Abstract
Background and purpose
The purpose of this study was to determine the distribution of 4-borono-2-18F-fluoro-phenylalanine (18F-BPA) and L-[methyl-11C] methionine (11C-Met) in normal organs and tumors and to evaluate the usefulness of 11C-Met/PET in screening potential candidates for boron neutron capture therapy (BNCT).
Material methods
Seven patients who had at least one histologically confirmed head and neck tumor were included in this study. They underwent both whole-body 18F-BPA-PET/CT and 11C-Met-PET/CT within a span of 6 months. Uptake was evaluated using the maximum standardized uptake value (SUVmax). Regions of interest (ROIs) were placed within the tumors and target organs of brain, thyroid, submandibular gland, lung, liver, esophagus, stomach pancreas, spleen, muscle, and bone marrow.
Results
The tumor SUVmax of FBPA and 11C-Met showed strong correlation (r 2 = 0.72, P = 0.015). Although 18F-BPA and 11C-Met showed markedly different uptake in some organs (submandibular gland, liver, heart, stomach pancreas, spleen, and bone marrow), the uptake of 11C-Met was consistently higher than that of 18F-BPA in these cases.
Conclusion
11C-Met PET/CT might be used instead of 18F-BPA PET/CT to predict the accumulation of 10B in tumors and to select candidates for BNCT. However, it would not be suitable for evaluating accumulation in some normal organs. Therefore, the 18F-BPA-PET study remains a prerequisite for BNCT. This is the first report of the correlation between 18F-BPA and 11C-Met accumulation.
Background
Boron neutron capture therapy (BNCT) has recently attracted attention and has been used for brain tumors, head and neck cancers, and melanoma [1–4]. It is a targeted radiotherapy method, based on the nuclear reaction of neutrons and 10B. After the injection of a 10B carrier, it accumulates in target tumor cells. The region to be treated is then exposed to thermal neutrons, and the nuclear reaction of these neutrons with 10B produces alpha particles and 7Li at very short range (<10 μm), causing lethal damage to tumor cells. The success of BNCT is dependent on the sufficient accumulation of 10B in tumor cells and the minimization of such accumulation in normal cells [1, 2, 5]. 10B accumulation is not consistent across cases, and is reported to depend upon tumor type. However, even tumors of the same grade may differ in terms of their biochemical properties. Therefore, it is necessary to assess 10B accumulation in each case prior to performing BNCT [6].
10B-borono-L-phenylalanine (BPA) is the most frequently used 10B carrier, and 18F-BPA, an 18F-labelled analog of BPA, has been developed to predict 10B accumulation in tumors and normal tissues by positron emission tomography (PET) [7, 8]. Before BNCT, a 18F-BPA-PET study is carried out to evaluate the tumor-to–normal tissue accumulation ratio (TNR). The TNR strongly influences the success of BNCT, and the ideal TNR has been reported to be greater than 2.5 to 5 [1, 5]. However, the synthesis of 18F-BPA, a radiolabeled amino acid, is limited due to low radio yield, low synthetic yield, and high cost. Therefore few hospitals or institutions can synthesize 18F-BPA in practice [9].
11C -labelled Methionine (11C-Met) is the most popular radiolabeled amino acid. Methionine is an essential amino acid and plays an important role in protein synthesis as it is coded for by the initiation codon. Radiolabeled methionine is a convenient radiochemical product because it can be obtained by rapid synthesis with high radiochemical yield [10]. 11C-Met is a highly sensitive tool capable of yielding considerable information on protein synthesis, and has been used widely in the assessment of various cancers [11, 12]. Compared to 18F-fluorodeoxyglucose, 11C-Met shows low uptake in the brain, so 11C-Met PET can evaluate head and neck tumors without interference from physiological accumulation in the brain [13, 14].
In this study, we evaluated the distribution of these two radiolabeled amino acids, 18F-BPA and 11C-Met, in patients with head and neck tumors, in order to evaluate whether 11C-Met PET can be used instead of 18F-BPA PET for screening potential candidates for BNCT.
Materials and methods
General
All chemical reagents were obtained from commercial sources. This study was conducted according to a protocol approved by the institutional review board/independent ethics committee of the National Cancer Center Hospital (Tokyo, Japan). All patients signed a written informed consent form before the initiation of the study.
Radiosynthesis of 18F-BPA and 11C-MET
18F-BPA was synthesized by direct electrophilic radiofluorination of BPA (Sigma-Aldrich, St. Louis, MO, USA) using 18F-acetyl hypofluorite as described previously [6]. Purification of 18F-BPA was performed by high performance liquid chromatography (HPLC) using a YMC-Pack ODS-A column (20 × 150 mm; YMC, Kyoto, Japan) eluted with 0.1% acetic acid at a flow rate of 10 ml/minute. The radiochemical purity of 18F-BPA as determined by HPLC was > 99.5%, while its specific activity was 25 MBq/μmol.
11C-Met was synthesized in the radiochemical laboratory of this institute by methylation with L-homocysteine thiolactone followed by isolation of the final product by solid phase extraction using L-homocysteine thiolactone (Sigma-Aldrich, St. Louis, MO, USA) [10]. The radiochemical purity of 11C-Met ranged from 95 to 98%.
Subjects
The seven patients included in this study underwent both 18F-BPA-PET/computed tomography (CT) and 11C-Met-PET/CT at least 24 h apart, but within 6 months of each other, between January 2014 and July 2016. Of the 116 patients who underwent 18F-BPA PET/CT, seven also underwent 11C-Met-PET/CT. These seven patients were retrospectively selected for this study. They had histologically confirmed head and neck tumors, Eastern Cooperative Oncology Group performance status (PS) of 0–1, adequate organ function (neutrophil count ≥ 1500 /μL, platelet count ≥ 75,000 /μL, hemoglobin concentration ≥ 9.0 g/dL, serum bilirubin ≤1.5 mg/dL, aspartate transaminase (AST) and alanine aminotransferase (ALT) ≤ 100 IU/L, serum creatinine ≤ 1.5 mg/dL, baseline left ventricular ejection fraction (LVEF) > 60%), and were older than age 20. For evaluating the distribution of 11C-Met and 18F-BPA in normal organs, we excluded patients with congestive heart failure, uncontrolled angina pectoris, arrhythmia, symptomatic infectious disease, severe bleeding, pulmonary fibrosis, obstructive bowel disease or severe diarrhea, and symptomatic peripheral or cardiac effusion.
Patients fasted for at least 4 h before examination.
PET/CT protocol
PET/CT images were acquired with a Discovery 600 scanner (GE Healthcare, Milwaukee, WI, USA). Whole-body 18F-BPA PET/CT imaging was carried out at 1 h after the injection of 18F-BPA (ca. 4 MBq/kg). The scan timing for 18F-BPA PET/CT was determined as in our previous work [6]. Whole-body 11C-Met PET/CT was carried out 10 min after the injection of 11C-Met (ca. 4 MBq/kg). The scan timing for11C-Met PET/CT was determined by a previous report [15]. A scout image was first acquired to determine the scanning field range from the head to the pelvis of the patient, using settings of 10 mA and 120 kV. Next, whole-body 16-slice helical CT and whole-body 3D PET were performed. PET images were acquired in 7–8 bed positions with 2-min acquisition durations per bed position, such that the images covered the same field as the whole-body CT image. The acquired data were reconstructed as 192 × 192 matrix images (3.65 × 3.65 mm) using a 3D ordered subsets-expectation maximization algorithm.
Tumor uptake of 18F-BPA and 11C-Met
PET image evaluation and quantification of the standardized uptake value (SUV) were performed using AW Volume Share 4.5 software (GE Healthcare, Milwaukee, WI, USA). Regions of interest (ROIs) were delineated on the axial 2-D image. SUV was defined as regional radioactivity divided by injected radioactivity normalized to body weight. 18F-BPA uptake was evaluated using the maximum SUV (SUVmax) 1 h after injection, while 11C-Met uptake was evaluated 10 min after injection. ROIs were placed within the tumor and target organs of brain (white matter), thyroid, submandibular gland, lung, liver, esophagus (cervical esophagus), stomach, pancreas, spleen, muscle (latissimus dorsi), and bone marrow (12th thoracic vertebra). Tumor ROIs were defined as the areas of highest activity. ROIs were also placed onto normal tissue surrounding the tumor to calculate the TNR of 18F-BPA. Clinically, dose planning is performed based on the TNR prior to the initiation of BNCT to avoid severe damage to normal tissues.
Statistical analysis
For statistical analysis of the data, JMP software (version 9.0, SAS Institute, Inc., Cary, NC) was used. A linear regression analysis was performed for the correlation study. Fisher’s exact test was used to estimate the concordance of the cut-off values of the two tracers. Probability values of P < 0.05 were considered significant. Since patients in previous studies were determined to be eligible for BNCT when the TNR of 18F-BPA was more than 2.5 [1, 6, 8, 16], we used a 18F-BPA TNR of more than 2.5 as a cut-off to distinguish positive from negative.
Results
Seven patients with head and neck tumors underwent both 18F-BPA-PET/CT and 11C-Met-PET/CT during the study period and were enrolled in this study (six males and one female; ages 20 to 66 years, median 47 years).
Patient and tumor characteristics are summarized in Table 1. In terms of the primary disease type, two patients had facial rhabdomyosarcoma, one had external auditory canal cancer, one had lingual cancer, one had malignant melanoma of the nasal cavity, one had parotid gland cancer, and one had adenoid cystic carcinoma of the lacrimal sac. Histological diagnoses included two patients with squamous cell carcinoma (SCC), two with adenoid cystic carcinoma (ACC), two with rhabdomyosarcoma (RBD), and one with malignant melanoma. Four patients experienced local recurrence (LR), one had distant metastases, and two had a newly diagnosed second malignancy. All patients had unresectable tumors. All lesions were located within the head and neck region (two lesions in the maxillary sinus, one in the external auditory canal, one in the nasal cavity, one in the tongue, one in the parotid gland, and one in the orbit). Tumor size scaled by PET/CT ranged from 2 to 5 cm. The tumor size did not change in the interval between PET scans in six cases, but in one case (No. 1) the tumor grew from 3 cm to 5 cm. Five patients underwent surgery and received radiotherapy and chemotherapy within 1 year before the PET scans, while two patients did not receive any treatment before the scans. Neither chemotherapy nor radiation therapy was performed in any patient in the interval between PET scans, but one patient (No. 1) underwent palliative surgery during this time. However, in case No. 1 the target tumor was almost unresectable and we were able to evaluate radioisotope accumulation in the tumor tissue. In four cases, 18F-BPA-PET/CT was performed prior to 11C-Met-PET/CT. In the remaining three cases, 18F-BPA-PET/CT was performed after 11C-Met-PET/CT. The interval between studies was less than 3 weeks in six cases, and more than 3 months in one case (No. 1). The interval ranged from 2 to 123 days (mean, 23 ± 41; median, 5).
Table 1.
Patient and tumor characteristics
| No. | Sex | Age | Primary disease | Presentation | Histology | Location | Past therapy | Size (cm) | Interval between studies (days) | Tumor SUVmax | Tumor TNR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11C-Met | 18F-BPA | 11C-Met | 18F-BPA | ||||||||||
| 1 | M | 20 | Facial RBD | NT | RBD | orbit | Noa | 3.2 × 3.0 to 4.5 × 5.0b | 123 | 3.7 | 3.6 | 3.8 | 3.9 |
| 2 | M | 20 | Facial RBD | LR | RBD | MS | PO/CRT | 2.5 × 1.5 | 5 | 1.5 | 1.6 | 1.1 | 1.3 |
| 3 | M | 27 | Lingual | LR | SCC | Lingual | PO/CRT | 3.2 × 4.1 | 16 | 6.6 | 4.2 | 4.6 | 3.1 |
| 4 | M | 50 | EAC cancer | LR | SCC | EAC | PO /CRT | 3.3 × 4.4 | 2 | 4.4 | 4.1 | 3.1 | 2.9 |
| 5 | M | 47 | Parotid gland ACC | LR | ACC | Parotid gland | PO /CRT | 2.0 × 2.3 | 2 | 5.6 | 5.6 | 5.1 | 2.7 |
| 6 | M | 47 | Nasal melanoma | NT | Melanoma | Nasal cavity | No | 3.1 × 3.2 | 4 | 5.8 | 4.7 | 4.1 | 3.9 |
| 7 | F | 66 | Lacrimal ACC | M | ACC | MS | PO /CRT | 3.3 × 2.2 | 6 | 4.7 | 3.6 | 2 | 2.2 |
| Average | 5 (median) |
4.6 ± 1.7 (mean) |
3.9 ± 1.2 (mean) |
3.4 ± 1.4 (mean) |
2.9 ± 0.9 (mean) |
||||||||
RBD rhabdomyosarcoma, EAC external auditory canal, ACC adenoid cystic carcinoma, NT newly diagnosed tumor, LR local recurrence, M Metastasis, SCC squamous cell carcinoma, MS maxillary sinus, PO postoperative, CRT chemoradiation therapy
aPalliative surgery was performed in the interval between PET scans. bTumor size increased in the interval between PET scans
Representative PET/CT imaging of 18F-BPA and 11C-Met is shown in Fig. 1. SUVmax values of 18F-BPA and 11C-Met in tumor tissue are summarized in Table 1. The accumulations of 18F-BPA and 11C-Met varied widely among tumor cases, even in those with the same pathology. The tumor SUVmax of 18F-BPA ranged from 1.6 to 5.6 (mean, 3.9 ± 1.4), while that of 11C-Met ranged from 1.5 to 5.8 (mean, 4.6 ± 1.7). The TNRs of both 18F-BPA and 11C-Met also varied widely. The TNR of 18F-BPA ranged from 1.3 to 3.9 (mean, 2.9 ± 0.9), while that of 11C-Met ranged from 1.1 to 5.1 (mean, 3.4 ± 1.4).
Fig. 1.
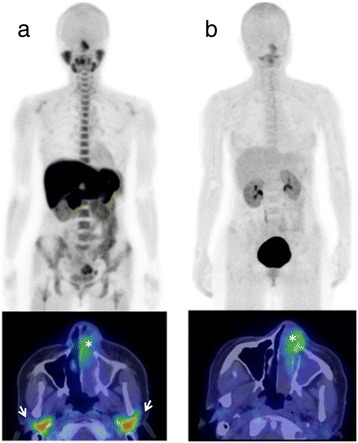
Representative 11C-Met and 18F-BPA PET/CT (patient No. 6). Upper panel: maximum intensity projection imaging. Lower panel: PET/CT fusion image. a 11C-Met-PET/CT at 10 min after injection. Tumor SUVmax was 5.8. (*). High physiological uptake is shown in the salivary glands (arrows), bone marrow, and some abdominal organs. b 18F-BPA-PET/CT at 1 h after injection. Tumor SUVmax was 4.7. (*). Physiological uptake is generally low
The TNRs of 18F-BPA and 11C-Met were weakly correlated (r 2 = 0.51), though statistical significance was not observed (P = 0.07). However, the SUVmax of 18F-BPA and 11C-Met within each tumor exhibited strong correlation (Fig. 2; r 2 = 0.72, P = 0.015).
Fig. 2.
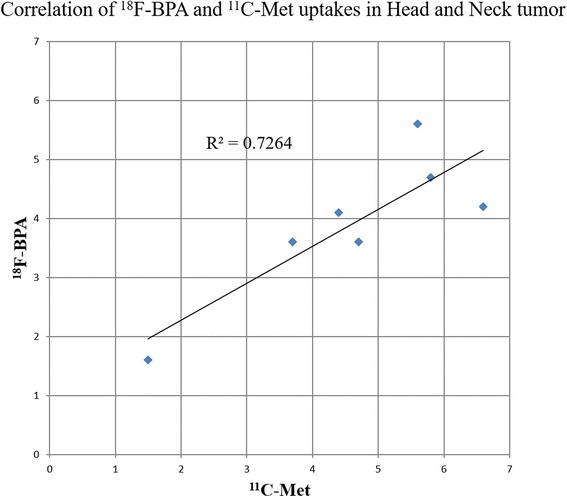
Correlation of SUVmax between 18F-BPA and 11C-Met in head and neck tumors. A close linear correlation is observed between FBPA uptake and MET uptake in head and neck tumors (r 2 = 0.72, P = 0.15)
Accumulations of 11C-Met and 18F-BPA in normal organs are summarized in Table 2. As expected, accumulations of 18F-BPA in normal organs showed smaller differences between patients than accumulations in tumors.
Table 2.
Average and standard deviation (SD) of 18F-BPA and 11C-Met uptake in normal organs
| 11C-Met | 18F-BPA | P value | |
|---|---|---|---|
| Brain | 1.5 ± 0.1 | 1.3 ± 0.3 | 0.31 |
| Submandibular gland | 5.2 ± 1.2 | 2.0 ± 0.5 | <<0.01 |
| Thyroid gland | 2.1 ± 0.4 | 1.4 ± 0.5 | 0.7 |
| Esophagus | 2.3 ± 0.6 | 1.9 ± 0.6 | 0.18 |
| Lung | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.76 |
| Heart | 2.5 ± 0.4 | 1.2 ± 0.1 | <<0.01 |
| Blood | 1.5 ± 0.2 | 1.3 ± 0.1 | 0.07 |
| Stomach | 8.8 ± 2.6 | 1.5 ± 0.2 | <<0.01 |
| Liver | 11 ± 1.8 | 2.0 ± 0.5 | <<0.01 |
| Pancreas | 12.6 ± 2.8 | 1.7 ± 0.3 | <<0.01 |
| Spleen | 3.1 ± 0.6 | 1.5 ± 0.3 | <<0.01 |
| Kidney | 4.0 ± 0.4 | 3.4 ± 0.9 | 0.18 |
| Muscle | 1.3 ± 0.1 | 1.2 ± 0.2 | 0.21 |
| Bone marrow | 3.8 ± 0.8 | 1.5 ± 0.3 | <<0.01 |
| Tumor | 4.6 ± 1.7 | 3.9 ± 1.2 | 0.39 |
Across all patients, the uptake of 18F-BPA and 11C-Met differed widely in the submandibular gland, liver, heart, stomach, pancreas, spleen, and bone marrow. In these organs, the uptake of 11C-Met was consistently higher than that of 18F-BPA. In all other organs, no significant difference was observed.
Discussion
It is well-known that accumulation of radioisotopes is affected by nature of tumors [17, 18]. In most cases in this study, PET/CTs were performed as preparation for therapy and the intervals between scans were less than 3 weeks. However, in one case (No. 1), the interval between 18F-BPA-PET/CT and 11C-Met-PET/CT was more than 3 months; palliative surgery was performed during the interscan interval, although the target tumor was almost unresectable. The long interval and palliative operation may have affected the results in this case. However, excluding the single long-interval case, the TNRs of 18F-BPA and 11C-Met showed a weak correlation (r 2 = 0.57 P = 0.08), and the SUVmax of 18F-BPA and 11C-Met within each tumor were strongly correlated (r 2 = 0.73, P = 0.03). As a result, we do not believe that the interscan interval or the palliative operation particularly influenced the results of this study. On the other hand, five patients underwent surgery and received radiotherapy and chemotherapy within 1 year before the PET scans, and it is unclear whether this treatment influenced our results.
Radiolabeled amino acids are among the most important tracers for identifying and examining tumors, since cellular proliferation requires protein synthesis. Amino acids are the natural building blocks of proteins, and high uptake of these precursors is a normal feature of rapidly proliferating cells such as tumor cells. Tumor cells take up amino acids by amino acid transporters, thus the numbers of such transporters is increased in most tumor types as compared to healthy tissue [19]. Studies have shown that there are a variety of amino acid transporters, such as System L, System A, System ASC, and System B [9, 20], and 18F-BPA is a System L–specific imaging agent [9]. System L is Na+-independent and is a major nutrient transport system responsible for the transport of neutral amino acids. System L includes four families, LAT1–LAT4.
18F-BPA uptake correlates with total LAT expression, but more specifically with that of LAT1 and LAT4, which are overexpressed in many tumors [9, 21–23]. On the other hand, 11C-Met is taken up not only by System L, but also by many other types of amino acid transporter such as System A, System ASC, and System B [20, 24, 25]. It has been previously reported that the expression of amino acid transporters in tumors varies widely, and it sometimes reflects proliferation speed and malignancy [26]. This may explain the wide variation in the tumor accumulation of 18F-BPA and 11C-Met in this study, regardless of pathology. Despite the fact that 11C-Met and 18F-BPA are affected by different amino acid transporters, we found that 11C-Met uptake correlated closely with 18F-BPA uptake. One possible explanation is that the rates of amino acid transport and protein synthesis are so rapid that differences in the types of amino acid transporter may not be significant. Further studies with larger numbers of participants and comparison of particular histological features should be performed to resolve this question.
In normal organs, we found that accumulation differed between 11C-Met and 18F-BPA in the submandibular gland, liver, heart, stomach, pancreas, spleen, and bone marrow, whereas it was similar in all other normal organs. Previous papers have also reported similar tendencies in 11C-Met distribution [15] . The uptake of 11C-Met and 18F-BPA in normal organs showed smaller individual differences than in tumors. The variation in relative accumulation between tumors and healthy organs may be attributed to the histological heterogeneity of tumors. Similarly, there was no significant difference between accumulations in brain, thyroid, lung, esophagus, and muscle. These organs had insufficient accumulations of both 11C-Met and 18F-BPA to reveal any difference, perhaps because there is less protein synthesis and cell proliferation in these organs, making the uptake of the amino acids themselves very low.
On the other hand, some normal organs showed higher uptake of 11C-Met than of 18F-BPA. This difference was especially pronounced in organs with high levels of protein synthesis or cell proliferation, such as the submandibular gland, pancreas, liver, stomach, and bone marrow. The heart produces several hormones, such as brain natriuretic peptide. The spleen sometimes has extramedullary hematopoietic functions in cases where the bone marrow has been damaged. Thus both organs can be considered to be involved in protein synthesis. Although these organs all require amino acids to synthesize proteins, the pancreas, liver, stomach, heart, and submandibular gland do not express LAT1 or LAT4, while the bone marrow and spleen express LAT1 but not LAT4 [27, 28]. These latter organs may exhibit less 18F-BPA uptake because is transported mainly by LAT1 and LAT4 [9]. As mentioned, 11C-Met can be transported by many types of amino acid transporter [9, 20], including System A, expressed by the stomach, liver, pancreas, heart, and spleen [29], and System B (0,+), expressed by the salivary glands [30]. This could explain the fact that the accumulation of 11C-Met in these organs was higher than that of 18F-BPA, and it may be that variation in the distribution of amino acid transporters causes differences in uptake. In other words, the greater accumulation of 11C-Met may suggest that its uptake reflects protein synthesis more generally, whereas the uptake of 18F-BPA also reflects the expression of LAT1 and LAT4.
Our study also revealed a low physiological accumulation of 18F-BPA in normal organs. This may be of great advantage, not only in the evaluation of 10B accumulation, but in tumor detection. It could be especially useful when evaluating tumors in the liver, the pancreas, or stomach. In particular, a study on bile duct carcinoma, including pancreatic cancer, found that the expression of LAT 1 correlated positively with degree of malignancy [26], thus the accumulation of 18F-BPA might predict the malignancy of these cancers.
Our findings suggest that the accumulation of 11C-Met may predict the accumulation of 18F-BPA in tumors. Nevertheless, for success of BNCT, performance of the TNR is more important than evaluation of SUVmax [1, 5].
In all cases where the tumor TNR of 11C-Met was positive, the tumor TNR of 18F-BPA was also positive. However, the correlation between the TNRs of 18F-BPA and 11C-Met was not statistically significant. It is inferred that the accumulation in normal tissues surrounding the tumor differed between 18F-BPA and 11C-Met. In this study, as the organs demonstrating high uptake of 11C-Met were located far from the tumors, or post resection, we were able to a certain extent to evaluate the TNR by 11C-Met PET/CT. However, if a tumor were near such organs, it would disturb the evaluation of the TNR.
Conclusion
Despite variations in tumor pathology and a small patient population, 18F-BPA accumulation in tumors showed a strong correlation with 11C-Met accumulation. Thus, 11C-Met PET/CT might be useful to select candidates for 18F-BPA PET/CT. However, 11C-Met PET/CT would not be suitable for evaluating accumulation in some normal organs, such as the submandibular gland, liver, heart, stomach pancreas, spleen, and bone marrow. Therefore, the 18F-BPA-PET study remains a prerequisite for BNCT. However, further studies are called for, using larger numbers of participants and selection of particular histological diagnostic criteria.
Limitations
As mentioned, some limitations should be acknowledged. First, the very small population and retrospective nature of this study may have led to selection bias. Second, the study included a variety of tumor pathologies.
Acknowledgements
The authors thank Mr. Takayuki Nanma and the staff of SHI Accelerator Service Ltd. for their technical support. We thank Mr. Akira Hirayama and the staff of GE healthcare for their technical support. We thank Dr. Atsushi Kono and Kitajima Kazuhiro for manuscript revision, and Mr. Paul Shelton for English proofreading.
We also thank Ms. Rieko Onoe for her secretarial support. Finally, we thank all the study participants and patients.
Funding
This work was supported by the Practical Research for Innovative Cancer Control Program from the Japan Agency for Medical Research and Development (AMED), the Cancer Research and Development Fund of NCC (#23-A-46), and JSPS KAKENHI Grant Number JP26461873.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
Author contributions were as follows. Conception and design: HK. Data acquisition: HK. Analysis and interpretation of data: YW, HK. Drafting of the manuscript or revising it critically for important intellectual content: all authors. Final approval of the submitted manuscript: all authors.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from all patients for publication of this study. A copy of the written consent is available from the Editor-in-Chief of this journal for review.
Ethics approval and consent to participate
This study was reviewed and approved by the National Cancer Center Hospital Research-Ethics Review Committee. The committee’s reference number is 2011–165.
Abbreviations
- 11C-Met
L-[methyl-11C] methionine
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- BNCT
Boron neutron capture therapy
- BPA
Borono-L-phenylalanine
- CT
Computed tomography
- HPLC
High-performance liquid chromatography
- LAT
System L amino acid transporter
- LVEF
Baseline left ventricular ejection fraction
- PET
Positron emission tomography
- PS
Eastern Cooperative Oncology Group performance status
- ROI
Region of interest
- SUVmax
The maximum standardized uptake value
- TNR
Tumor-to–normal tissue accumulation ratio
Contributor Information
Yoshiaki Watanabe, Email: ds14094@gmail.com.
Hiroaki Kurihara, Phone: +81-3-3542-2511, Email: hikurihancc@gmail.com.
Jun Itami, Email: jitami@ncc.go.jp.
Ryohei Sasaki, Email: yuunasasaki@hotmail.com.
Yasuaki Arai, Email: arai-y3111@mvh.biglobe.ne.jp.
Kazuro Sugimura, Email: sugimura@med.kobe-u.ac.jp.
References
- 1.Barth RF, Vicente MG, Harling OK, Kiger WS, 3rd, Riley KJ, Binns PJ, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrera MS, Gonzalez SJ, Minsky DM, Kreiner AJ. Evaluation of performance of an accelerator-based BNCT facility for the treatment of different tumor targets. Phys Med. 2013;29:436–46. doi: 10.1016/j.ejmp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Kankaanranta L, Seppala T, Koivunoro H, Saarilahti K, Atula T, Collan J, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2012;82:e67–75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 4.Menendez PR, Roth BM, Pereira MD, Casal MR, Gonzalez SJ, Feld DB, et al. BNCT for skin melanoma in extremities: updated Argentine clinical results. Appl Radiat Isot. 2009;67:S50–3. doi: 10.1016/j.apradiso.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Henriksson R, Capala J, Michanek A, Lindahl SA, Salford LG, Franzen L, et al. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA) Radiother Oncol. 2008;88:183–91. doi: 10.1016/j.radonc.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Tani H, Kurihara H, Hiroi K, Honda N, Yoshimoto M, Kono Y, et al. Correlation of (18)F-BPA and (18)F-FDG uptake in head and neck cancers. Radiother Oncol. 2014;113:193–7. doi: 10.1016/j.radonc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Imahori Y, Ueda S, Ohmori Y, Kusuki T, Ono K, Fujii R, et al. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J Nucl Med. 1998;39:325–33. [PubMed] [Google Scholar]
- 8.Imahori Y, Ueda S, Ohmori Y, Sakae K, Kusuki T, Kobayashi T, et al. Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: part II. Clin Cancer Res. 1998;4:1833–41. [PubMed] [Google Scholar]
- 9.Yoshimoto M, Kurihara H, Honda N, Kawai K, Ohe K, Fujii H, et al. Predominant contribution of L-type amino acid transporter to 4-borono-2-18 F-fluoro-phenylalanine uptake in human glioblastoma cells. Nucl Med Biol. 2013;40:625–9. doi: 10.1016/j.nucmedbio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Långström B, Antoni G, Gullberg P, Halldin C, Malmborg P, Någren K, et al. Synthesis of L-and D-[methyl-11C] methionine. J Nucl Med. 1987;28:1037–40. [PubMed] [Google Scholar]
- 11.Bergström M, Lundqvist H, Ericson K, Lilja A, Johnström P, Långström B, et al. Comparison of the accumulation kinetics of L-(methyl-11C)-methionine and D-(methyl-11C)-methionine in brain tumors studied with positron emission tomography. Acta Radiol. 1987;28:225–9. [PubMed] [Google Scholar]
- 12.Kubota K. From tumor biology to clinical PET: a review of positron emission tomography (PET) in oncology. Ann Nucl Med. 2001;15:471–86. doi: 10.1007/BF02988499. [DOI] [PubMed] [Google Scholar]
- 13.Leskinen-Kallio S, Nagren K, Lehikoinen P, Ruotsalainen U, Teras M, Joensuu H. Carbon-11-methionine and PET is an effective method to image head and neck cancer. J Nucl Med. 1992;33:691–5. [PubMed] [Google Scholar]
- 14.Lindholm P, Leskinen S, Lapela M. Carbon-11-methionine uptake in squamous cell head and neck cancer. J Nucl Med. 1998;39:1393–7. [PubMed] [Google Scholar]
- 15.Isohashi K, Shimosegawa E, Kato H, Kanai Y, Naka S, Fujino K, et al. Optimization of [11 C] methionine PET study: appropriate scan timing and effect of plasma amino acid concentrations on the SUV. EJNMMI Res. 2013;3:1. doi: 10.1186/2191-219X-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyashita M, Miyatake S-I, Imahori Y, Yokoyama K, Kawabata S, Kajimoto Y, et al. Evaluation of fluoride-labeled boronophenylalanine-PET imaging for the study of radiation effects in patients with glioblastomas. J Neurooncol. 2008;89:239–46. doi: 10.1007/s11060-008-9621-6. [DOI] [PubMed] [Google Scholar]
- 17.Nakasone Y, Inoue T, Oriuchi N, Takeuchi K, Negishi A, Endo K, et al. The role of whole-body FDG-PET in preoperative assessment of tumor staging in oral cancers. Ann Nucl Med. 2001;15:505–12. doi: 10.1007/BF02988503. [DOI] [PubMed] [Google Scholar]
- 18.Alluri KC, Tahari AK, Wahl RL, Koch W, Chung CH, Subramaniam RM. Prognostic value of FDG PET metabolic tumor volume in human papillomavirus-positive stage III and IV oropharyngeal squamous cell carcinoma. AJR Am J Roentgenol. 2014;203:897–903. doi: 10.2214/AJR.14.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–35. doi: 10.1007/s00259-012-2295-5. [DOI] [PubMed] [Google Scholar]
- 20.Stevens BR. Vertebrate intestine apical membrane mechanisms of organic nutrient transport. Am J Physiol Regul Integr Comp Physiol. 1992;263:R458–63. doi: 10.1152/ajpregu.1992.263.3.R458. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–66. [DOI] [PubMed]
- 22.Haase C, Bergmann R, Fuechtner F, Hoepping A, Pietzsch J. L-type amino acid transporters LAT1 and LAT4 in cancer: uptake of 3-O-methyl-6-18F-fluoro-L-dopa in human adenocarcinoma and squamous cell carcinoma in vitro and in vivo. J Nucl Med. 2007;48:2063–71. doi: 10.2967/jnumed.107.043620. [DOI] [PubMed] [Google Scholar]
- 23.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophysica Acta. 2001;1514:291–302. doi: 10.1016/S0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 24.Coope DJ, Čížek J, Eggers C, Vollmar S, Heiss W-D, Herholz K. Evaluation of primary brain tumors using 11C-methionine PET with reference to a normal methionine uptake map. J Nucl Med. 2007;48:1971–80. doi: 10.2967/jnumed.107.043240. [DOI] [PubMed] [Google Scholar]
- 25.Jager PL, Vaalburg W, Pruim J, De Vries EG, Langen K-J, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432–45. [PubMed] [Google Scholar]
- 26.Kaira K, Sunose Y, Ohshima Y, Ishioka NS, Arakawa K, Ogawa T, et al. Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer. 2013;13:1. doi: 10.1186/1471-2407-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodoy S, Fotiadis D, Stoeger C, Kanai Y, Palacín M. The small SLC43 family: facilitator system l amino acid transporters and the orphan EEG1. Mol Aspects Med. 2013;34:638–45. doi: 10.1016/j.mam.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Fotiadis D, Kanai Y, Palacín M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34:139–58. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Schiöth HB, Roshanbin S, Hägglund MG, Fredriksson R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Aspects Med. 2013;34:571–85. doi: 10.1016/j.mam.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


