Abstract
The E. coli fpg gene encodes the formamido-pyrimidine-DNA-glycosylase (FPG protein) which specifically removes the formamido-pyrimidine and C8-oxoGuanine residues from gamma-irradiated DNA. The fpg gene was ligated in the psV2 vector and transfected into the Chinese hamster CHO and V-79 cells. The transfected cells expressed a formamido-pyrimidine-DNA-glycosylase activity 30 to 40-fold over the constitutive level. The resistance of CHO and V-79 cells to the lethal effect of gamma-rays was similar in control and transfected cells. Furthermore CHO cells expressing the fpg gene had the same resistance to the lethal effect of hydrogen peroxide as control cells. However, the sensitivity to the mutagenic effect of gamma-rays, measured as 6-thioguanine resistance, decreased both in CHO and V-79 transfected cells. Since the lethal effect of gamma-rays was not modified in cells overproducing the FPG protein, the results suggest that this protein protects the cells against the mutagenic lesions formed by ionizing radiations, and among them C8-oxoguanine.
Full text
PDF
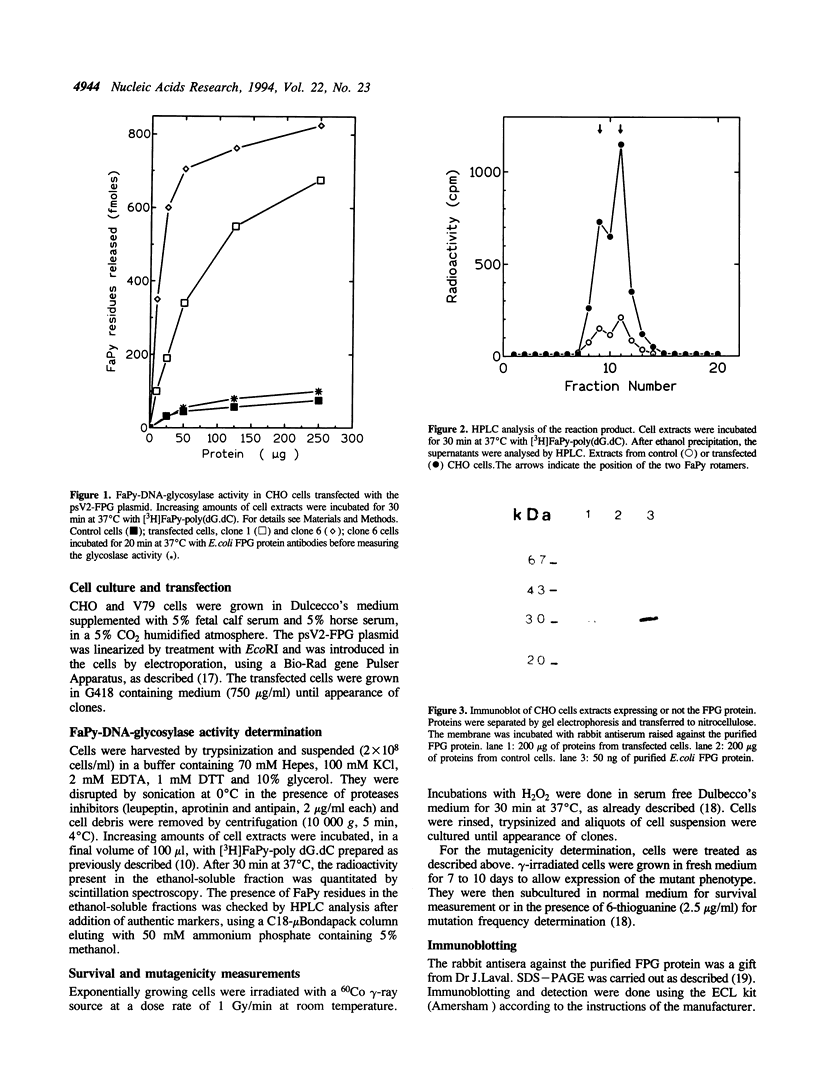
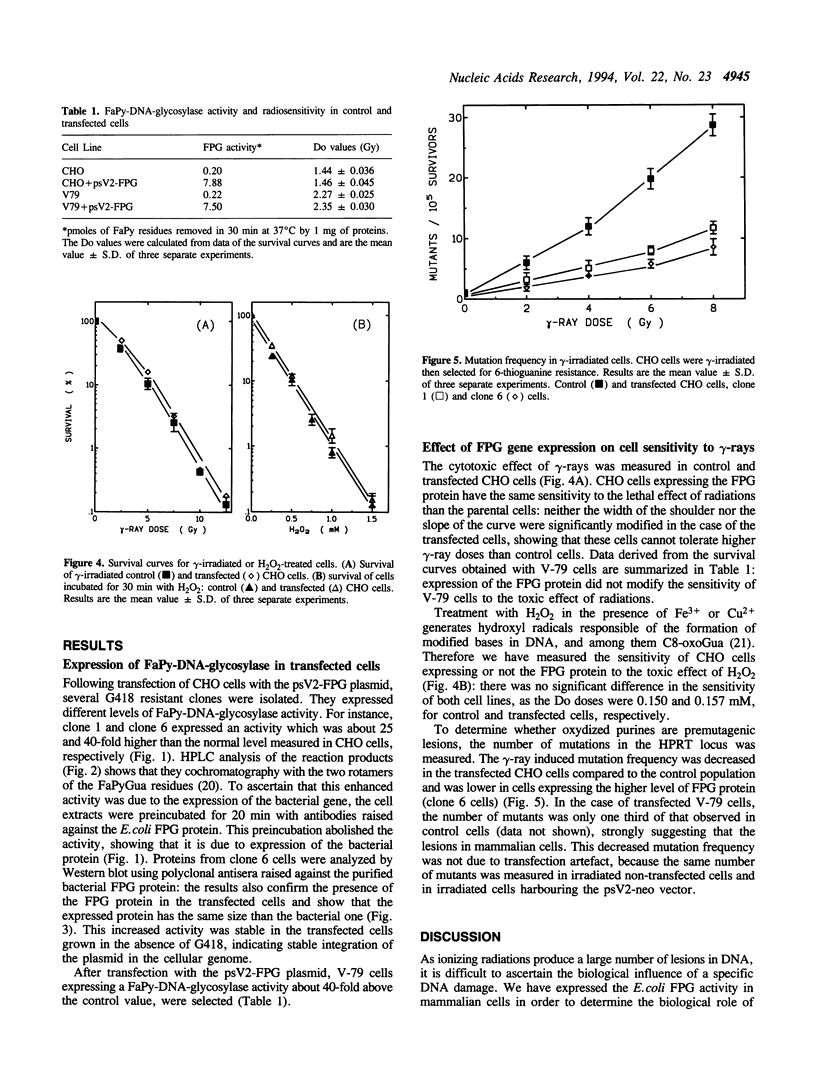

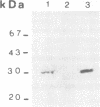
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessho T., Tano K., Kasai H., Ohtsuka E., Nishimura S. Evidence for two DNA repair enzymes for 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in human cells. J Biol Chem. 1993 Sep 15;268(26):19416–19421. [PubMed] [Google Scholar]
- Bessho T., Tano K., Nishmura S., Kasai H. Induction of mutations in mouse FM3A cells by treatment with riboflavin plus visible light and its possible relation with formation of 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in DNA. Carcinogenesis. 1993 May;14(5):1069–1071. doi: 10.1093/carcin/14.5.1069. [DOI] [PubMed] [Google Scholar]
- Boiteux S., Belleney J., Roques B. P., Laval J. Two rotameric forms of open ring 7-methylguanine are present in alkylated polynucleotides. Nucleic Acids Res. 1984 Jul 11;12(13):5429–5439. doi: 10.1093/nar/12.13.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S., Gajewski E., Laval J., Dizdaroglu M. Substrate specificity of the Escherichia coli Fpg protein (formamidopyrimidine-DNA glycosylase): excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry. 1992 Jan 14;31(1):106–110. doi: 10.1021/bi00116a016. [DOI] [PubMed] [Google Scholar]
- Boiteux S., O'Connor T. R., Lederer F., Gouyette A., Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990 Mar 5;265(7):3916–3922. [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Dizdaroglu M. Measurement of radiation-induced damage to DNA at the molecular level. Int J Radiat Biol. 1992 Feb;61(2):175–183. doi: 10.1080/09553009214550791. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Rao G., Halliwell B., Gajewski E. Damage to the DNA bases in mammalian chromatin by hydrogen peroxide in the presence of ferric and cupric ions. Arch Biochem Biophys. 1991 Mar;285(2):317–324. doi: 10.1016/0003-9861(91)90366-q. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Gajewski E., Rao G., Nackerdien Z., Dizdaroglu M. Modification of DNA bases in mammalian chromatin by radiation-generated free radicals. Biochemistry. 1990 Aug 28;29(34):7876–7882. doi: 10.1021/bi00486a014. [DOI] [PubMed] [Google Scholar]
- Graves R. J., Felzenszwalb I., Laval J., O'Connor T. R. Excision of 5'-terminal deoxyribose phosphate from damaged DNA is catalyzed by the Fpg protein of Escherichia coli. J Biol Chem. 1992 Jul 15;267(20):14429–14435. [PubMed] [Google Scholar]
- Habraken Y., Laval F. Increased resistance of the Chinese hamster mutant irs1 cells to monofunctional alkylating agents by transfection of the E. coli or mammalian N3-methyladenine-DNA-glycosylase genes. Mutat Res. 1993 Mar;293(3):187–195. doi: 10.1016/0921-8777(93)90069-s. [DOI] [PubMed] [Google Scholar]
- Homma Y., Tsunoda M., Kasai H. Evidence for the accumulation of oxidative stress during cellular ageing of human diploid fibroblasts. Biochem Biophys Res Commun. 1994 Sep 15;203(2):1063–1068. doi: 10.1006/bbrc.1994.2290. [DOI] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laval F. Pretreatment with oxygen species increases the resistance of mammalian cells to hydrogen peroxide and gamma-rays. Mutat Res. 1988 Sep;201(1):73–79. doi: 10.1016/0027-5107(88)90112-1. [DOI] [PubMed] [Google Scholar]
- Mori T., Hori Y., Dizdaroglu M. DNA base damage generated in vivo in hepatic chromatin of mice upon whole body gamma-irradiation. Int J Radiat Biol. 1993 Dec;64(6):645–650. doi: 10.1080/09553009314551881. [DOI] [PubMed] [Google Scholar]
- Moriya M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G.C-->T.A transversions in simian kidney cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1122–1126. doi: 10.1073/pnas.90.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T. R., Laval J. Physical association of the 2,6-diamino-4-hydroxy-5N-formamidopyrimidine-DNA glycosylase of Escherichia coli and an activity nicking DNA at apurinic/apyrimidinic sites. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5222–5226. doi: 10.1073/pnas.86.14.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinski R., Zastawny T., Budzbon J., Skokowski J., Zegarski W., Dizdaroglu M. DNA base modifications in chromatin of human cancerous tissues. FEBS Lett. 1992 Sep 7;309(2):193–198. doi: 10.1016/0014-5793(92)81093-2. [DOI] [PubMed] [Google Scholar]
- Tudek B., Laval J., Boiteux S. SOS-independent mutagenesis in lacZ induced by methylene blue plus visible light. Mol Gen Genet. 1993 Jan;236(2-3):433–439. doi: 10.1007/BF00277144. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Esteve A., Morningstar M. L., Kuziemko G. M., Essigmann J. M. Genetic effects of oxidative DNA damage: comparative mutagenesis of 7,8-dihydro-8-oxoguanine and 7,8-dihydro-8-oxoadenine in Escherichia coli. Nucleic Acids Res. 1992 Nov 25;20(22):6023–6032. doi: 10.1093/nar/20.22.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]