Abstract
Atrial natriuretic peptide (ANP)-mediated natriuretic response is a well-established cardiac endocrine function. Corin is a transmembrane protease that activates ANP in the heart. Corin expression has been detected in non-cardiac tissues including the kidney. Here we examined corin, pro-ANP/ANP and natriuretic peptide receptor-A (NPR-A) expression in human renal segments. By immunostaining and in situ hybridization, we found similar corin, pro-ANP/ANP and NPR-A protein and mRNA expression in human renal segments. The expression was most abundant in the proximal convoluted tubules and the medullary connecting ducts. In the proximal tubules, corin protein was present in the apical membrane region underneath the brush border where the ANP-degrading protease neprilysin was abundant. These results suggest that corin-mediated pro-ANP activation may occur in renal segments and that locally produced ANP may act in an autocrine manner to regulate sodium and water reabsorption in situ. Our results also point to the proximal convoluted tubules as a major site for local ANP action. Such a renal corin/ANP autocrine mechanism may differ from the cardiac corin/ANP endocrine mechanism in regulating sodium homeostasis under physiological and pathological conditions.
Keywords: atrial natriuretic peptide, corin, natriuretic peptide receptor, neprilysin
INTRODUCTION
Maintenance of sodium homeostasis is essential for normal blood pressure. Atrial natriuretic peptide (ANP) is a cardiac hormone that promotes natriuresis and diuresis, thereby regulating salt-water balance [1, 2]. The ANP function is mediated by the natriuretic peptide receptor-A (NPR-A), also called guanylyl cyclase-A, which contains an intracellular guanylyl cyclase domain [3]. The binding of ANP to NPR-A stimulates the guanylyl cyclase activity, increasing cyclic guanosine monophosphate (cGMP) production [3]. Elevated intracellular cGMP levels activate cGMP-dependent protein kinases, which in turn increase the glomerular filtration rate, reduce sodium and water reabsorption, and inhibit renin release in the kidney [4–7].
ANP is synthesized as a precursor, i.e. pro-ANP, which is converted to active ANP by corin, a transmembrane serine protease [8]. In mice, corin deficiency prevents pro-ANP processing, impairs renal sodium excretion, and causes salt-sensitive hypertension and cardiac hypertrophy [9–12]. In humans, CORIN variants have been identified in hypertensive patients [13–17]. Most recently, we have identified proprotein convertase subtilisin/kexin-6 (PCSK6) as a primary corin activator [18]. In mice, PCSK6 deficiency leads to salt-sensitive hypertension [18]. These data indicate that the corin-ANP pathway is critical for sodium homeostasis and normal blood pressure [19–22].
The heart is the main site for ANP and corin production [5, 23, 24]. The primary ANP action in promoting natriuresis and diuresis occurs in the kidney [6, 7]. It is well established that this endocrine function is carried out primarily by circulating ANP of heart origin [5, 25]. Previously, in situ hybridization and immunostaining detected pro-ANP/ANP mRNA and protein expression in renal segments [26–30], although the significance of such expression was unclear.
Corin expression in mouse and rat kidneys has been reported [24, 28]. Similarly, corin is also expressed in human kidneys [31, 32]. To date, however, corin distribution in human renal segments remains unclear. In patients with chronic kidney disease, reduced renal corin expression and urine soluble corin levels were reported [31]. Low levels of renal corin expression also were found in rat models of proteinuric kidney diseases [28]. These results suggest that local corin expression may play a role in kidney function and disease [33]. Here we examined corin, pro-ANP/ANP and NPR-A expression in human renal segments. We also examined renal expression of neprilysin and ADAM10 (a disintegrin and metalloproteinase 10), which are involved in ANP degradation and corin shedding, respectively [34, 35].
MATERIALS AND METHODS
Human kidney samples
Tissues were obtained from five patients (3 males and 2 females, 30–73 years of age) with renal cell carcinoma who underwent nephrectomy. The study was approved by the ethics committee at Soochow University and conducted in accordance with the approved guidelines. Informed consent was obtained from all subjects. After the removal of the tumor, non-cancerous tissues were fixed in 4% (v/v) formaldehyde. The histology was verified by licensed pathologists. No fibrosis, hemorrhage and necrosis were observed in hematoxylin and eosin (H&E)-stained sections.
Immunostaining
For immunohistochemistry (IHC), tissues were fixed in 4% (v/v) formaldehyde and embedded in paraffin. Serial sections (4 μm in thickness) were mounted on chrome alum gelatin-coated slides and used alternately for H&E staining and IHC. The sections were de-paraffinized with xylene, re-hydrolyzed with graded ethanol solutions, and boiled in a solution (10 mM sodium citrate, pH 6.0) for antigen retrieval. Endogenous peroxidase activity was inhibited by 3% hydrogen peroxide in a methanol solution. The sections were incubated with 5% bovine serum albumin (BSA) in phosphate buffered solution (PBS) and then primary antibodies at 4°C overnight. After washing, horseradish peroxidase-conjugated secondary antibodies were added. The polyclonal anti-human corin antibody was made in our laboratory [13], which showed positive staining in heart sections (positive control) but not liver sections (negative control) (Supplementary Figure 1). The antibody also detected corin in kidneys by Western blotting (Supplementary Figure 2). The anti-ANP antibody was from Millipore. In Western analysis, the antibody detected pro-ANP in human kidneys (Supplementary Figure 3). Consistent with our previous studies in hearts, ANP was likely secreted and undetectable in tissues by Western analysis [18]. Other antibodies used were anti-NPR-A (Abcam), anti-AQP1 (Abcam), anti-CK7 (Maixin) and anti-neprilysin (Maixin) antibodies. In negative controls, the primary antibody was replaced by 5% BSA (Supplementary Figure 4). Sections were examined under a microscope (Leica DM2000; Olympus DP73). Immunostaining intensity was quanti ed using Image-Pro Plus software (Media Cybernetics) [31]. Immunofluorescent staining was done with similarly treated sections and secondary antibodies labeled with Alexa Flour-488 or -594 (Invitrogen). The slides were mounted with a solution containing DAPI (4-6-diamidino-2-phenylindole). The images were taken using a confocal microscope (Olympus, FV1000). Similar results were observed in tissue sections from all five individuals. Presented results were representative and from tissue sections of four individuals.
In situ hybridization
To detect corin, NPPA and NPR-A mRNAs, antisense oligonucleotide probes were labeled with digoxin using an In Situ Hybridization kit (Boster, China). Sequences of the oligonucleotide probes were: CORIN: 5′-AAT TAC TCC TGG CCG GAT TTC CTC AGA TGC TCC CA-3′; NPPA: 5′-CAG ACC AGA GCT AAT CCC ATG TAC AAT GCC GTG TC-3′; and NPR-A: 5′-TTC ATG CGG GTC CGC GAC CGC CTC AAT ATT ACG GT-3′. Human kidney sections (3 μm in thickness) were treated with proteinase K at room temperature for 20 min. After washing in PBS with 0.1% diethy pyrocarbonate, slides were fixed in 4% paraformaldehyde for 15 min. A pre-hybridization solution was added at 40°C. After 2 h, slides were incubated with 20-μl probes for 16 h. The sections were washed with a sodium citrate solution, incubated with a blocking solution at 37 °C for 30 min and biotin anti-digoxigenin at 37 °C for 1 h. After washing with PBS, the sections were incubated with streptavidin-biotin for 30 min and biotin peroxidase for 30 min. Hybridization signal was visualized using 3,3′-diaminobenzidine. As a negative control, pre-hybridization solution without labeled probes was used in the procedure (Supplementary Figure 5).
Immuno-Electron Microscopy (EM)
Immuno-EM was performed to examine corin subcellular distribution in renal proximal tubules. Kidneys from 6-week-old C57BL/6J male mice were fixed in 4% (w/v) formaldehyde in 0.1 M phosphate buffer, dehydrated, and embedded in LR White medium (Ted Pella). Ultrathin sections were mounted on Formvar carbon-coated nickel grids (Ted Pella) and incubated with an anti-corin primary antibody (1:100 dilution) overnight. After washing with PBS, 10 nm gold-conjugated goat anti-rabbit IgG (1:50) (Ted Pella) was added and incubated for 45 min. Grids were stained with uranyl acetate and lead citrate and examined under an electron microscope (FEI Tecnai G2 Spirit). As a negative control, procedures were done without the primary antibody (Supplementary Figure 6). All animal procedures were carried out in accordance with the NIH guideline for the ethical treatment and handling of animals in research, and approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Cell culture and plasmid constructs
Corin-expressing HEK293 cells were cultured in DMEM with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. Human corin protein was expressed with the plasmid pcDNACorin (Invitrogen) [36]. The protein encoded by this plasmid contained a C-terminal V5 tag for detection in Western blotting using an anti-V5 antibody (Invitrogen). Recombinant human neprilysin protein was expressed with the plasmid PCMV3 (Sino), which contained a C-terminal flag tag for detection using an anti-flag antibody (Invitrogen).
Transfection and Western analysis
HEK293 cells were transfected with plasmids using TurboFect reagents (Thermo Scientific). After the transfection, the cells were cultured in DMED with 10% FBS. After 18 h at 37°C, conditioned medium was collected and the cells were lysed in a buffer with 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10% glycerol (v/v) and 1% Triton X-100 (v/v). Experimental procedures for immunoprecipitation and Western analysis for corin proteins in the conditioned medium and cell lysate were described previously [37].
Neprilysin activity assay
Neprilysin activity was examined using an assay kit (AnaSpec). The kit used a fluoro-peptide substrate, which is cleaved by neprilysin into two fragments, resulting in the release of fluorescence to be detected at 490 and 520 nm (excitation and emission) with a microplate reader (Molecular Devices). In these experiments, tested samples included cell lysates from neprilysin plasmid transfected HEK293 cells and control parental HEK293 cells. An assay buffer and purified human neprilysin protein (AnaSpec) were used as negative and positive controls, respectively.
Statistical analysis
Data were analyzed using the Prism 5 software (GraphPad). Comparisons among three groups were made using one-way ANOVA followed by Bonferroni’s post analysis. All data presented are mean ± S.D. A P value <0.05 is considered significant.
RESULTS
Corin protein expression
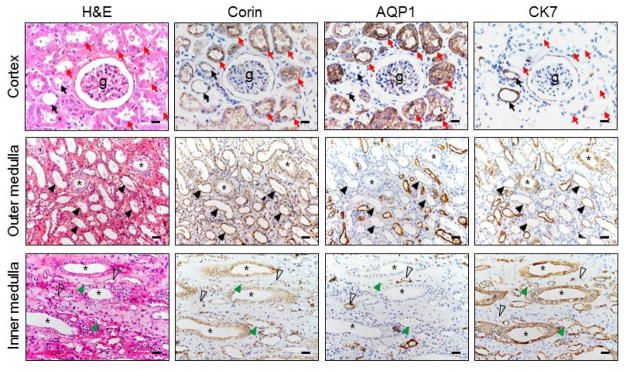
We examined renal corin expression by IHC. We included antibodies against aquaporin-1 (AQP1) and cytokeratin7 (CK7) as markers for the proximal and distal tubules, respectively [38, 39]. In glomeruli, corin staining was mostly negative (Figure 1, top panels). Strong corin staining appeared in proximal tubular epithelial cells (AQP1-positive and CK7-negative) (Figure 1, top panels). In distal tubular epithelial cells, (AQP1-negative and CK7-positive), corin staining was weak (Figure 1, top panels). In the outer medulla (Figure 1, middle panels), corin staining was positive in epithelial cells of both collecting ducts and thick ascending limbs (AQP1-negative and CK7-positive). In the inner medulla (Figure 1, lower panels), positive corin staining was mostly in the collecting ducts (AQP1-negative and CK7-positive), although weak corin staining was found in thin limbs (AQP1- or CK7-positive). Based on quantitative imaging analysis, corin expression in the proximal convoluted tubules was higher than those in the distal convoluted tubules and the collecting ducts (relative optical densities: 0.16 ± 0.01 vs. 0.10 ± 0.01 and 0.07 ± 0.02, respectively, n=3 per group, both p values <0.01).
Figure 1. Corin protein expression in renal segments.
Human kidney sections were stained by H&E. Immunohistochemistry was performed using an anti-corin antibody or control antibodies against AQP1 and CK7, respectively. Proximal and distal convoluted tubules around glomeruli (g) are indicated by red and black arrows, respectively. Asterisks indicate collecting ducts. Black arrowheads indicate thick ascending limbs. Open arrowheads indicate descending thin limbs. Green arrowheads indicate ascending thin limbs. Scale bars: 40 μm.
ANP and NPR-A protein expression
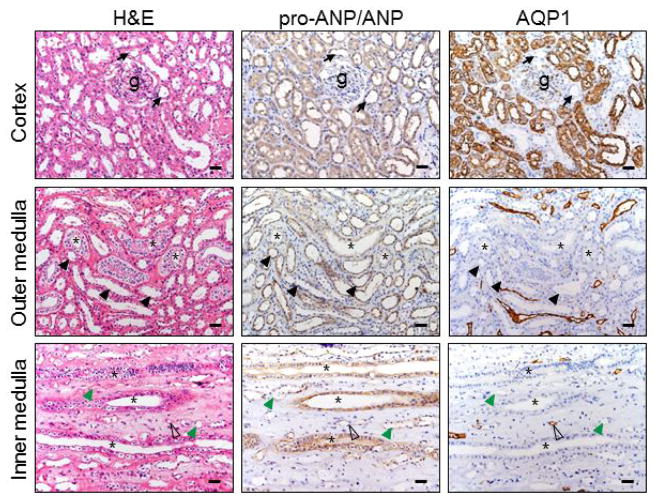
In parallel, we immunostained ANP and NPR-A proteins in kidney sections. The ANP antibody used did not distinguish ANP from pro-ANP. The antigen recognized by the antibody is referred to as pro-ANP/ANP hereafter. We found little pro-ANP/ANP staining in glomeruli but was positive in both proximal (AQP1-positive) and distal (AQP1-negative) convoluted tubules (Figure 2, top panels). In the outer medulla (middle panels), pro-ANP/ANP staining was positive in collecting ducts and thick ascending limbs. In the inner medulla (lower panels), most positive pro-ANP/ANP staining was in the collecting ducts, although weak staining was present in thin limbs.
Figure 2. ANP protein expression in renal segments.
Human pro-ANP/ANP protein in the renal cortex and medullary sections was stained by immunohistochemistry. AQP1 staining was included as a control. Black arrows indicate distal convoluted tubules near glomeruli (g) (top panels). Asterisks indicate collecting ducts (middle and lower panels). Black arrowheads indicate thick ascending limbs (middle panels). Open arrowheads indicate descending thin limbs. Green arrowheads indicate ascending thin limbs. Scale bars: 40 μm.
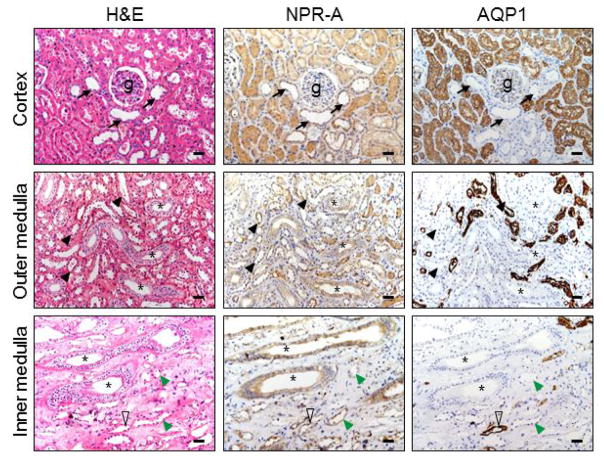
The overall NPR-A staining patterns in renal segments were similar to that of corin and pro-ANP/ANP (Figure 3). In the cortex (top panels), positive NPR-A staining was stronger in epithelial cells of the proximal convoluted tubules than in the distal tubules. In the medulla, similar positive staining was present in the collecting ducts, distal tubules and thin limbs (middle and lower panels).
Figure 3. NPR-A protein expression in renal segments.
Human NPR-A protein in the renal cortex and medullary sections was stained by immunohistochemistry. AQP1 staining was included as a control. Black arrows indicate distal convoluted tubules near glomeruli (g) (top panels). Asterisks indicate collecting ducts (middle and lower panels). Black arrowheads indicate thick ascending limbs (middle panels). Open arrowheads indicate descending thin limbs. Green arrowheads indicate ascending thin limbs. Scale bars: 40 μm.
Corin, NPPA and NPR-A mRNA expression
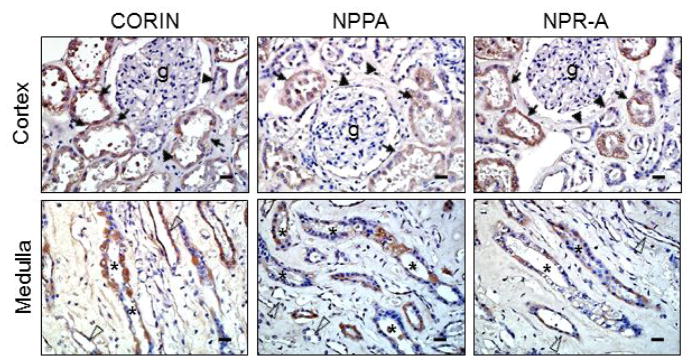
To verify our findings, we did in situ hybridization to examine corin, NPPA (encoding pro-ANP) and NPR-A mRNA expression. In the cortex (Figure 4, top panels), high levels of corin, NPPA and NPR-A mRNA expression were detected in proximal tubular epithelial cells, whereas the expression was weaker in the distal tubular epithelial cells. In the medulla (lower panels), positive corin, NPPA and NPR-A mRNA expression was detected in collecting duct epithelial cells. Lower levels of the expression also appeared in thin limb epithelial cells.
Figure 4. Corin, NPPA and NPR-A mRNA expression in human renal segments.
In situ hybridization was performed to detect corin, NPPA and NPR-A mRNAs in human renal cortex (top panels) and medulla (lower panels). Proximal and distal convoluted tubules around glomeruli (g) are indicated by arrows and arrowheads, respectively (top panels). Medullary collecting ducts and thin limbs are indicated by asterisks and open arrowheads, respectively (lower panels). Scale bars: 20 μm.
Neprilysin protein expression
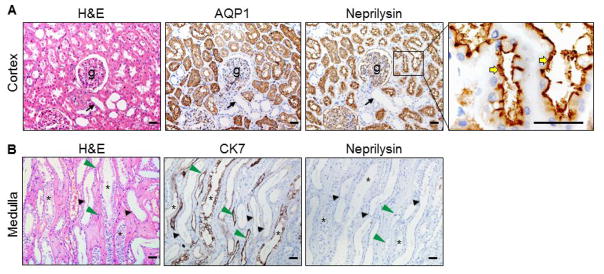
Neprilysin is a metalloproteinase that inactivates ANP [35]. Neprilysin staining was positive in the parietal epithelial cells of Bowman’s capsules and in the podocytes surrounding glomerular capillaries (Figure 5A). In the proximal convoluted tubules (AQP1-positive), neprilysin staining was highly positive in the brush border (right panels), whereas the staining was negative in the distal convoluted tubules (AQP1-negative). In the medulla (Figure 5B), neprilysin staining was negative in the collecting ducts, distal tubules and thin limbs (CK7-positive).
Figure 5. Neprilysin expression in renal segments.
Neprilysin protein in human renal cortex (A) and medullary (B) sections was stained by immunohistochemistry. AQP1 and CK7 staining was included as controls. Black arrows indicated distal convoluted tubules near glomeruli (g). Yellow arrows indicate the brush border. Asterisks indicate collecting ducts. Black arrowheads indicate thick ascending limbs. Green arrowheads indicate ascending thin limbs. Scale bars: 40 μm.
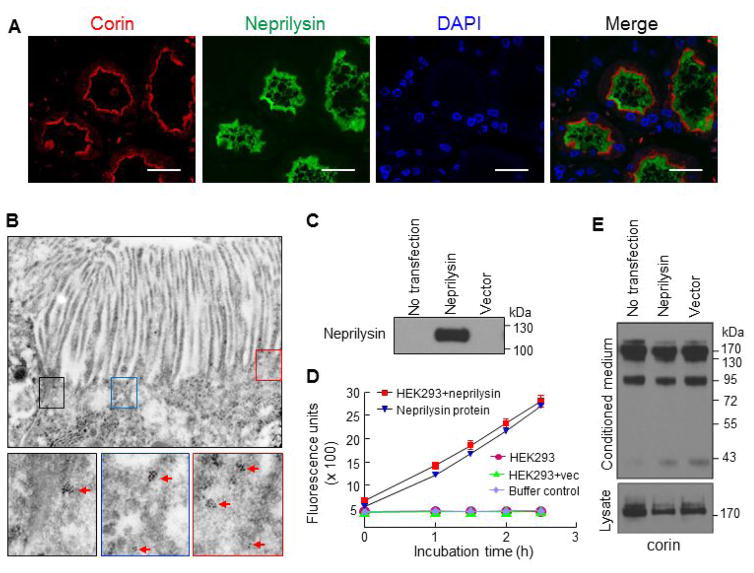
We next did co-staining to understand the subcellular distribution of corin and neprilysin proteins. In the proximal convoluted tubules, neprilysin staining was in the brush border region, whereas corin staining was in the apical membrane region underneath the brush border (Figure 6A). We verified this result by immune-EM. Because the immuno-EM experiment requires highly fresh tissues, which could be technically challenging for human samples, we therefore did immune-EM with mouse kidneys. The results confirmed apical corin distribution in proximal tubular epithelial cells (Figure 6B). One possible explanation for the distinct corin and neprilysin expression patterns in the proximal tubules is that neprilysin degrades or sheds corin in the brush border. To test this hypothesis, we expressed corin with or without neprilysin in HEK293 cells, as shown in Western analysis (Figure 6C). The neprilysin activity in the transfected cells was verified by a neprilysin activity assay (Figure 6D). In these transfected cells, neprilysin expression did not alter corin protein levels in the conditioned medium, which contained corin shedding fragments of ~190, ~160, ~100 and ~40-kDa [34] (Figure 6E, top panel), or in cell lysates, which contained corin zymogen of ~190-kDa (Figure 6E, lower panel).
Figure 6. Corin and neprilysin expression in proximal tubules and transfected HEK293 cells.
(A) Human renal cortex sections were co-stained for corin (red), neprilysin (green), and nuclei (DAPI). Scale bar: 20 μm. (B) Apical corin expression in proximal tubular epithelial cells of mouse kidneys was verified by EM. Red arrows indicate antibody-conjugated gold particles. Original power magnifications of the top panel and lower panels were x34003 and x91568, respectively. (C and D) Plasmid expressing human neprilysin or a control vector was transfected in HEK293 cells. Neprilysin protein in cell lysates from the transfected cells was examined by Western analysis (C). Neprilysin activity in the transfected cells was verified by a fluogenic assay (D). Additional controls in the neprilysin activity assay included parental HEK293 cell lysate (no transfection), buffer (negative control), and purified neprilysin protein. (E) Corin protein fragments in the conditioned medium (top panel) and cell lysates (lower panel) from the transfected HEK293 cells were analyzed by Western blotting.
In similar experiments, we co-stained neprilysin and pro-ANP/ANP or NPR-A in proximal tubular epithelial cells. Neprilysin staining was strong in the brush border, whereas pro-ANP/ANP staining appeared defuse mostly in the cytoplasm. In contrast, NPR-A staining was mostly in the brush border, overlapping with that of neprilysin (Supplementary Figure 7).
ADAM10 protein expression
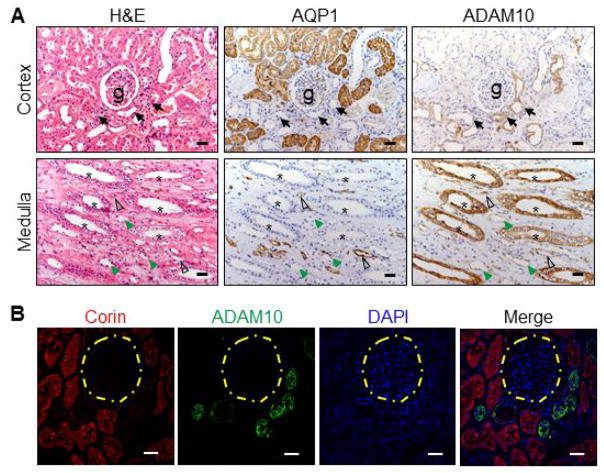
Soluble corin has been detected in human urine [31], indicating corin shedding in the kidney. In cardiomyocytes, ADAM10 sheds corin from the cell surface [34]. We examined ADAM10 expression in human kidneys. In the cortex (Figure 7A, top panels), ADAM10 was mostly positive in the distal convoluted tubules (AQP1-negative). In the medulla (lower panels), ADAM10 was positive mostly in the collecting ducts. In co-staining (Figure 7B), corin expression was mostly in the proximal convoluted tubules, whereas ADAM10 staining was in the distal convoluted tubules, consistent with the findings in Figure 7A.
Figure 7. ADAM10 expression in renal segments.
(A) Human ADAM10 protein in renal cortex and medullary sections was immune-stained. AQP1 staining was used as a control. Black arrows indicate distal convoluted tubules near glomeruli (g) (top panels). Asterisks indicate collecting ducts (lower panels). Open arrowheads indicate descending thin limbs. Green arrowheads indicate ascending thin limbs. Scale bar: 40 μm. (B) Immunofluorescent staining of corin (red), ADAM10 (green) and nuclei (DAPI) in proximal and distal tubules. Yellow dashed circles indicate glomeruli. Scale bars: 40 μm.
DISCUSSION
Corin is a key enzyme in the natriuretic peptide system [8]. Here we examined corin protein and mRNA expression in human kidneys. We detected corin protein and mRNA in most renal segments. The expression levels were abundant in the proximal convoluted tubules and the medullary collecting ducts, where pro-ANP/ANP and NPR-A levels were also high. Our results are consistent with previous findings of corin expression in rat renal tubules [28]. More importantly, our findings of similar corin, pro-ANP/ANP and NPR-A expression in the same renal segments suggest that locally produced and activated ANP may act in an autocrine manner to regulate sodium homeostasis. Although the antibody used in our study did not distinguish pro-ANP from ANP, the detection of NPPA mRNA by in situ hybridization and pro-ANP protein by Western analysis does support the idea of pro-ANP production in the renal segments.
Neprilysin, also called neutral endopeptidase, is a major ANP-degrading protease [35, 40]. Neprilysin inhibition increases ANP levels in vivo [41]. In clinical trials, inhibitors for both neprilysin and the angiotensin II receptor type 1 reduced the mortality and morbidity in heart failure patients [42–44]. In agreement with previous reports [45], we found abundant neprilysin expression in the brush border of the proximal tubular epithelial cells. In these cells, corin protein was in the apical membrane region, suggesting that corin-activated ANP may not pass through the brush border where neprilysin is abundant. Consistently, previous studies showed ANP staining in the apical endocytic compartment, but not in the brush border region [29, 30]. We also found a defuse pattern of pro-ANP/ANP staining in the cytoplasm of the proximal tubular epithelial cells. Given the similar corin, pro-ANP/ANP and NPR-A expression patterns in the same renal segment, corin-generated ANP is likely to act in situ in the proximal convoluted tubules.
The primary ANP action is believed to inhibit sodium reabsorption in the inner medullary collecting ducts [7, 46–48]. Consistently, we found corin, pro-ANP/ANP and NPR-A expression in epithelial cells of the medullary collecting ducts, indicating that locally activated ANP may act in situ in the collecting ducts. Our results also raise a critical question regarding the physiological significance of corin, pro-ANP/ANP and NPR-A expression in the proximal convoluted tubules. As discussed above, abundant neprilysin in the brush border of the proximal tubules makes it difficult, if not impossible, for locally produced ANP to escape and travel downstream to the inner medullary collecting ducts. Previously, ANP was reported to inhibit angiotensin-stimulated sodium reabsorption in the proximal tubules [49] but the significance of such a finding remained uncertain [7, 50]. In the kidney, the majority of sodium reabsorption occurs in the proximal convoluted tubules [51]. Our findings of high corin, pro-ANP/ANP and NPR-A levels in the proximal convoluted tubules suggest an ANP-dependent autocrine mechanism at this strategic location to regulate sodium reabsorption. Additional studies are needed to determine if and how this renal ANP autocrine mechanism differs from the cardiac ANP endocrine mechanism in response to homeostatic challenges.
Sodium retention is common in kidney diseases. In rat models of proteinuric kidney diseases, decreased renal corin expression contributed to sodium retention [28, 33]. We also found reduced renal corin expression and urinary soluble corin levels in patients with chronic kidney disease [31]. These results support the idea that decreased renal corin expression prevents local ANP production and hence the ANP autocrine function. If the renal ANP autocrine mechanism is not compensated by heart-derived circulating ANP, reduced renal corin function may impair sodium homeostasis and cause sodium retention in kidney diseases. It will be important to determine if compromised renal ANP autocrine function contributes to the apparent natriuretic peptide resistance in patients who exhibit sodium retention despite high levels of circulating natriuretic peptides [52–56].
Currently, the regulation of renal corin expression remains unknown. The transcriptional control of renal corin expression probably differs from that in cardiomyocytes, where GATA-4 is involved [57]. ADAM10-mediated shedding is another mechanism to regulate corin expression in cardiomycytes [34, 58]. Corin shedding also occurs in the kidney, as indicated by soluble corin in human urine [31]. In this study, we did not detect neprilysin-dependent corin degradation or shedding in cultured cells. We found strong ADAM10 expression in the distal convoluted tubules and weak expression in the proximal convoluted tubules, which mirrors weak corin staining in the distal tubules and strong staining in the proximal tubules. Possibly, ADAM10-mediated shedding reduces corin levels in the selected renal segments. Further studies are required to understand how renal corin expression is regulated under physiological and pathological conditions.
In summary, we found similar renal segmental patterns of corin, pro-ANP/ANP and NPR-A mRNA and protein expression. The highest levels were in the proximal convoluted tubules and the inner medullary collecting ducts. These results suggest that corin-mediated pro-ANP activation and subsequent ANP signaling in these renal segments may serve as an autocrine mechanism to regulate sodium homeostasis. ANP was discovered in the early 1980s [59]. ANP-mediated natriuresis has been considered primarily as a cardiac endocrine function [25]. Our findings of a possible renal corin-ANP autocrine mechanism may revise this concept. Our results also point to the proximal convoluted tubules as a major site for renal ANP production and action. In kidney diseases, reduced renal corin expression may impair the ANP autocrine function, thereby contributing to sodium retention and the ANP resistance phenotype in patients. Our findings should encourage further studies to elucidate the role of corin and ANP in renal physiology and disease.
Supplementary Material
Summary Statement.
ANP-dependent regulation of salt-water balance is a well-established cardiac endocrine function. This study challenges this long-standing concept, suggesting that locally produced ANP by corin in the kidney may act as an autocrine function to regulate sodium homeostasis.
CLINICAL PERSPECTIVES.
ANP is essential for salt-water balance and normal blood pressure. This study suggests that locally produced ANP by corin in the kidney may act in an autocrine mechanism to regulate sodium homeostasis and that the proximal convoluted tubules may be a major site for ANP action.
The results challenge the long-standing concept that ANP-mediated natriuretic response is primarily a cardiac endocrine function.
In kidney diseases, reduced renal corin function may impair sodium homeostasis and contribute to sodium retention.
Acknowledgments
We thank Drs. Meng Liu, Dongrong Yang and Tiantian Zhou for their help. We also thank Mei Yin for her assistance in immuno-EM.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (81370718, 81570457 and 31500636), the Ministry of Education of China (213016A), and the Priority Academic Program Development of Jiangsu Higher Education Institutes.
Abbreviations
- ADAM10
a disintegrin and metalloproteinase-10
- ANP
atrial natriuretic peptide
- BSA
bovine serum albumin
- cGMP
cyclic guanosine monophosphate
- DEPC
diethy pyrocarbonate
- EM
electron microscope
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- NPR-A
natriuretic peptide receptor-A
- PBS
phosphate buffered solution
- PCSK6
proprotein convertase subtilisin/kexin-6
- SSC
sodium citrate solution
Footnotes
Declarations of interest
None.
Author contributions
Liang Dong planed and performed the experiments, analyzed data and wrote the paper. Hao Wang performed the experiments. Ningzheng Dong planed the experiments and analyzed data. Ce Zhang performed the experiments. Boxin Xue collected the patient samples. Qingyu Wu planed the experiments, analyzed data and wrote the paper. All authors read and approved the paper.
References
- 1.Rubattu S, Sciarretta S, Volpe M. Atrial natriuretic peptide gene variants and circulating levels: implications in cardiovascular diseases. Clin Sci (Lond) 2014;127:1–13. doi: 10.1042/CS20130427. [DOI] [PubMed] [Google Scholar]
- 2.Song W, Wang H, Wu Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA) Gene. 2015;569:1–6. doi: 10.1016/j.gene.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol Rev. 2016;96:751–804. doi: 10.1152/physrev.00022.2015. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–9. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 5.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 6.Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol. 2015;308:F1047–F1055. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeidel ML. Renal actions of atrial natriuretic peptide: regulation of collecting duct sodium and water transport. Annu Rev Physiol. 1990;52:747–59. doi: 10.1146/annurev.ph.52.030190.003531. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–6. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley CL, Stokes AJ. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul Pept. 2011;172:44–50. doi: 10.1016/j.regpep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–90. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong N, Fang C, Jiang Y, Zhou T, Liu M, Zhou J, Shen J, Fukuda K, Qin J, Wu Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J Biol Chem. 2013;288:7867–74. doi: 10.1074/jbc.M112.411512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 16.Stepanian A, Alcais A, de Prost D, Tsatsaris V, Dreyfus M, Treluyer JM, Mandelbrot L. Highly significant association between two common single nucleotide polymorphisms in CORIN gene and preeclampsia in Caucasian women. PLoS One. 2014;9:e113176. doi: 10.1371/journal.pone.0113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Li H, Zhou J, Wang A, Yang J, Wang C, Liu M, Zhou T, Zhu L, Zhang Y, Dong N, Wu Q. A corin variant identified in hypertensive patients that alters cytoplasmic tail and reduces cell surface expression and activity. Sci Rep. 2014;4:7378. doi: 10.1038/srep07378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Cao P, Dong N, Peng J, Zhang C, Wang H, Zhou T, Yang J, Zhang Y, Martelli EE, Naga Prasad SV, Miller RE, Malfait AM, Zhou Y, Wu Q. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med. 2015;21:1048–53. doi: 10.1038/nm.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armaly Z, Assady S, Abassi Z. Corin: a new player in the regulation of salt-water balance and blood pressure. Curr Opin Nephrol Hypertens. 2013;22:713–22. doi: 10.1097/01.mnh.0000435609.35789.32. [DOI] [PubMed] [Google Scholar]
- 20.Dries DL. Process matters: Emerging concepts underlying impaired natriuretic peptide system function in heart failure. Circ Heart Fail. 2011;4:107–10. doi: 10.1161/CIRCHEARTFAILURE.111.960948. [DOI] [PubMed] [Google Scholar]
- 21.Ichiki T, Huntley BK, Burnett JC., Jr BNP molecular forms and processing by the cardiac serine protease corin. Adv Clin Chem. 2013;61:1–31. doi: 10.1016/b978-0-12-407680-8.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep. 2014;16:415. doi: 10.1007/s11906-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–7. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 24.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 25.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–77. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa CD, Lewis HM, MacIver AG, Mackenzie JC, Bhoola KD. Cellular localisation of atrial natriuretic factor in the human kidney. Nephrol Dial Transplant. 1990;5:25–31. doi: 10.1093/ndt/5.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Lai FJ, Hsieh MC, Hsin SC, Lin SR, Guh JY, Chen HC, Shin SJ. The cellular localization of increased atrial natriuretic peptide mRNA and immunoreactivity in diabetic rat kidneys. J Histochem Cytochem. 2002;50:1501–8. doi: 10.1177/002215540205001110. [DOI] [PubMed] [Google Scholar]
- 28.Polzin D, Kaminski HJ, Kastner C, Wang W, Kramer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, Bachmann S, Theilig F. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–9. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez G, Saba SR, Dietz JR, Vesely DL. Immunocytochemical localization of proANF 1–30, proANF 31–67 and atrial natriuretic factor in the kidney. Kidney Int. 1992;41:334–41. doi: 10.1038/ki.1992.46. [DOI] [PubMed] [Google Scholar]
- 30.Saba SR, Ramirez G, Vesely DL. Immunocytochemical localization of ProANF 1–30, ProANF 31–67, atrial natriuretic factor and urodilatin in the human kidney. Am J Nephrol. 1993;13:85–93. doi: 10.1159/000168596. [DOI] [PubMed] [Google Scholar]
- 31.Fang C, Shen L, Dong L, Liu M, Shi S, Dong N, Wu Q. Reduced urinary corin levels in patients with chronic kidney disease. Clin Sci (Lond) 2013;124:709–17. doi: 10.1042/CS20120517. [DOI] [PubMed] [Google Scholar]
- 32.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC., Jr Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–7. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 33.Klein JD. Corin: an ANP protease that may regulate sodium reabsorption in nephrotic syndrome. Kidney Int. 2010;78:635–7. doi: 10.1038/ki.2010.223. [DOI] [PubMed] [Google Scholar]
- 34.Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, Wu Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenny AJ, Stephenson SL. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988;232:1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- 36.Dong N, Zhou T, Zhang Y, Liu M, Li H, Huang X, Liu Z, Wu Y, Fukuda K, Qin J, Wu Q. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J Biol Chem. 2014;289:17909–16. doi: 10.1074/jbc.M114.551424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–35. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 38.Bondy C, Chin E, Smith BL, Preston GM, Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993;90:4500–4. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P, Young A, de Peralta-Venturina M, Amin MB. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol. 2005;29:747–54. doi: 10.1097/01.pas.0000163362.78475.63. [DOI] [PubMed] [Google Scholar]
- 40.Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–9. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant. 2015;30:738–743. doi: 10.1093/ndt/gfu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bavishi C, Messerli FH, Kadosh B, Ruilope LM, Kario K. Role of neprilysin inhibitor combinations in hypertension: insights from hypertension and heart failure trials. Eur Heart J. 2015;36:1967–73. doi: 10.1093/eurheartj/ehv142. [DOI] [PubMed] [Google Scholar]
- 43.Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–7. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 44.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 45.Schulz WW, Hagler HK, Buja LM, Erdos EG. Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest. 1988;59:789–97. [PubMed] [Google Scholar]
- 46.Light DB, Schwiebert EM, Karlson KH, Stanton BA. Atrial natriuretic peptide inhibits a cation channel in renal inner medullary collecting duct cells. Science. 1989;243:383–5. doi: 10.1126/science.2463673. [DOI] [PubMed] [Google Scholar]
- 47.Sonnenberg H, Honrath U, Chong CK, Wilson DR. Atrial natriuretic factor inhibits sodium transport in medullary collecting duct. Am J Physiol. 1986;250:F963–6. doi: 10.1152/ajprenal.1986.250.6.F963. [DOI] [PubMed] [Google Scholar]
- 48.Zhao D, Pandey KN, Navar LG. ANP-mediated inhibition of distal nephron fractional sodium reabsorption in wild-type and mice overexpressing natriuretic peptide receptor. Am J Physiol Renal Physiol. 2010;298:F103–8. doi: 10.1152/ajprenal.00479.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature. 1987;326:697–8. doi: 10.1038/326697a0. [DOI] [PubMed] [Google Scholar]
- 50.Liu FY, Cogan MG. Atrial natriuretic factor does not inhibit basal or angiotensin II-stimulated proximal transport. Am J Physiol. 1988;255:F434–7. doi: 10.1152/ajprenal.1988.255.3.F434. [DOI] [PubMed] [Google Scholar]
- 51.Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev. 2006;86:709–46. doi: 10.1152/physrev.00016.2005. [DOI] [PubMed] [Google Scholar]
- 52.Charloux A, Piquard F, Doutreleau S, Brandenberger G, Geny B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur J Clin Invest. 2003;33:769–78. doi: 10.1046/j.1365-2362.2003.01222.x. [DOI] [PubMed] [Google Scholar]
- 53.Humphreys MH. Mechanisms and management of nephrotic edema. Kidney Int. 1994;45:266–81. doi: 10.1038/ki.1994.33. [DOI] [PubMed] [Google Scholar]
- 54.Koepke JP, DiBona GF. Blunted natriuresis to atrial natriuretic peptide in chronic sodium-retaining disorders. Am J Physiol. 1987;252:F865–71. doi: 10.1152/ajprenal.1987.252.5.F865. [DOI] [PubMed] [Google Scholar]
- 55.Perico N, Remuzzi G. Edema of the nephrotic syndrome: the role of the atrial peptide system. Am J Kidney Dis. 1993;22:355–66. doi: 10.1016/s0272-6386(12)70137-3. [DOI] [PubMed] [Google Scholar]
- 56.Peterson C, Madsen B, Perlman A, Chan AY, Myers BD. Atrial natriuretic peptide and the renal response to hypervolemia in nephrotic humans. Kidney Int. 1988;34:825–31. doi: 10.1038/ki.1988.256. [DOI] [PubMed] [Google Scholar]
- 57.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–8. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 58.Dong N, Chen S, Wang W, Zhou Y, Wu Q. Corin in clinical laboratory diagnostics. Clin Chim Acta. 2012;413:378–83. doi: 10.1016/j.cca.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–70. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.