Abstract
Background
Use of left ventricular assist devices (LVADs) for treatment of advanced heart failure has expanded significantly over the past decade. However, concomitant use of heart failure medical therapies after implant is poorly characterized.
Methods and Results
We examined the use of heart failure medications before and after LVAD implant in adult patients enrolled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) between 2008 and 2013 (N = 9359). Using logistic regression, we examined relationships between patient characteristics and medication use at 3 months after implant. Baseline rates of heart failure therapies before implant were 38% for angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), 55% for β-blockers, 40% for mineralocorticoid receptor antagonists (MRAs), 87% for loop diuretics, 54% for amiodarone, 11% for phosphodiesterase inhibitors, 22% for warfarin, and 54% for antiplatelet agents. By 3 months after implant, the rates were 50% for ACE inhibitors or ARBs, 68% for β-blockers, 33% for MRAs, 68% for loop diuretics, 42% for amiodarone, 21% for phosphodiesterase inhibitors, 92% for warfarin, and 84% for antiplatelet agents. In general, age, pre-implant INTERMACS profile, and prior medication use were associated with medication use at 3 months.
Conclusions
Overall use of neurohormonal antagonists was low after LVAD implant, whereas use of loop diuretics and amiodarone remained high. Heart failure medication use is highly variable but appears to generally increase after LVAD implantation. Low neurohormonal antagonist use may reflect practice uncertainty in the clinical utility of these medications post-LVAD.
Keywords: Heart-Assist Devices, Heart Failure, Outcome and Process Assessment (Health Care)
Introduction
Patients with advanced heart failure have limited treatment options, namely heart transplant or left ventricular assist devices (LVADs). Because of the relatively fixed availability of heart transplants per year nationwide, the use of LVADs for patients with advanced heart failure has grown tremendously over the past decade.1 As a result, understanding of the optimal management of this evolving technology has also grown. However, unlike medical management of heart failure, for which there are volumes of data to guide clinicians in the appropriate treatment of individual patients, there remain large gaps in knowledge regarding the medical management of patients after LVAD implant.
The role of traditional heart failure medications—angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), β-blockers, mineralocorticoid antagonists (MRAs), and diuretics—in the care of patients with an LVAD is largely unknown. Factors such as right heart failure,2 atrial and ventricular arrhythmias,3 renal dysfunction,4 and pulmonary hypertension5 can compromise the efficiency of an LVAD, resulting in ineffective cardiac support. Neurohormonal antagonists may help to improve cardiac function, which could allow for greater myocardial recovery. Moreover, some medications such as loop diuretics or amiodarone may have long-term adverse side effects.
With more patients receiving LVADs, there is a pressing need to better understand medical therapy in these patients to address opportunities for improvement or areas where more evidence is needed. We sought to describe the current landscape and trends in heart failure medical therapy from before LVAD implant to 6 months after implant and the factors associated with post-implant medical therapy.
Methods
Data Source
The study cohort was derived from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), a national, audited, US Food and Drug Administration (FDA)–approved registry for patients who receive mechanical circulatory support devices for treatment of advanced heart failure. The registry was created in June 2005 as a joint effort of the National Heart, Lung, and Blood Institute, the FDA, clinicians, scientists, and industry representatives. It is housed and managed at the University of Alabama at Birmingham. The Joint Commission requires that patients with a chronically implanted ventricular assist device have their health data entered into a national audited registry,6 and INTERMACS is the only registry that meets their requirements. Institutional review board approval is required at participating centers before data collection begins at the center. Centers transmit data using a Web-based system. The data are stored on a secure server provided by the United Network for Organ Sharing and are checked for completeness by the central collection facility. Data outliers are validated with the site of origin, but source documents are not checked against the submitted data. A medical events committee reviews major events, including cause of death, neurological dysfunction, bleeding, device malfunction, and infection.
Study Population
We included all patients with data entered into INTERMACS between 2008 and 2013 who received a durable continuous-flow LVAD. For patients in the registry with multiple LVAD implants, we included only the first implant in the analysis. Approximately 10% of eligible patients are not enrolled because of inability or refusal to consent. We included patients who received either biventricular support (i.e., both an LVAD and a right ventricular assist device) (n = 248) or an isolated LVAD. We excluded patients who received a total artificial heart (n = 216) because of the marked differences in anatomy and the potential benefit of heart failure medications. Patient-level data were transmitted securely to the Duke Clinical Research Institute for analysis.
Statistical Analysis
We describe pre-implant patient characteristics and both pre- and post-implant medication use using frequencies and percentages for categorical variables and medians with interquartile ranges for continuous variables. Characteristics of the study population included demographic characteristics (i.e., age and sex), implant information (i.e., year of implant, receipt of biventricular support, strategy, and INTERMACS profile), vital signs (i.e., body mass index and blood pressure), and laboratory test results (i.e., albumin, total bilirubin, blood urea nitrogen, creatinine, hemoglobin, international normalized ratio, potassium, aspartate aminotransferase, alanine aminotransferase, and sodium). We report medication use for all patients before implant and at 1 week, 1 month, 3 months, and 6 months after implant, including ACE inhibitors, ARBs, β-blockers, MRAs, loop diuretics, antiplatelet agents, warfarin, amiodarone, and phosphodiesterase inhibitors. We plotted longitudinal medication use through 6 months after implant to ensure a consistent population at each time point. We then examined associations between patient characteristics and use of ACE inhibitors or ARBs, β-blockers, MRAs, and loop diuretics at 3 months after implant (a time of relative clinical stability) using logistic regression. We report the associations using odds ratios with 95% confidence intervals (CIs). We included follow-up data in the analysis for as long as the patient was still alive with an LVAD in place.
We used SAS version 9.3 (SAS Institute Inc, Cary, North Carolina) for all analyses. The institutional review board of the Duke University Health System and the INTERMACS executive research committee approved the study.
Results
Between 2008 and 2013, 9359 patients received an implantable ventricular assist device and were enrolled in INTERMACS. Approximately 60% of patients in the study were aged 50 to 69 years, and 80% were men (Table 1). The number of implants increased annually during the study period. Fewer than 3% of patients received biventricular support. LVAD designations were 36% destination therapy, 26% bridge to transplant, 36% possible bridge to transplant, and 1% other. Eighty percent of implants were in patients classified as INTERMACS profiles 1 through 3. Comorbidity data were available for 42% of patients in the analysis. Notable comorbid conditions included chronic renal disease (22%), atrial arrhythmia (21%), smoking (30%), high body mass index (15%), and pulmonary hypertension (26%) [data not shown].
Table 1.
Pre-Implant Characteristics of the Study Population
| Variable | Patients (N = 9359) |
|---|---|
| Age, No. (%) | |
| 19-29 y | 432 (4.6) |
| 30-39 y | 632 (6.8) |
| 40-49 y | 1335 (14.3) |
| 50-59 y | 2535 (27.1) |
| 60-69 y | 3126 (33.4) |
| 70-79 y | 1237 (13.2) |
| ≥ 80 y | 62 (0.7) |
| Men | 7391 (79.0) |
| Implant characteristics | |
| Implant year | |
| 2008 | 459 (4.9) |
| 2009 | 865 (9.2) |
| 2010 | 1581 (16.9) |
| 2011 | 1837 (19.6) |
| 2012 | 2204 (23.5) |
| 2013 | 2413 (25.8) |
| Biventricular assist device | 248 (2.6) |
| Strategy | |
| Bridge to Transplant | 2473 (26.4) |
| Possible Bridge to Transplant | 3404 (36.4) |
| Destination Therapy | 3369 (36.0) |
| Other/Unknown | 113 (1.2) |
| INTERMACS profile | |
| 1: Critical Cardiogenic Shock | 1384 (14.8) |
| 2: Progressive Decline | 3596 (38.4) |
| 3: Stable but Inotrope Dependent | 2591 (27.7) |
| 4: Resting Symptoms | 1311 (14.0) |
| 5: Exertion Intolerant | 276 (2.9) |
| 6: Exertion Limited | 128 (1.4) |
| 7: Advanced NYHA Class 3 | 73 (0.8) |
| Vital signs and laboratory test results, median (interquartile range) | |
| Body mass index, kg/m | 27.8 (24.0-32.2) |
| Blood pressure, mm Hg | |
| Systolic | 102.0 (93.0-113.0) |
| Diastolic | 63.0 (56.0-71.0) |
| Mean arterial pressure | 76.0 (70.0-83.7) |
| Albumin, g/dL | 3.4 (3.1-3.8) |
| Bilirubin, mg/dL | 1.1 (0.7-1.5) |
| Blood urea nitrogen, mg/dL | 25.0 (18.0-37.0) |
| Creatinine, mg/dL | 1.3 (1.0-1.6) |
| Hemoglobin, g/dL | 11.2 (9.9-12.7) |
| International normalized ratio | 1.2 (1.1-1.4) |
| Potassium, mEq/L | 4.1 (3.8-4.4) |
| Aspartate aminotransferase, u/L | 32.0 (23.0-43.0) |
| Alanine aminotransferase, u/L | 31.0 (20.0-47.0) |
| Sodium, mEq/L | 135.0 (132.0-138.0) |
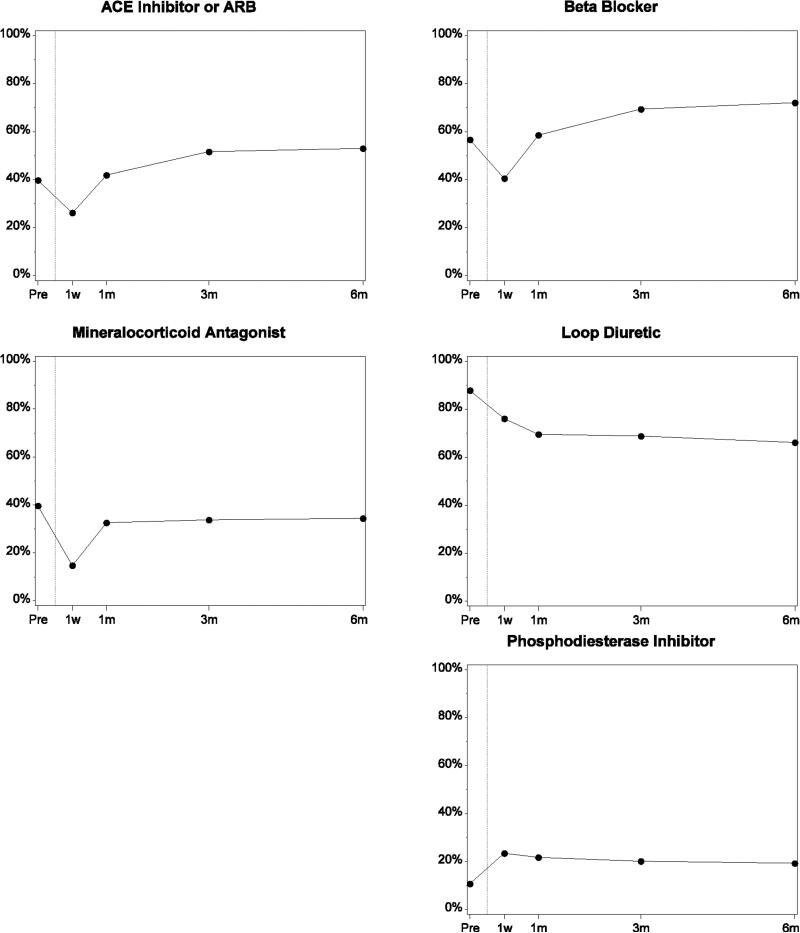
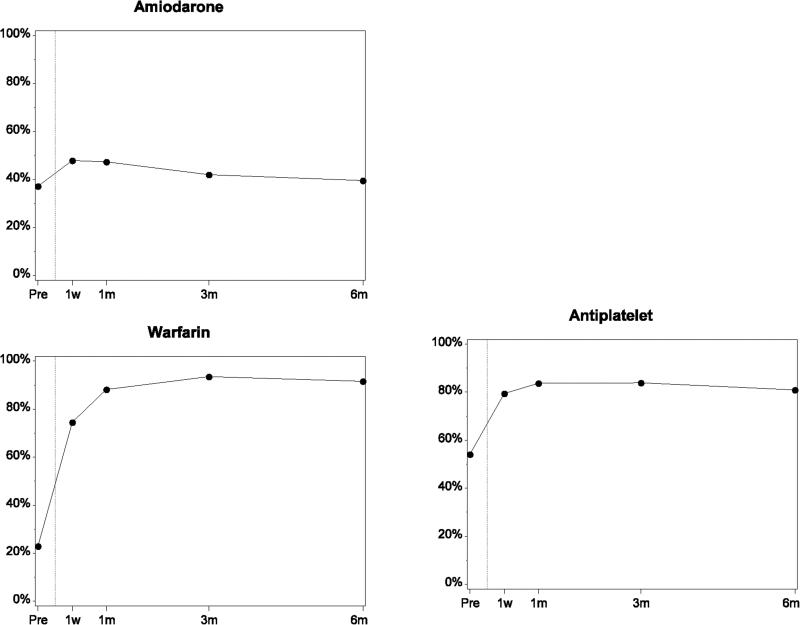
Rates of use of many heart failure medical therapies were low before implant (ACE inhibitor or ARB, 38%; β-blocker, 55%; MRA, 40%; Figure 1; Table 2). These rates dropped at 1 week after implant. Use of ACE inhibitors or ARBs, β-blockers, and MRAs increased and plateaued 1 to 3 months after implant (ACE inhibitor or ARB, 50%; β-blocker, 70%; MRA, 33%). Rates of ACE inhibitor or ARB and β-blocker use were higher after implant than at baseline, whereas MRA rates were lower. Use of loop diuretics was high before implant (87%) and declined to 67% at 3 to 6 months after implant. Amiodarone use was slightly less than 40% before implant, approximately 48% at 1 week to 1 month after implant, and approximately 40% at 3 to 6 months. Phosphodiesterase inhibitor use was 11% before implant, 24% at 1 week after implant, and 20% at 3 to 6 months. Warfarin and antiplatelet use, respectively, were 22% and 54% before implant, increased substantially in the first week, and leveled off at 92% and 82% by 3 to 6 months. Lovenox use was less than 3% throughout the implant period. (Data on lovenox use was collected after 2011 only). Pre-implant rates of ACE inhibitors or ARBs, β-blockers, and MRAs were lower for patients classified as INTERMACS profiles 1 and 2 (Table 3). In contrast, amiodarone use was higher in this group.
Figure.
Medication Use From Before Implant to 6 Months After Implant Among Patients With Data Through the 6-Month Follow-up Period
Table 2.
Medication Use From Before Implant to 6 Months After Implant Among Patients With Data Through the 6-Month Follow-up Period
| Medication | Overall (N = 9359) | Follow-up | |||
|---|---|---|---|---|---|
| 1 Week (n = 9108) | 1 Month (n = 5389) | 3 Months (n = 7241) | 6 Months (n = 5840) | ||
| ACE inhibitor or ARB | 3562 (38.1) | 2251 (24.7) | 2110 (39.2) | 3625 (50.1) | 3098 (53.0) |
| Amiodarone | 3584 (38.3) | 4371 (48.0) | 2516 (46.7) | 3054 (42.2) | 2313 (39.6) |
| Antiplatelet | 5082 (54.3) | 7184 (78.9) | 4470 (82.9) | 6046 (83.5) | 4722 (80.9) |
| β-Blocker | 5162 (55.2) | 3442 (37.8) | 2997 (55.6) | 4890 (67.5) | 4203 (72.0) |
| Enoxaparin | — | 92 (2.1) | 102 (2.6) | 94 (2.5) | 77 (2.4) |
| Loop diuretic | 8139 (87.0) | 6720 (73.8) | 3662 (68.0) | 4930 (68.1) | 3862 (66.1) |
| Mineralocorticoid receptor antagonist | 3707 (39.6) | 1302 (14.3) | 1694 (31.4) | 2408 (33.3) | 2008 (34.4) |
| Phosphodiesterase inhibitora | 500 (11.1) | 1069 (24.1) | 902 (23.0) | 776 (20.9) | 609 (19.2) |
| Warfarin | 2095 (22.4) | 6516 (71.5) | 4577 (84.9) | 6655 (91.9) | 5347 (91.6) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
The denominator differs because these medications were added to the data collection forms recently.
Table 3.
Medication Use by INTERMACS Profile
| Variable | Profile 1 (n = 1384) | Profile 2 (n = 3596) | Profile 3 (n = 2591) | Profile 4 (n = 1311) | Profile 5 (n = 276) | Profile 6 (n = 128) | Profile 7 (n = 73) |
|---|---|---|---|---|---|---|---|
| Pre-implant medications | |||||||
| ACE inhibitor or ARB | 298 (21.5) | 1167 (32.5) | 1099 (42.4) | 719 (54.8) | 165 (59.8) | 73 (57.0) | 41 (56.2) |
| MRA | 316 (22.8) | 1424 (39.6) | 1154 (44.5) | 604 (46.1) | 120 (43.5) | 59 (46.1) | 30 (41.1) |
| Amiodarone | 643 (46.5) | 1442 (40.1) | 897 (34.6) | 435 (33.2) | 96 (34.8) | 42 (32.8) | 29 (39.7) |
| Antiplatelet agent | 706 (51.0) | 1900 (52.8) | 1430 (55.2) | 749 (57.1) | 171 (62.0) | 82 (64.1) | 44 (60.3) |
| β-Blocker | 418 (30.2) | 1773 (49.3) | 1581 (61.0) | 1031 (78.6) | 211 (76.4) | 101 (78.9) | 47 (64.4) |
| Loop diuretic | 1043 (75.4) | 3211 (89.3) | 2298 (88.7) | 1165 (88.9) | 252 (91.3) | 109 (85.2) | 61 (83.6) |
| Phosphodiesterase inhibitora | 48 (7.4) | 208 (12.8) | 180 (13.3) | 52 (7.6) | 6 (4.7) | 2 (4.3) | 4 (11.1) |
| Warfarin | 147 (10.6) | 788 (21.9) | 616 (23.8) | 394 (30.1) | 87 (31.5) | 38 (29.7) | 25 (34.2) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist.
Profile 1, critical cardiogenic shock; Profile 2, progressive decline; Profile 3, stable but inotrope-dependent; Profile 4, resting symptoms; Profile 5, exertion-intolerant; Profile 6, exertion-limited; Profile 7, advanced New York Heart Association functional classification 3.
b The denominator differs because these medications were added to the data collection forms recently.
After adjustment for demographic characteristics, device type, and implant date, younger age was associated with use of ACE inhibitors or ARBs, β-blockers, and MRAs at 3 months, but it was not associated with use of loop diuretics (Table 4). Patients with more recent implants (i.e., 2010 and later) were more likely to use MRAs at 3 months than patients with implants in 2008. Pre-implant medication use had the strongest association with post-implant use.
Table 4.
Adjusted Associations Between Patient Characteristics and Medication Use at 3 Months After Implant
| Characteristic | ACE Inhibitor or ARB | B-Blocker | MRA | Loop Diuretic | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted RR (95% CI) | P Value | Adjusted RR (95% CI) | P Value | Adjusted RR (95% CI) | P Value | Adjusted RR (95% CI) | P Value | |
| Age group | ||||||||
| 19-29 y | 1.10 (1.00, 1.20) | .04 | 1.09 (1.01, 1.16) | .02 | 1.22 (1.07, 1.39) | .003 | 0.92 (0.85, 1.01) | .07 |
| 30-39 y | 1.08 (1.00, 1.17) | .049 | 1.07 (1.01, 1.13) | .03 | 1.24 (1.11, 1.39) | < .001 | 1.02 (0.96, 1.09) | .45 |
| 40-49 y | 1.01 (0.95, 1.08) | .68 | 1.03 (0.98, 1.08) | .21 | 1.12 (1.01, 1.23) | .02 | 1.02 (0.97, 1.07) | .45 |
| 50-59 y | 1.00 [Reference] | — | 1.00 [Reference] | — | 1.00 [Reference] | — | 1.00 [Reference] | — |
| 60-69 y | 0.85 (0.81, 0.91) | < .001 | 0.97 (0.94, 1.02) | .22 | 0.90 (0.83, 0.98) | .02 | 0.97 (0.93, 1.01) | .11 |
| 70-79 y | 0.79 (0.72, 0.86) | < .001 | 0.97 (0.91, 1.02) | .24 | 0.86 (0.76, 0.98) | .02 | 0.96 (0.91, 1.02) | .22 |
| ≥ 80 y | 0.44 (0.26, 0.76) | .003 | 1.00 (0.83, 1.20) | .97 | 0.57 (0.31, 1.07) | .08 | 0.79 (0.60, 1.03) | .08 |
| Men | 1.00 (0.94, 1.05) | .87 | 1.01 (0.97, 1.05) | .65 | 0.97 (0.90, 1.05) | .47 | 1.03 (0.99, 1.07) | .19 |
| Destination therapy v. BTT | 0.90 (0.84, 0.95) | < .001 | 0.96 (0.92, 1.00) | .04 | 0.88 (0.81, 0.96) | .005 | 1.00 (0.95, 1.04) | .83 |
| Possible BTT v. BTT | 0.91 (0.86, 0.96) | < .001 | 0.94 (0.90, 0.98) | .002 | 0.90 (0.84, 0.98) | .009 | 1.01 (0.97, 1.05) | .76 |
| INTERMACS profile | ||||||||
| 7: Advanced NYHA class 3 | 1.06 (0.82, 1.36) | .67 | 0.82 (0.65, 1.04) | .10 | 1.00 (0.68, 1.47) | > .99 | 1.01 (0.82, 1.25) | .91 |
| 6: Exertion Intolerant | 1.10 (0.95, 1.26) | .20 | 1.00 (0.91, 1.10) | .98 | 0.78 (0.62, 0.98) | .03 | 1.13 (1.02, 1.24) | .01 |
| 5: Exertion Limited | 0.99 (0.81, 1.22) | .94 | 1.01 (0.89, 1.14) | .94 | 0.91 (0.68, 1.23) | .55 | 1.05 (0.91, 1.21) | .49 |
| 4: Progressive Decline | 1.01 (0.94, 1.09) | .77 | 0.96 (0.92, 1.01) | .16 | 0.99 (0.89, 1.09) | .77 | 1.09 (1.04, 1.16) | .001 |
| 3: Resting Symptoms | 1.13 (1.03, 1.23) | .008 | 1.00 (0.94, 1.06) | .95 | 0.92 (0.81, 1.04) | .17 | 1.14 (1.08, 1.22) | < .001 |
| 2: Stable but Inotrope Dependent | 1.12 (1.03, 1.21) | .005 | 1.03 (0.98, 1.09) | .20 | 1.00 (0.90, 1.10) | .93 | 1.08 (1.02, 1.14) | .01 |
| 1: Critical Cardiogenic Shock | 1.00 [Reference] | — | 1.00 [Reference] | — | 1.00 [Reference] | — | 1.00 [Reference] | — |
| Implant year | ||||||||
| 2008 | 1.00 [Reference] | — | 1.00 [Reference] | — | 1.00 [Reference] | — | 1.00 [Reference] | — |
| 2009 | 1.08 (0.95, 1.23) | .22 | 0.94 (0.85, 1.03) | .19 | 1.17 (0.93, 1.47) | .18 | 1.02 (0.93, 1.12) | .70 |
| 2010 | 1.09 (0.97, 1.23) | .15 | 1.06 (0.97, 1.15) | .20 | 1.51 (1.23, 1.87) | < .001 | 1.08 (0.99, 1.17) | .08 |
| 2011 | 1.15 (1.02, 1.30) | .02 | 1.11 (1.02, 1.20) | .01 | 1.64 (1.34, 2.02) | < .001 | 1.08 (0.99, 1.17) | .08 |
| 2012 | 1.18 (1.05, 1.33) | .005 | 1.13 (1.04, 1.22) | .004 | 1.81 (1.47, 2.22) | < .001 | 1.06 (0.97, 1.15) | .17 |
| 2013 | 1.19 (1.06, 1.34) | .004 | 1.07 (0.98, 1.16) | .13 | 1.74 (1.41, 2.14) | < .001 | 1.10 (1.01, 1.19) | .03 |
| Pre-implant use of the same medication | 1.31 (1.25, 1.38) | < .001 | 1.23 (1.19, 1.28) | < .001 | 1.70 (1.59, 1.81) | < .001 | 1.20 (1.13, 1.28) | < .001 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BTT, Bridge to Transplant; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; NYHA, New York Heart Association; RR, relative risk.
Discussion
As focus on advanced heart failure has turned toward mechanical support, little has been done to understand the role of continued medical therapy. To our knowledge, ours is the first analysis to characterize heart failure medication use in this population using data from the largest registry for mechanical circulatory support. Our study had 3 important findings. First, rates of many heart failure medical therapies were low before implant. Second, rates of use of ACE inhibitors, ARBs, and β-blockers were higher after implant than before implant, and rates of MRA use were lower. Third, use of other medications, such as loop diuretics and amiodarone, was common even months after LVAD implant.
Although LVADs mechanically unload the left ventricle, heart failure medical therapy may be an important adjunct in the long-term care of these patients.7-15 Guidelines from the International Society for Heart and Lung Transplantation do not recommend continued use of heart failure medications for all patients (because of a lack of evidence of benefit in patients with mechanical circulatory support), but they do recommend the use of ACE inhibitors or ARBs for the treatment of hypertension and cardiovascular risk reduction in diabetes and vascular disease, β-blockers for hypertension and tachyarrhythmia, MRAs for potassium-sparing and antifibrotic effects, and diuretics for volume overload and right ventricular dysfunction (all class I, level of evidence C).16 However, some evidence supports the use of neurohormonal antagonists such as ACE inhibitors or ARBs, MRAs, and β-blockers to improve remodeling, prevent further fibrosis, and potentially reduce the risk of arrhythmias.7-9,11 Thus, there may be clinical benefit in continuing these medications after implant that are not yet realized.
We found that pre-implant use of ACE inhibitors or ARBs, β-blockers, and MRAs in clinical practice was lower than previously documented for clinically stable, ambulatory patients with heart failure.17 This finding was not unexpected given that patients with advanced systolic heart failure often have renal or circulatory limitations because of continued use of β-blockers and ACE inhibitors or ARBs, and these agents often are discontinued before consideration of LVAD implant. In fact, intolerance of these drugs is considered an early marker of American Heart Association stage D heart failure and may trigger referral to an advanced heart failure center.18-21
However, given the enhanced circulatory support and typical improvement in renal function after LVAD implant, one might expect to see an increase in the use of neurohormonal antagonists. We observed only modest increases in the use of ACE inhibitors or ARBs (12% increase) and β-blockers (15% increase) at 3 to 6 months compared with pre-implant rates and a surprising 7% decrease in the use of MRAs. This finding may reflect clinical uncertainty in the use of neurohormonal antagonists or adverse effects in post-LVAD settings.22 Hypotension or difficulty in blood pressure measurement may make it more difficult to assess patients clinically outside of specialized centers, therefore creating a barrier to titration of these therapies. For example, patients who received an LVAD after 2010 were more likely to receive an MRA compared with those who received an implant in 2008.
Loop diuretics are a cornerstone of heart failure therapy for management of volume overload and congestion.23 As expected, we observed a high rate of pre-implant loop diuretic use in this analysis. However, the continued use of loop diuretics months after implant was unexpected. Although we could not account for dosages, the continued requirement for loop diuretics suggests that patients may still have signs and symptoms of excess volume. This may represent persistent or late right ventricular failure, which has emerged as a major reason for readmission and morbidity after treatment with isolated left ventricular support.24-31 Thus, it remains important to identify patients who are at risk for persistent right ventricular failure and volume overload and carefully examine the role of heart failure therapies in this growing population.
Overall, relatively few factors were associated with post-implant use of heart failure medications. Pre-implant medication use was found to have the strongest association with post-implant use and may reflect physician inertia regarding alterations in medical treatment.32 Because patients who receive an LVAD are subject to frequent hospital readmission33-35 and experience multiple transitions in care, there may be opportunities to intervene and initiate neurohormonal antagonists after implant, depending on residual problems. The reason for admission for these patients may not be heart failure. Both gastrointestinal bleeding and device-associated infection often lead to hemodynamic compromise and may necessitate reduction or discontinuation of neurohormonal antagonists where heart failure therapies are reduced or discontinued. Future initiatives in the management of these patients should take into account the common reasons for hospital admission and consider the impact on continuation or discontinuation of heart failure drug therapy. There also may be important downstream consequences of heart failure therapies in this population that have yet to be identified.
The majority of LVAD implants in this analysis were under the designation of bridge to transplant or possible bridge to transplant. Interestingly, the destination therapy and possible bridge to transplant groups were less likely to be taking ACE inhibitors, ARBs, β-blockers, or MRAs than the bridge to transplant group. This could be because patients listed as destination or possible bridge to transplant are often older, have more comorbidities, and are less likely to tolerate these medications compared with many patients listed for bridge to transplant LVADs. These differences could also reflect practice variations in the medical care of patients intended for destination therapy versus transplantation.
With nearly a third of all heart transplants now performed in patients on mechanical circulatory support,1,36 there may be important consequences of β-blockers, ACE inhibitors, ARBs, and other agents such as amiodarone on heart transplant. In the era before LVADs as a bridge to transplant, heart transplant was infrequent among patients who could tolerate these agents, and operative and post-procedural practices have yet to adapt to this change. It is conceivable that an alternate pharmacological approach may need to be adopted in patients receiving an LVAD as a bridge to transplant. Finally, although the concept of complete myocardial recovery after LVAD implant is still under investigation, certain patients supported with LVAD therapy may receive neurohormonal antagonists for aggressive ventricular reverse remodeling. Younger patients with shorter duration of heart failure have the highest rates of recovery and may be a group targeted for the most aggressive drug titration. This concept is particularly intriguing given that the highest incidence of β-blocker and ACE inhibitor or ARB therapy was among younger patients in our analysis.
Our study has limitations. First, the INTERMACS registry does not include patients who refused to consent or were too ill to consent. Medical management in these patients, both before and after LVAD implant, remains unknown. Second, some variables were not collected in the registry. For example, heparin use was not included, and enoxaparin was not added until 2011. Third, INTERMACS does not collect data on type of cardiomyopathy (i.e., ischemic vs nonischemic). Knowledge of the etiology of heart failure would have been helpful in our analysis, because much of the research on myocardial recovery has been in nonischemic cardiomyopathy. Fourth, we were unable to determine whether patients were eligible for medications, and we could not account for dosage or adherence. In addition, this was an observational, retrospective analysis. Finally, unmeasured confounders may have influenced the results. In particular, we lacked site or hospital identifiers and could not account for confounding due to site effects. Variation in neurohormonal antagonist prescription may be associated with local practice patterns rather than the clinical status of patients.37 Thus, future availability of such data would be important for quality improvement and proper long-term outcomes analysis.
Conclusion
In a large, multicenter registry of patients receiving mechanical circulatory support, we found that the use of many medical therapies for heart failure was low before LVAD implant and increased only modestly over time. Pre-implant medication use had the strongest association with post-implant use. In particular, loop diuretic use remained common even months after implant. Medication use, particularly neurohormonal antagonist use, may play a role in myocardial remodeling and optimal functioning of the LVAD. Future studies should focus on the importance of continuing these medications after implant—in terms of mortality, hospital admission, and quality of life—so that that optimal medical therapy can be understood and uniformly applied.
Highlights.
Use of heart failure medical therapies is poorly characterized after LVAD implantation.
In this analysis of a large, multicenter LVAD registry, we found that use of neurohormonal antagonists was low after LVAD implant but that use of loop diuretics and amiodarone remained high.
Future studies should examine associations of heart failure medication use post-LVAD implant in terms of mortality, readmission, and quality of life so that optimal medical therapy can be better understood in this population.
Acknowledgments
Funding Source: This project was supported in part by grant U19HS021092 from the Agency for Healthcare Research and Quality. Dr Khazanie was supported in part by grant T32HL069749 from the National Heart, Lung, and Blood Institute. Dr Arnold was supported by career development grant K23HL116799 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Contributions: Damon M. Seils, MA, Duke University, assisted with manuscript preparation. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Disclaimer: The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality, the National Heart, Lung, and Blood Institute, or the National Institutes of Health.
Role of the Sponsor: The Agency for Healthcare Research and Quality and the National Heart, Lung, and Blood Institute had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript. The manuscript was reviewed and approved by the INTERMACS publications committee.
Disclosures: Drs Patel and Kiernan reported receiving consulting fees from Thoratec Corporation and HeartWare Inc. No other authors reported having conflicts of interest.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Miller MA, Timothy Baldwin J, Young JB. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The Right Ventricular Failure Risk Score: a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–2172. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziv O, Dizon J, Thosani A, Naka Y, Magnano AR, Garan H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005;45:1428–1434. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59:26–36. doi: 10.1016/j.jacc.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Atluri P, Fairman AS, MacArthur JW, Goldstone AB, Cohen JE, Howard JL, Zalewski CM, Shudo Y, Woo YJ. Continuous flow left ventricular assist device implant significantly improves pulmonary hypertension, right ventricular contractility, and tricuspid valve competence. J Card Surg. 2013;28:770–775. doi: 10.1111/jocs.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:1–30. doi: 10.2174/1568006033337276. [DOI] [PubMed] [Google Scholar]
- 8.Klotz S, Foronjy RF, Dickstein ML, Gu A, Garrelds IM, Danser AH, Oz MC, D'Armiento J, Burkhoff D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation. 2005;112:364–374. doi: 10.1161/CIRCULATIONAHA.104.515106. [DOI] [PubMed] [Google Scholar]
- 9.Matsumiya G, Monta O, Fukushima N, Sawa Y, Funatsu T, Toda K, Matsuda H. Who would be a candidate for bridge to recovery during prolonged mechanical left ventricular support in idiopathic dilated cardiomyopathy? J Thorac Cardiovasc Surg. 2005;130:699–704. doi: 10.1016/j.jtcvs.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Burkhoff D, Klotz S, Mancini DM. LVAD-induced reverse remodeling: basic and clinical implications for myocardial recovery. J Card Fail. 2006;12:227–239. doi: 10.1016/j.cardfail.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Klotz S, Danser AH, Foronjy RF, Oz MC, Wang J, Mancini D, D'Armiento J, Burkhoff D. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–1174. doi: 10.1016/j.jacc.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 12.Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AH. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensinaldosterone system: Therapeutic consequences? Eur Heart J. 2009;30:805–812. doi: 10.1093/eurheartj/ehp012. [DOI] [PubMed] [Google Scholar]
- 13.Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, Lehmkuhl HB, Knosalla C, Hetzer R. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J. 2011;32:1148–1160. doi: 10.1093/eurheartj/ehq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler CR, Jugdutt BI. The paradox of left ventricular assist device unloading and myocardial recovery in end-stage dilated cardiomyopathy: implications for heart failure in the elderly. Heart Fail Rev. 2012;17:615–633. doi: 10.1007/s10741-012-9300-8. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad T, Wang T, O'Brien EC, Samsky MD, Pura JA, Lokhnygina Y, Rogers JG, Hernandez AF, Craig D, Bowles DE, Milano CA, Shah SH, Januzzi JL, Felker GM, Patel CB. Effects of left ventricular assist device support on biomarkers of cardiovascular stress, fibrosis, fluid homeostasis, inflammation, and renal injury. JACC Heart Fail. 2015;3:30–39. doi: 10.1016/j.jchf.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J. International Society for Heart and Lung Transplantation. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 18.Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XH, Deswal A, Bozkurt B. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J. 2011;161:567–573. doi: 10.1016/j.ahj.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosy AP, Vaduganathan M, Mentz RJ, Greene SJ, Subačius H, Konstam MA, Maggioni AP, Swedberg K, Gheorghiade M. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J. 2013;165:216–225. doi: 10.1016/j.ahj.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Lampert BC, Eckert C, Weaver S, Scanlon A, Lockard K, Allen C, Kunz N, Bermudez C, Bhama JK, Shullo MA, Kormos RL, Dew MA, Teuteberg JJ. Blood pressure control in continuous flow left ventricular assist devices: efficacy and impact on adverse events. Ann Thorac Surg. 2014;97:139–146. doi: 10.1016/j.athoracsur.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 23.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 24.Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, Horton K, Haddad F, Li DY, Renlund DG, Fisher PW. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105:1030–1035. doi: 10.1016/j.amjcard.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ. HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol. 2012;60:521–528. doi: 10.1016/j.jacc.2012.02.073. [DOI] [PubMed] [Google Scholar]
- 27.Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, Acker AL, Hiesinger W, Howard JL, Acker MA, Woo YJ. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. 2013;96:857–863. doi: 10.1016/j.athoracsur.2013.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazar JF, Swartz MF, Schiralli MP, Schneider M, Pisula B, Hallinan W, Hicks GL, Jr, Massey HT. Survival after left ventricular assist device with and without temporary right ventricular support. Ann Thorac Surg. 2013;96:2155–2159. doi: 10.1016/j.athoracsur.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Patlolla B, Beygui R, Haddad F. Right-ventricular failure following left ventricle assist device implantation. Curr Opin Cardiol. 2013;28:223–233. doi: 10.1097/HCO.0b013e32835dd12c. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K, Naka Y, Yang JA, Uriel N, Colombo PC, Jorde UP, Takayama H. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant. 2014;33:141–148. doi: 10.1016/j.healun.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–2172. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krantz MJ, Ambardekar AV, Kaltenbach L, Hernandez AF, Heidenreich PA, Fonarow GC. Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients (from Get With the Guidelines-Heart Failure). Am J Cardiol. 2011;107:1818–1823. doi: 10.1016/j.amjcard.2011.02.322. [DOI] [PubMed] [Google Scholar]
- 33.Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG, Milano CA, Curtis LH, Hernandez AF. Trends in the use and outcomes of ventricular assist devices among medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol. 2014;63:1395–1404. doi: 10.1016/j.jacc.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smedira NG, Hoercher KJ, Lima B, Mountis MM, Starling RC, Thuita L, Schmuhl DM, Blackstone EH. unplanned hospital readmissions after heartmate ii implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail. 2013;1:31–39. doi: 10.1016/j.jchf.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Stulak JM, Joyce L, Daly R, Park SJ, Kushwaha SS. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 36.Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller CD, Callahan ER, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2013 Annual Data Report: heart. Am J Transplant. 2015;15:1–28. doi: 10.1111/ajt.13199. [DOI] [PubMed] [Google Scholar]
- 37.Peterson PN, Chan PS, Spertus JA, Tang F, Jones PG, Ezekowitz JA, Allen LA, Masoudi FA, Maddox TM. Practice-level variation in use of recommended medications among outpatients with heart failure: insights from the NCDR PINNACLE program. Circ Heart Fail. 2013;6:1132–1138. doi: 10.1161/CIRCHEARTFAILURE.113.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]