection in Golestan Province, South East of Caspian Sea, Iran. Iran J Basic Med Sci. 2013;16(3):225–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Alter MJ. Epidemiology and prevention of hepatitis B. Semin Liver Dis. 2003;23(1):39–46. doi: 10.1055/s-2003-37583. [DOI] [PubMed] [Google Scholar]
- 15.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–9. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad B, Ali S, Ali I, Azam S, Bashir S. Response rates of standard interferon therapy in chronic HCV patients of Khyber Pakhtunkhwa (KPK). Virol J. 2012;9:18. doi: 10.1186/1743-422X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadi Ghezeldasht S, Hassannia T, Rafatpanah H, Hekmat R, Valizadeh N, Ghayour Mobarhan M, et al. Oncogenic Virus Infections in the General Population and End-stage Renal Disease Patients With Special Emphasis on Kaposi's Sarcoma Associated Herpes Virus (KSHV) in Northeast of Iran. Jundishapur J Microbiol. 2015;8(3):ee14920. doi: 10.5812/jjm.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali A, Lal A. False positivity of serological tests for hepatitis C virus. J Ayub Med Coll Abbottabad. 2010;22(2):43–5. [PubMed] [Google Scholar]
- 19.Cobo F. Application of molecular diagnostic techniques for viral testing. Open Virol J. 2012;6:104–14. doi: 10.2174/1874357901206010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storch GA. Diagnostic virology. Clin Infect Dis. 2000;31(3):739–51. doi: 10.1086/314015. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Martinez JL, Coreno-Juarez MO, Montano-Estrada LF, Attlan M, Gomez-Dantes H. Seroprevalence of hepatitis B in pregnant women in Mexico. Salud Publica Mex. 2003;45(3):165–70. doi: 10.1590/s0036-36342003000300005. [DOI] [PubMed] [Google Scholar]
- 22.King's College London. World prison population list 2007. 2007 Available from: http://www.kcl.ac.uk/depsta/law/research/icps/downloads/World-prison-pop-seventh.pdf.
- 23.UNAIDS. Report on the global AIDS epidemics. UNAIDS 10th Anniversary Special editio. 2006. [Google Scholar]
- 24.Javadi AA, Avijgan M, Hafizi M. Prevalence of HBV and HCV infections and associated risk factors in addict prisoners. Iran J Public Health. 2006;35(4):33–6. [Google Scholar]
- 25.Burek V, Horvat J, Butorac K, Mikulic R. Viral hepatitis B, C and HIV infection in Croatian prisons. Epidemiol Infect. 2010;138(11):1610–20. doi: 10.1017/S0950268810000476. [DOI] [PubMed] [Google Scholar]
- 26.Mahfoud Z, Kassak K, Kreidieh K, Shamra S, Ramia S. Prevalence of antibodies to human immunodeficiency virus (HIV), hepatitis B and hepatitis C and risk factors in prisoners in Lebanon. J Infect Dev Ctries. 2010;4(3):144–9. doi: 10.3855/jidc.517. [DOI] [PubMed] [Google Scholar]
- 27.Nelwan EJ, Van Crevel R, Alisjahbana B, Indrati AK, Dwiyana RF, Nuralam N, et al. Human immunodeficiency virus, hepatitis B and hepatitis C in an Indonesian prison: prevalence, risk factors and implications of HIV screening. Trop Med Int Health. 2010;15(12):1491–8. doi: 10.1111/j.1365-3156.2010.02655.x. [DOI] [PubMed] [Google Scholar]
- 28.Saiz de la Hoya P, Marco A, Garcia-Guerrero J, Rivera A, Prevalhep study G. Hepatitis C and B prevalence in Spanish prisons. Eur J Clin Microbiol Infect Dis. 2011;30(7):857–62. doi: 10.1007/s10096-011-1166-5. [DOI] [PubMed] [Google Scholar]
- 29.Treso B, Barcsay E, Tarjan A, Horvath G, Dencs A, Hettmann A, et al. Prevalence and correlates of HCV, HVB, and HIV infection among prison inmates and staff, Hungary. J Urban Health. 2012;89(1):108–16. doi: 10.1007/s11524-011-9626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin-Esmaeili M, Rahimi-Movaghar A, Razaghi EM, Baghestani AR, Jafari S. Factors Correlated With Hepatitis C and B Virus Infections Among Injecting Drug Users in Tehran, IR Iran. Hepat Mon. 2012;12(1):23–31. doi: 10.5812/kowsar.1735143X.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azarkar Z, Sharifzadeh G. Evaluation of the Prevalence of Hepatitis B, Hepatitis C, and HIV in Inmates with Drug-Related Convictions in Birjand, Iran in 2008. Hepat Mon. 2010;10(1):26–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 33.Yasunaga J, Jeang KT. Viral transformation and aneuploidy. Environ Mol Mutagen. 2009;50(8):733–40. doi: 10.1002/em.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coelho HC, de Oliveira SA, Miguel JC, Oliveira Mde L, Figueiredo JF, Perdona GC, et al. Predictive markers for hepatitis C virus infection among Brazilian inmates. Rev Soc Bras Med Trop. 2009;42(4):369–72. doi: 10.1590/s0037-86822009000400002. [DOI] [PubMed] [Google Scholar]
- 35.Vescio MF, Longo B, Babudieri S, Starnini G, Carbonara S, Rezza G, et al. Correlates of hepatitis C virus seropositivity in prison inmates: a meta-analysis. J Epidemiol Community Health. 2008;62(4):305–13. doi: 10.1136/jech.2006.051599. [DOI] [PubMed] [Google Scholar]
- 36.Jalilvand S, Shoja Z, Mokhtari-Azad T, Nategh R, Gharehbaghian A. Seroprevalence of Human herpesvirus 8 (HHV-8) and incidence of Kaposi's sarcoma in Iran. Infect Agent Cancer. 2011;6:5. doi: 10.1186/1750-9378-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fathimoghaddam F, Hedayati-Moghaddam MR, Bidkhori HR, Ahmadi S, Sima HR. The prevalence of hepatitis B antigen-positivity in the general population of Mashhad, Iran. Hepat Mon. 2011;11(5):346–50. [PMC free article] [PubMed] [Google Scholar]
- 38.Alavian SM. Hepatitis C virus infection: Epidemiology, risk factors and prevention strategies in public health in IR IRAN. Gastroenterol Hepatol Bed Bench. 2009;3(1) [Google Scholar]
- 39.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299(5613):1713–6. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 40.Ansaldi F, Comar M, D'Agaro P, Grainfenberghi S, Caimi L, Gargiulo F, et al. Seroprevalence of HTLV-I and HTLV-II infection among immigrants in northern Italy. Eur J Epidemiol. 2003;18(6):583–8. doi: 10.1023/a:1024655228893. [DOI] [PubMed] [Google Scholar]
- 41.Catalan-Soares BC, Almeida RT, Carneiro-Proietti AB. Prevalence of HIV-1/2, HTLV-I/II, hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum and Trypanosoma cruzi among prison inmates at Manhuacu, Minas Gerais State, Brazil. Rev Soc Bras Med Trop. 2000;33(1):27–30. doi: 10.1590/s0037-86822000000100004. [DOI] [PubMed] [Google Scholar]
- 42.Fialho M, Messias M, Page-Shafer K, Farre L, Schmalb M, Pedral-Sampaio D, et al. Prevalence and risk of blood-borne and sexually transmitted viral infections in incarcerated youth in Salvador, Brazil: opportunity and obligation for intervention. AIDS Behav. 2008;12(4 Suppl):S17–24. doi: 10.1007/s10461-008-9409-x. [DOI] [PubMed] [Google Scholar]
- 43.Sabbatani S, Giuliani R, Fulgaro C, Paolillo P, Baldi E, Chiodo F. [HIVAb, HCVAb and HBsAg seroprevalence among inmates of the prison of Bologna and the effect of counselling on the compliance of proposed tests]. Epidemiol Prev. 2004;28(3):163–8. [PubMed] [Google Scholar]
- 44.Adoga MP, Banwat EB, Forbi JC, Nimzing L, Pam CR, Gyar SD, et al. Human immunonodeficiency virus, hepatitis B virus and hepatitis C virus: sero-prevalence, co-infection and risk factors among prison inmates in Nasarawa State, Nigeria. J Infect Dev Ctries. 2009;3(7):539–47. doi: 10.3855/jidc.472. [DOI] [PubMed] [Google Scholar]
- 45.Anwar MS, Nafees M, Nabi U. Sero-prevalence of HCV and associated infections with HIV and HBV among prisoners in Lahore. Biomedica. 2011;27(2):119–1122. [Google Scholar]