Abstract
Objective
Polymicrogyria (PMG) is a malformation of cortical development characterized by formation of an excessive number of small gyri. Sixty percent to 85% of patients with PMG have epilepsy that is refractory to medication, but surgical options are usually limited. We characterize a cohort of patient with polymicrogyria who underwent epilepsy surgery and document seizure outcomes.
Methods
A retrospective study of all patients with PMG who underwent epilepsy surgery (focal seizure foci resection and/or hemispherectomy) at our center was performed by review of all clinical data related to their treatment.
Results
We identified 12 patients (7 males and 5 female) with mean age of 18 (ranging from 3 months to 44 years) at time of surgery. Mean age at seizure onset was 8 years, with the majority (83%) having childhood onset. Six patients had focal, five had multifocal, and one patient had diffuse PMG. Perisylvian PMG was the most common pattern seen on magnetic resonance imaging (MRI). Eight patients had other cortical malformations including hemimegalencephaly and cortical dysplasia. Scalp electroencephalography (EEG) often showed diffuse epileptic discharges that poorly lateralized but were focal on intracranial electrocorticography (ECoG). Eight patients underwent seizure foci resection and four underwent hemispherectomy. Mean follow-up was 7 years (ranging from one to 19 years). Six patients (50%) were seizure-free at last follow-up. One patient had rare seizures (Engel class II). Three patients were Engel class III, having either decreased seizure frequency or severity, and two patients were Engel class IV. Gross total resection of the PMG cortex trended toward good seizure control.
Significance
Our study shows that even in patients with extensive or bilateral PMG malformations, some may still be good candidates for surgery because the epileptogenic zone may involve only a portion of the malformation. Intracranial ECoG can provide additional localizing information compared to scalp EEG in guiding resection of epileptogenic foci.
Keywords: Polymicrogyria, Cortical malformation, Cortical dysplasia, Malformation of cortical development, Schizencephaly, Hemimegalencephaly, Hemispherectomy, Refractory epilepsy, Surgical treatment, Seizure outcome
Polymicrogyria (PMG) is a cortical malformation characterized by an excessive number of small irregular gyri leading to the appearance of an abnormal cortical surface. It arises from disturbances during cerebral cortical development, most likely during the late stages of neuronal migration and early stages of cortical organization.1 Causes of PMG are diverse and include congenital infection (in particular cytomegalovirus infection),2 in utero ischemia, and genetic mutations (e.g., WDR62, NDE1, and TBR2).3
PMG can be focal, multifocal, or diffuse, affecting different areas and portions of the cerebral cortex in a unilateral or bilateral pattern. The most common locations are the insula and the perisylvian cortex. Patients with PMG may have a variety of clinical presentations including hemiparesis, intractable epilepsy, as well as speech and developmental delays. Although the incidence of PMG is unknown, it is one of the most common malformations of cortical development and an important contributor to medically refractory epilepsy,4 as 60–85% of all patients with the diagnosis of PMG have epilepsy.5 Onset is typically during childhood but can present in the second decade. Seizure types are multiple and include focal with or without alteration of awareness, secondary generalized, epileptic spasms, and atonic seizures, among others. Associated epileptic syndromes are generally defined as those symptomatic of malformations of cortical development, and status epilepticus of sleep may be a feature.6,7 Most patients with PMG are refractory to medication.8
Clinical management of epilepsy patients with PMG can be difficult. Often, they are not good surgical candidates given the extent and often multifocal involvement of the lesion. There are limited data on surgical outcomes of patients with PMG.9–12 A few case reports have described successful surgical treatment of patients with focal PMG by resection of epileptic foci,5,13 but there are few systematic studies to date that examine surgical outcomes for patients with PMG who have undergone epilepsy surgery.
In this study, we characterize 12 cases of PMG with medically refractory epilepsy that underwent surgical resection for management of seizures. Surgical interventions lead to a reduction in seizure frequency or severity, and in some cases, complete seizure freedom.
Methods
Patient selection
Patients were queried from neuroimaging and pathology databases at University of California San Francisco (UCSF) between 1987 and 2014. Patients were selected based on either radiographic or pathologic findings of PMG. We also included patients with PMG with other malformations of cortical development including cortical dysplasia, schizencephaly, heterotopia, and hemimegalencephaly. We identified 34 patients and included 19 who underwent surgical resection for management of their epilepsy. Only 12 patients had sufficient clinical data to be in the study. All research protocols were approved by the UCSF institutional review board for human research (Committee for Human Research).
Clinical data review
Preoperative data included date of seizure onset, type of seizure (focal with or without alteration of awareness, secondary generalized, atonic, infantile spasms, and so on), seizure frequency, seizure duration, medications, and other associated developmental symptoms. Other preoperative tests included scalp electroencephalography (EEG) studies, magnetic resonance (MR) imaging, and neuropsychological testing when available. Data pertaining to patient demographics, seizure-related history, neuroimaging, surgical details, pathology reports, and seizure outcome were obtained from hospital and departmental records. Available scalp EEG was independently reviewed by an experienced epileptologist (SBC). Available preoperative MRI was independently reviewed by an experienced neuroradiologist (AJB). PMG lesions were considered to be focal if they were restricted to a single lobe or well-circumscribed adjacent gyri (i.e., perisylvian), multifocal if they were multiple discrete areas (i.e., bilateral hemispheric involvement), and diffuse if the abnormal cortices expanded to multiple lobes or involved the whole hemisphere.
Resection of epileptogenic foci
Operative data, including the use of cortical and subcortical language and motor mapping and intracranial electrocorticography (ECoG), were reviewed. Most cases utilized Stealth (Medtronic, Minneapolis, MN, U.S.A.) or BrainLab (BrainLab North America, Westchester, IL, U.S.A.) frameless neuronavigation to assist with intraoperative localization. Intraoperative electrocorticography (ECoG) was obtained with use of a customized crown of 16 ball-tipped carbon electrodes referred to a common cerebral reference. When necessary, additional coverage was obtained by using subdural electrode strips and depth electrodes. Patients underwent implantation of chronic subdural electrode arrays for extraoperative seizure monitoring and electrical stimulation mapping when there is no clear localization of epileptic foci on scalp EEG and/or the lesion was adjacent to eloquent cortical areas. Extraoperative ECoG was used in patients 4, 6, 10, and 12 to guide resection, and intraoperative ECoG was typically not repeated prior to resection. The anatomic resection was tailored according to the extent of the intracranial ECoG abnormalities and the functional anatomy. Epileptogenic tissue was defined by those contacts with active epileptiform discharges on intracranial ECoG during the interictal or ictal period from either intraoperative or extraoperative recordings. Epileptic tissue was considered to be focal if epileptiform discharges arose from contacts within a well-circumscribed region of the cortex or within a lobe, and diffuse if discharges were seen throughout contacts spanning multiple lobes.
Patients with defined seizure foci on scalp EEG and/or intracranial ECoG underwent focal resection of their electrographically active regions as first line of treatment. The exceptions are patients with hemimegalencephaly or with nonfocal ECoG findings, who received hemispherectomies as their initial surgery. Extent of resection was designated as gross-total or subtotal according to review of available preand postoperative MR images and the pre- and postexcision intracranial ECoG recordings. Resection of epileptogenic tissue determined by ECoG was considered complete if all tissue exhibiting critical patterns was resected. Gross total resection was designated for patients who underwent functional hemispherectomy given complete disconnection of cortical and subcortical structures.
Postoperative outcome and statistical analysis
Clinic visits and phone interviews were conducted for follow-up. Each patient's outcome was assessed using the Engel Seizure Outcome Classification Scheme14: Engel class I, free from disabling seizures (Ia, completely seizure-free since surgery; Ib, nondisabling simple partial seizures only since surgery; Ic, some disabling seizures since surgery, but free from disabling seizures for ≥2 years; Id, generalized convulsions with discontinuation of antiepileptic drugs only); Engel class II, rare disabling seizures (IIa, initially free from disabling seizures, but still has rare seizures; IIb, rare disabling seizures since surgery; IIc, occasional disabling seizures since surgery, but rare seizures for the past 2 years; IId, nocturnal seizures only); Engel class III, worthwhile improvement (IIIa, worthwhile seizure reduction; IIIb, prolonged seizure-free intervals amounting to >50% of follow-up period, but not <2 years); and Engel class IV, no worthwhile improvement (IVa, significant seizure reduction; IVb, no appreciable change, IVc, seizures worse). Postoperative motor and cognitive deficits were extracted from clinical notes, and when available, from outpatient physical/occupational therapy visits and school performance notes.
For univariate analysis for predictors of seizure control, we used chi-square test for categorical variables and t-tests for continuous variables.
Results
Patient demographic and seizure characteristics
Our study included 12 patients (7 male and 5 female) with ages ranging from 3 months to 44 years at time of surgery (mean age 18.7 years, median 13.1). Seizure onset ranged from birth to 26 years of age (mean age 8.5 years, median 5.5) with the majority (10 of 12 patients, 83%) having childhood onset. Mean epilepsy duration until surgery was 10.2 years. Seizure types included focal with or without alteration in awareness, secondarily generalized tonic–clonic, epileptic spasms, and atypical absence seizures. All patients had poor response to antiepileptic drugs (AED) after a trial of multiple (three to six) medications. After review of clinical semiology, scalp EEG, and imaging features, nine patients were classified as having medically refractory epilepsy symptomatic of malformation of cortical development, and one patient had electrical status epilepticus during sleep (ESES). Two patients were uncategorized given either normal or unavailable scalp EEG. In addition to seizures, three patients were found to have congenital hydrocephalus (patients 3, 8, and 9), and one had infantile spasms (patient 11). Of note, patient 2 had a genetic syndrome known as CLOVES-like syndrome, which is congenital disorder characterized by lipomatous overgrowth, vascular malformations, epidermal nevus, and spinal anomalies. Patient demographics are summarized in Table 1.
Table 1.
Patient preoperative epilepsy syndrome, PMG characteristics, surgery description, and seizure control outcome
| Pt | Age, sex | Age onset | Preop seizure type | PMG type and associated imaging findings | Epilepsy syndrome | Surgery performed | Engel |
|---|---|---|---|---|---|---|---|
| 1 | 3 month, F | Birth | Focal dyscognitive | Multifocal left PMG of frontal pole, convexity and frontal and parietal opercula with transmantle cortical dysplasia and hemimegalencephaly | Not available | Left hemispherectomy | Ia |
| 2 | 1 year, F | Birth | ES, focal motor | Diffuse left PMG with hemimegalencephaly, pachygyria and subependymal heterotopia | Focal refractory with ES, symptomatic of MCD | Left functional hemispherectomy | Ia |
| 3 | 9 year, M | 6 year | Focal motor and dyscognitive, SGTCS, SE, atypical absences, and ESES | Bilateral temporal occipital polymicrogyria, bilateral parietal and occipital while matter volume loss and thinning of the posterior corpus callosum | ESES | Right occipital seizure foci resection | Ia |
| 4 | 34 year, M | 26 year | Focal ± dyscognitive, motor or SGTCS | Right occipital polymicrogyria extending from occipital pole to calcarine sulcus and to posteromedial aspect of occipital horn of lateral ventricle | Focal refractory, symptomatic of MCD | Right occipital seizure foci resection | Ia |
| 5 | 30 year, F | 11 year | Focal ± dyscognitive, motor or SGTCS | Focal right perisylvian polymicrogyria with involvement of the frontal, parietal and temporal lobes | Not available | 1. Right frontal seizure foci resection | Ic |
| 2. VNS | |||||||
| 6 | 38 year, M | 26 year | Focal dyscognitive ± SGTCS | Right temporal lobe polymicrogyria with cortical dysplasia | Focal refractory, symptomatic of MCD | Right frontoparietal and temporal seizure foci resection | Ic |
| 7 | 36 year, F | Child hood | Focal dyscognitive with aphasia and motor symptoms ± SGTCS | Bilateral perisylvian polymicrogyria with left closed-lip schizencephaly | Focal refractory, symptomatic of MCD | Left frontotemporal seizure foci resection | IIc |
| 8 | 4 month, M | 4 month | Focal motor ± SGTCS | Focal right frontal polymicrogyria involving operculum, anterior insula, orbital gyri, reduced white matter volume | Focal refractory, symptomatic of MCD | 1. Right frontal seizure foci resection | IIIa |
| 2. Right functional hemispherectomy | |||||||
| 9 | 10 year, M | 5 year | Focal sensory and motor ± SGTCS | Bilateral occipital lobe polymicrogyria with left parietal gross architectural distortion likely from in utero insult | Focal refractory, symptomatic of MCD | Left functional hemispherectomy | IIIa |
| 10 | 44 year, F | 18 year | Focal dyscognitive with aphasia ± SGTCS | Left greater than right bilateral posterior perisylvian polymicrogyria extending to parietal operculum | Focal refractory, symptomatic of MCD | Right anterior temporal, inferior frontal and parietal seizure foci resection | IIIa |
| 11 | 2 year, M | 2 month | ES, focal motor | Focal right perisylvian PMG and hemimegalencephaly | Focal refractory with ES, symptomatic of MCD | Right functional hemispherectomy | IVb |
| 12 | 16 year, M | 3.5 year | Focal motor ± dyscognitive, ±SGTCS | Focal left perisylvian polymicrogyria with left hippocampal atrophy and nodular heterotopia of the occipital horn | Focal refractory, symptomatic of MCD | Left superior temporal, peri-insula, and parietal seizure foci resection | IVb |
PMG, polymicrogyria; FU, follow-up; SGTCS, secondary generalized tonic–clonic seizures; ES, epileptic spasm; SE, status epilepticus; ESES, electrical status epilepticus during sleep; MCD, malformation of cortical development; VNS, vagus nerve stimulator.
Preoperative imaging characteristics
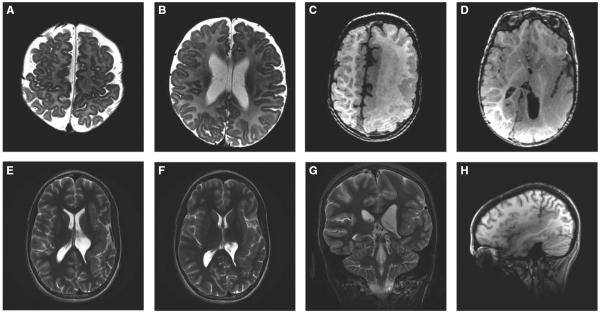
As demonstrated in our patient population, the location of PMG can be unilateral or bilateral, and associated with other central nervous system (CNS) cortical development abnormalities. The PMG lesion was found in one or more of the following lobes: frontal lobe (two patients) (Fig. 1A,B), temporal lobe (three patients), and occipital lobe (three patients). The most common location was the perisylvian region, which was the site in six patients (50%) (Fig. 1E–H). Eight patients had unilateral PMG (three left hemispheric, five right hemispheric), and four patients had bilateral involvement. Those patients with bilateral involvement included two with bilateral perisylvian and two with bilateral occipital lobe PMG. In addition to PMG, eight patients had other cortical malformations, including three patients with hemimegalencephaly, two patients with cortical dysplasia, two patients with heterotopia/architectural distortion, and one patient with closed-lip schizencephaly. Of the three patients with hemimegalencephaly, PMG was in the affected hemisphere in all cases (Fig. 1C,D). The preoperative MRI findings are described in Table 1.
Figure 1.
(A and B) Axial inversion recovery images of patient 8 showing very fine polymicrogyria of the right frontal operculum, right anterior insula and right orbital gyri. (C and D) Axial T1 MRI images of patient 2 who has left hemimegalencephaly with diffuse polymicrogyria involving most of the left hemisphere. There is abnormal white matter containing several layers of heterotopic neurons and enlarged hemisphere with dysmorphic ventricles including enlarged trigone/occipital horn. (E–H) Left perisylvian polymicrogyria as demonstrated in patient 7. Axial and coronal T2 (E, F, and G) images showing left posterior perisylvian PMG involving the entire insula and temporal and parietal operculum, up to the parietal convexity. Sagittal T1 sequence demonstrates the extent of the perisylvian polymicrogyria (H).
Epilepsia © ILAE
Seizure localization
All patients underwent video scalp electroencephalography (EEG) for preoperative seizure localization. Of the 11 available scalp EEG reports prior to surgery, eight had focal seizures recorded from the scalp and five were well lateralized to the affected hemisphere (Table 2). Of the eight patients with unilateral PMG, three patients had poorly lateralized seizures (patients 4, 5, and 6) and one had status epilepticus of sleep, or ESES (patient 3). For the four patients with bilateral PMG, scalp EEG was useful in determining seizure lateralization in two patients (patients 3 and 7). Patient 3 had bilateral occipital lobe PMG with scalp EEG showing spikes arising from right occipital and posterior parietal regions. Patient 7 had bilateral perisylvian PMG but also closed-lip schizencephaly in the left parietal region and had focal seizures arising from the corresponding inferior parietal and superior temporal region.
Table 2.
Scalp EEG and intracranial electrocorticography characteristics
| Scalp EEG features |
Intracranial ECoG features |
||||||
|---|---|---|---|---|---|---|---|
| Pt | Scalp EEG description | Laterality | Focal/diffuse | Seizure focus | Focal/diffuse | Concord with PMG on MRI | Concord with other MCD on MRI |
| 1 | NA | Left | Diffuse | – | – | Yes | Yes |
| 2 | Focal seizures arising from the left posterior region with multifocal left hemisphere interictal epileptiform transients and left hemisphere slowing | Left | Diffuse | – | – | Yes | Yes |
| 3 | Frequent 2.5–3 Hz spike and wave with a right parietal predominance enhanced during sleep consistent with ESES | Right | Diffuse | Right occipital and posterior parietal region | Focal | Yes | Yes |
| 4 | Focal seizures with evolving theta poorly lateralized or localized but with a right sided emphasis | Nonlateral | Diffuse | Right occipital lobea | Focal | Yes | na |
| 5 | Routine normal EEG | – | – | Right suprasylvian, frontoparietal region | Focal | Yes | na |
| 6 | Focal seizures, poorly lateralized with a right temporal emphasis | Nonlateral | Diffuse | Right posterior suprasylvian and temporal regiona | Focal | Yes | Yes |
| 7 | Focal seizures arising from the left temporal region | Left | Focal | Left inferior parietal and superior temporal region | Focal | Yes | Yes |
| 8 | Focal seizure arising from the right frontal region with concordant, focal interictal epileptiform transients in wakefulness and sleep | Right | Focal | Right frontal region | Focal | Yes | Yes |
| 9 | Generalized, asymmetric slowing of the background affecting the left greater than right hemisphere | Nonlateral | Diffuse | Left parietal region and hemisphere | Diffuse | No | Yes |
| 10 | Focal seizures with poor lateralization or localization | Nonlateral | Diffuse | Right suprasylvian and temporal regiona | Diffuse | Yes | na |
| 11 | Focal seizures arising broadly from the right hemisphere with right interictal discharges | Right | Diffuse | – | – | Yes | Yes |
| 12 | Focal seizures arising from the left hemisphere | Left | Diffuse | Left postcentral cortex and superior temporal regiona | Focal | Yes | Yes |
EEG, electroencephalography; ECoG, electrocorticography; Concord, concordance; PMG, polymicrogyria; MCD, malformation of cortical development; ESES, electrical status epilepticus during sleep; na; not applicable.
Patients 4, 6, 10, and 12 underwent extraoperative ECoG recording with subdural grid depth electrodes.
Nine of the 12 patients who underwent epilepsy surgery had either intraoperative or extraoperative intracranial ECoG for further delineation of their seizure foci (Table 2). The three patients who did not undergo intracranial ECoG underwent either functional or anatomic hemispherectomies for hemimegalencephaly (patients 1, 2, and 11). Patients 4, 6, 10, and 12 underwent extraoperative ECoG recording with chronic subdural grid and depth electrodes for seizure localization. ECoG was useful in defining the epileptogenic tissue in all cases and demonstrated good concordance with all four lateralizing scalp EEGs (patients 3, 7, 8, and 12). Notably, intracranial ECoG was valuable in localizing epileptogenic tissue in patients with diffuse and nonlateralizing scalp EEG findings (patients 4, 6, 9, and 10), including a patient with normal EEG (patient 5). Based on intracranial ECoG, seizure foci were focal in seven patients and diffuse in two patients (Table 2).
Operative outcome
Eight patients underwent focal resection of epileptic foci and four underwent either anatomic or functional hemispherectomy as their first operation for seizure control. Two patients who had focal resection of seizure foci had subsequent surgeries for additional seizure control (patient 5 had a vagus nerve stimulator placed and patient 9 underwent functional hemispherectomy). Mean length of follow-up was 7 years (ranging from 1 to 19 years). Six patients became seizure-free (Engel class I) at last follow-up. One patient had rare seizures following surgery (Engel class II). Three patients had significant improvement in their seizures frequency and severity (Engel class III), and two patients had the same seizure frequency and no worthwhile improvement (Engel class IV). Seizure control outcome and Engel subclassifications are summarized in Table 4.
Table 4.
Univariate analysis of factors affecting seizure outcome for all patients
| All patients | Seizure-free | Residual seizures | p-Value | |
|---|---|---|---|---|
| N (%) | 12 | 6 (50) | 6 (50) | |
| Age (mean ± SD) | 18.7 ± 16.8 | 19.2 ± 17.1 | 18.2 ± 18.0 | 0.9233 |
| Seizure onset (mean ± SD) | 8.5 ± 9.7 | 11.5 ± 12.0 | 5.5 ± 6.6 | 0.3139 |
| Seizure duration (mean ± SD) | 10.2 ± 10.3 | 7.7 ± 7.3 | 12.7 ± 12.8 | 0.4301 |
| MRI location | ||||
| Perisylvian | 6 | 2 (33) | 4 (67) | 0.2482 |
| Other lobe (frontal, parietal, temporal, or occipital) | 6 | 4 (67) | 2 (33) | |
| MRI laterality | ||||
| Bilateral | 4 | 1 (25) | 3 (75) | 0.2207 |
| Unilateral | 8 | 3 (38) | 5 (64) | |
| Type of PMG | ||||
| Focal | 6 | 3 (50) | 3 (50) | 0.5488 |
| Multifocal | 5 | 2 (40) | 3 (60) | |
| Diffuse | 1 | 1 (100) | 0 (0) | |
| Other cortical malformations | ||||
| Hemimegalencephaly | 3 | 2 (67) | 1 (33) | 0.2548 |
| Cortical dysplasia | 2 | 2 (100) | 0 (0) | |
| Schizencephaly | 1 | 0 (0) | 1 (100) | |
| Heterotopia/architectural distortion | 2 | 0 (0) | 2 (100) | |
| Scalp EEG: seizure-onset zone | ||||
| Diffuse | 9 | 5 (56) | 4 (44) | 0.1535 |
| Focal | 2 | 0 (0) | 2 (100) | |
| Intracranial ECoG: epileptogenic tissue | ||||
| Diffuse | 2 | 0 (0) | 2 (100) | 0.1515 |
| Focal | 7 | 4 (57) | 3 (43) | |
| Type of surgery | ||||
| Hemispherectomy | 4 | 2 (50) | 2 (50) | 1.000 |
| Seizure foci resection | 8 | 4 (50) | 4 (50) | |
| Extent of PMG resection | ||||
| Gross total resectiona | 7 | 5 (71) | 2 (29) | 0.0790 |
| Subtotal resection | 5 | 1 (20) | 4 (80) | |
| Extent of MCD resection | ||||
| Gross total resectiona | 5 | 3 (60) | 2 (40) | 0.4652 |
| Subtotal resection | 3 | 1 (33) | 2 (67) | |
| Extent of ECoG resection | ||||
| Gross total resection | 6 | 4 (67) | 2 (33) | 0.1025 |
| Subtotal resection | 2 | 0 (0) | 2 (100) |
PMG, polymicrogyria; EEG, electroencephalography; ECoG, electrocorticography; MCD, malformation of cortical development.
Including those patients with functional hemispherectomy.
Common postoperative deficits included hemiparesis (six patients), hemianopsia (four patients), and language and cognitive delay (four patients). Of patients who had postoperative hemiparesis, four had mild preoperative hemiparesis (patients 1, 2, 11, and 12). All patients who underwent hemispherectomies (patients 1, 2, 8, 9, and 11) had expected postoperative contralateral hemiparesis. When able to assess, patients who underwent hemispherectomy or occipital lobectomy had expected hemianopsia. Of the four patients who had recognizable cognitive, language, and/or social and emotional delay, two had resections in the left hemisphere and two in the right hemisphere. One patient developed permanent diabetes insipidus postoperatively. These findings are summarized in Table 3.
Table 3.
Extent of resection and outcome
| Extent of surgical resection |
Engel outcome | Seizure reduction | Follow-up (years) | |||||
|---|---|---|---|---|---|---|---|---|
| Pt | PMG | Cortical malform | ECoG | STR reason | Postoperative deficits | |||
| 1 | GTR | GTR | – | Ia | Seizure-free | 1.5 | Mild right hemiparesis, unknown cognitive outcome | |
| 2 | GTRa | GTRa | – | Ia | Seizure-free | 2.4 | Right arm>leg weakness; Mild motor and cognitive delay | |
| 3 | STR | STR | GTR | Bilateral | Ia | Seizure-free | 1.5 | Mild cognitive, social, emotional delay |
| 4 | GTR | – | GTR | Ia | Seizure-free | 0.9 | Left homonymous hemianopsia, no cognitive deficits | |
| 5 | GTR | – | GTR | Ia | Seizure-free | 9.2 | No motor, language, or cognitive deficits | |
| 6 | GTR | GTR | GTR | Ic | Seizure-free after 5 months | 15.2 | Mild left hand apraxia, no cognitive deficits | |
| 7 | STR | STR | STR | Bilateral, eloquent: language | IIc | Monthly focal seizures | 17.7 | No motor, language, or cognitive deficits |
| 8 | GTRa | – | GTR | IIIa | Clusters of seizures, reduced severity | 6.4 | Left arm>leg weakness; language delay: able to comprehend but has slowed expressive language | |
| 9 | STRa | GTRa | – | Bilateral | IIIa | Daily seizures, reduced frequency and severity | 19.2 | Right hemiparesis; homonymous hemianopsia |
| 10 | STR | – | STR | Bilateral, eloquent: motor | IIIa | Rare seizures | 1.0 | No motor, language, or cognitive deficits |
| 11 | GTRa | GTRa | – | IVb | Same daily seizure frequency, different type | 2.3 | Left arm>leg weakness, oromotor dysfunction, DI, gross developmental delay, tracks, nonverbal | |
| 12 | STR | STR | GTR | No discharge in other parts of PMG | IVb | Same daily seizure frequency | 6.9 | Very mild right hemiparesis, no language or cognitive deficits |
PMG, polymicrogyria; malform, malformation; ECoG, electrocorticography; GTR, gross total resection; STR, subtotal resection; DI, diabetes insipidus; na, not available.
Including patients who underwent functional hemispherectomy.
We performed univariate analysis to find predictors of favorable seizure-free outcome. The location, focality, or laterality of the PMG region were not predictors of seizure control; neither was the presence of other cortical malfor mations (Table 4). The focality of preoperative scalp EEG or intracranial ECoG abnormalities did not appear to correlate with seizure-free outcome. The extent of resection for areas of PMG, other cortical malformation, and electrographic abnormalities was described for each patient (Table 3). We found that gross total resection or disconnection (in case of functional hemispherectomy) of PMG cortex was associated with improved seizure control, although it did not reach statistical significance (p = 0.0790, Table 4). Of interest, the extent of resection for other associated cortical malformations or intracranial ECoG foci did not significantly affect seizure control rates (Table 4). We also performed our univariate analysis to exclude those patients with hemimegalencephaly, given that it is a different malformation of cortical development for which hemispherectomy is an established surgical option for treating medically refractory seizures.15,16 We also did not find any statistically significant factors associated with seizure freedom, after evaluation of the location of PMG, focality of imaging, and electrographic abnormalities, as well as the extent of resection (Table 5).
Table 5.
Univariate analysis of factors affecting seizure outcome excluding patients with hemimegalencephaly
| Excluding HMG patients | Seizure-free | Residual seizures | p-Value | |
|---|---|---|---|---|
| N(%) | 9 | 4 (44) | 5 (56) | |
| Age (mean ± SD) | 24.4 ± 15.4 | 28.2 ± 12.7 | 21.4 ± 18.2 | 0.5455 |
| Seizure onset (mean ± SD) | 11.3 ± 9.7 | 21.45 ± 18.2 | 6.6 ± 6.7 | 0.1017 |
| Seizure duration (mean ± SD) | 13.1 ± 10.3 | 11.0 ± 6.7 | 12.2 ± 13.0 | 0.6109 |
| MRI location | ||||
| Perisylvian | 4 | 1 (25) | 3 (75) | 0.2937 |
| Other lobe (frontal, parietal, temporal, or occipital) | 5 | 3 (60) | 2 (40) | |
| MRI laterality | ||||
| Bilateral | 4 | 1 (25) | 3 (75) | 0.2937 |
| Unilatera | 5 | 3 (60) | 2 (40) | |
| Type of PMG | ||||
| Focal | 5 | 3 (60) | 2 (40) | 0.2937 |
| Multifocal | 4 | 1 (25) | 3 (75) | |
| Other cortical malformations | ||||
| Cortical dysplasia | 2 | 2 (100) | 0 (0) | 0.0821 |
| Schizencephaly | 1 | 0 (0) | 1 (100) | |
| Heterotopia/architectural distortion | 2 | 0 (0) | 2 (100) | |
| Scalp EEG: seizure-onset zone | ||||
| Diffuse | 6 | 3 (50) | 3 (50) | 0.2059 |
| Focal | 2 | 0 (0) | 2 (100) | |
| Intracranial ECoG: seizure-onset zone | ||||
| Diffuse | 2 | 0 (0) | 2 (100) | 0.1515 |
| Focal | 7 | 4 (57) | 3 (43) | |
| Type of surgery | ||||
| Hemispherectomy | 2 | 0 (0) | 2 (100) | 0.1515 |
| Seizure foci resection | 7 | 4 (57) | 3 (43) | |
| Extent of PMG resection | ||||
| Gross total resectiona | 4 | 3 (75) | 1 (25) | 0.0989 |
| Subtotal resection | 5 | 1 (20) | 4 (80) | |
| Extent of MCD resection | ||||
| Gross total resectiona | 2 | 1 (50) | 1 (50) | 0.7094 |
| Subtotal resection | 3 | 1 (33) | 2 (67) | |
| Extent of ECoG resection | ||||
| Gross total resection | 6 | 4 (67) | 2 (33) | 0.1025 |
| Subtotal resection | 2 | 0 (0) | 2 (100) |
PMG, polymicrogyria; EEG, electroencephalography; ECoG, electrocorticography; MCD, malformation of cortical development.
Including those patients with functional hemispherectomy.
Discussion
In this report, we described the surgical management and seizure outcomes in a series of 12 patients with PMG. We found that 83% of patients had childhood onset of epilepsy secondary to PMG. Perisylvian PMG was the most common pattern seen, and most patients had unilateral presentations. Despite the diffuse and often nonlateralizing scalp EEG abnormalities, intracranial ECoG was effective in defining the epileptogenic tissue in these cases. Surgical intervention led to a significant reduction in seizures in 33% of patients, and 50% of patients became seizure-free.
Sixty percent to 85% of patients with PMG present with epilepsy, which may be one of the earliest clinical manifestations. The majority of the patients in our cohort had childhood onset of epilepsy, with mean onset at around 8 years of age, but the mean time between diagnosis and surgery was >10 years. This means that patients often have years or even decades of medically refractory epilepsy prior to surgical evaluation. A previous report of 66 patients with unilateral PMG, congenital hemiparesis, and ESES syndrome showed good response to AED in terms of seizure reduction, but only three patients were able to become seizure-free with medication alone.6 Several other studied demonstrated that in medically refractory cases of PMG, surgery provided excellent seizure control, and one report demonstrated developmental and cognitive improvements after epilepsy surgery.9,10,17 Therefore, surgical intervention may offer the best chance for long-term seizure freedom for patients with medically refractory PMG, and evaluation for surgery should be performed early.
The main imaging feature of PMG is an excessive number of small cortical gyri separated by shallow sulci; the distribution of abnormality is highly variable. As pointed out previously, PMG may result from different mechanisms of disturbance of cortical development, including multiple genetic mutations, in utero insults, or both.3,18 One large study examined 328 patients with PMG and found 5 main patterns that accounted for 93% of all cases reviewed: perisylvian, generalized, polymicrogyria with periventricular gray matter heterotopia, frontal, and parasagittal parietooccipital.19 Sometimes polymicrogyria can be seen in association with hemimegalencephaly, as demonstrated by three patients in our study. Reports shows that disorders of the RTK-PI3K-AKT signaling cascade can cause megalencephaly syndromes with associated PMG.20 Despite the heterogeneity of PMG disorders and types of associated cortical malformations, we did not find an association in surgical outcome between PMG patients with and without associated cortical malformations, even after excluding those with hemimegalencephaly from our analysis. We also found that complete resection of these other cortical malformation is not necessarily required for favorable seizure control outcome.
Polymicrogyria is a highly epileptogenic lesion with approximately 85% of patients eventually developing seizures. A large study consisting of 328 patients with imaging characteristics of PMG found no correlation between incidence of epilepsy and different patterns of PMG, suggesting that epileptogenicity of PMG cortex is relatively consistent regardless of the topography, extent, or laterality.19 We found diffuse electrographic abnormalities on scalp EEG in nearly all patients, which mostly showed concordance with the location of PMG lesions. Our results are similar to a large cohort study of PMG patients, where 76 patients with available scalp EEG showed that 71.4% had seizures with laterality concordant, with the laterality of PMG involvement.5 However, when scalp EEG findings were nonlateralizing, intra- or extraoperative intracranial ECoG could locate or lateralize the seizure foci in all cases based on interictal or ictal abnormalities. Therefore, intracranial ECoG can be extremely valuable in identifying the epileptogenic tissue and directing the type and extent of surgical resection.
In our series, patients with defined seizure foci on either scalp EEG or intracranial ECoG typically underwent tailored focal resection of their electrographically active regions as first-line treatment. The exceptions are patients with diffuse unilateral cortical abnormalities (i.e., hemimegalencephaly) or with nonfocal ECoG findings, who received hemispherectomies as their initial surgery. One patient had changes in his seizure type and seizure focus after the initial lesionectomy, and therefore underwent hemispherectomy as a second procedure. Six of 12 patients were seizure-free at last follow-up, and four patients had significant reduction of seizure frequency and severity. Previously reported success of PMG surgery had been variable: one study reported 12 patients who underwent epilepsy surgery for unilateral focal PMG with three patients (25%) with Engel class I outcome.12 Another case series reported nine patients who underwent epilepsy surgery (partial or total lesionectomy) for PMG where, at 1 year follow-up, seven were Engel class I, one patient had no improvement, and one patient had worsening of seizures.5 The differences could be attributed to our longer length of follow-up and capturing more patients with seizure recurrence. Of interest, we were unable to identify any significant predictors of seizure freedom. However, gross total resection of the PMG lesion trended toward favorable outcome. Due to our small sample size, we may be underpowered to find statistically significant predictors. In addition, intraoperative ECoG was not used in every case, nor was a postexcisional ECoG performed in all cases. Therefore, it is difficult to make conclusions regarding the extent of ECoG resection on seizure outcome.
As with all epilepsy surgeries, one key consideration in epilepsy surgery for PMG is defining the epileptogenic zone (EZ) and whether it resides purely within the abnormal cortex. We found a high incidence of abnormal ECoG or EEG activity arising from the region of PMG, concordant with a study that used ictal SPECT to identify hyperperfusion in PMG cortex of all patients, suggesting the involvement of PMG cortex in seizure generation or early seizure propagation.12 However, not all parts of the PMG cortex are epileptogenic, as Maillard and colleagues showed using a combination of magnetic source imaging and stereo-EEG in a patient with schizencephaly and polymicrogryia.21 We also demonstrated this as patients with bilateral PMG usually had a unilateral seizure-onset zone, and even in case of unilateral focal PMG, only a portion of the PMG cortex had active epileptic discharges. Furthermore, other groups have found that the epileptogenic network not only resides within the abnormal tissue, but also extends beyond the area of PMG using stereo-EEG in patients with unilateral focal PMG.9,10 These studies suggest that PMG can be part of a larger epileptogenic network and that multimodal imaging and neurophysiologic tests should be performed to define the epileptic zone.
Surgical treatment options for patients with PMG are usually limited. However, our study provides evidence that, even in patients with diffuse and bilateral lesions, seizures may arise from focal regions within or around the PMG, and surgical resection may provide significant benefit in seizure control outcome. Intracranial ECoG can provide additional localizing information compared to scalp EEG. Our study has its limitations, given the small patient sample size and heterogeneity of PMG lesions. However, our data are consistent with previous studies and argue that patients with medically refractory epilepsy secondary to PMG should undergo a detailed presurgical evaluation with volumetric magnetic resonance imaging (MRI)22 and epileptic foci mapping with scalp EEG, stereo-EEG,9,21,23 or intracranial ECoG. PMG abnormalities as visualized on MRI are frequently extensive and ambiguous with regard to actual seizure-onset localization, and magnetoencephalography (MEG) and single-proton emission computed tomography (SPECT) offer additive noninvasive functionally relevant localization for optimal surgical planning.24–27 Epilepsy surgery, with the aid of multimodal imaging and neurophysiologic tests, may provide excellent long-term seizure control for patients with PMG.
Key Points.
In our cohort of 12 patients with polymicrogyria who underwent epilepsy surgery, 83% of patients had childhood onset of epilepsy secondary to polymicrogyria
Perisylvian polymicrogyria was the most common pattern seen, and most patients had unilateral polymicrogyria
Despite extensive abnormalities on MRI and broad epileptiform abnormalities on scalp EEG, intracranial electrocorticography can provide additional localizing information for guiding surgical planning
Surgical intervention led to significant seizure reduction in 33% of patients, and 50% of patients became seizure-free
Biography

Dr. Doris Wang is a neurosurgery resident at the University of California, San Francisco.
Footnotes
Disclosure The authors report no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Barkovich AJ, Kuzniecky RI, Jackson GD, et al. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Lindan CE. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am J Neuroradiol. 1994;15:703–715. [PMC free article] [PubMed] [Google Scholar]
- 3.Barkovich AJ. Current concepts of polymicrogyria. Neuroradiology. 2010;52:479–487. doi: 10.1007/s00234-009-0644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronica E, Becker AJ, Spreafico R. Malformations of cortical development. Brain Pathol. 2012;22:380–401. doi: 10.1111/j.1750-3639.2012.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shain C, Ramgopal S, Fallil Z, et al. Polymicrogyria-associated epilepsy: a multicenter phenotypic study from the Epilepsy Phenome/Genome Project. Epilepsia. 2013;54:1368–1375. doi: 10.1111/epi.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caraballo RH, Cersosimo RO, Fortini PS, et al. Congenital hemiparesis, unilateral polymicrogyria and epilepsy with or without status epilepticus during sleep: a study of 66 patients with long-term follow-up. Epileptic Disord. 2013;15:417–427. doi: 10.1684/epd.2013.0612. [DOI] [PubMed] [Google Scholar]
- 7.Loddenkemper T, Cosmo G, Kotagal P, et al. Epilepsy surgery in children with electrical status epilepticus in sleep. Neurosurgery. 2009;64:328–337. doi: 10.1227/01.NEU.0000336767.14252.76. discussion 337. [DOI] [PubMed] [Google Scholar]
- 8.Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues Clin Neurosci. 2008;10:47–62. doi: 10.31887/DCNS.2008.10.1/rjleventer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassoux F, Landre E, Rodrigo S, et al. Intralesional recordings and epileptogenic zone in focal polymicrogyria. Epilepsia. 2008;49:51–64. doi: 10.1111/j.1528-1167.2007.01267.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramantani G, Koessler L, Colnat-Coulbois S, et al. Intracranial evaluation of the epileptogenic zone in regional infrasylvian polymicrogyria. Epilepsia. 2013;54:296–304. doi: 10.1111/j.1528-1167.2012.03667.x. [DOI] [PubMed] [Google Scholar]
- 11.Sisodiya SM. Surgery for malformations of cortical development causing epilepsy. Brain. 2000;123(Pt 6):1075–1091. doi: 10.1093/brain/123.6.1075. [DOI] [PubMed] [Google Scholar]
- 12.Wichert-Ana L, de Azevedo-Marques PM, Oliveira LF, et al. Ictal technetium-99 m ethyl cysteinate dimer single-photon emission tomographic findings in epileptic patients with polymicrogyria syndromes: a subtraction of ictal-interictal SPECT coregistered to MRI study. Eur J Nucl Med Mol Imaging. 2008;35:1159–1170. doi: 10.1007/s00259-007-0655-3. [DOI] [PubMed] [Google Scholar]
- 13.Brodtkorb E, Torbergsen T, Nakken KO, et al. Epileptic seizures, arthrogryposis, and migrational brain disorders: a syndrome? Acta Neurol Scand. 1994;90:232–240. doi: 10.1111/j.1600-0404.1994.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 14.Wieser HG, Blume WT, Fish D, et al. ILAE Commission Report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42:282–286. [PubMed] [Google Scholar]
- 15.Schramm J, Delev D, Wagner J, et al. Seizure outcome, functional outcome, and quality of life after hemispherectomy in adults. Acta Neurochir (Wien) 2012;154:1603–1612. doi: 10.1007/s00701-012-1408-z. [DOI] [PubMed] [Google Scholar]
- 16.Schramm J, Kuczaty S, Sassen R, et al. Pediatric functional hemispherectomy: outcome in 92 patients. Acta Neurochir (Wien) 2012;154:2017–2028. doi: 10.1007/s00701-012-1481-3. [DOI] [PubMed] [Google Scholar]
- 17.Isik U, Dincer A, Ozek MM. Surgical treatment of polymicrogyria with advanced radiologic and neurophysiologic techniques. Childs Nerv Syst. 2007;23:443–448. doi: 10.1007/s00381-006-0262-9. [DOI] [PubMed] [Google Scholar]
- 18.Guerrini R, Dobyns WB, Barkovich AJ. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–162. doi: 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Leventer RJ, Jansen A, Pilz DT, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133:1415–1427. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riviere JB, Mirzaa GM, O'Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maillard L, Koessler L, Colnat-Coulbois S, et al. Combined SEEG and source localisation study of temporal lobe schizencephaly and polymicrogyria. Clin Neurophysiol. 2009;120:1628–1636. doi: 10.1016/j.clinph.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 22.De Ciantis A, Barkovich AJ, Cosottini M, et al. Ultra-high-field MR imaging in polymicrogyria and epilepsy. AJNR Am J Neuroradiol. 2015;36:309–316. doi: 10.3174/ajnr.A4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rikir E, Koessler L, Gavaret M, et al. Electrical source imaging in cortical malformation-related epilepsy: a prospective EEG-SEEG concordance study. Epilepsia. 2014;55:918–932. doi: 10.1111/epi.12591. [DOI] [PubMed] [Google Scholar]
- 24.Burneo JG, Bebin M, Kuzniecky RI, et al. Electroclinical and magnetoencephalographic studies in epilepsy patients with polymicrogyria. Epilepsy Res. 2004;62:125–133. doi: 10.1016/j.eplepsyres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Burneo JG, Kuzniecky RI, Bebin M, et al. Cortical reorganization in malformations of cortical development: a magnetoencephalographic study. Neurology. 2004;63:1818–1824. doi: 10.1212/01.wnl.0000144179.87918.2f. [DOI] [PubMed] [Google Scholar]
- 26.Knowlton RC, Elgavish RA, Bartolucci A, et al. Functional imaging: II. Prediction of epilepsy surgery outcome. Ann Neurol. 2008;64:35–41. doi: 10.1002/ana.21419. [DOI] [PubMed] [Google Scholar]
- 27.Knowlton RC, Elgavish RA, Limdi N, et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008;64:25–34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]