Abstract
Background
Thymic-derived regulatory T cells (tTreg) are critical regulators of the immune system. Adoptive tTreg transfer is a curative therapy for murine models of autoimmunity, graft rejection, and graft versus host disease (GVHD). We previously completed a “first-in-human” clinical trial using in vitro expanded umbilical cord blood (UCB) derived tTreg to prevent GVHD in patients undergoing UCB hematopoietic stem cell transplantation (HSCT). tTreg were safe and demonstrated clinical efficacy, but low yield prevented further dose escalation.
Methods
To optimize yield, we investigated the use of KT64/86 artificial antigen presenting cells (aAPC) to expand tTreg and incorporated a single re-stimulation after day 12 in expansion culture.
Results
aAPC increased UCB tTreg expansion >8-fold over CD3/28 stimulation. Re-stimulation with aAPC increased UCB tTreg expansion an additional 20–30 fold. Re-stimulated human UCB tTreg ameliorated GVHD disease in a xenogeneic model. Following cGMP validation, a trial was conducted with tTreg. tTreg doses up to >30-fold higher compared to that obtained with anti-CD3/28 mAb coated-bead expansion and Foxp3 expression was stable during in vitro expansion and following transfer to patients. Increased expansion did not result in a senescent phenotype and GVHD was significantly reduced.
Discussion
Expansion culture with cGMP aAPC and re-stimulation reproducibly generates sufficient numbers of UCB tTreg that exceeds the numbers of T effector cells in an UCB graft. The methodology supports future tTreg banking and is adaptable to tTreg expansion from HSC sources. Furthermore, since HLA matching is not required, allogeneic UCB tTreg may be a useful strategy for prevention of organ rejection and autoimmune disease.
Keywords: cGMP production, graft versus host disease, regulatory T cell
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative therapy for many types of cancer. Unfortunately, graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following HSCT1,2. Thymic-derived regulatory T cells (tTreg), whose natural function is to prevent autoimmunity by suppressing self-reactive lymphocytes 3–5, may be a useful strategy for preventing autoimmunity as well as graft rejection and GVHD with proof of concept demonstrated in xenogeneic models of disease 6–8. However, therapeutic translation for clinical testing has been hampered by the inability to consistently manufacture sufficient numbers of tTreg.
We recently completed a “first-in-human” clinical trial to assess the safety and efficacy of in vitro expanded UCB tTreg in treating GVHD in patients undergoing allogeneic HSCT 9–12. While adoptively transferred UCB tTreg were safe and showed clinical efficacy in reducing the risk of GVHD, tTreg yields were highly variable with 28% of products not reaching the targeted tTreg cell dose. Based on results in murine models, the optimal number of tTreg would equal or exceed the number of T effector cells in the HSC graft. Therefore, we sought to optimize the expansion of functional tTreg. Toward that end, we developed a more robust expansion strategy, using the K562 cell-line that stably expressed the high affinity Fc receptor (CD64) to allow loading with anti-CD3 for T cell signaling and CD86 for T cell co-stimulation with a single re-stimulation13,14. In addition, we found that UCB tTreg expanded with aAPC were more effective on a per cell basis in vivo than those expanded with anti-CD3/28 beads13.
Here we describe a novel cGMP in vitro expansion protocol for manufacturing large numbers of UCB-derived tTreg, which incorporates a combination of aAPC and re-stimulation that increases yield >30-fold, compared to our initial cGMP method using anti-CD3/28 monoclonal antibody (mAb)-coated beads. In addition, the variability in range of yield declined from >1000-fold to ~8-fold over a range of cell products used for clinical testing. All UCB tTreg products passed lot release criteria and were safely infused into patients.
Methods
UCB tTreg purification
tTreg were purified from UCB units by positive selection as previously described 10. Briefly, frozen UCB were thawed and tTreg enriched using directly conjugated anti-CD25 magnetic microbeads (Miltenyi Biotec) and CliniMACS (Enrichment Program 3.20). The CD25+ cells were volume reduced by centrifugation at 200×g for ten minutes at room temperature, and a sample was sent for flow cytometry to determine purity. UCB units used for developmental research experiments were collected under approved IRB protocol under the American Red Cross Cord Blood Program (D. McKenna). UCB units used for the clinical trial were obtained from the St. Louis Cord Blood Bank. All tTreg UCB units were partially HLA matched with the patient at 4–6/6 HLA loci 10.
UCB tTreg Culture
CD25+ selected cells were washed, re-suspended in culture medium, transferred to the appropriate vessel based upon seeding density and media depth, stimuli added (either anti-CD3/28 beads or anti-CD3 -loaded aAPC, see below), and cultured at 37°C/5% CO2. UCB tTreg culture medium consisted of X-VIVO 15 (BioWhittaker, Walkersville, MD) with 10% heat inactivated human AB serum (Valley Biomedical, Winchester, VA), L-glutamine (Invitrogen), and N-acetylcysteine (American Regent, Shirley, NY). IL-2 (Proleuken®, Chiron Corporation, Emeryville, CA) was added on day 3 (300U/ml final) and re-constituted every 2–3 days for the full volume of media. If the viable nucleated cells (NC)/mL was 0.4 × 106 – 0.6 × 106, only IL-2 was added. If the viable NC/mL was ≤0.4 × 106, then the product was volume reduced to obtain a final concentration of 0.5 × 106 viable NC/ml.
tTreg grow optimally at a concentration of 0.5×106/ml with significantly reduced expansion observed at higher concentrations (e.g. >1.5×106/ml). Therefore, by day +6 to +8, cells were typically transferred to Cell Factories/CellSTACKs (CF/CS; Corning Inc., One Riverfront Plaza Corning, NY, 14831, USA). On day 12±1, UCB tTreg were re-stimulated with either anti-CD3/28 mAb-coated beads [or cryopreserved aAPCs (see below for details) at a ratio of 1:1 (aAPC:nucleated cell)] with IL-2 maintained.
UCB tTreg stimulation
anti-CD3/28 mAb-coated beads were prepared by conjugating anti-CD3 (OKT3, Orthoclone, Janssen-Cilag) and anti-CD28 (CD28.2) to tosylated beads (Dynal Biotech, Oslo, Norway) and were manufactured by Dr. Bruce Levine (Clinical Cell and Vaccine Production Facility; University of Pennsylvania) as previously described 14,15. Beads were washed 3x and added to UCB tTreg at a ratio of 3:1 (bead:cell). aAPC were K562 cells transduced to express the high affinity Fc receptor (CD64) and the endogenous ligand for CD28 (CD86), referred to as KT64/86 14,15. aAPC were obtained from a cGMP-qualified master cell bank. aAPC were cultured for 10–14 days at ≤0.5×106/ml to generate 2–5 × 109 cells total. KT64/86 were then incubated overnight in serum free media (to allow serum antibody to dissociate from Fc receptors), volume reduced, irradiated (≥10,000 cGy from an X-ray source), and loaded with anti-CD3 (OKT3, Miltenyi Biotec). Cleared, irradiated and loaded (CIL) KT64/86 were then frozen down in aliquots of either 25 or 250×106 cells at 12–60×106 NC/ml. KT cells were thawed immediately prior to use with an inoculum of KT64/86 cells at a 1:1 ratio with tTreg in the culture.
Cell washing/Volume reduction
Depending on yield/volume, cells were washed and volume reduced with either a Sorvall centrifuge (1000rpm; 300 × g) for 5 minutes at room temperature (if <500ml) or a COBE 2991 (if >500ml) using the manual mode with the following settings: centrifuge speed: 1500rpm (310 × g), super out rate: 450 mL/min, minimum agitate time: 30 seconds and super out volume: 600 ml. The product is then centrifuged for 3 minutes/spin until the entire product has been volume reduced. For cultures with > 0.6 × 106 viable NC/mL, Treg UCB culture media was added to achieve a cell concentration of 0.5 × 106 viable NC/ml.
Final Clinical Processing
Cells were cultured for a total of 18 +/−1 days and then harvested. The product was volume reduced using the COBE 2991 using the same protocol as above, and then transferred to a 1L Lifecell bag, diluted to 150–200 mL with 5% HSA washed using the COBE 2991. The final product was released for infusion after successful lot release testing (Table III).
Table III.
Comparison of GMP production of UCB tTreg produced with KT64/86 and re-stimulation and a single stimulation with CD3/28 beads.
| aAPC + re-stimulation (n=12) | αCD3/28 bead (n=23) | |
|---|---|---|
| Avg ± SEM (range) | Avg ± SEM (range) | |
| Fold tTreg expansion | 15061 ± 2820 (1352–33645) | 418 ± 98 (13–1796) |
| Total # tTreg (CD4+127-Foxp3+) | 29 ± 13 × 109 (0.8–166 × 109) | 2.4 ± 0.8 × 109 (0.1–7.5 × 109) |
Quality Control/Product Testing
Lot release testing of all clinical products included viability (7-AAD ≥70%), specified phenotype (CD4−CD8+, ≤10%; CD4+25+, ≥60%), Gram stain with no organisms and endotoxin of ≤5 EU/kg. Sterility and mycoplasma testing were verified after release.
UCB tTreg clinical products and patient blood were phenotyped by flow cytometry to determine expression of: CD4, CD127, Foxp3, Helios, CD45RA, CD45RO, CCR7, CD27 and KLRG1. In addition, in vitro suppressive function of UCB tTreg was determined by a carboxyfluorescein diacetate succinimidyl ester (CFSE)-based suppression assay as previously reported 14. Briefly, human peripheral blood mononuclear cells (PBMC) from buffy coats were labeled with CFSE according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and plated at 105 cells per well in 96-well U-bottom plates with graded titrations of expanded Treg (1:2 to 1:32 tTreg:PBMC) in 200μL Treg culture media. Cells were stimulated with anti-CD3 conjugated Dynal Magnetic Beads (Invitrogen, Carlsbad, CA) at a bead:PBMC ratio of 1:1. On day 4, cells were harvested, stained with antibodies to CD8, and the amount of CFSE remaining determined by flow cytometry. Acquired data were analyzed using the proliferation platform in FlowJo software (Treestar Inc., Ashland, OR).
Cryopreservation and thawing of tTregs for assessment of in vitro suppressive function
An important consideration for UCB tTreg quality control was developing freezing/thawing conditions that preserve the phenotype and in vitro suppressive function of UCB tTreg 14. Specifically, UCB tTreg cultures are washed twice and resuspended at 50×106/ml in X-Vivo 15. An equal volume of cold 2x freezing medium (80% human AB serum + 20% DMSO) is added to the cells, and 25×106 cells (i.e. 1 ml) were aliquoted into pre-chilled cryotubes (Nunc), which were immediately transferred to a rate-controlled freezer. After freezing, samples were stored in liquid nitrogen. tTreg were thawed by incubating cryotubes at 37°C until liquid was visible around a solid ice core. Cells were diluted in pre-warmed supplemented X-Vivo 15 (10 ml), layered onto a cushion of 200μl 25% Human serum Albumin (CSL Behring), and centrifuged at 300×g for 10 minutes. Cells were collected from the interface and washed once more before being tested for phenotype and suppressive function.
Xenogeneic model of GVHD
A published xenogeneic GVHD model was used 16. Briefly, T-, B- and NK-deficient NOD/Scid/γc−/− mice (Jackson) were housed in a pathogen-free facility in micro-isolator cages and on day 0, animals were irradiated (200cGray from an X-ray source) and human PBMCs (15×106) were injected with or without expanded UCB tTreg at a tTreg:PBMC of 1:1 (i.e. 15×106 Treg). Mice were assessed for signs of GVHD daily and weighed thrice weekly. To assess in vivo persistence of fresh and frozen/directly thawed tTreg, mice were bled on day 7 and PBMC stained with antibodies to detect the expanded tTreg (CD45, HLA-A2, CD4 and Foxp3). All animal protocols were approved by IACUC at the University of Minnesota.
Patient samples
Patients receiving re-stimulated UCB tTreg products (described in 17) had blood drawn on day 8 and, if at least 200,000 cells were present following phenotyping, aliquots of 200,000 were frozen down for additional analysis. An informative HLA marker (unique to the tTreg UCB unit) was available in 7 of 11 patients, of which additional aliquots were available for 2. The presence of the tTreg product in the peripheral blood (PB) was tracked by flow cytometry, and assessed for purity (CD4+CD127-Foxp3+Helios+) and stage of differentiation.
Statistical analysis
Data were analyzed by ANOVA or Student’s t-test. Survival effects were assessed by Mantel-Cox (Prism 5). Probability (P) values ≤0.05 were considered statistically significant.
Results
Artificial APC increase UCB tTreg expansion compared to anti-CD3/28 mAb-coated beads under cGMP-compatible conditions
We initially compared UCB tTreg expansion using anti-CD3/28 mAb-coated beads and a K562 cell line that had been transduced with the low-affinity Fc receptor (CD32), to which antibodies to anti-CD3 and anti-CD28 Abs were bound 13. Under these low Fc receptor affinity conditions, there would be an increased likelihood for release of aAPC bound anti-CD3 mAb that could result in more variability in expansion properties. Therefore, subsequent studies were performed using a K562 cells which were transduced with the high affinity Fc Receptor (CD64) and the endogenous ligand for CD28 (CD86) (KT64/86) (depicted in Figure S1A) 14,15. This later variant has the added benefit of being able to be frozen as a mAb (OKT3) pre-loaded, irradiated, product; and was subsequently qualified for cGMP use.
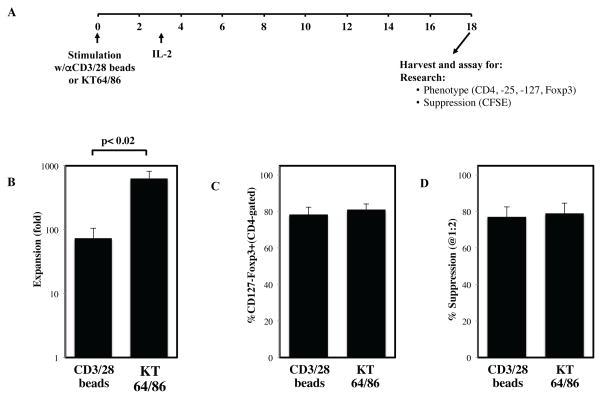
Since K562 cell lines express endogenous co-stimulatory molecules such as 4.1BBL 13, we reasoned that such cell-based aAPC-based stimulation expand UCB tTreg better than anti-CD3/28 mAb-coated. Indeed in prior preclinical studies, we observed enhanced expansion of flow cytometry sorted peripheral blood (PB) tTregs cultured in rapamycin and exposed to KT64/86 cells as compared to mAb-coated bead-based aAPC expansion. In an effort to build on our prior clinical study of mAb-bead expanded UCB tTregs10, we compared UCB tTreg expansion using clinical grade anti-CD3/28 beads to anti-CD3-loaded KT64/86. To simulate clinical production as closely as possible, tTreg were purified from frozen/thawed UCB units in a GMP facility using clinical-grade reagents. Similar to our findings in the previous clinical trial employing anti-CD3/28 bead expanded UCB tTreg, purified tTreg were 87±2% CD4+ and 63±2% of the CD4+ T cells expressed the tTreg phenotype of CD127 (IL7 receptor)neg Foxp3+, markers of tTreg purity 18. Purified UCB tTreg were stimulated with either anti-CD3/28 mAb-coated beads or frozen/thawed anti-CD3 mAb-loaded KT64/86 and were expanded over 18±1 days using culture conditions as used for the clinical trial (Figure 1A). Stimulation with KT64/86 increased UCB tTreg expansion over anti-CD3/28 mAb-coated beads (643±183-fold vs. 73±32-fold; p<0.02), while not adversely affecting phenotype (81±3 vs. 78±4% CD127-Foxp3+) or suppressive function (79±6 vs. 77±6% at 1:2) (Figure 1B–D).
Figure 1. Artificial APC increase UCB tTreg expansion compared to anti-CD3/28 beads.
tTreg were purified from frozen umbilical cord blood units under cGMP conditions using anti-CD25 magnetic beads and CliniMACS and were expanded in vitro with either anti-CD3/28 mAb-coated beads or aAPC (KT64/86). (A) Schematic representation of UCB tTreg culture. (B) Fold tTreg expansion (average ± SEM). (C) Percentage of cultured cells (CD4-gated) that are CD127–Foxp3+ after stimulation. (D) Percent suppression of in vitro, anti-CD3–mediated CD8+ T cell proliferation at 1:2 (tTreg/PBMC) as determined by CFSE dye dilution. From n=3 experiments.
Re-stimulation of UCB tTreg with cell-based aAPCs greatly increases expansion without compromising phenotype or in vitro suppressive function
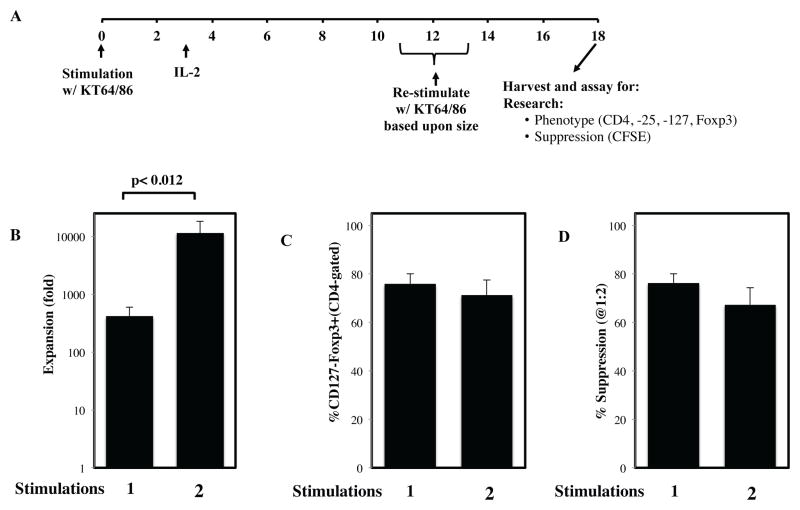
While in vitro tTreg expansion can be greatly increased through re-stimulation, some cultures lose Foxp3 expression and suppressive function, depending on tTreg purity at the time of re-stimulation 14,19. Because previous studies did not use cGMP reagents and were conducted on sorted PB tTreg instead of UCB tTreg, we conducted 4 experiments (2 in a research facility and 2 in a cGMP facility) to determine whether re-stimulation also increased expansion of UCB tTreg cells. We further determined whether such re-stimulation affected Foxp3 expression and suppressive function (Figure 2A). To maximize yield, UCB tTreg were re-stimulated with KT64/86 after they had returned to resting size (≤8.5 mm), which we have shown maximizes CD4+ T cell expansion 14,15.
Figure 2. Re-stimulation of UCB tTreg greatly increases expansion without compromising phenotype or suppressive function.
tTreg were purified from frozen umbilical cord blood units under cGMP conditions using anti-CD25 magnetic beads and CliniMACS and were expanded in vitro with one or two rounds of stimulation with anti-CD3 loaded aAPC (KT64/86). (A) Schematic representation of UCB tTreg culture. (B) Fold tTreg expansion (average ± SEM). (C) Percentage of cultured cells (CD4-gated) that are CD127negFoxp3+ after stimulation. (D) Percent suppression of in vitro, anti-CD3–mediated CD8+ T cell proliferation at 1:2 (tTreg/PBMC) as determined by CFSE dye dilution. From n=4 experiments.
As shown in Figure 2B, re-stimulation increased UCB tTreg expansion 15–30 fold compared to a single stimulation (114422±680 vs. 416±180-fold; p<0.012). Importantly, Foxp3 expression was not significantly decreased in re-stimulated UCB tTreg (71±6 vs. 76±4% of CD4+ cells were CD127-Foxp3+). Re-stimulation also did not affect the in vitro suppressive function of the UCB tTreg (67±7% vs. 76±4% suppression at a tTreg:PBMC ratio of 1:2). Finally, none of the re-stimulated UCB tTreg cultures contained >1.5% contaminating CD8+ T cells, CD19+ B cells or CD14+ macrophages (data not shown).
Cell-based aAPC re-stimulated UCB tTreg maintain in vivo suppressive function
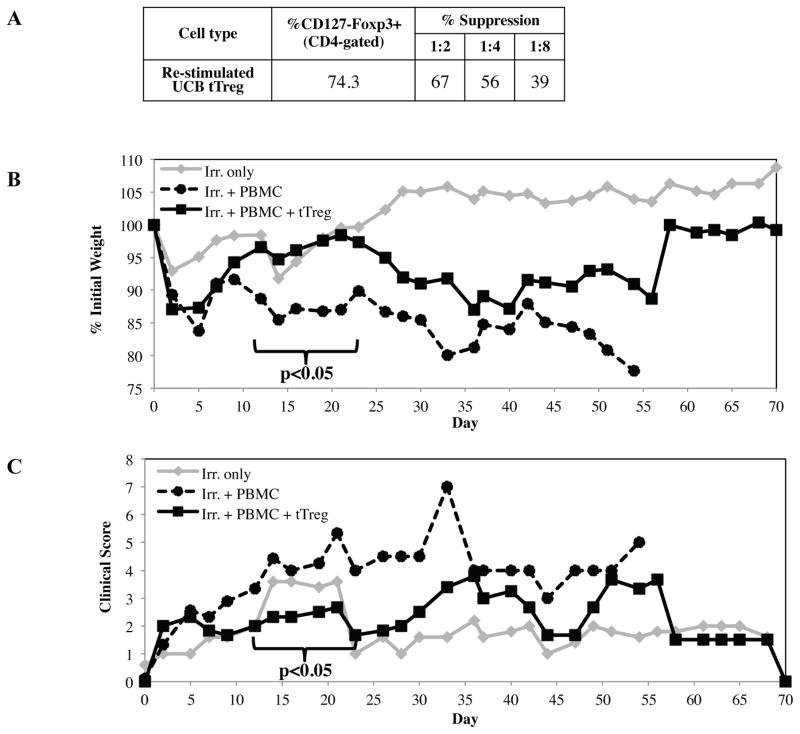
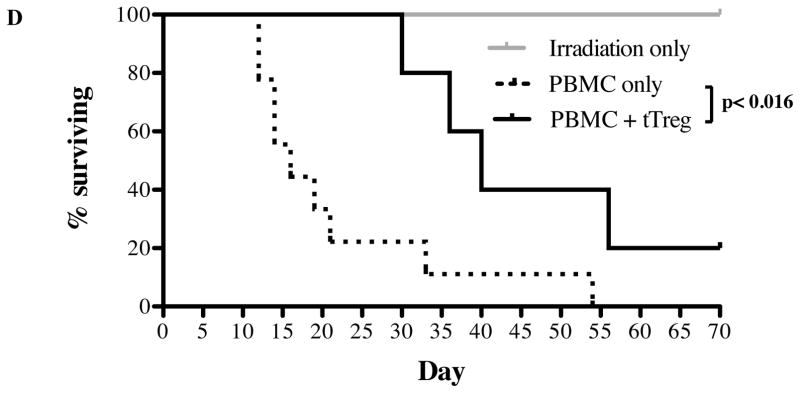
Several groups have reported that tTregs are not terminally differentiated and can be reprogrammed into helper T-cells 20 and effector T-cells (Teff) 21,22, capable of inducing pro-inflammatory cytokines and disease 22. Since our ultimate goal was to use this protocol for clinical products, we tested the safety and efficacy of re-stimulated UCB tTreg cultures in a xenogeneic model of human GVHD. Three experiments were performed with replicate findings. In a representative example shown in Figure 3, mice receiving PBMC only (15 × 106) characteristically lost weight, had increasing clinical GVHD scores, and all succumbed to GVHD by day 54 (median survival of 16 days). In contrast, mice receiving UCB tTreg (1:1 tTreg to PBMC) expanded with KT64/86 and re-stimulation showed decreased disease-associated weight loss and overall clinical pathology between days 12 and 21 (Figure 3B and C; p<0.05 for each day). UCB tTreg treated mice also had a significant increase in survival (p<0.016), with 60% of mice surviving past day 36, compared to 11% for mice receiving PBMC only.
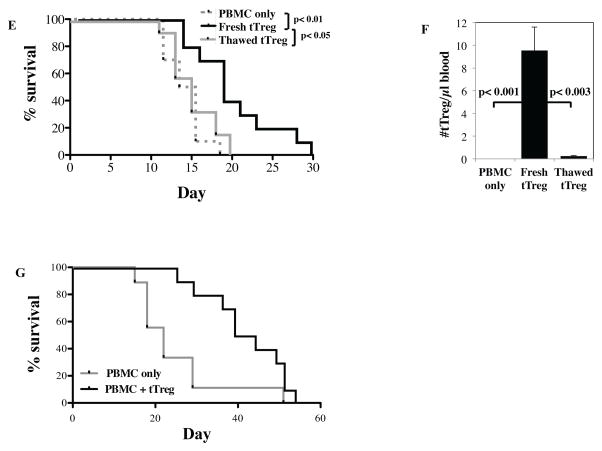
Figure 3. UCB tTreg expanded with aAPC and re-stimulation maintain in vivo suppressive function.
Re-stimulated UCB tTreg (15 × 106 cells) were co-transferred with allogeneic PBMCs (15 × 106 cells) into NOD/Scid/γc−/− mice to assess the ability to ameliorate xenogeneic GVHD. (A) Characteristics of the UCB tTreg cultures that were used. (B) Average weight (percentage of initial) for mice surviving on a given day for different groups of mice (*P < 0.05 for tTreg from days 12 to 23 for UCB tTreg). (C) Average clinical score for mice surviving on a given day for different groups of mice (*P < 0.05 for tTreg from days 12 to 23 for UCB tTreg). (D) Kaplan– Meier survival curves for mice receiving PBMCs only or PBMCs plus adoptive transfer of re-stimulated UCB tTreg. Three independent experiments were performed with re-stimulated UCB tTreg with similar results. Re-stimulated UCB tTreg were either kept in culture or frozen in a rate-controlled freezer and stored overnight. UCB tTreg were processed (washed or thawed/washed), and were co-transferred (15 × 106 cells) with allogeneic PBMCs (15 × 106 cells) into irradiated (50 cGy) NOD/Scid/γc−/− mice to compare their ability to ameliorate xenogeneic GVHD. (E) Kaplan– Meier survival curves for mice receiving PBMCs only or PBMCs plus adoptive transfer of re-stimulated UCB tTreg (n=10). To assess tTreg persistence, animals were bled on day 7 and the number of allotype-specific tTreg/μl blood determined. (G) UCB tTreg were purified, stimulated with KT64/86, cultured for 14 days, and then frozen. After several weeks, tTreg were thawed, re-stimulated with KT64/86, expanded for 7 days and then used as prophylaxis in the xenoGVHD model as described above. Kaplan– Meier survival curves for mice receiving PBMCs only or PBMCs plus adoptive transfer of frozen/thawed/re-stimulated UCB tTreg (n=10).
We and others have shown that frozen/directly-thawed tTreg are significantly less effective than fresh tTreg (Figure 3E and 23). Decreased in vivo persistence could explain the decreased efficacy of frozen/thawed tTreg, so animals were bled on day 6 and tTreg quantitated (Figure 3F). We found that mice receiving frozen/thawed cells had >10-fold fewer tTreg in the PB than those receiving fresh tTreg, suggesting that this is likely the case. To determine whether frozen UCB tTreg regain suppressive function following re-stimulation, in vitro expanded UCB tTreg were frozen down on day 12, and were then thawed, re-stimulated with KT64/86, expanded another 7 days, and were then transferred into NSG mice along with allogeneic PBMC as before. As shown in Figure 3G, thawed/re-stimulated UCB tTreg effectively suppressed xenoGVHD lethality.
Qualification and clinical production of cell-based, aAPC re-stimulated UCB tTreg
To facilitate the use of KT64/86 aAPCs, an efficient approach could entail pre-loading of irradiated cells with anti-CD3 mAb that would be thawed, washed and used. Because tTreg expansion requires more robust T cell receptor (TCR) signaling than that required for Teff, process improvements were necessary to consistently produce lots of KT64/86 aAPC capable of expanding UCB tTreg. A cell size-based prompt for re-stimulation would greatly complicate cGMP production of UCB tTreg, largely due to the need for daily sampling and monitoring. Fortunately, in 8 translational experiments using cell size-based UCB tTreg re-stimulation, a consistent size minima was observed between days 11–13 (data not shown). Therefore, re-stimulation was performed on day 12 ± 1 for all clinical products.
To validate the clinical production of aAPC re-stimulated UCB tTreg, CD25++ cells were purified from a frozen UCB unit using a previously published GMP protocol 10. From 1.7×109 total cells in the unit, 6.3×106 CD25++ cells (yield 0.37%) were isolated and incubated with frozen/thawed KT64/86 cells that had been previously irradiated and loaded with anti-CD3 mAb at a ratio of 1:1 (CD25++:KT). UCB tTreg were expanded at the University of Minnesota GMP facility with GMP compliant reagents using the protocol developed for the clinical trial employing anti-CD3/28 mAb-coated bead expanded UCB tTreg 10. After 12 days, expanded cells were washed, counted and re-suspended with additional frozen/thawed, pre-loaded and irradiated KT64/86 cells at a ratio of 1:1 (CD25++:KT). After 7 days, re-stimulated UCB tTreg were harvested and tested for phenotype, function and potential contaminants.
Table 1 compares the expansion, phenotype and function of the research scale cultures (n=4) and validation culture conducted at the GMP facility, and shows that the validation run would have generated >200×109 UCB tTreg (i.e. ~6×106 purified UCB tTreg x 33,000-fold expansion). The validation product also passed all lot release criteria previously established for anti-CD3/28 bead-expanded UCB tTreg (Table II). Using this protocol, 11 patients with high-risk lympho-hematopoietic malignancy who were receiving a double UCB HSC transplant were treated with increasing doses of in vitro expanded UCB tTreg (from 3- to 100×106 cells/kg) purified from a third UCB donor 17.
Table I.
Comparison of UCB tTreg produced with KT64/86 under research versus cGMP conditions
| Research (n=4) | Validation | ||
|---|---|---|---|
| QC Testing | Avg ± SEM (range) | Avg ± SEM | |
| Fold expansion | # of stim. | 416 ± 179 (178–868) | 897 |
| 1 | |||
| 2 | 11422 ± 6789 (2818–28495) | 33645 | |
| % CD4+ | 92 ± 2% (89–96%) | 91.7% | |
| %CD127-Foxp3+ (CD4-gated) | 71±6% (61–81%) | 85% | |
| % Suppression @1:2 | 67±7% (66–81%) | 79% | |
Table II.
Summary of quality control testing.
| QC Testing | Specification | Validation | |
|---|---|---|---|
| Lot release | TR Phenotype (CD4+/CD25+) | ≥60% | 71.5% |
| % CD4−/CD8+ | ≤10% | 0.9% | |
| Viability (7-AAD) | ≥70% | 98.5% | |
| Gram Stain | No organisms | No organisms | |
| Contaminants | Endotoxin (LAL Method) | <5 EU/kg | <0.05 EU/kg |
Cell-based aAPC expanded UCB tTreg maintain phenotype after adoptive transfer into patients
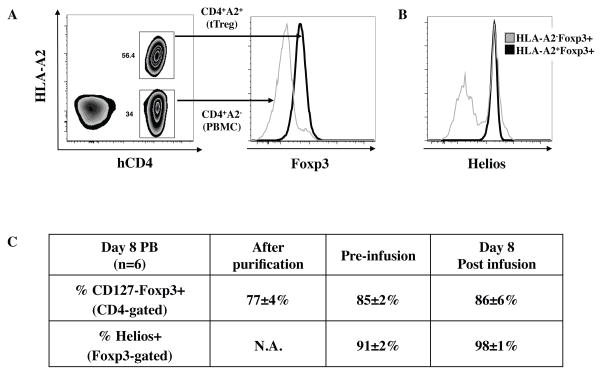
Although tTreg were shown to maintain their phenotype after adoptive transfer in the xenogeneic model of GVHD 13,14, it was still possible that de-differentiation or outgrowth of non-Treg in that model was prevented by external factors (e.g. murine cytokines that do not activate human receptors). Therefore, it was imperative to determine whether aAPC expanded UCB tTreg also maintained phenotype after adoptive transfer into patients. As in the previous trial using anti-CD3/28 bead expanded UCB tTreg 10, we used HLA disparate UCB units (HLA-A2 or HLA-B7) as the source for tTreg and HSC, if possible, which distinguished the contribution from the cultured tTreg lot (tTreg and contaminating cells) and those derived from the UCB hematopoietic cell graft. Of the 11 patients receiving UCB tTreg, 7 were mismatched for HLA-A2 or HLA-B7, and cultured cells were detected in the PB of 6 patients. Since we have previously shown that adoptively transferred UCB tTreg are nearly undetectable in the PB after day 14, tTreg phenotype and relative outgrowth of non-Treg in PB were assessed on day 8. As shown in Figure 4, the only cultured cells observed in the periphery were CD4+ T cells, and no decrease in Foxp3 expression (compared to cultured cells prior to infusion) was seen. Helios is a critical transcription factor expressed by a subset of tTreg that stabilizes Foxp3 expression, and Foxp3-specific deletion of Helios causes tTreg instability and loss of suppressive function24–26. The limited number of tTreg following purification from UCB (6±1×106)10,17 precluded analysis of Helios expression for clinical products. However, previous reports found >90% of Foxp3+ cells in UCB expressed Helios27. We stained for this marker on similarly purified UCB tTreg, and 77±4% of Foxp3+ cells expressed Helios (n=4). In contrast to sorted PB tTreg cultures, which lose Helios expression over time28, >90% of Foxp3+ cells in the six HLA-disparate UCB tTreg cultures remained Helios+ (Figures 4B, C). Importantly, Helios expression was observed in nearly all (98±1%) UCB tTreg detected in the PB 8 days after transfer (Figure 4C). In conclusion, UCB tTreg preferentially express the transcription factor Helios, which marks a stable population of tTreg, and UCB tTreg maintain Helios expression during in vitro expansion and after adoptive transfer into patients.
Figure 4. aAPC expanded UCB tTreg can be detected in vivo, and maintain phenotype.
In order to follow in vitro expanded UCB tTreg after adoptive transfer to patients, UCB units chosen for HSC and tTreg were purposely mismatched at either HLA-A2 or –B7. (A) Representative example showing how re-stimulated UCB tTreg are differentiated from HSC-derived T cells. (B) Representative example of Helios staining in CD4+Foxp3+ T cells derived from UCB used for tTreg culture (HLA-A2−) or HSC (HLA-A2+). (C) Summary of Foxp3 and Helios expression amongst the adoptively transferred UCB tTreg.
Differentiative state of aAPC expanded UCB tTreg products prior to and after adoptive transfer
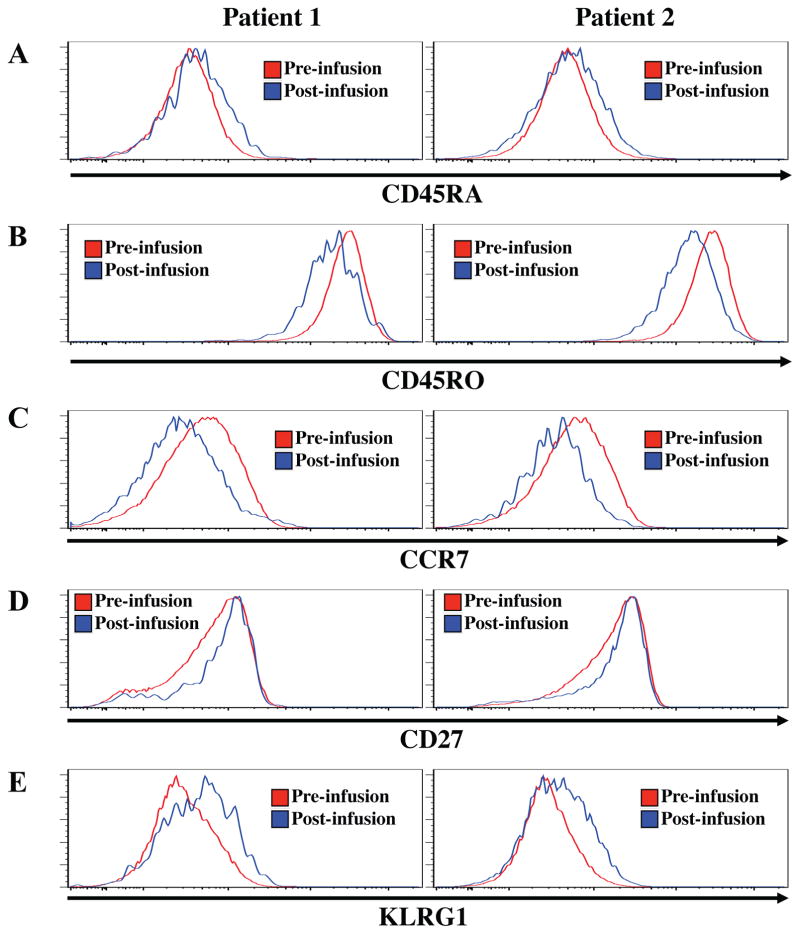
Upon antigen exposure, CD4 T cells differentiate from naïve (CCR7+CD27+CD45RA+), to central memory (CCR7+CD27+CD45RO+) and/or effector memory (CCR7−CD27+/−CD45RO+), and to short-lived effector T cells (CCR7−CD27−CD45RA+) 29–31. With continued antigen exposure, T cells can acquire a senescent phenotype, characterized by the graded loss of co-stimulatory molecules (e.g. CD27) and increasing expression of inhibitory molecules (e.g. KLRG1) 29. Since large-scale in vitro expansion of CD4 and CD8 T cells, as in the context of adoptive immunotherapy, has been linked with senescence and decreased in vivo function 32–34, we sought to determine the differentiative state of UCB tTreg following expansion, and to assess whether further differentiation takes place in the patients following adoptive transfer. Of the 6 patients with detectable HLA-disparate tTreg discussed above, additional aliquots of day 8 PBMC existed for 2 patients. Therefore, the products and day 8 post-UCB PBMC (n=2) were stained with antibodies to CD45RA, CD45RO, CCR7, KLRG1 and CD27 (Figure 5A–E).
Figure 5. Phenotyping the differentiative state of aAPC expanded UCB tTreg.
UCB tTreg were assessed for differentiation antigens directly after in vitro expansion (pre-infusion) and 8 days after adoptive transfer into patients (post-infusion). As in Figure 4, expanded UCB tTreg were identified in vivo by HLA mismatching. Cryopreserved samples of expanded UCB tTreg and day 8 PBMC were stained with antibodies to CD45RA (A), CD45RO (B), CCR7 (C), KLRG1 (D) and CD27 (E) and expression determined by flow cytometry.
CD45RA and CD45RO expression distinguishes naïve and effector cells from central memory and effector memory T cells. As seen in Figures 5A and B, in vitro expanded UCB tTreg expressed CD45RO, and not CD45RA, indicating they are memory Treg. In addition, UCB tTreg in the PB on day 8 maintained this CD45RA/RO expression pattern, indicating that they retain a memory phenotype. As T cells transit from central to effector memory cells, they lose expression of the chemokine receptor involved in homing to secondary lymphoid organs (CCR7). Based upon decreasing CCR7 expression, UCB tTreg appeared to differentiate from TCM after in vitro expansion to TEM following adoptive transfer (Figure 5C). Although tTreg in the PB of patients maintained high expression of CD27 (Figure 5D), tTreg showed low-level expression of KLRG1 suggesting they are on the path to senescence.
Discussion
Despite strong preclinical evidence for the therapeutic potential of Treg cells, clinical translation of this cell type has been slowed greatly because: 1) Treg are rare, and need to be highly purified; 2) Treg are relatively hypo-proliferative compared to non-Treg T cells; and 3) relatively high doses (i.e. up to a 1:1 ratio with transferred non-Treg cells) may be needed for clinical efficacy in allogeneic HSCT patients. Our initial clinical trial using UCB tTreg expanded in vitro with anti-CD3/28 mAb-coated beads and high dose IL-2 was very encouraging but, due to tTreg yield, the maximal Teff:Treg achieved was significantly less than 1:1 (averaging ~1:6). Another predominant limiting factor was the significant variability in product yield which prevented 5 out of 18 (28%) patients from receiving even a relatively low dose of tTreg (i.e. 2x at 3×106/kg) 10. The current study demonstrates that UCB tTreg expansion can be increased almost 100-fold using a cGMP aAPC and re-stimulation. Re-stimulation did not decrease Foxp3 expression or suppressive function, and re-stimulated tTreg maintained the ability to suppress disease in a xenogeneic model of GVHD. In addition, less variability was observed in clinical production using aAPC re-stimulated UCB tTreg compared to those expanded with anti-CD3/28 beads (6- vs. 138-fold), and all products generated >1.3×109 Treg. UCB tTreg are primarily Foxp3+Helios+, which marks a stable subset of Treg 24–26, and this phenotype was maintained throughout expansion and adoptive transfer. In vitro expanded UCB tTreg had a central memory phenotype, and while they exhibited some signs of further differentiation after adoptive transfer, they do not appear to be short-lived TEFF cells or overtly senescent.
Like all therapies, clinical use of ex vivo expanded Tregs is associated with potential risks. Despite early concerns, Treg cellular therapy has not caused any infusional toxicity, and preliminary studies to date have suggested in allogeneic HSCT patients safety with regard to risk of infection, relapse or early mortality 9,10,17. However, all tTreg cultures contain some number of Foxp3neg cells, which have the potential, especially after re-stimulation, to become Teff and exacerbate disease. We have previously shown that ≤1% of Foxp3− cells in the aAPC re-stimulated UCB tTreg clinical products secrete effector cytokines, including IL-2, IL-4, IL-17 and IFNγ 17. Here we show that, following adoptive transfer, Foxp3neg cells did not preferentially expand in the PB.
Another concern surrounding tTreg cellular therapy is the notion of Treg plasticity, whereby expanded Tregs can revert to conventional T cells 35,36. We and others have shown that PB tTreg cultures initiated with even highly purified populations are susceptible to losing Foxp3 expression and suppressive function following re-stimulation 14,19,37. One difference between PB and UCB tTreg is that Foxp3+ Treg purified from UCB uniformly express Helios, a transcription factor required for tTreg stability 25, whereas this marker is only expressed on 50–70% of Treg purified from PB (data not shown). Helios was still expressed on ≥90% of re-stimulated UCB tTreg in the clinical products 17, and this ratio remained constant in the day 8 PB samples.
For therapeutic use, a banking system for UCB tTreg would be able to greatly reduce cost, the time to transplant, and increase consistency. Although frozen/directly thawed UCB tTreg were not effective at suppressing xenoGVHD, we found that frozen/thawed/re-stimulated UCB tTreg did ameliorate disease. Therefore, UCB tTreg re-stimulation can be a convenient banking protocol in that UCB tTreg can be frozen down at the end of their first stimulation (i.e. day 12–14). Aliquots could then be thawed, re-stimulated, and given to patients on day 7, which is also more in line with HSCT preparatory regimen (i.e. chemotherapy) than the full 19(±1)-day expansion protocol. Freezing a portion of the UCB tTreg product prior to re-stimulation could also be used to support multiple UCB tTreg doses, or even as a treatment for acute GVHD. Freeze/thaw/re-stimulation can also be helpful with unexpected disruptions during the HSCT process (e.g. infections), and was used for 1 of the patients on the clinical trial 17.
In conclusion, we present a protocol for in vitro UCB tTreg expansion capable of generating yields of >25 billion cells. Preliminary studies of re-stimulated UCB tTreg were shown to be safe with respect to infusional toxicity in humans receiving a double cord blood HSCT, and they ameliorated disease in a xenogeneic model of GVHD. In addition, this protocol is very amenable for banking UCB tTreg, which would expand its utility for GVHD prevention in the context of HSCT. Finally, since no inter-unit HLA matching was required between the UCB tTreg donor unit and the two UCB HSC graft units, an off-the-shelf allogeneic, ex vivo expanded UCB tTreg may show efficacy in preventing organ rejection and autoimmune disease.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the expertise of the staff of the M Health Clinical Cell Therapy Laboratory and Molecular & Cellular Therapeutics, the cGMP facility of the University of Minnesota and contribution of UCB units from the American Red Cross (research units) and St. Louis Cord Blood Bank (clinical units).
Funding
This work was supported in part by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute contract HHSN268201000008C (J.S.M., D.H.M., K.L.H., J.C., J.E.W.) and grant R01 HL11879 (B.R.B.), National Cancer Institute grants P01 CA65493 (C.G.B., J.S.M., D.H.M., K.L.H., B.R.B., J.E.W.), and P30 CA77598 (J.S.M., J.E.W.), Leukemia and Lymphoma Society Scholar in Clinical Research Award CDP-2417-11 (C.G.B.), Leukemia and Lymphoma Translational Research grant R6029-07 (B.R.B.), and the Earl E. Bakken Charitable Trust and Children’s Cancer Research Fund. This work was also supported in part by NIH P30 CA77598 utilizing the shared resource Flow Cytometry Core from the Masonic Cancer Center, University of Minnesota.
Abbreviations used
- tTreg
Thymic-derived regulatory T cells
- GVHD
graft versus host disease
- UCB
umbilical cord blood
- HSCT
Allogeneic hematopoietic stem cell transplantation
- aAPC
artificial antigen presenting cells
- PBMC
peripheral blood mononuclear cells
- PB
peripheral blood
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- Teff
effector T-cells
- TCR
T cell receptor
Footnotes
Conflict of interest disclosure: The authors share a patent entitled “Methods to expand a T Regulatory Cell Master Cell Bank”; US Patent # 13/639,927.
References
- 1.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363–73. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7:280rv2. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–4. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews Immunology. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008;28:677–84. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- 7.Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Seminars in immunology. 2011;23:462–8. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen KL, McKenna DH, Curtsinger J, McGlave PB, Wagner JE. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol Blood Marrow Transplant. 2013;19:1271–3. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley PJ, Bollard CM, Brunstein CG. Adoptive immunotherapy with the use of regulatory T cells and virus-specific T cells derived from cord blood. Cytotherapy. 2015;17:749–55. doi: 10.1016/j.jcyt.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawitzki B, Brunstein C, Meisel C, Schumann J, Vogt K, Appelt C, Curtsinger JM, Verneris MR, Miller JS, Wagner JE, Blazar BR. Prevention of graft-versus-host disease by adoptive T regulatory therapy is associated with active repression of peripheral blood Toll-like receptor 5 mRNA expression. Biol Blood Marrow Transplant. 2014;20:173–82. doi: 10.1016/j.bbmt.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, Wagner JE, June CH, Riley JL, Blazar BR. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–57. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Science Translational Medicine. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–30. [PubMed] [Google Scholar]
- 16.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1148–57. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, Wagner JE. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044–51. doi: 10.1182/blood-2015-06-653667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–97. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 20.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, Blazar BR, Mellor AL, Munn DH. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–54. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–9. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181:3137–47. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Peters JH, Preijers FW, Woestenenk R, Hilbrands LB, Koenen HJ, Joosten I. Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS One. 2008;3:e3161. doi: 10.1371/journal.pone.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, Cantor H. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350:334–9. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ. Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci U S A. 2016;113:6248–53. doi: 10.1073/pnas.1604765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian M, Lopez-Ocasio M, Metidji A, Rieder SA, Shevach EM, Thornton AM. Helios Controls a Limited Subset of Regulatory T Cell Functions. J Immunol. 2016;196:144–55. doi: 10.4049/jimmunol.1501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayyoub M, Raffin C, Valmori D. Comment on “helios+ and helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans”. J Immunol. 2013;190:4439–40. doi: 10.4049/jimmunol.1390018. [DOI] [PubMed] [Google Scholar]
- 28.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, Shevach EM. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–8. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–95. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–84. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Curr Opin Immunol. 2013;25:556–63. doi: 10.1016/j.coi.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crompton JG, Clever D, Vizcardo R, Rao M, Restifo NP. Reprogramming antitumor immunity. Trends Immunol. 2014;35:178–85. doi: 10.1016/j.it.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol Rev. 2014;257:264–76. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamphorst AO, Ahmed R. CD4 T-cell immunotherapy for chronic viral infections and cancer. Immunotherapy. 2013;5:975–87. doi: 10.2217/imt.13.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–33. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.