Abstract
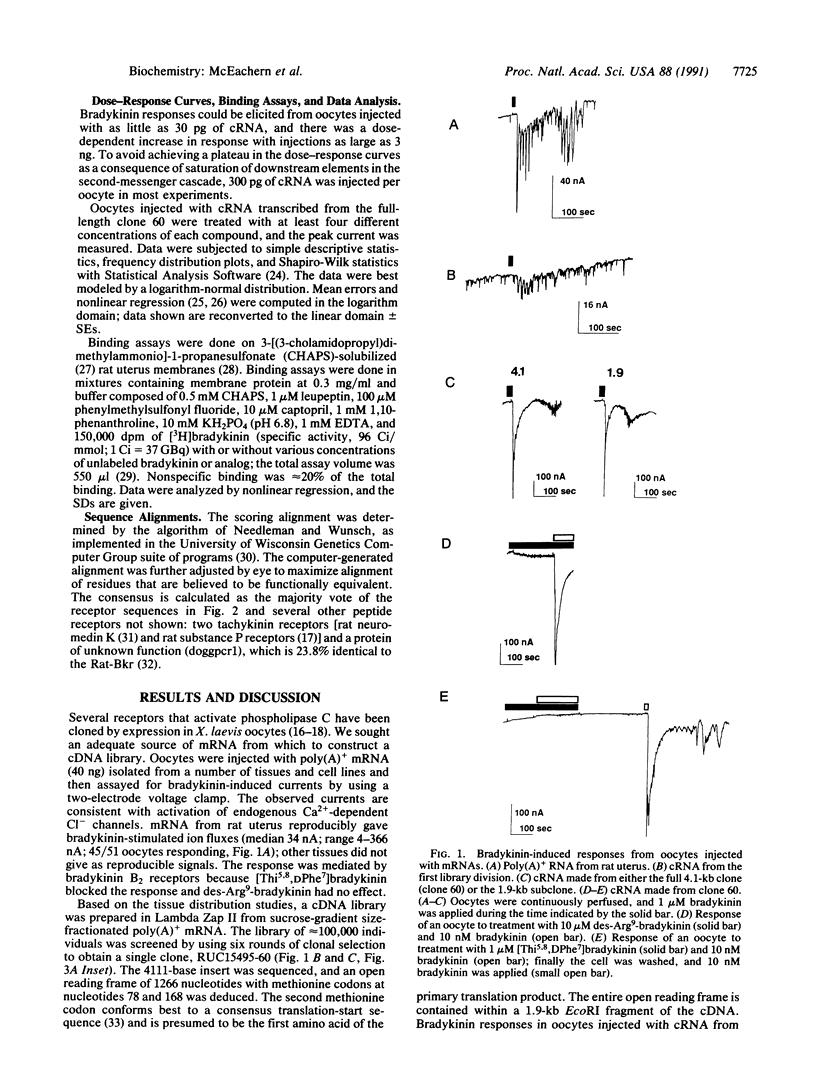
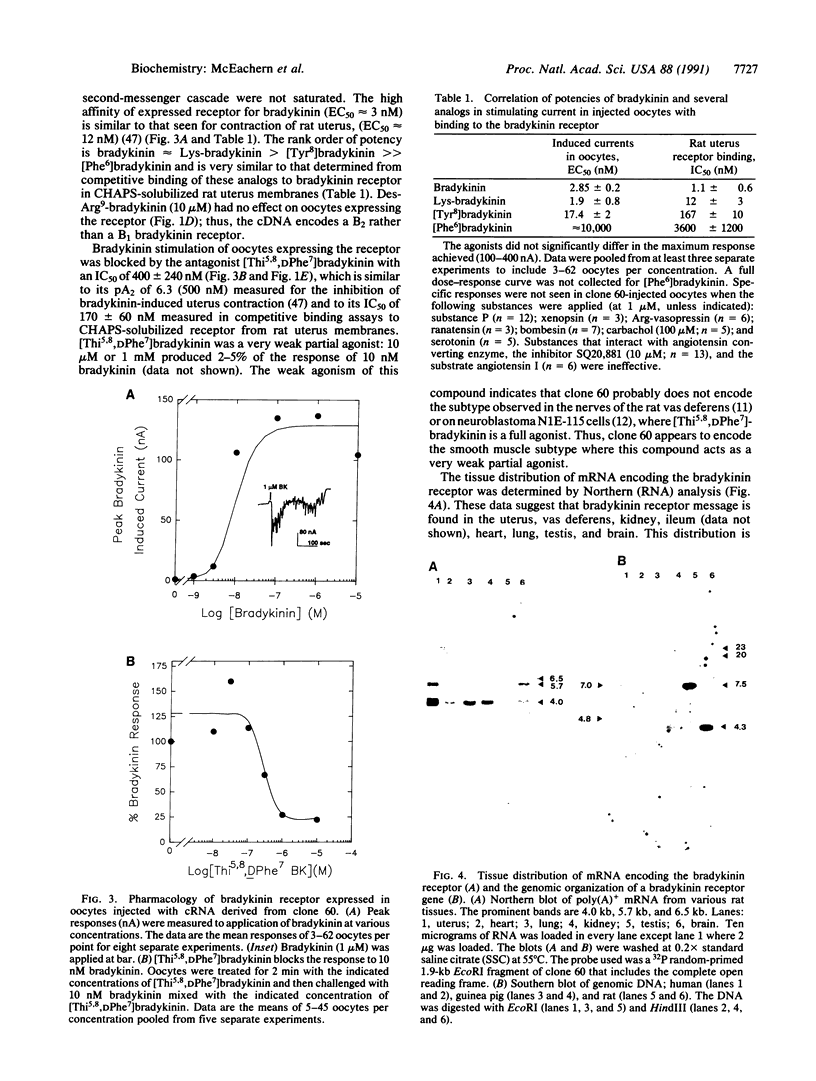
A cDNA encoding a functional bradykinin receptor was isolated from a rat uterus library by a clonal selection strategy using Xenopus laevis oocytes to assay for expression of bradykinin responses. The predicted protein is homologous to the seven transmembrane G protein-coupled superfamily of receptors. Bradykinin and its analogs stimulate a Cl- current oocytes expressing the receptor with the rank order of potency: bradykinin approximately Lys-bradykinin greater than [Tyr8]-bradykinin much greater than [Phe6]bradykinin. This is the rank order of potency observed for these compounds in competitive binding assays on soluble receptor from rat uterus. Des-Arg9-bradykinin (10 microM) elicits no response when applied to oocytes expressing the receptor; thus, the cDNA encodes a B2 type bradykinin receptor. [Thi5,8,DPhe7]bradykinin, where Thi is beta-(2-thienyl)-alanine, is a very weak partial agonist and inhibits the bradykinin-mediated ion flux, suggesting the cDNA encodes a smooth muscle, rather than a neuronal, B2 receptor subtype. Receptor message has a distribution consistent with previous reports of bradykinin function and/or binding in several tissues and is found in rat uterus, vas deferens, kidney, lung, heart, ileum, testis, and brain. Receptor subtypes are a possibility because several tissues contain two or three message species (4.0, 5.7, and 6.5 kilobases). Southern blot high-stringency analysis demonstrated that the rat, guinea pig, and human genomes contain a single gene. As bradykinin is a key mediator of pain, knowledge of the primary structure of this receptor will allow a molecular understanding of the receptor and aid the design of antagonists for pain relief.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIENS E. J., VAN ROSSUM J. M., SIMONIS A. M. A theoretical basis of molecular pharmacology. I. Interactions of one or two compounds with one receptor system. Arzneimittelforschung. 1956 May;6(5):282–293. [PubMed] [Google Scholar]
- Becherer P. R., Mertz L. F., Baenziger N. L. Regulation of prostaglandin synthesis mediated by thrombin and B2 bradykinin receptors in a fibrosarcoma cell line. Cell. 1982 Aug;30(1):243–251. doi: 10.1016/0092-8674(82)90030-7. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Braas K. M., Manning D. C., Perry D. C., Snyder S. H. Bradykinin analogues: differential agonist and antagonist activities suggesting multiple receptors. Br J Pharmacol. 1988 May;94(1):3–5. doi: 10.1111/j.1476-5381.1988.tb11492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lawson-Wendling K., Pugsley T. A. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983 Jul 1;132(1):74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Burch R. M., Steranka L. R., Axelrod J. Distinct bradykinin receptors mediate stimulation of prostaglandin synthesis by endothelial cells and fibroblasts. J Pharmacol Exp Ther. 1988 Feb;244(2):646–649. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Sigal I. S., Candelore M. R., Register R. B., Scattergood W., Rands E., Strader C. D. Structural features required for ligand binding to the beta-adrenergic receptor. EMBO J. 1987 Nov;6(11):3269–3275. doi: 10.1002/j.1460-2075.1987.tb02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrick M. J., Vavrek R. J., Stewart J. M., Odya C. E. Further studies of myometrial bradykinin receptor-like binding. Biochem Pharmacol. 1984 Sep 15;33(18):2887–2892. doi: 10.1016/0006-2952(84)90212-0. [DOI] [PubMed] [Google Scholar]
- Gantz I., Schäffer M., DelValle J., Logsdon C., Campbell V., Uhler M., Yamada T. Molecular cloning of a gene encoding the histamine H2 receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):429–433. doi: 10.1073/pnas.88.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocayne J., Robinson D. A., FitzGerald M. G., Chung F. Z., Kerlavage A. R., Lentes K. U., Lai J., Wang C. D., Fraser C. M., Venter J. C. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8296–8300. doi: 10.1073/pnas.84.23.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Manning D. C., Stewart J. M., Snyder S. H. [3H]Bradykinin receptor binding in mammalian tissue membranes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2630–2634. doi: 10.1073/pnas.78.4.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S. S., Khorana H. G. Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. J Biol Chem. 1990 Oct 15;265(29):17520–17524. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M. J., Dumont J. E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989 May 5;244(4904):569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Llona I., Vavrek R., Stewart J., Huidobro-Toro J. P. Identification of pre- and postsynaptic bradykinin receptor sites in the vas deferens: evidence for different structural prerequisites. J Pharmacol Exp Ther. 1987 May;241(2):608–614. [PubMed] [Google Scholar]
- Loosfelt H., Misrahi M., Atger M., Salesse R., Vu Hai-Luu Thi M. T., Jolivet A., Guiochon-Mantel A., Sar S., Jallal B., Garnier J. Cloning and sequencing of porcine LH-hCG receptor cDNA: variants lacking transmembrane domain. Science. 1989 Aug 4;245(4917):525–528. doi: 10.1126/science.2502844. [DOI] [PubMed] [Google Scholar]
- Mahan L. C., Burch R. M. Functional expression of B2 bradykinin receptors from Balb/c cell mRNA in Xenopus oocytes. Mol Pharmacol. 1990 Jun;37(6):785–789. [PubMed] [Google Scholar]
- Manning D. C., Snyder S. H., Kachur J. F., Miller R. J., Field M. Bradykinin receptor-mediated chloride secretion in intestinal function. Nature. 1982 Sep 16;299(5880):256–259. doi: 10.1038/299256a0. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- Olsen R., Santone K., Melder D., Oakes S. G., Abraham R., Powis G. An increase in intracellular free Ca2+ associated with serum-free growth stimulation of Swiss 3T3 fibroblasts by epidermal growth factor in the presence of bradykinin. J Biol Chem. 1988 Dec 5;263(34):18030–18035. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Lys-bradykinin stimulates Na+ influx and DNA synthesis in cultured human fibroblasts. Cell. 1983 Mar;32(3):979–985. doi: 10.1016/0092-8674(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Nonlinearity and facilitation in phosphoinositide signaling studied by the use of caged inositol trisphosphate in Xenopus oocytes. J Neurosci. 1989 Nov;9(11):4068–4077. doi: 10.1523/JNEUROSCI.09-11-04068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perney T. M., Miller R. J. Two different G-proteins mediate neuropeptide Y and bradykinin-stimulated phospholipid breakdown in cultured rat sensory neurons. J Biol Chem. 1989 May 5;264(13):7317–7327. [PubMed] [Google Scholar]
- Plevin R., Owen P. J. Multiple B2 kinin receptors in mammalian tissues. Trends Pharmacol Sci. 1988 Nov;9(11):387–389. doi: 10.1016/0165-6147(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Roberts R. A. Bradykinin receptors: characterization, distribution and mechanisms of signal transduction. Prog Growth Factor Res. 1989;1(4):237–252. doi: 10.1016/0955-2235(89)90013-6. [DOI] [PubMed] [Google Scholar]
- Roscher A. A., Manganiello V. C., Jelsema C. L., Moss J. Autoregulation of bradykinin receptors and bradykinin-induced prostacyclin formation in human fibroblasts. J Clin Invest. 1984 Aug;74(2):552–558. doi: 10.1172/JCI111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Nakanishi S. Molecular characterization of rat substance K receptor and its mRNAs. Biochem Biophys Res Commun. 1989 Dec 15;165(2):695–702. doi: 10.1016/s0006-291x(89)80022-1. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Yokota Y., Tsuchida K., Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990 Jan 15;265(2):623–628. [PubMed] [Google Scholar]
- Steranka L. R., Manning D. C., DeHaas C. J., Ferkany J. W., Borosky S. A., Connor J. R., Vavrek R. J., Stewart J. M., Snyder S. H. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988 May;85(9):3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Masu M., Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990 Jun;4(6):847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Vavrek R. J., Stewart J. M. Competitive antagonists of bradykinin. Peptides. 1985 Mar-Apr;6(2):161–164. doi: 10.1016/0196-9781(85)90033-6. [DOI] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with Shaker mRNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7243–7247. doi: 10.1073/pnas.86.18.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. G., Phillips E., Snell C. R., Snell P. H., Webb M. Construction of a physiologically active photoaffinity probe based on the structure of bradykinin: labelling of angiotensin converting enzyme but not candidate bradykinin receptors on NG108-15 cells. J Neurochem. 1989 May;52(5):1508–1516. doi: 10.1111/j.1471-4159.1989.tb09201.x. [DOI] [PubMed] [Google Scholar]