Abstract
Caveolae are specialized, invaginated plasma membrane domains that are defined morphologically and by the expression of signature proteins called, caveolins. Caveolae and caveolins are abundant in a variety of cell types including vascular endothelium, glia, and fibroblasts where they play critical roles in transcellular transport, endocytosis, mechanotransduction, cell proliferation, membrane lipid homeostasis, and signal transduction. Given these critical cellular functions, it is surprising that ablation of the caveolae organelle does not result in lethality suggesting instead that caveolae and caveolins play modulatory roles in cellular homeostasis. Caveolar components are also expressed in ocular cell types including retinal vascular cells, Müller glia, retinal pigment epithelium (RPE), conventional aqueous humor outflow cells, the corneal epithelium and endothelium, and the lens epithelium. In the eye, studies of caveolae and other membrane microdomains (i.e., “lipid rafts”) have lagged behind what is a substantial body of literature outside vision science. However, interest in caveolae and their molecular components has increased with accumulating evidence of important roles in vision-related functions such as blood-retinal barrier homeostasis, ocular inflammatory signalling, pathogen entry at the ocular surface, and aqueous humor drainage. The recent association of CAV1/2 gene loci with primary open angle glaucoma and intraocular pressure has further enhanced the need to better understand caveolar functions in the context of ocular physiology and disease. Herein, we provide the first comprehensive review of literature on caveolae, caveolins, and other membrane domains in the context of visual system function. This review highlights the importance of caveolae domains and their components in ocular physiology and pathophysiology and emphasizes the need to better understand these important modulators of cellular function.
Keywords: Keywords: caveolin, caveolae, lipid rafts, blood-retinal barrier, vascular permeability, neuroinflammation, glaucoma, ocular hypertension
1. INTRODUCTION: Caveolae as specialized lipid rafts, a historical perspective
The “nautical” description of cell membranes as a sea of lipids with randomly-distributed protein “buoys” defined by Singer and Nicolson’s fluid mosaic model (Singer and Nicolson, 1972) was a major advance in the understanding of cellular membrane organization. This maritime definition of cell membranes was later expanded (disputed?) to incorporate observations of thermodynamically-stable clusters of lipids (Karnovsky et al., 1982; Lee et al., 1974) that were eventually described formally as “lipid rafts” (Simons and Ikonen, 1997). The lateral heterogeneity of model membranes is essentially without question but the challenge of defining such domains in a cellular context has consumed cell biologists, biochemists, and biophysicists since the raft hypothesis was “floated” (pun intended). The purpose of this review is not to argue the evidence supporting the existence of lipid rafts in cell membranes as this has been reviewed extensively (Carquin et al., 2015; Munro, 2003; Pike, 2006, 2009; Shogomori and Brown, 2003). Instead, we will focus on caveolae, specialized lipid rafts, that by virtue of their ability to be visualized ultrastructurally and to be defined molecularly, have been the best studied and accepted membrane platforms traveling on the lipid seas. We will further focus our discussion on caveolae and their molecular components in the context of vertebrate visual system function and dysfunction.
With the advent of electron microscopy to elucidate cellular ultrastructure, George Palade, in 1953, first described plasma membrane invaginations in capillary endothelial cells, which he referred to as plasmalemmal vesicles (Fig. 1) (Palade, 1953). Shortly thereafter, a similar vesicular structure was observed in epithelial cells and named “caveola intracellularis” or “intracellular cave” (YAMADA, 1955). The appearance of caveolae led to the early postulation that they could be pinched off from the plasma membrane to mediate the transport of fluid or other molecules across the cell, thus representing a mechanism for transcellular capillary permeability (Palade, 1953, 1961). Based on the electron microscopic description, caveolae possess the following morphological characteristics: 1) flask-shaped membrane invaginations that are typically associated with the plasma membrane and occasionally seen with a narrow neck or a diaphragm; 2) typical sizes are of 50–100 nm without an apparent electron-dense coating compared clathrin-coated pits (Palade, 1953; Palade and Bruns, 1968; YAMADA, 1955). Further electron microscopic experiments demonstrated that morphologically identifiable caveolae could be found in almost every cell type, but were particularly abundant in adipocytes, fibroblasts, smooth muscle cells, and endothelial cells (Gabella, 1976; NAPOLITANO, 1963; Palade, 1953). However, they were reportedly absent in certain cell types (lymphocyte cell lines (Fra et al., 1994) and most neurons (Gorodinsky and Harris, 1995)).
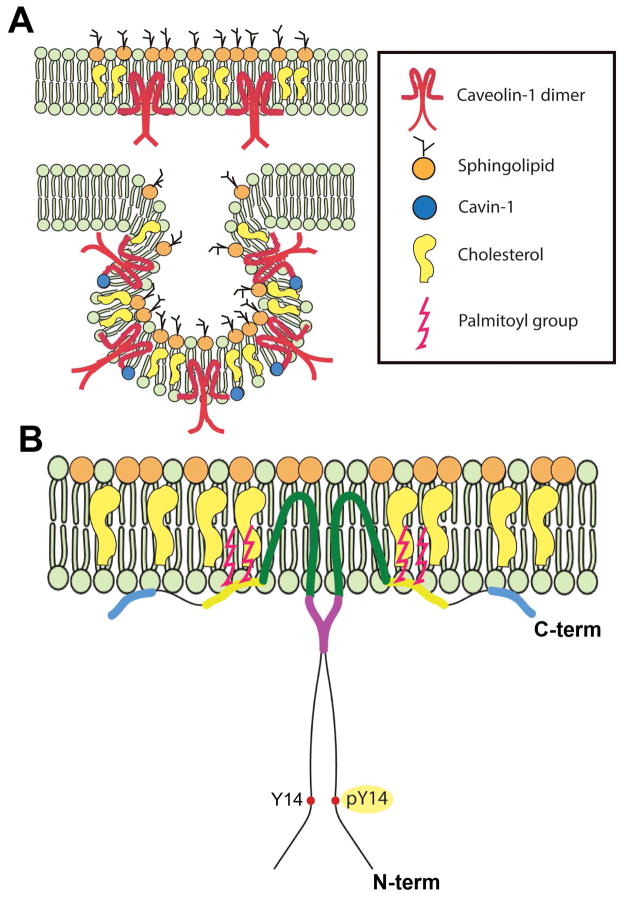
Fig. 1.
Caveolae membrane domains and the signature Cav-1 protein. (A) Illustrations of a planar Cav-1-enriched membrane microdomains with “extracaveolar” caveolin-1 and a typical caveola. (B) Model of the insertion of a caveolin-1 homodimer with the CSD (purple) and the Tyr-14 which can be phosphorylated by several tyrosine kinases.
The initial discovery of caveolae in the 1950’s relied on ultrastructural analyses which did not provide details on their molecular composition. Nearly 40 years passed from their initial identification until the first protein component of caveolae, now known as “caveolin-1” (Cav-1) was identified. Cav-1 was discovered as a phosphorylated substrate with a molecular weight of 22 kDa in an antibody screen of phosphotyrosine-modified proteins in Rous sarcoma virus-transformed chicken fibroblasts (Glenney and Zokas, 1989). Importantly, the authors of this breakthrough noted the concentrated punctate immunolocalization of the protein at cell margins (Glenney and Zokas, 1989). The eventual localization of this 22-kDa protein to morphological caveolae by immuno-electron microscopy resulted in the naming of this protein, “caveolin” (Rothberg et al., 1992) which was subsequently changed to “caveolin-1” after the Lisanti laboratory identified a second caveolin family member (Scherer et al., 1996). Despite the later discovery of cytosolic and secreted forms (Liu et al., 1999; Moon et al., 2014; Tahir et al., 2001), Cav-1 normally behaves as an integral protein embedded as a hairpin loop into the inner leaflet of the lipid bilayer with both the N- and C-termini facing the cytoplasm (Fig. 1) (reviewed in (Cohen et al., 2004; Parton and Simons, 2007)). It contains a cholesterol-binding domain (Murata et al., 1995), a highly conserved caveolin scaffolding domain (CSD) (Li et al., 1996a) and the previously identified tyrosine phosphorylation site which are collectively thought to play important roles in modulating cell signaling. The CSD was originally discovered in an in vitro experiment as a short peptide of 20 amino acids (82–101 residues of Cav-1) (Li et al., 1996a). In a large body of literature (reviewed in (Cohen et al., 2004; Patel et al., 2008)), Cav-1 has been shown to interact with G proteins, receptor tyrosine kinases (e.g., epidermal growth factor receptor, vascular endothelial growth factor receptor), non-receptor protein kinases (e.g., Src family, protein kinase C), other enzymes (e.g., endothelial nitric oxide synthase) and cytoskeletal proteins that localize to caveolae. Importantly, CSD interactions tend to negatively regulate the activities of many associated proteins (Couet et al., 1997a; Couet et al., 1997b; García-Cardeña et al., 1997; Labrecque et al., 2003; Li et al., 1996a; Li et al., 1995; Lisanti et al., 1994a; Lisanti et al., 1994b). These interacting partners share caveolin binding motifs present in their protein sequence: ΦXΦXXXXΦ and ΦXXXXΦXXΦ (or combined ΦXΦXXXXΦXXΦ), where Φ is an aromatic residue (Phe, Tyr, or Trp) and X is any amino acid (Couet et al., 1997a). Caveolae and caveolin-rich domains were proposed to act as cell signaling regulatory platforms (Lisanti et al., 1994a). Although the overarching hypothesis that the CSD is a general regulator of signal transduction has recently been challenged (Collins et al., 2012), there is clear evidence that the CSD is involved in endogenous inhibition of endothelial nitric oxide synthase (eNOS) in vascular endothelium and that mechanical uncoupling of eNOS from Cav-1 results in NO production (García-Cardeña et al., 1997; Yu et al., 2006). Recent evidence using an eNOS-activating CSD mutant (Bernatchez et al., 2011) has allowed for the separation of this CSD signal regulation from the formation of caveolae domains (Kraehling et al., 2015). Besides the CSD, the originally-identified phosphotyrosine modification at Tyr14 provides an additional means for Cav-1 to modulate signaling (Glenney and Zokas, 1989; Li et al., 1996b). The Tyr14 phosphorylation of Cav-1 is tightly regulated in normal cells and may serve as a docking site for SH2 domain signaling proteins (Cao et al., 2002; Lee et al., 2000; Li et al., 1996b).
In addition to Cav-1, two additional caveolin family members, caveolin-2 (Cav-2) and caveolin-3 (Cav-3) were subsequently discovered (Scherer et al., 1996; Tang et al., 1996; Way and Parton, 1996). Cav-2 generally coexists with Cav-1 to form hetero-oligomeric complexes in caveolae (Scherer et al., 1997; Scherer et al., 1996) and Cav-2 requires Cav-1 for proper membrane localization/stabilization such that ablation of Cav-1 results in downregulation of Cav-2 (Razani et al., 2001). The third family member, Cav-3, is exclusively expressed in muscle cells where it is essential for caveolae formation (Tang et al., 1996; Way and Parton, 1996). The discovery that caveolins also bind cholesterol (Murata et al., 1995) and that caveolae are enriched in cholesterol and glycosphingolipids (Ortegren et al., 2004) led to the definition of caveolae as specialized lipid rafts (Fig. 1).
As mentioned above, several lymphocyte cell lines do not express caveolins nor do they possess morphologically identifiable caveolae (Fra et al., 1994). Astonishingly, they were capable of making caveolae when Cav-1 was introduced, suggesting that Cav-1 is a key element for caveolar biogenesis (Fra et al., 1995). The generation of each of the caveolin knockout (Cav-1−/−) mouse lines has unequivocally confirmed that Cav-1 but not Cav-2 is essential for the formation of caveolae in non-muscle cells (Drab et al., 2001; Razani et al., 2001; Razani et al., 2002) and that Cav-3 is essential for making caveolae in muscle cells (Galbiati et al., 2001). However, although Cav-1 is necessary for caveolae biogenesis, it is not sufficient to generate morphologically identifiable caveolae and requires a more recently discovered family of proteins called “cavins” which have not yet received attention in the eye. Cavin-1, originally named “polymerase I and transcript release factor” (PTRF) because of its role in transcription, was subsequently found to be essential to stabilize mature caveolin and form morphologically identifiable caveolae (Hill et al., 2008; Liu et al., 2008). Lack of cavin-1 destabilizes membrane-bound Cav-1 and promotes its lysosomal degradation (Hill et al., 2008; Liu et al., 2008). Therefore, the necessity of caveolins and cavins in caveolae biogenesis is likely critical to understanding why certain types of cells (e.g., Müller glia) do not possess caveolae but still abundantly express Cav-1, likely in planar rafts (Fig. 1) (Nelson et al., 2011; Roesch et al., 2008). In fact, the elucidation of the functions of extra-caveolar caveolins is only recently being realized in the context of cell migration and cancer (Hill et al., 2012; Moon et al., 2014; Nassar et al., 2015; Tahir et al., 2013). Given that Müller glial cells in the retina only appear to express extra-caveolar Cav-1, future studies of Cav-1 function in this cell type could provide fundamental insight into novel Cav-1 functions.
Since their discovery in the 1950s, the functions of caveolae and caveolins have been extensively studied outside of the visual system. In this introduction, we have focused on a historical perspective and several groundbreaking research contributions. For more information on general caveolae functions, the interested reader is pointed to several outstanding reviews that highlight the myriad functions attributed to caveolae/caveolins including lipid trafficking, transcytosis, extracellular matrix (ECM) remodeling, mechanotransduction/mechanoprotection and cell signaling (Cheng and Nichols, 2016; Chidlow and Sessa, 2010; Cohen et al., 2004; Echarri and Del Pozo, 2015; Nassoy and Lamaze, 2012; Parton and del Pozo, 2013; Parton and Simons, 2007; Sotgia et al., 2012). The remainder of this review will focus on putative caveolae/caveolin functions with specific relevance to visual physiology and pathophysiology. We will concentrate broadly on caveolins/caveolae in: 1) the retina and blood-retinal barrier; 2) primary open angle glaucoma and intraocular pressure/aqueous humor outflow; and 3) the ocular surface and lens.
2. Caveolins/caveolae in the neuroretina and RPE
2.1. Localization of caveolins in the adult retina
Caveolins and/or caveolae have been observed widely in retinal vascular cells (Caldwell and Slapnick, 1992; Chen et al., 2007; Feng et al., 1999a; Feng et al., 1999b; Gardiner and Archer, 1986a; Gu et al., 2014a; Opreanu et al., 2011; Raviola and Butler, 1983; Sagaties et al., 1987; Stitt et al., 2000a; Stitt et al., 2000b), Müller glia (Gu et al., 2014b; Hauck et al., 2010; Li et al., 2012; Nelson et al., 2011; Reagan et al., 2016; Roesch et al., 2008), RPE (Bridges et al., 2001; Chen et al., 2003; Chowers et al., 2004; Forbes et al., 2007; Kook et al., 2008; Mora et al., 2006; Omri et al., 2011; Sethna et al., 2016), and photoreceptors (Berta et al., 2011; Boesze-Battaglia et al., 2002; Corley and Albert, 2011; Elliott et al., 2003; Elliott et al., 2008; Kachi et al., 2001; Nair et al., 2002; Senin et al., 2004). In the adult mouse retina, Cav-1 is the major detectable caveolin protein and is abundant in retinal and choroidal vascular beds and in Müller glia with detectable but lower expression in the retinal pigmented epithelium (RPE) (Li et al., 2012)(Fig. 2a). When visualized en face in neuroretinal wholemounts (Fig. 2b), Cav-1 immunoreactivity is apparent in all branches of the vasculature with the most intense immunoreactivity detected in retinal venules (Gu et al., 2014a). The vascular localization is consistent with ultrastructural studies showing caveolae in retinal vascular endothelium and mural cells (Caldwell and Slapnick, 1992; Gardiner and Archer, 1986a; Gu et al., 2014a; Raviola and Butler, 1983; Sagaties et al., 1987) (Fig. 2c). The strong Cav-1 immunoreactivity in Müller glia in the neuroretinal compartment agrees well with transcriptional data indicating that Cav-1 is a Müller cell-enriched transcript compared to retinal neurons (Roesch et al., 2008). Although enriched in Müller glia relative to photoreceptors, Cav-1 protein has been found in biochemical isolates of photoreceptor membranes (Boesze-Battaglia et al., 2002; Elliott et al., 2008; Martin et al., 2005; Nair et al., 2002; Senin et al., 2004) and in isolated photoreceptor preparations where it is preferentially observed in inner segment blebs (Elliott et al., 2003). Consistent with this, Cav-1 has also been found associated with rod photoreceptor ribbon synapses by immunogold EM (Kachi et al., 2001). As discussed later, the function of photoreceptor-intrinsic Cav-1 is unclear as photoresponse deficits resulting from Cav-1 ablation do not seem to result from loss of photoreceptor-intrinsic Cav-1 (Li et al., 2012). Instead, it is more likely that the loss of Cav-1 in photoreceptor support cells (e.g., RPE and Müller cells) contribute to secondary photoreceptor functional deficits (Sethna et al., 2016). Cav-1 expression in native and cultured RPE can support the formation of rare ultrastructurally-identifiable caveolae with both apical and basolateral localizations (Mora et al., 2006) but immunogold localization (Bridges et al., 2001) and later functional studies (Sethna et al., 2016) suggest that Cav-1 may function outside of caveolae in these cells.
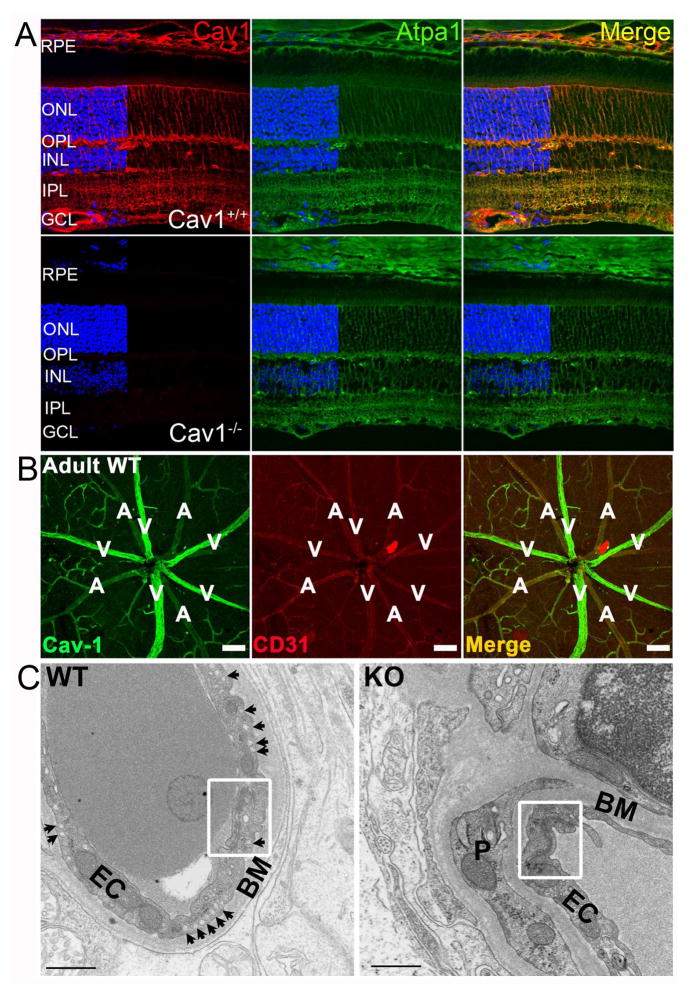
Fig. 2.
Localization of caveolin-1 and caveolae in the murine retina. (A) Retinal cross-sections from Cav-1+/+ (upper panel) and Cav-1−/− mice (lower panel) stained for Cav-1 (red) and the Na/K-ATPase. Cav-1 immunoreactivity is predominantly localized to Müller glia, retinal and choroidal vasculature, and to the RPE (modified from (Li et al., 2012)). (B) Vascular localization of Cav-1 (green) and CD31 (red) revealed in retinal whole mount. Cav-1 is detected throughout the retinal vasculature with enhanced immunoreactivity in large retinal veins. Scale bar = 100 μm; “A”, artery; “V”, vein. (C) Ultrastucture of Cav-1+/+ and Cav-1−/− retinal vessels. Note the numerous abluminal caveolae (arrows in the left panel) and the absence of caveolae in Cav-1−/− vessel. Well-developed tight junctions (white boxes) are visible in both genotypes. BM, basement membrane; EC, endothelial cell; P, pericyte. Scale bar = 500 nm. Panels B and C from (Gu et al., 2014a).
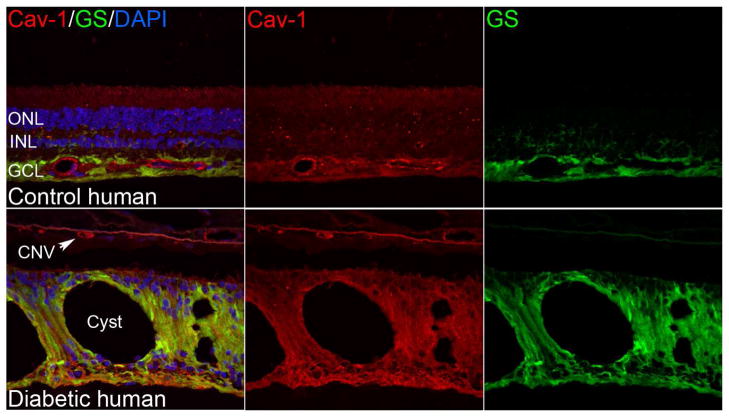
In human retina (Fig. 3), the localization of Cav-1 is consistent with that observed in murine (Li et al., 2012; Reagan et al., 2016) and other species (Berta et al., 2011; Berta et al., 2007b). Immunoreactivity is enhanced in the nerve fiber layer, presumably in Müller glial endfeet (Berta et al., 2007a)(Fig. 3). Of note, Müller glial immunoreactivity is increased in diabetic retina around areas with cystic lesions (Fig. 3). Whether this represents increased expression in the diabetic retina or more efficient immunolabeling is not clear at present.
Fig. 3.
Localization of caveolin-1 and caveolae in control and diabetic human retinae. Like the murine retina, Cav-1 immunoreactivity is detectable in retinal vasculature and Müller glia (labeled with glutamine synthetase, “GS”, in green). In the cystic, diabetic retina in the lower panels Cav-1 immunoreactivity is enhanced in Müller glia and is also present in the choroidal neovascular (CNV) lesion. (Elliott laboratory, unpublished images).
2.2. Developmental expression of caveolins in the retina
The developmental expression of caveolins has been addressed in several important studies. In zebrafish, Cav-1 plays a critical role in development as its depletion by morpholinos resulted in significant reductions of caveolae and profound systemic developmental abnormalities (Fang et al., 2006; Nixon et al., 2007). In the zebrafish eye, Cav-1 silencing resulted in defects in RPE differentiation, eye pigmentation, and ocular cell organization (Fang et al., 2006). This essential role of Cav-1 in eye development in fish is not recapitulated in mammals as Cav-1−/− mice, which also lack caveolae, develop structurally normal RPE and retinae (Li et al., 2012). However, subtle defects in ocular development have not been rigorously tested in Cav-1−/− mice.
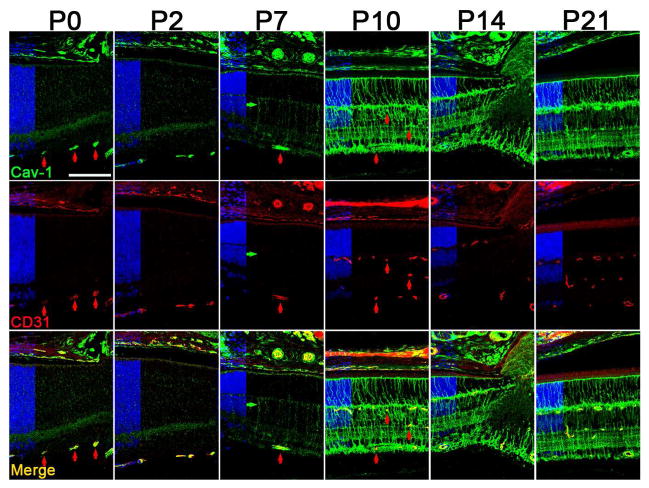
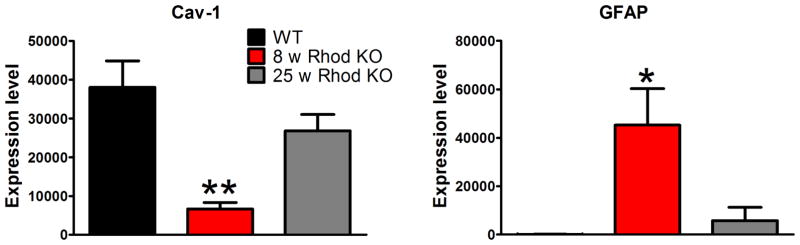
The expression of caveolins during postnatal retinal development has been studied in mice (Gu et al., 2014b; Nelson et al., 2011) and hamster (Berta et al., 2011), respectively, with largely consistent findings. As represented in Fig. 4 (reproduced from (Gu et al., 2014b)), Cav-1 expression at postnatal (P) days 0 and 2 is most pronounced in the newly developing retinal vasculature and in the choroidal vasculature with detectable expression also localized to the RPE at these early timepoints. Cav-1 expression in the mouse RPE declines from P0–P21 consistent with western blotting data from rat RPE (Mora et al., 2006). Expression in the neuroretinal compartment is weak until P7–P10 at which point the localization appears in cells with Müller glial morphology. This coincides with increased expression of glutamine synthetase in these same cells (Gu et al., 2014b). The predominant Müller glial expression of Cav-1 is maintained into adulthood which agrees with the relative enrichment of Cav-1 mRNA in these cells compared to retinal neurons (Roesch et al., 2008). These findings are also consistent with increased expression of Cav-1 and Cav-2 mRNAs at the time when Müller cells terminally differentiate (Nelson et al., 2011). Further evidence that Cav-1 expression is associated with Müller glia differentiation state comes from analysis of expression in gliotic/dedifferentiated cells responding to retinal insult. Following up on their elegant transcriptomic analysis of isolated Müller cells (Roesch et al., 2008), the Cepko laboratory performed gene expression studies of Müller glia at the peaks of rod and cone degeneration in rhodopsin knockout mice (Roesch et al., 2012). Using their raw data, we plotted the expression of Cav-1 and glial fibrillary acidic protein (GFAP), a marker of Müller gliosis (Fig. 5). Intriguingly, Cav-1 mRNAs were dramatically reduced in Müller cells at the peak of rod degeneration and returned to control levels by the peak of cone degeneration. These expression changes negatively correlated with the expression of GFAP. Collectively, these findings imply that Cav-1 is predominantly expressed in differentiated Müller glia and not in cells that have either dedifferentiated or have not yet differentiated. The role that Cav-1 plays in Müller cell differentiation is as yet unexplored and merits rigorous attention.
Fig. 4.
Developmental expression of Cav-1 in the murine retina. Cav-1 (green) is expressed in developing vasculature (colabeled with CD31 in red) as early as P0. In the neuroretinal compartment, Cav-1 expression increases dramatically from P7–P10 in cells with Müller glial morphology. Reproduced from (Gu et al., 2014b).
Fig. 5.
Cav-1 mRNA expression is significantly reduced in isolated Müller glia at the peak of rod degeneration in rhodopsin knockout mice. The Müller gliotic gene, GFAP, shows the opposite pattern of expression. These results suggest that Cav-1 expression is associated with differentiated Müller glia. The gray bar shows expression in Müller glia at the peak of cone degeneration in the rhodopsin knockout model. These data were plotted from the raw data presented in (Roesch et al., 2012).
2.3. Functional consequences of Cav-1 deficiency in the retina
The expression and localization of caveolins and morphological caveolae in the retina suggest that they make important contributions to retinal function. At this time, our working hypothesis was that photoreceptor-intrinsic Cav-1 played an important role in modulating phototransduction either through direct interactions with phototransduction machinery or via control of photoreceptor lipid composition. This hypothesis was based on biochemical and biophysical data showing that phototransduction components either associated with Cav-1 or were present in cholesterol-rich lipid microdomains and that the heterotrimeric G-protein, transducin, was sequestered in such domains in an activity-dependent manner (Boesze-Battaglia et al., 2002; Ding et al., 2008; Elliott et al., 2003; Elliott et al., 2008; Martin et al., 2005; Nair et al., 2002; Senin et al., 2004; Seno et al., 2001; Wang et al., 2008). Earlier work had indicated that the cholesterol content of rod photoreceptor disks is spatially heterogeneous with newly formed, basal disks having a higher cholesterol content than older, apical disks (Boesze-Battaglia et al., 1990; Boesze-Battaglia et al., 1989) and that a higher cholesterol content impairs rhodopsin activation (Niu et al., 2002). Given Cav-1’s established role in cholesterol transport, we predicted that Cav-1 deficiency would perturb photoreceptor lipid content leading to functional deficits.
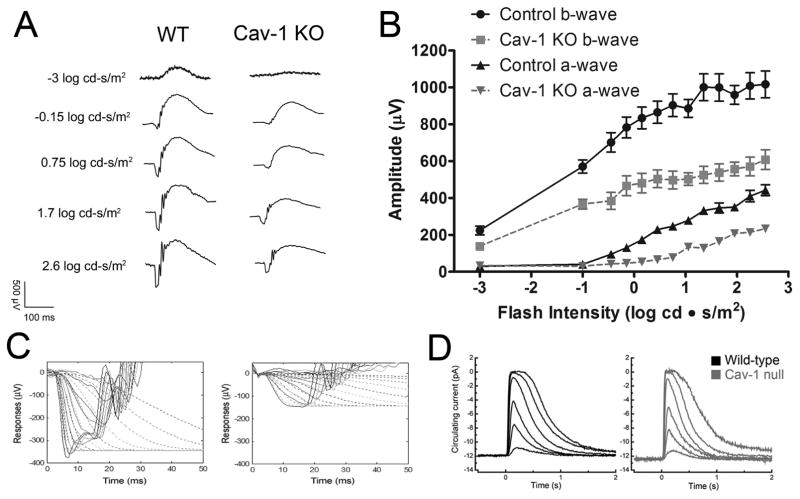
To test this prediction, electrophysiological studies were carried out on Cav-1-deficient mice (Li et al., 2012). As shown in Fig. 6A–C, genetic ablation of Cav-1 results in significant deficits in both a- and b-wave responses as measured by scotopic electroretinography (ERG). To assess rod photoreceptor function in more detail, a computational model of phototransduction (Hood and Birch, 1994) was fit to the leading edges of a-wave responses revealing a significant impairment in phototransduction amplification. Using a second in vivo functional test, manganese-enhanced magnetic resonance imaging, Li et al. (2012) found significantly suppressed ion uptake under dark-adapted conditions in Cav-1−/− mice implying a reduction in photoreceptor dark current. These results supported the initial hypothesis that photoreceptor-intrinsic Cav-1 is important to support photoreceptor function. However, rigorous analyses of photoreceptor structure and biochemistry, including the finding that cholesterol content and fatty acid compositions were indistinguishable between Cav-1−/− and control mice, failed to yield a photoreceptor-intrinsic explanation for reduced retinal function. The penultimate experiment that eliminated a significant contribution of photoreceptor-intrinsic Cav-1 was performed in collaboration with Gordon Fain’s laboratory at UCLA where isolated photoresponses were measured from Cav-1−/− and control rods by the suction electrode method revealing that dark current and flash intensity/response relationships were indistinguishable between genotypes (Fig. 6D). A subtle defect in the dim flash (single photon) response was noted but did not explain the more significant functional deficits revealed by ERG analysis. The observation of functional deficits, in vivo, without significant deficits in isolated rod responses led to the revised hypothesis that photoreceptor-extrinsic Cav-1 is important in the maintenance of a retinal environment conducive to neuronal function.
Fig. 6.
Electrophysiological studies from Cav-1−/− mice. ERG analysis (A–C) revealed significantly reduced photoreceptor a-wave and second order neuronal (b-wave) responses, in vivo. (D) Suction electrode recordings of rod light responses to graded series of flash intensities reveal normal photoresponses from isolated rods. (left panel) Mean current traces in wildtype rods (black traces) to flashes at intensities of 4, 17, 43, 160, 450, and 1122 photons μm−2. The average dark current in wildtype rods was 14.1 ± 0.6 pA (n = 45). (right panel) mean current traces from Cav-1−/− to the same flash intensities. The average dark current in Cav-1 null rods was 12.5 ± 1.1pA(n = 9), not significantly different from wildtype (p = 0.24, Student’s t-test). The flash sensitivity of the Cav-1−/− rods was 0.26 ± 0.03 pA/photon/μm2, not significantly different (p = 0.20) from wildtype rods (0.31 ± 0.02 pA/photon/μm2. These results indicate that retinal function in situ is defective but that the functional deficit is not intrinsic to rods. Reproduced from (Li et al., 2012).
One possibility is that Cav-1 deficiency alters the ionic milieu surrounding photoreceptors, in situ. Ionic and fluid homeostasis in the interphotoreceptor space is largely controlled by transport activities in the apical RPE including the activity of the α1-sodium/potassium ATPase (Na/K-ATPase) (reviewed in (Strauss, 2005)). The Na/K-ATPase contains consensus Cav-1 binding motifs, interacts with Cav-1 in non-ocular cells, and its activity is sensitive to cholesterol (Wang et al., 2004; Yeagle, 1983). In Cav-1-deficient RPE, the Na/K-ATPase has reduced affinity for potassium in the concentration range found in the subretinal space (Li et al., 2012). Thus, the observed retinal functional deficits in Cav-1−/− mice could result from defects in the ability of the RPE to support photoreceptor function. Additional evidence of RPE dysfunction due to specific ablation of Cav-1 in the RPE will be discussed in the next section. The lack of evidence that photoreceptor-intrinsic Cav-1 modulates phototransduction does not eliminate the potential impact of ribbon synapse-localized Cav-1 (Kachi et al., 2001). This could be addressed through rod-specific conditional deletion of Cav-1 but remains an open question.
2.4. The role of RPE-specific Cav-1 in phagolysosomal digestion
The RPE fulfills several critical functions to maintain photoreceptor health and function including epithelial transport, formation of the outer blood-retinal barrier (BRB), maintenance of the retinoid visual cycle, polarized secretion of anti- and pro-angiogenic factors, and removal and clearance of debris from the diurnal shedding of photoreceptor outer segments (POSs) (reviewed in (Strauss, 2005)). RPE cells are post-mitotic professional phagocytes that efficiently engulf and digest large quantities of photoreceptor material in the process of photoreceptor renewal (Young and Bok, 1969). Unique among most epithelia is the bipolar localization of caveolae and caveolins with a significant proportion of caveolae found on the apical, photoreceptor-facing surface (Mora et al., 2006). When cultured RPE cells are challenged with POS material, Cav-1 displays time-dependent increases in expression suggesting a role in the phagocytic process (Chowers et al., 2004). Cav-1 deficiency/impairment in another professional phagocyte, macrophages, reduces their phagocytic capacity (Fu et al., 2012; Li et al., 2005; Rodriguez et al., 2006; Tsai et al., 2011).
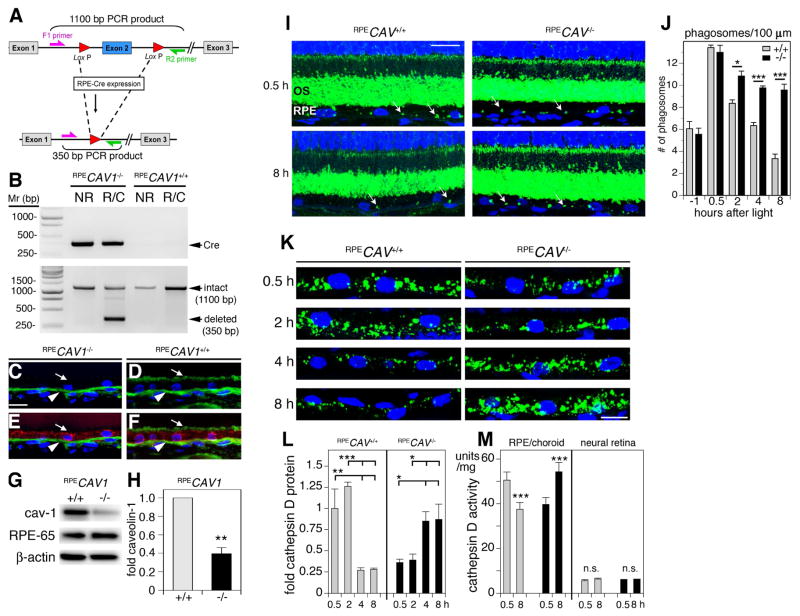
To begin to assess cell-specific Cav-1 functions, in vivo, we recently generated the first conditional, tissue-specific Cav-1 knockout mice including a model with Cav-1 specifically deleted within the RPE (Fig. 7A–H modified from (Sethna et al., 2016)). Ablation of Cav-1 specifically in the RPE resulted in significant reductions in both rod photoreceptor-driven a- and b-wave responses as assessed by ERG (see (Sethna et al., 2016)). The reduction in ERG amplitudes in RPE-specific Cav-1 mutants at least partially explains the photoreceptor-extrinsic functional deficits observed in global Cav-1−/− mice (Li et al., 2012). To better understand the RPE dysfunction induced by loss of Cav-1 in the RPE, we collaborated with Silvia Finnemann’s laboratory to assess the potential role of Cav-1 in RPE phagocytosis. RPE-specific Cav-1 ablation, in vivo, did not affect the RPE’s ability to bind or engulf POS but instead resulted in a delay in phagosome clearance from POS laden RPE (Fig. 7I,J). This delayed digestion resulted from defects in the upregulation and activity of the lysosomal enzymes, cathepsin D (Fig. 7K–M) and β-N-acetyl-glucosaminidase (see (Sethna et al., 2016)) which are essential for POS-opsin digestion and deglycosylation, respectively. Intriguingly, the upregulation of cathepsin D immediately after diurnal POS shedding observed in control mouse RPE, was significantly delayed in RPE with specific Cav-1 ablation. Similar delays in phagosome clearance and upregulation of cathepsin D levels and activity were found in POS-challenged cultured RPE-J cells in which Cav-1 expression was silenced. Overexpression of both wildtype and a CSD mutant of Cav-1 that fails to target to the plasma membrane accelerated POS clearance indicating that Cav-1’s impact on digestion was independent of its scaffolding domain. As chronic accumulation of indigestible debris is pathological to the RPE in conditions such as age-related macular degeneration (AMD), these findings suggest that increasing Cav-1 expression in the RPE could be a therapeutic strategy to facilitate debris removal. These findings may also be relevant to other professional phagocytes in the eye (e.g., trabecular meshwork cells, retinal microglia) but this remains to be studied.
Fig. 7.
RPE-specific deletion of Cav-1 impairs phagosome clearance by RPE cells, in vivo. (A) Schematic representation of Cre/lox mediated deletion of Cav-1 via RPE-specific Cre recombinase expression. (B) Representative gel of PCR products from genomic DNA from neural retina (NR) and RPE/choroid (R/C) from littermate RPE-Cre-expressing and RPE-Cre-negative mice showing Cre (top panel) and caveolin-1 floxed products. The 350 bp CAV1 deletion product (bottom panel) is detected only in RPE/choroid from Cre-carrying mice following doxycyline induction. (C–F) Representative images showing cross sections of RPE/choroid from RPECAV1−/− (C, E) and RPECAV1+/+ (D, F) labeled with caveolin-1 (green; C–F) and RPE-65 (red; E, F). Arrows indicate apical RPE surface showing absence or presence of caveolin-1 in RPECAV1−/− (C, E) and RPECAV1+/+ (D, F), respectively. Arrowhead indicates caveolin-1 signal in the choroid. (G) Representative western blot showing RPE ablation of caveolin-1 in RPE/choroid lysates from RPECAV1+/+ and RPECAV1−/− mice. RPE-65 and β-actin are loading controls. (H) Quantification of experimental conditions as indicated in (G). (I–J) Representative images showing cross sections of retina from RPECAV1+/+ and RPECAV1−/− mice as indicated sacrificed at 0.5 h (top panels) or 8 h (bottom panels) after light onset labeled with opsin N-terminus antibody B6-30 (green) and nuclei counterstain (blue). Arrows indicate POS phagosomes residing in the RPE. ONL, outer nuclear layer; OS, outer segment layer. Bar = 10 μm. (I) Quantification of phagosome content of 100 μm-stretches of RPE counted from images and samples as shown in A. Bars show mean ± s.e.m.; n = 4 mice per group with phagosomes counted in at least 6 images per mouse. Gray bars: RPECAV1+/+ mice, black bars: RPECAV1−/− mice. (K–M) RPE-specific deletion of Cav-1 reverses the diurnal rhythm in activity of phagolysosomal enzymes in the RPE in situ. (K) Representative images showing close-up views of RPE in retina cross sections from RPECAV1+/+ and RPECAV1−/− mice as indicated sacrificed between 0.5 and 8 h after light onset as indicated labeled with cathepsin D antibody (green) and nuclei counterstain (blue). Bar = 10 μm. (L) Quantification of total cathepsin D protein levels in RPE in situ from images and samples as shown in A. Bars show mean ± s.e.m.; n = 4 mice per group with cathepsin D signal quantified in 4 images per mouse. Gray bars: RPECAV1+/+ mice, black bars: RPECAV1−/− mice. M, Comparison of cathepsin D enzyme activity at 0.5 and 8 h after light onset in posterior eyecups enriched in the RPE and neural retina as indicated from RPECAV1+/+ (gray bars) and RPECAV1−/− mice (black bars). Bars show mean ± s.e.m., n = 4 mice per condition. Reproduced from (Sethna et al., 2016).
2.5. Caveolins/caveolae in the blood-retinal barrier (BRB)
The BRB consists of two distinct but analogous interfaces between the neuroretina and the circulatory system. The “inner” BRB is analogous to the blood-brain barrier originally described by Reese and Karnovsky (Reese and Karnovsky, 1967) and is formed by the retinal vascular endothelial cells which have well-developed tight junctions and reduced transcellular transport. The second “outer” BRB is formed by the well-developed tight junctions of the RPE which is adjacent to the fenestrated and inherently “leaky” choroidal vasculature. We will focus our discussion on the inner BRB as unpublished studies from our laboratory using RPE-specific Cav-1−/− mice suggest that caveolae do not contribute significantly to outer, RPE barrier integrity. The roles that caveolae play in the control of retinal vascular permeability has been reviewed extensively in a previous volume of this journal (Klaassen et al., 2013). In this section, we will expand upon this previous review to include additional studies that implicate caveolae in BRB maintenance under basal and pathological conditions. We begin with a general review of the evidence of caveolar participation in endocytosis and transcytosis in non-barrier endothelium before focusing on studies of the BRB.
Caveolae have long been thought to be key players in clathrin-independent endocytosis (reviewed recently in (Cheng and Nichols, 2016)). However, the surprising stability of caveolae at the plasma membrane (Thomsen et al., 2002) and the small proportion of caveolae that actually undergo endocytosis under steady state conditions (Shvets et al., 2015) suggests that their direct contribution to large-scale endocytosis is relatively low. Regardless of their direct contribution, caveolins and caveolae clearly influence clathrin-independent endocytosis as ablation of Cav-1 or PTRF/cavin-1 suppresses this endocytic pathway (Chaudhary et al., 2014). Due to this crosstalk and the relatively small direct contribution of caveolae to endocytosis, a clear understanding of the importance of caveolar endocytosis is as yet incomplete.
Caveolae have also been implicated in transcytosis, a specialized form of endocytosis, to deliver cargoes across vascular endothelia via either caveolae vesicles or transendothelial channels formed by the fusion of caveolae (Ghitescu et al., 1986; Milici et al., 1987; Predescu et al., 1994). There is also evidence that the caveolar pathway can be engaged for rapid transendothelial transport, in vivo (Oh et al., 2007). Furthermore, recent evidence suggests that caveolae transcytosis participates in the early stages of blood-brain barrier breakdown following ischemic injury (Knowland et al., 2014). The contribution of caveolae to transcytosis remains controversial (Cheng and Nichols, 2016; Rippe et al., 2002). If caveolae are key players in endothelial transcytosis, then why are paradoxical increases in vascular permeability widely reported in mice in which Cav-1 is deleted or silenced (Gu et al., 2014a; Miyawaki-Shimizu et al., 2006; Schubert et al., 2002)? One mechanism proposed to explain the paradoxically increased permeability caused by caveolae deficiency is a compensatory increase in paracellular permeability via loss of junctional integrity due to crosstalk between the caveolae and junctional pathways (Komarova and Malik, 2010). Alternatively, the increased permeability could be explained by increased capillary hydraulic pressure due to upstream vasodilation induced by eNOS hyperactivity secondary to caveolae loss (Rosengren et al., 2006).
In the retina, the question of whether caveolae contribute to transendothelial permeability has also been debated. Classical EM studies have revealed that caveolae in the retinal vascular endothelium (like that in the brain) are largely polarized to the abluminal surface facing the basement membrane rendering them less accessible to blood components in circulation (for a qualitative example of this localization, see the left panel of Fig. 2C) (Caldwell and Slapnick, 1992; Raviola and Butler, 1983; Sagaties et al., 1987). In addition, the numbers of caveolae in the retinal vascular endothelium is reduced compared to non-barrier endothelia (Sagaties et al., 1987). Collectively, these results imply that under normal conditions, caveolae in the retinal vascular endothelium are unlikely to support blood-to-tissue transendothelial transport. This is in keeping with the concept that a relatively impermeable BRB is formed by: (1) well-developed endothelial cell junctions and (2) reduced numbers of caveolae that do not participate in transendothelial transport (Raviola, 1977). In fact, when horseradish peroxidase (HRP) was injected into the circulation of several mammalian species, HRP reaction product failed to cross to the endothelial basal lamina (Gu et al., 2014a; Raviola and Butler, 1983) nor was it taken up by morphologically-identifiable caveolae within the endothelium (Gardiner and Archer, 1986b). When HRP was injected intravitreally, HRP reaction product filled the vascular basal lamina and abluminal caveolae (Gardiner and Archer, 1986a; Raviola and Butler, 1983). In one study, HRP appeared within endothelial vesicles as well as rare caveolae open to the lumen suggesting the presence of a caveolae-dependent unidirectional transport from the neuropil to the blood (Raviola and Butler, 1983) but this interpretation was later questioned (Gardiner and Archer, 1986a). Collectively, these results question the contribution of caveolae to transendothelial transport under normal conditions.
What contribution does caveolar transcytosis make to increased permeability during pathological BRB breakdown? As indicated above, ischemic brain injury induces an initial increase in transcellular, caveolae-associated permeability that precedes any paracellular, junctional leakage (Knowland et al., 2014). In the retina, several lines of evidence suggest that caveolae transcytosis is upregulated in pathological conditions such as diabetes. In the early 1980’s, Ishibashi and colleagues examined retinal vascular permeability to HRP in streptozotocin (STZ)-induced diabetic rats and found a dramatic increase in the number of HRP-laden vesicles in the diabetic compared to control group (Ishibashi et al., 1980). An elegant freeze fracture EM study of retinal vasculature from STZ-induced diabetic rats revealed that while the number of luminal plasmalemmal vesicles (caveolae) did not increase, the size of these vesicles was abnormally large and the number of “double vesicles” that appear to represent fused caveolae was increased compared to controls (Caldwell and Slapnick, 1992). Of note, the number of caveolae on the abluminal surface of pericytes did increase dramatically in diabetic animals. In agreement with these earlier studies, the expression of molecular components of caveolae, including Cav-1 (Klaassen et al., 2009) and plasmalemmal vesicle-associated protein (PLVAP, also known as PV-1 or PAL-E antigen), are upregulated in hyper-permeable, diabetic retinal vasculature (Schlingemann et al., 1997; Schlingemann et al., 1999; Wisniewska-Kruk et al., 2014). In cultured retinal microvascular endothelial cells, advanced glycation end-product-modified proteins, which are implicated in diabetic complications, bind to receptors within, and are internalized by, caveolae (Stitt et al., 2000b). In vivo, the delivery of AGE-modified proteins to normoglycemic rats induced BRB breakdown that coincided with increases in endothelial caveolae (Stitt et al., 2000a). Treatment of retinal vascular endothelial cells with vascular endothelial growth factor (VEGF), a potent inducer of vascular permeability (Senger et al., 1983), induces permeability via increased caveolae-associated transcytosis (Feng et al., 1999a). Intriguingly, VEGF receptor-2 (VEGFR-2) is localized to caveolae (Feng et al., 1999b; Labrecque et al., 2003). A recent study shows that retinal vascular endothelium displays polarized responsiveness to VEGF with more pronounced VEGFR-2-mediated responses localized to the abluminal endothelium (Hudson et al., 2014) where caveolae are most abundant. Intraocular injection of VEGF in monkeys resulted in increased vascular permeability that did not result from tight junction opening but instead was associated with a dramatic increase in the number of caveolae on the luminal surface of the retinal endothelium (Hofman et al., 2000). Similar VEGF-induced increases in caveolae numbers have recently been reported in human retinal explants and this correlates with increased PLVAP expression which is not normally expressed in barrier-forming endothelia (Wisniewska-Kruk et al., 2016). Importantly, silencing PLVAP expression blocked this VEGF-induced caveolae formation without reducing the basal numbers of caveolae. Silencing PLVAP, in vivo, also reduced BRB permeability in a model of oxygen-induced retinopathy. Thus, pathological upregulation of PLVAP in retinal vasculature likely increases BRB permeability through upregulation of caveolae transcytosis. Collectively, these results suggest that transendothelial permeability mediated by caveolae is an important contributor to pathological BRB breakdown.
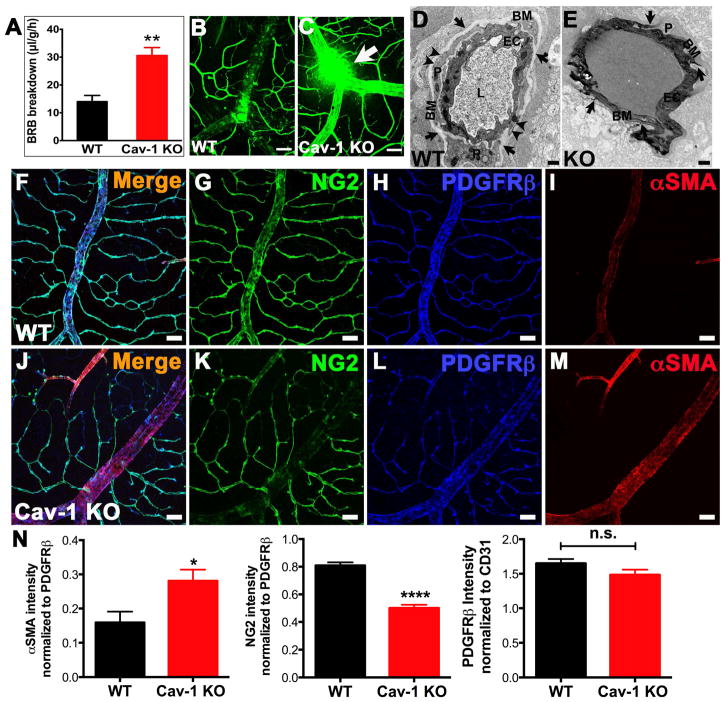
To determine the role of Cav-1/caveolae in the BRB directly, Gu et al. (2014) examined the integrity of the retinal vascular barrier in mice globally deficient in Cav-1. Ablation of Cav-1 resulted in a complete loss of morphologically-identifiable caveolae in the retinal vasculature (see Fig. 2C, right panel). Using several methodologies including measurements of endogenous albumin extravasation and permeability to intravenously delivered contrast agents, a consistent and significant increase in BRB permeability was observed (Fig. 8). These results agree with studies showing increased vascular permeability in non-barrier endothelium from Cav-1−/− mice (Schubert et al., 2002). In retinal wholemounts, where the entire retinal vasculature can be examined, en face, the location of leakage was most pronounced in large retinal veins (Fig. 8C). At the ultrastructural level, we observed extravasation of intravenously administered HRP only in Cav-1−/− vessels that stained the vascular basement membrane and the cytoplasm of Cav-1-deficient endothelium (Fig. 8D,E). In wildtype endothelium, numerous caveolae, completely devoid of HRP reaction product (arrowheads in Fig. 8D), were apparent on the abluminal side of both the endothelium (“EC”) and pericyte (“P”) in wildtype mice and basement membranes were free of HRP reaction product. Although in some cases we observed HRP extravasation at junctional sites in Cav-1−/− vessels, no obvious changes in junction protein localization was observed. These findings suggest that under normal conditions caveolae do not participate in transcellular transport in the retinal vascular endothelium and, in fact, indicated that the absence of caveolae renders retinal vessels hyper-permeable.
Fig. 8.
Loss of Cav-1 results in BRB hyperpermeability (A–E), venous enlargement and a transition of contractile phenotype (increased alpha-SMA and decreased NG2) in mural cells (F–N). Reproduced from (Gu et al., 2014a).
Careful analysis of retinal veins revealed a significant increase in venous caliber (compare Fig. 8F to 8J for example) as well as phenotypic changes in venous smooth muscle cells. These include increased expression of alpha smooth muscle actin (αSMA) (Fig. 8F–N) and a concomitant decreased expression of the mural cell marker, chondroitin sulfate proteoglycan-4 (also known as NG2) (Fig. 8F–N). These results imply that Cav-1 deficiency leads to phenotypic alterations in the contractile properties of venous smooth muscle cells. Currently, we favor the hypothesis that increased BRB permeability in Cav-1-deficient retinal vasculature results from reduced retinal blood flow resulting in venous stasis and subsequent passive leakage from pooled venous blood. The enlarged venous caliber may result from blood engorgement due to venous stasis and suggests that the increase in contractile phenotype of venous smooth muscle cells may be a compensatory response to venous engorgement. It is becoming increasingly apparent from work outside the eye that caveolae regulate resistance vessel autoregulation in part through regulation of eNOS activity (Murata et al., 2007; Yu et al., 2006). Loss of Cav-1 results in eNOS hyperactivity and consequent dilation of resistance vessels. Surprisingly, although eNOS hyperactivity is expected to reduce myogenic tone and, consequently, systemic blood pressure, the mean arterial pressure in Cav-1−/− mice is not different from controls (Rosengren et al., 2006). This apparent paradox is reconciled by the finding that small arterioles in Cav-1−/− mice are more responsive to adrenergic stimulation than controls which compensates for the reduced myogenic tone resulting from eNOS hyperactivity (Albinsson et al., 2007). In the retina, this presents a dilemma as retinal arterioles lack autonomic innervation and thus rely on intrinsic mechanisms such as myogenic autoregulation to regulate retinal blood flow (reviewed in (Kur et al., 2012)). Thus, the retinal vasculature is likely to be intrinsically more reliant on caveolae-regulated myogenic control and thus more sensitive to caveolae deficiency than systemic vasculature. This hypothesis is consistent with our current data and merits more attention given the association of CAV1 polymorphisms with primary open angle glaucoma (POAG) and the potential of vascular tone dysregulation to play a role in POAG (Kang et al., 2014; Pasquale, 2016).
2.6. Caveolins/caveolae in choriocapillaris transport
The role of caveolae in transcellular transport across the choroidal vasculature has not been as well studied as that in the inner retinal vasculature. Although caveolae are observed in fenestrated vascular beds, including the choroid (Bernstein and Hollenberg, 1965; Gardiner and Archer, 1986b), caveolae are not necessary for fenestrae formation (Sorensson et al., 2002). An early HRP tracer study suggested that caveolae do not participate in transendothelial transport across the choroid and that such transport would be unnecessary due to the rapid exchange pathway provided by choroidal fenestrae (Gardiner and Archer, 1986b). However, while HRP, by virtue of its relatively small size (40 kDa, Einstein-Stokes radius of 30 Å), can readily pass through fenestrae of the choriocapillaris, larger molecules, such as albumin, hemoglobin, and lactoperoxidase are largely restricted from passage (Pino, 1985; Pino and Essner, 1981). Thus, a recent study from the Lutty laboratory directly assessed the role of caveolae in regulated transendothelial transport of albumin across the choriocapillaris (Nakanishi et al., 2016). They found that the absence of caveolae in Cav-1-deficient mice dramatically reduced the transport of albumin into the outer retina. These results have implications for AMD as a previous quantitative mass spectrometric analysis revealed a significant reduction in both Cav-1 and PLVAP protein abundances in Bruch’s membrane/choroid complexes from AMD donor eyes compared to age-matched controls (Yuan et al., 2010). Given the crucial role caveolae play in lipoprotein transport (Frank et al., 2009) and that lipoprotein deposits in the Bruch’s membrane/choroid complex are a feature of AMD pathogenesis (Curcio et al., 2010), the role that caveolae transendothelial transport plays in AMD merits further study.
2.7. Caveolar transcytosis: A route for retinal drug delivery?
Delivery of systemically-administered drugs to central nervous system locations that reside behind a tight barrier remains a major pharmacological challenge. Given their role in endothelial transcytosis, caveolae represent attractive routes to bypass blood-CNS barriers. Groundbreaking work from Jan Schnitzer’s laboratory demonstrated the power using caveolae for targeted delivery to the lung. They originally screened and identified candidate molecules (e.g., aminopeptidase-P) that were specific components of lung endothelial caveolae (Oh et al., 2004) and subsequently demonstrated rapid and lung-specific uptake of intravenously delivered antibody against aminopeptidase-P (Oh et al., 2007). Although a similar targeting strategy has not been used in the CNS, phosphodiesterase 5 (PDE5) inhibitors have been found to improve drug delivery to metastatic brain tumors, in part, via caveolae-mediated transport (Hu et al., 2010). Other mechanism to bypass the BRB have been tested, including transient disruption of endothelial tight junctions (Campbell et al., 2011; Campbell et al., 2009), but, to our knowledge, hijacking caveolae transcytosis has not yet been attempted. A potential hurdle to utilizing caveolae targeting in the retinal vasculature is the relatively low number of caveolae that face the luminal endothelium surface (Gardiner and Archer, 1986a; Raviola, 1977; Raviola and Butler, 1983; Sagaties et al., 1987). However, as the number of luminal endothelial caveolae in retinal vasculature may increase under pathological conditions (Hofman et al., 2000), targeting caveolae for ocular drug delivery may still be worth pursuing.
2.8. Caveolins/caveolae and retinal inflammation
As the first line of host defense, the innate immune system uses pattern recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs) present on the surfaces of foreign invaders, to elicit rapid host immune responses to resolve the invasion (Janeway and Medzhitov, 2002). One class of PRRs are Toll-like receptors (TLRs), which are transmembrane proteins with an extracellular leucine-rich repeat (LRR) domain and an intracellular Toll/IL-1 receptor (TIR) domain (Medzhitov, 2001). The TIR domain is also found in MyD88 and TIR domain-containing adaptor protein (TIRAP), which both function as adaptor proteins in TLR signaling pathways (Akira et al., 2001; Kawai and Akira, 2010; Kumar et al., 2009; Medzhitov, 2001). Gram-negative bacterial lipopolysaccharide (LPS) is a classical PAMP that is recognized and bound by TLR4 to initiate inflammatory signaling (Beutler and Rietschel, 2003). The binding of TLR4 and LPS recruits MyD88 through the TIR domain to initiate signaling via the MyD88-dependent pathway, culminating in the early-phase activation of NF-κB (Akira et al., 2001; Medzhitov, 2001). Alternatively, the complex is endocytosed into endosomes where it triggers the TRIF-dependent pathway that signals to induce late-phase NF-κB activation (Akira et al., 2001; Kawai and Akira, 2010). NF-κB activation is required for the production of a battery of inflammatory cytokines (Akira et al., 2001; Beutler and Rietschel, 2003; Kawai and Akira, 2010; Kumar et al., 2009; Medzhitov, 2001). Notably, the TRIF-dependent pathway additionally activates IRF3 to induce type I interferon expression (Kawai and Akira, 2010).
There is a growing body of evidence that caveolins and caveolae can regulate innate immune responses (Chidlow and Sessa, 2010; Jin et al., 2011). Importantly, TLR4 (and other TLRs) contain a consensus Cav-1 binding motif in the TIR domain suggesting that Cav-1 could directly modulate TLR function (Wang et al., 2009). In the immune system, caveolins are widely expressed in myeloid cells and have been best studied in macrophages where they are important for monocyte/macrophage differentiation (Fu et al., 2012), inflammatory signaling (Tsai et al., 2011; Wang et al., 2009), and phagocytosis (Li et al., 2005). However, no simple picture has yet evolved to indicate whether caveolin expression generally promotes or suppresses inflammation.
Caveolins seem to exert cell-specific inflammatory regulatory properties best exemplified in several elegant studies of acute lung injury (reviewed in (Jin et al., 2011). In a model of pulmonary sepsis, Garrean and colleagues (Garrean et al., 2006) found that Cav-1-deficiency blunted the NF-κB response to systemic lipopolysaccharide (LPS) challenge. Cav-1−/− mice challenged with LPS showed attenuation of immune cell infiltration, microvascular leakage, and edema and had reduced LPS-induced mortality compared to controls. In lung alveolar cells, overexpression of Cav-1 aggravated LPS injury and increased the production of the TLR4-induced, pro-inflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) providing positive evidence that Cav-1 promotes TLR4 signaling (Lv et al., 2010). Two non-exclusive mechanisms have been proposed to describe how Cav-1 promotes TLR4 signaling in pulmonary inflammation: (1) an indirect, nitric oxide-dependent mechanism whereby eNOS hyper-activity secondary to loss Cav-1 induces nitration of TLR4 signaling components and suppression of downstream signaling (Mirza et al., 2010); (2) a direct effect whereby tyrosine phosphorylated Cav-1 interacts with TLR4 and promotes the assembly of the TLR4-MyD88 signaling complex (Jiao et al., 2013). Surprisingly, in RAW264.7 macrophages, Cav-1 seems to have an opposite effect on TLR4 signaling where it acts as a pro-inflammatory effector in immune cells (Wang et al., 2009). This effect occurs through the direct interaction of Cav-1 with the TIR domain which blocks the formation of the TLR4-MyD88 complex. Thus, the combined effect of anti-inflammatory action in tissue and pro-inflammatory effects in immune cells clearly illustrates the importance of understanding caveolin/caveolae inflammatory regulation in cell-specific contexts to determine if Cav-1 might represent a therapeutic target for inflammatory disease.
In 2010, Hauck and colleagues published results of an elegant quantitative proteomic screen of differentially-expressed retinal membrane proteins in equine autoimmune uveitis (Hauck et al., 2010). Cav-1 was found to be upregulated by a remarkable 16-fold in uveitic versus healthy retinas. The uveitis-induced upregulation was found predominantly in Müller glial cells which may, in part, reflect their relatively high levels of Cav-1 expression and their abundance in the retina (Reagan et al., 2016). The expression of PTRF/cavin-1, which is necessary along with Cav-1 to build caveolae (Hill et al., 2008), is dramatically increased in uveitis. This suggests that Müller cells may acquire the ability to build caveolae under pathological conditions but this remains to be determined. The dramatic upregulation of caveolae components in uveitis implies that retina-intrinsic Cav-1 may play a role in chronic inflammation in the retina.
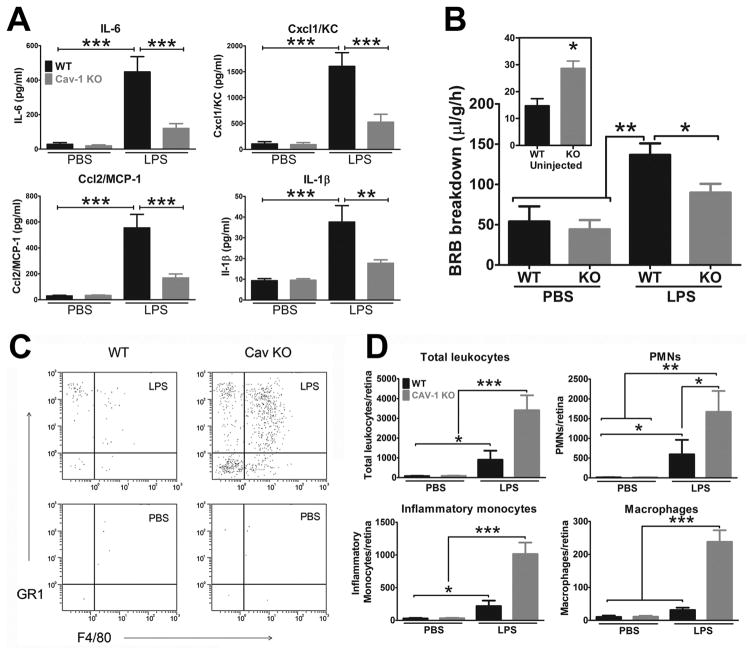
Given the results in the lung inflammation model and the intriguing suggestion that Cav-1 may be induced during retinal inflammation, we set out to interrogate the Cav-1-TLR4 axis in the endotoxin-induced uveitis model (Li et al., 2014). Similar to the pulmonary sepsis model, we found that global Cav-1 deficiency significantly blunted the production of pro-inflammatory cytokines in the retina after intraocular delivery of LPS (Fig. 9A). In naïve, uninjected eyes, BRB permeability was increased in Cav-1−/− eyes in agreement with previous results (Gu et al., 2014a). However, while delivery of LPS to wildtype eyes induced dramatically increased permeability, this response was largely blunted in Cav-1−/− mice (Fig. 9B). Thus, the presence of Cav-1 promotes inflammatory signaling and pathological BRB breakdown, in vivo. It is unclear if the reduction in BRB permeability results from inflammatory suppression or from the lack of ability to upregulate caveolae transcytosis under inflammatory conditions (Knowland et al., 2014). To our surprise, we observed a paradoxical increase in recruitment of innate immune cells to the retina even in the context of significant suppression of pro-inflammatory cytokine production (Fig. 9C,D). The explanation behind this paradox is not yet understood but we speculate that the pro-inflammatory effects of Cav-1 deficiency in immune cells (Wang et al., 2009) and systemic immune dysfunction may contribute. This is supported by increased numbers of circulating immune cells in Cav-1−/− mice even without LPS challenge (Fu et al., 2012; Li et al., 2014). Alternatively, venous stasis or vascular endothelial dysfunction from Cav-1 ablation may allow more efficient immune cell extravasation even in the context of the blunted cytokine response. Understanding this paradox is key to the development of rational, Cav-1-targeting therapeutics to modulate local immune responses.
Fig. 9.
(A) Cav-1 deficiency suppresses LPS-induced pro-inflammatory cytokine production. (B) Although basal BRB permeability is higher in Cav-1−/− retinas (inset), inflammatory BRB breakdown induced by LPS is significantly reduced with Cav-1 deficiency. (C–D) Flow cytometry shows that immune cell infiltration in the retina in response to LPS is paradoxically increased in Cav-1−/− retinas. Reproduced from (Li et al., 2014)
The suppression of local cytokine production that results from Cav-1 deficiency could also impact retinal neuroprotection. Interleukin-6 family cytokines (e.g., ciliary neurotrophic and leukemia inhibitory factors) are potent neuroprotective cytokines in the retina that mediate protection through the gp130/STAT3 signaling pathway (Ueki et al., 2009; Wen et al., 2012). Retina-specific Cav-1 deficiency results in suppression of STAT3 phosphorylation following retinal injury (Reagan et al., 2016). The blunted STAT3 response likely results from suppressed IL-6 family cytokine production rather than an effect of Cav-1 on the gp130/STAT3 pathway as STAT3 activation in response to exogenous IL-6 family cytokine stimulation is not affected by Cav-1 ablation. This highlights the possibility that endogenous PAMPs produced during retinal injury may activate TLR4 in the absence of pathogen and that Cav-1 may modulate this response.
The retinal cell type(s) in which Cav-1 might modulate TLR4 signaling are not yet identified. Likely cellular candidates include microglia, astrocytes, Müller glia, and RPE, all of which are reported to express TLR4 (Jiang et al., 2009; Karlstetter et al., 2015; Kindzelskii et al., 2004; Kumar and Shamsuddin, 2012). Although TLR4 is expressed in retinal astrocytes and microglia, we have not observed detectable Cav-1 immunoreactivity in either cell type in retinal flatmounts even following LPS activation (unpublished observation). Thus, at present we favor Müller glia and/or RPE, both of which express both Cav-1 and TLR4, as the cell types in which Cav-1 may modulate TLR4 signaling. Our newly developed tissue-specific conditional Cav-1−/− mice should help us to answer this question in future studies.
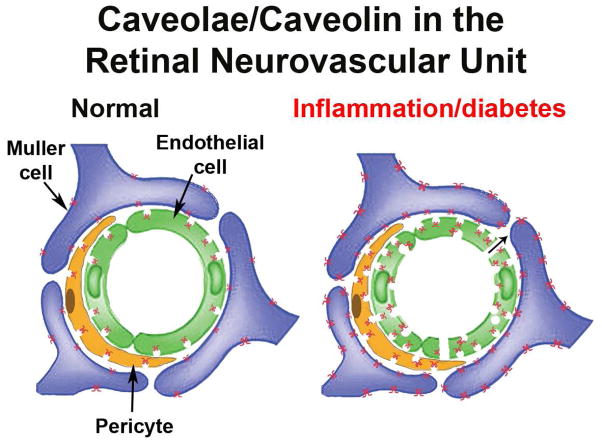
As demonstrated in the previous sections, caveolins and caveolae make several important contributions to retinal inflammatory signaling and BRB maintenance under both normal and inflammatory conditions. As shown in the model in Fig. 10, under normal conditions caveolae are predominantly localized to the abluminal side in both vascular endothelium and pericytes/smooth muscle cells and Müller glia are decorated with extra-caveolar caveolin-1. Under these conditions, caveolae do not contribute significantly to transendothelial transport and the BRB is essentially impermeable. Upon inflammation (e.g., diabetic insult, uveitis, ischemic injury), caveolin-1 expression is increased in all of the cell types of the neurovascular unit, caveolae numbers increase and their localization in vascular cells becomes more bipolar. The permeability of the BRB increases via both transcellular and paracellular pathways and the Müller glia with increased caveolin-1 expression can potentiate TLR signaling and pro-inflammatory cytokine release.
Fig. 10.
Illustration of Cav-1/caveolae in the neurovascular unit of normal and inflamed retinal vasculature. Under normal conditions, caveolae show a predominant abluminal localization in vascular endothelium (green) and mural cells (orange). Cav-1 protein, but no caveolae are detectable in Müller glia (purple). During inflammatory conditions (e.g., diabetic retinopathy), caveolae increase in number and show bipolar localization in both endothelial and mural cells possibly promoting transcellular permeability. Cav-1 expression in Müller glia is dramatically increased.
3. Caveolins/caveolae in primary open angle glaucoma (POAG) and intraocular pressure (IOP)
3.1. Introduction to POAG, IOP regulation, and aqueous outflow
There are several excellent recent reviews on glaucoma risk factors, genes, and IOP control and the interested reader is pointed to these (Janssen et al., 2013; Nickells et al., 2012; Stamer et al., 2015; Wang and Wiggs, 2014). In this review will briefly introduce a few important concepts before focusing on recent evidence of the genetic and functional links of caveolins/caveolae to POAG. Glaucoma is a complex group of diseases, with POAG being the most prevalent, that is a major cause of blindness (Quigley and Broman, 2006) resulting from progressive damage to the optic nerve and loss of retinal ganglion cells (reviewed in (Nickells et al., 2012)). Although the vision-compromising insult impacts retinal neurons, the primary risk factor for POAG is IOP(Janssen et al., 2013) and lowering IOP is currently the primary therapeutic strategy even in the case of normal tension glaucoma (Anderson and Study, 2003).
IOP is maintained by the balanced production of aqueous humor from the ciliary body and its drainage from the eye by two pathways, the conventional and uveoscleral outflow tracts (Tamm et al., 2015). The conventional outflow pathway consists of the trabecular meshwork (TM) and a unique drainage vessel called the Schlemm’s canal (SC) (Kizhatil et al., 2014; Tamm, 2009). The conventional outflow pathway is pressure-dependent and thus maintains IOP in a normal range by varying its resistance to aqueous outflow in response to IOP fluctuations. Conventional outflow is the major pathway for normal drainage and is the pathological site of ocular hypertension in POAG making it a key target for the development of IOP-lowering therapeutics (Stamer and Acott, 2012). The TM is anatomically organized into three regions: the innermost uveal meshwork (close to the anterior chamber), the middle corneoscleral meshwork and the deep juxtacanalicular connective tissue (JCT; adjacent to the endothelial lining of Schlemm’s canal). Unlike the uveal and corneoscleral regions that are formed of regular trabecular lamellae, the JCT is a loose connective tissue with scattered cells surrounded by irregular fibrillar extracellular matrix (Tamm, 2009; Tian et al., 2000). Given that the uveal and corneoscleral tissues are highly porous (thus providing little resistance to aqueous humor outflow), it is thought that IOP originates from resistance at the JCT and/or the endothelial lining of SC (Overby et al., 2009; Tamm, 2009). A unifying hypothesis for how this region of tissue generates resistance has been proposed that takes into account fluid dynamics, cellular organization and biomechanics (Overby et al., 2009). The inner wall of the SC (the wall closest to the anterior chamber) and the JCT are subject to large and fluctuating mechanical loads and must have mechanisms to withstand these loads and alter outflow resistance to maintain IOP. Likely as a result of the large gradient associated with aqueous outflow, the inner endothelial lining of the SC can generate large (0.1 to 2 μm) giant vacuoles in which the SC endothelium is stretched thin and frequently forms pores that allow aqueous humor egress (Overby et al., 2014). Pore density is lower in glaucoma patients (Johnson et al., 2002) and the dynamic process of pore formation is impaired in glaucomatous SC cells (Overby et al., 2014). Pathological outflow resistance in POAG is also likely the result of extracellular matrix remodeling in the TM (recently reviewed in (Vranka et al., 2015)).
3.2. Genetic studies associating CAV1/2 loci with POAG and IOP
Among the multifactorial risk factors for POAG there are clear genetic risk variants. Genome-wide association studies (GWAS) by genotyping single nucleotide polymorphisms (SNPs) have emerged as powerful, efficient, unbiased tools to identify disease-associated genes in complex diseases, such as POAG (Fan et al., 2006; Hirschhorn and Daly, 2005). Using this method, Thorleifsson et al. (2010), located a common risk variant (rs4236601) for POAG to chromosome 7q31, near a region that encodes CAV1 and CAV2 (Thorleifsson et al., 2010). This association was originally made in a large cohort from Iceland and confirmed in additional cohorts of POAG patients and controls from Sweden, United Kingdom, Australia, and China (Thorleifsson et al., 2010). The association of rs4236601 with POAG has been replicated in several subsequent studies and new SNPs in the same genetic region have also been associated (Huang et al., 2014; Loomis et al., 2014; Rong et al., 2016; Wiggs et al., 2011). In addition, several of these CAV1/2 POAG-associated SNPs have been recently associated with IOP (Chen et al., 2015; Hysi et al., 2014; Kim et al., 2015; Ozel et al., 2014; van Koolwijk et al., 2012). Although replicated in multiple populations, associations of the CAV1/2 loci with POAG have not been found in all populations (Abu-Amero et al., 2012; Burdon et al., 2015; Kuehn et al., 2011; Liu et al., 2013; Williams et al., 2015).
Vascular tone is an additional contributor to POAG risk and impaired autoregulation and blood flow has been reported (reviewed in (Flammer et al., 2002; Hayreh, 2001; Pasquale, 2016). Genes (including CAV1) involved in local control of vascular tone are more strongly associated with POAG with early paracentral visual field loss (Kang et al., 2014; Loomis et al., 2014). Patients with this POAG subtype are more likely to have systemic vascular dysfunction (Park et al., 2012). The association of this subtype of glaucoma with CAV1 polymorphisms is interesting given the retinal vascular phenotypes observed in Cav-1−/− mice (Gu et al., 2014a) which are suggestive of defects in local autoregulation. Furthermore, Cav-1−/− mice display systemic vascular dysfunction resulting in pulmonary hypertension and heart failure (Cohen et al., 2003; Zhao et al., 2002).
3.3. The role of caveolae in IOP maintenance and aqueous humor outflow
Caveolae are abundant features of the SC endothelium and TM (Tamm, 2009). Caveolins are also expressed in the human and murine outflow pathway, in situ, and in outflow pathway cell in culture (Aga et al., 2014; Elliott et al., 2016; Gonzalez et al., 2000; Kuehn et al., 2011; Surgucheva and Surguchov, 2011) In fact, treatments of TM cells with dexamethasone or soluble CD44 induce expression (Clark et al., 2013) or phosphorylation (Giovingo et al., 2013) of Cav-1, respectively. Recently, Aga et al. (2014) transiently silenced Cav-1 and Cav-2 in ex vivo anterior segment organ cultures and in TM cells and found that silencing CAV1 increased outflow facility (aqueous humor drainage) while silencing CAV2 reduced facility. These authors also found overall increased matrix turnover in Cav-1/Cav-2 silenced TM cells (Aga et al., 2014). In contrast, in Cav-1-deficient mice which chronically lack caveolae and have reduced expression of Cav-2, we observed significantly increased IOP and significantly reduced outflow facility (Elliott et al., 2016). The John laboratory has found similarly increased IOP and reduced aqueous humor drainage in Cav-1−/− mice (Dr. Simon John, The Jackson Laboratory, personal communication) and a recent study reported similar findings (Lei et al., 2016). The apparent conflict between results from Cav-1−/− mice and those of Aga et al. are likely due to differences between chronic caveolae deficiency and transient silencing. Transient loss of caveolins/caveolae may uncover immediate cellular responses such as rapid hyperactivation of eNOS that would be expected to transiently increase aqueous drainage as eNOS activity lowers IOP and increases outflow facility (Chang et al., 2015; Stamer et al., 2011). Chronic Cav-1/caveolae loss may lead to the development of outflow pathway dysfunction and our recent results imply that caveolae deficiency renders the conventional outflow tract more susceptible to IOP-induced injury (Elliott et al., 2016). Furthermore, Lei et al. (Lei et al., 2016) propose that eNOS hyperactivity resulting from Cav-1 ablation may lead to nitration of outflow tract proteins. Collectively, these results indicate that caveolins/caveolae play important roles in IOP maintenance and, specifically, in aqueous humor outflow.
Mechanistically, what Cav-1-dependent actions might be at play to explain the ocular hypertension and reduced outflow resulting from Cav-1 deficiency? In cells in which they are abundant, caveolae act as mechanosensors and membrane reservoirs that can donate membrane to buffer mechanical perturbation and provide mechanoprotection (Albinsson et al., 2008; Cheng et al., 2015; Dulhunty and Franzini-Armstrong, 1975; Joshi et al., 2012; Lo et al., 2015; Prescott and Brightman, 1976; Sinha et al., 2011; Yu et al., 2006). Reduced IOP-dependent outflow observed in Cav-1−/− eyes indicates that caveolae influence the ability of the outflow tissue to respond to IOP fluctuations. It is also possible that the observed functional defects in the SC and TM result as a consequence of the inability of caveolae-deficient tissue to withstand chronic fluctuations in mechanical load. This hypothesis remains to be tested rigorously in future experiments but recent results suggest that caveolae participate in buffering mechanical perturbations of outflow pathway cells.
4. Caveolins/caveolae in other ocular cells
4.1. Caveolae functions in the cornea
To date, caveolins and caveolae have received less attention in the cornea. The limited literature on corneal caveolins has focused in two main areas: epithelial wound healing and pathogen uptake/inflammatory modulation. Caveolin-1 is normally expressed weakly in the basal corneal epithelium and more strongly in the endothelium (Amino et al., 1997). Although not normally abundant in corneal keratocytes in the stroma, caveolae seem to be induced in corneal fibroblasts and myofibroblasts suggesting that they may be upregulated in stromal injury (Guo et al., 2007; Karamichos et al., 2011). In the corneal epithelium, Cav-1 may play a crucial role in re-epithelialization following injury. An early study demonstrated that the caveolar localization of a plasmalemmal calcium pump is reduced in regenerating epithelium indicating that caveolae composition is modified during epithelial wound healing (Amino et al., 1997). In human corneal epithelial cells, Cav-1 expression increases with age and this increased expression is negatively-associated with wound healing capacity (Rhim et al., 2010). While this association does not indicate causation, work from our laboratory (Griffith GL et al., IOVS 2014; 55: ARVO E-Abstract 4698) indicates that Cav-1 deficiency does, in fact, accelerate re-epithelialization in agreement with the original correlational study (Rhim et al., 2010). In fact, the faster re-epithelialization results, at least in part, from increased proliferation of Cav-1-deficient corneal epithelium. We also found that healing could be accelerated in wildtype mice by transiently silencing Cav-1 via RNA interference (RNAi). These results highlight the exciting possibility that Cav-1 could be targeted therapeutically to accelerate wound healing.
A small but growing body of evidence indicates that caveolae are also involved in pathogen uptake and inflammatory responses at the ocular surface. Recent work indicates that adenoviruses utilize a caveolae-mediated pathway for entry into corneal cells (Mukherjee et al., 2015; Yousuf et al., 2013). Importantly, disruption of caveolae and cholesterol-rich membrane domains by cholesterol depletion dramatically reduced viral infection (Yousuf et al., 2013). Studies designed to examine viral-mediated gene transfer to the corneal epithelium revealed improved transduction efficiency of recombinant adenovirus to the corneal epithelium by the PDE5 inhibitor, sildenafil, which acted by redistributing caveolin-1 expression from the normal basal epithelial location to suprabasal epithelium thus facilitating viral uptake (Saghizadeh et al., 2010). Sequestration of cholesterol by filipin abrogated the effect of sildenafil on viral transduction. Collectively, these results demonstrate that both pathological adenovirus infection and adenoviral gene transfer utilize caveolae to infect the corneal epithelium. In addition to viral pathogens, infection of the corneal epithelium with Pseudomonas aeruginosa drives inflammatory signaling via macrophage migration inhibitory factor (MIF) by assembling caveolin-1-rich signaling platforms (Reidy et al., 2013). The Pseudomonas aeruginosa and MIF-dependent activation of the pro-inflammatory cytokine, interleukin-8, was suppressed by silencing of Cav-1. This finding agrees with studies in the retina (Li et al., 2014) and lung (Garrean et al., 2006) suggesting that Cav-1 promotes pro-inflammatory cytokine production and highlights the potential of targeting Cav-1 to reduce inflammation in the eye.
Caveolin-1 plays an important role in modulating angiogenic signaling. As indicated previously, VEGFR-2 is localized to caveolae (Feng et al., 1999a; Labrecque et al., 2003). Outside of the eye, studies on the effect of caveolae on angiogenesis have been confounding with some studies suggesting that Cav-1 promotes angiogenesis (Chang et al., 2009; Sonveaux et al., 2004; Woodman et al., 2003) while others indicate anti-angiogenic activity (Bauer et al., 2005; Lin et al., 2007). A study using the corneal VEGF pocket assay in Cav-1−/− mice showed that Cav-1 expression promotes VEGF-induced corneal angiogenesis (Morais et al., 2012). In these studies, Cav-1−/− mice had a significantly blunted angiogenic response to VEGF. In agreement with this work in cornea, another study silenced Cav-1 expression with inhibitory RNAs delivered into the vitreous and showed that both vascular permeability and pathological angiogenesis were suppressed in retinas in the oxygen-induced retinopathy model (Tian et al., 2012). Our work also suggests that inflammation-induced retinal vascular permeability is blunted by global Cav-1 deletion (Li et al., 2014). However, as noted previously, Cav-1 deficiency promotes a modest increase in basal blood-retinal barrier permeability (Gu et al., 2014a). Although the mechanism by which Cav-1 inhibition suppresses pathological angiogenic responses in unknown, additional studies are warranted to assess the anti-angiogenic potential of Cav-1 inhibition in the cornea and retina.
4.2. Caveolae/caveolins in the lens
Like the corneal epithelium and RPE both morphological caveolae and Cav-1 have been found in the lens epithelium and lens fiber cells (Cenedella et al., 2006; Lo et al., 2004; Rujoi et al., 2003; Sexton et al., 2004). In the lens epithelium, caveolae have been implicated in the transcytosis of albumin (Sabah et al., 2007) and in the structure and function of lens epithelial gap junctions (Lin et al., 2004; Lin et al., 2003). In lens fiber cells, Cav-1 may regulate aquaporin-0 membrane particles to form square array junctions (Biswas et al., 2014). In Cav-1−/− lenses, fiber cells form abnormally large square array junctions possibly due to defective cholesterol trafficking as a result of Cav-1 deficiency. However, these dramatic fiber cell alterations do not result in induction of lens opacity and Cav-1−/− lenses retain their transparency. Thus, although Cav-1 is expressed in lens cells its function has yet to be completely elucidated.
5. Conclusions and future directions
Since their discovery in the 1950s, caveolae and their associated molecular components have received extensive attention outside of the eye (reviewed in (Cheng and Nichols, 2016; Chidlow and Sessa, 2010; Cohen et al., 2004; Echarri and Del Pozo, 2015; Nassoy and Lamaze, 2012; Parton and del Pozo, 2013; Parton and Simons, 2007; Sotgia et al., 2012). Given the expression of caveolins in important ocular cell types and the myriad functional tests to assess visual system function, the eye provides a unique model system to advance our basic understanding of these membrane domains. Furthermore, the association of polymorphisms in the CAV1/2 gene loci with POAG highlights the clinical importance of understanding caveolin/caveolae function in the aqueous outflow pathway, retina, and optic nerve. With the advent of new mouse models to conditionally manipulate the Cav1 gene using Cre/lox technology (Reagan et al., 2016; Sethna et al., 2016), there are now unique opportunities to study cell-specific caveolin/caveolae functions, in vivo. As caveolae domains provide a nexus for a variety of signaling pathways relevant to ocular physiology and pathophysiology (e.g., mechanotransduction, VEGF signaling, G-protein signaling), the understanding of caveolin function in ocular contexts provides an opportunity to develop novel therapeutic strategies to modulate IOP, retinal vascular permeability, neuroinflammation, and corneal infection and injury.
The cell biology community has focused recently on the function of caveolae in mechanotransduction and mechanoprotection as growing evidence indicates that these domains can directly induce cellular responses to mechanical perturbations (reviewed recently in (Cheng and Nichols, 2016; Echarri and Del Pozo, 2015; Nassoy and Lamaze, 2012; Parton and del Pozo, 2013)). Cellular responses to biomechanical fluctuations are likely key to our understanding of glaucoma-relevant processes such as IOP regulation by the conventional aqueous outflow pathway and the protection of the optic nerve head at the lamina cribrosa. Caveolae-dependent, flow-mediated activation of eNOS, a well-studied mechanism to modulate vascular autoregulation and vascular remodeling (Yu et al., 2006), has potentially profound relevance to ocular blood flow and BRB permeability. Much research has focused on the maintenance of BRB integrity via regulation of paracellular, junctional permeability (reviewed in (Antonetti et al., 2012; Kaur et al., 2008; Runkle and Antonetti, 2011)) but much less is known about the role of caveolar, transcellular permeability in the context of retinal vascular disease (Klaassen et al., 2013). Historical evidence that there are fewer caveolae in barrier-forming endothelium of the retina and brain compared to more permeable non-barrier endothelia (Sagaties et al., 1987; Stewart and Tuor, 1994) and that caveolae numbers and caveolin expression positively correlate with increased pathological BRB permeability (Feng et al., 1999a; Hofman et al., 2000; Klaassen et al., 2009; Stitt et al., 2000a) suggest that upregulation of transcellular permeability via caveolar vesicles contribute to pathological BRB breakdown. The concept that caveolae contribute to basal transcellular permeability in the barrier-forming retinal endothelium is controversial (Gardiner and Archer, 1986a, b). However, it remains an open question if caveolae transcytosis plays a role in pathological BRB breakdown that deserves careful study. Finally, caveolae seem to play important roles at the ocular surface both in pathogen uptake (Mukherjee et al., 2015; Yousuf et al., 2013), corneal inflammation (Reidy et al., 2013), and wound healing (Amino et al., 1997; Rhim et al., 2010) but these areas are yet to receive further attention.
5.1. Therapeutic potential of targeting caveolins/caveolae
As highlighted throughout this review, caveolins, by virtue of their ability to modulate myriad cellular responses in cell-specific contexts, represent challenging but intriguing therapeutic targets for a variety of ocular disorders. On one hand, because of their ability to modulate the activity of multiple signaling pathways, targeting caveolae to suppress inflammation and vascular dysfunction could provide broader therapeutic efficacy than targeting individual signaling pathways. On the other hand, this comprehensive targeting approach has the potential to cause unwanted side effects if critical caveolae functions are affected. The fact that global ablation of caveolae either by deletion of caveolins or PTRF/cavin-1 does not induce lethality in mice (although it is acknowledged that the health of such animals is compromised) highlights the potential of caveolae targeting as a rational therapeutic intervention. Most current efficacious therapies (e.g., steroids, anti-VEGF, anti-ocular hypertensives) are not without potential side effects but the ability to target delivery and dosages for the ocular environment (topical or intraocular administration) make the eye an ideal organ to assess therapeutic benefit while reducing risk of systemic side effects. Furthermore, several experimental results have suggested the possibility of targeting pathological but not basal caveolae functions. Thus, we conclude this review by highlighting potential means to manipulate caveolae functions for therapeutic benefit.
There are several potential strategies available to inhibit caveolins/caveolae functions. There has been significant work on in vivo delivery of Cav-1 silencing RNAs to target lung vasculature (Sparks et al., 2012). Silencing of Cav-1 expression in lungs has been shown to paradoxically suppress inflammation-induced permeability (Hu et al., 2008) while increasing basal permeability (Miyawaki-Shimizu et al., 2006) as we have previously observed in retinas from global Cav-1 knockout mice (Gu et al., 2014a; Li et al., 2014). Silencing of Cav-1 expression by intravitreal delivery of inhibitory RNAs can suppress angiogenesis and vascular permeability induced by the oxygen-induced retinopathy model (Tian et al., 2012). Besides targeting caveolin expression by RNAi, a recent study showed that suppressing expression of PLVAP significantly reduced VEGF-induced upregulation of caveolae and reduced BRB leakage (Wisniewska-Kruk et al., 2016). Thus, silencing expression of caveolae components by inhibitory RNAs could target inflammation and pathological BRB breakdown.
In addition to RNAi-based approaches, several plant-derived polyphenolic compounds (e.g., daidzein, quercetin, and green tea polyphenols) can suppress Cav-1 expression in non-ocular cells (Arya et al., 2011; Choi et al., 2010; Dasari et al., 2006; Kamada et al., 2016; Li et al., 2009; Sharma et al., 2012; Sobey et al., 2004; Woodman et al., 2004). Quercetin also suppresses oxidant-induced expression of Cav-1 in RPE cells and protects them from oxidative stress (Kook et al., 2008). Intriguingly, quercetin has also recently been shown to reduce fibrotic changes in human corneal fibroblasts, in vitro (McKay et al., 2015). Whether the anti-inflammatory, anti-oxidant, and anti-fibrotic properties of polyphenolic compounds are mediated through suppression of caveolin expression remains to be determined. Furthermore, the mechanism by which these polyphenolic compounds suppress caveolin expression has not been fully established. It is clear that the CAV1 gene has two oxidative-stress response elements that bind the transcription factor, Sp1 and that quercetin blocks oxidant-induced Cav-1 expression (Dasari et al., 2006). These results suggest that possibility that polyphenolic compounds could specifically target Cav-1 upregulation in response to pathologic stimulation without affecting basal Cav-1 expression levels. Thus, a clearer understanding of the mechanism(s) by which such compounds modulate Cav-1 expression could lead to the development of more specific therapeutics to manipulate caveolins.
Downregulating the expression of caveolae components may be beneficial under some circumstances (e.g., inflammation and inflammatory vascular barrier breakdown, corneal re-epithelialization). However, under some conditions it may be important to therapeutically augment caveolin expression. For example, Cav-1 expression in the brain declines with age and neuron-targeted re-expression promotes neuronal structural and functional improvement (Head et al., 2011; Head et al., 2010; Mandyam et al., 2015). Furthermore, there is a significant body of evidence that caveolins are necessary for ischemic- and anesthetic-induced preconditioning in the heart and brain (reviewed in (Schilling et al., 2015; Stary et al., 2012)). As preconditioning by light and ischemia can induce endogenous neuroprotection in the retina (Caprara and Grimm, 2012; Kast et al., 2016; Liu et al., 1998; Ueki et al., 2009), determining whether the mechanisms of retinal preconditioning require caveolins is warranted. Strategies to induce expression of caveolins are less well-understood and most experimental approaches have relied on transgenic overexpression (e.g., (Head et al., 2011; Murata et al., 2007)). One notable exception is evidence that Cav-1 expression is induced by peroxisome proliferator-activated receptor-gamma (PPARγ) (Chen et al., 2010). Cav-1 expression is induced by thiazolidinediones such as rosiglitazone, a PPARγ agonist used to treat type 2 diabetes (Llaverias et al., 2004; Tencer et al., 2008). In the retina, these compounds suppress retinal and choroidal neovascularization (Higuchi et al., 2010; Murata et al., 2001; Murata et al., 2000), reduce diabetes-induced retinal vascular inflammation and BRB breakdown (Muranaka et al., 2006), and promote neuroprotection (Doonan et al., 2009; Li et al., 2011; Normando et al., 2016). It remains to be determined whether these effects are mediated through Cav-1 expression in the retina.
The approaches described above focus on methods to manipulate the expression of caveolae components but another potential therapeutic strategy is to manipulate caveolae signaling via peptides based on caveolin protein sequences. The Sessa laboratory at Yale University has developed a compelling rationale for the use of peptide sequences derived from the caveolin scaffolding domain (CSD) to suppress inflammation, inhibit or activate eNOS, and block fibrosis. In their early studies, they developed a chimeric peptide containing the CSD sequence fused to a cell-permeable, “Trojan Horse” peptide (called “cavtratin”), that could be delivered, in vivo, to suppress inflammation and vascular permeability predominantly through its ability to inhibit eNOS (Bucci et al., 2000; Gratton et al., 2003). Through mutational analysis of the CSD they were able to define the essential amino acid, phenylalanine-92 (F92), that mediates eNOS inhibition (Bernatchez et al., 2005) and subsequently to generate a F92A mutant peptide (called “cavnoxin”) that activates eNOS (Bernatchez et al., 2011). It remains to be determined whether CSD-derived peptides could be beneficial in suppressing inflammation and vascular hyperpermeability in the eye. Furthermore, given the potential of nitric oxide to facilitate aqueous humor outflow and reduce IOP (Chang et al., 2015; Stamer et al., 2011), there may be therapeutic potential to activating endogenous eNOS activity via delivery of cavnoxin to the outflow tract.
There is a large body of literature describing caveolin functions and the potential of caveolae-targeted therapies outside of the eye but much less is known in eye-specific contexts. In this review, we have provided comprehensive coverage of this topic to facilitate further research in the hopes of taking advantage of diverse caveolae functions to identify novel therapeutics for ocular disorders.
Acknowledgments
The work from the Elliott laboratory presented herein was funded in part by NIH grants R01-EY019494, P30-EY021725 (Core Grant, OUHSC), the BrightFocus Foundation, the Alcon Research Institute, Sybil B. Harrington Special Scholar Award for Macular Degeneration Research and by an unrestricted grant to the OUHSC Department of Ophthalmology from Research to Prevent Blindness, Inc. The authors wish to thank the laboratories of several current and former collaborators including Drs. Steven Fliesler, Silvia Finnemann, Dan Stamer, Ernst Tamm, Masaki Tanito, Timothy Lyons, Gene Anderson, Daniel Carr, Michelle Callegan, Nawajes Mandal, Bill Stallcup, You-Yang Zhao, Richard Minshall, Yun Le, and Timothy Thompson. We also thank Dr. Simon John for kindly sharing unpublished data on IOP and outflow facility from Cav-1−/− mice.
Abbreviations
- AMD
age-related macular degeneration
- αSMA
alpha smooth muscle actin
- BRB
blood-retinal barrier
- Cav-1/CAV1
caveolin-1
- CSD
caveolin scaffolding domain
- eNOS
endothelial nitric oxide synthase
- ERG
electroretinogram/electroretinography
- GFAP
glial fibrillary acidic protein
- GWAS
genome-wide association studies
- HRP
horseradish peroxidase
- IL
interleukin
- LPS
lipopolysaccharide
- Na/K-ATPase
alpha-1 sodium/potassium ATPase
- NG2
chondroitin sulfate proteoglycan-4
- PAMPs
pathogen-associated molecular patterns
- PDE5
phosphodiesterase 5
- PLVAP
plasmalemmal vesicle-associated protein
- POAG
primary open angle glaucoma
- PRRs
pattern recognition receptors
- POS
photoreceptor outer segment
- PTRF
polymerase I and transcript release factor
- RNAi
RNA interference
- RPE
retinal pigment epithelium
- SC
Schlemm’s canal
- SNPs
single nucleotide polymorphisms
- STZ
streptozotocin
- TLRs
Toll-like receptors
- TM
trabecular meshwork
- VEGF
vascular endothelial growth factor
- VEGFR-2
vascular endothelial growth factor receptor-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Amero KK, Kondkar AA, Mousa A, Osman EA, Al-Obeidan SA. Lack of association of SNP rs4236601 near CAV1 and CAV2 with POAG in a Saudi cohort. Mol Vis. 2012;18:1960–1965. [PMC free article] [PubMed] [Google Scholar]
- Aga M, Bradley JM, Wanchu R, Yang YF, Acott TS, Keller KE. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2014;55:5497–5509. doi: 10.1167/iovs.14-14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Albinsson S, Nordstrom I, Sward K, Hellstrand P. Differential dependence of stretch and shear stress signaling on caveolin-1 in the vascular wall. Am J Physiol Cell Physiol. 2008;294:C271–279. doi: 10.1152/ajpcell.00297.2007. [DOI] [PubMed] [Google Scholar]
- Albinsson S, Shakirova Y, Rippe A, Baumgarten M, Rosengren BI, Rippe C, Hallmann R, Hellstrand P, Rippe B, Sward K. Arterial remodeling and plasma volume expansion in caveolin-1-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1222–1231. doi: 10.1152/ajpregu.00092.2007. [DOI] [PubMed] [Google Scholar]
- Amino K, Honda Y, Ide C, Fujimoto T. Distribution of plasmalemmal Ca(2+)–pump and caveolin in the corneal epithelium during the wound healing process. Curr Eye Res. 1997;16:1088–1095. doi: 10.1076/ceyr.16.11.1088.5098. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Study NTG. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Arya A, Yadav HN, Sharma PL. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Mol Cell Biochem. 2011;354:57–66. doi: 10.1007/s11010-011-0805-6. [DOI] [PubMed] [Google Scholar]
- Bauer PM, Yu J, Chen Y, Hickey R, Bernatchez PN, Looft-Wilson R, Huang Y, Giordano F, Stan RV, Sessa WC. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci U S A. 2005;102:204–209. doi: 10.1073/pnas.0406092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest. 2011;121:3747–3755. doi: 10.1172/JCI44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc Natl Acad Sci U S A. 2005;102:761–766. doi: 10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein MH, Hollenberg MJ. Fine structure of the choriocapillaris and retinal capillaries. Invest Ophthalmol. 1965;4:1016–1025. [PubMed] [Google Scholar]
- Berta AI, Boesze-Battaglia K, Magyar A, Szél A, Kiss AL. Localization of caveolin-1 and c-src in mature and differentiating photoreceptors: raft proteins co-distribute with rhodopsin during development. J Mol Histol. 2011;42:523–533. doi: 10.1007/s10735-011-9360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta AI, Kiss AL, Kemeny-Beke A, Lukats A, Szabó A, Szél A. Different caveolin isoforms in the retina of melanoma malignum affected human eye. Mol Vis. 2007a;13:881–886. [PMC free article] [PubMed] [Google Scholar]
- Berta AI, Kiss AL, Lukáts A, Szabó A, Szél A. Distribution of caveolin isoforms in the lemur retina. J Vet Sci. 2007b;8:295–297. doi: 10.4142/jvs.2007.8.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Brako L, Lo WK. Massive formation of square array junctions dramatically alters cell shape but does not cause lens opacity in the cav1-KO mice. Exp Eye Res. 2014;125:9–19. doi: 10.1016/j.exer.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Dispoto J, Kahoe MA. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with triton X-100-resistant membrane rafts from rod outer segment disk membranes. J Biol Chem. 2002;277:41843–41849. doi: 10.1074/jbc.M207111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, Albert AD. Relationship of cholesterol content to spatial distribution and age of disc membranes in retinal rod outer segments. J Biol Chem. 1990;265:18867–18870. [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Hennessey T, Albert AD. Cholesterol heterogeneity in bovine rod outer segment disk membranes. J Biol Chem. 1989;264:8151–8155. [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, El-Sherbeny A, Roon P, Ola MS, Kekuda R, Ganapathy V, Camero RS, Cameron PL, Smith SB. A comparison of caveolae and caveolin-1 to folate receptor alpha in retina and retinal pigment epithelium. Histochem J. 2001;33:149–158. doi: 10.1023/a:1017991925821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- Burdon KP, Mitchell P, Lee A, Healey PR, White AJ, Rochtchina E, Thomas PB, Wang JJ, Craig JE. Association of open-angle glaucoma loci with incident glaucoma in the Blue Mountains Eye Study. Am J Ophthalmol. 2015;159:31–36. e31. doi: 10.1016/j.ajo.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RB, Slapnick SM. Freeze-fracture and lanthanum studies of the retinal microvasculature in diabetic rats. Invest Ophthalmol Vis Sci. 1992;33:1610–1619. [PubMed] [Google Scholar]
- Campbell M, Humphries MM, Kiang AS, Nguyen AT, Gobbo OL, Tam LC, Suzuki M, Hanrahan F, Ozaki E, Farrar GJ, Kenna PF, Humphries P. Systemic low-molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol Med. 2011;3:235–245. doi: 10.1002/emmm.201100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Nguyen AT, Kiang AS, Tam LC, Gobbo OL, Kerskens C, Ni Dhubhghaill S, Humphries MM, Farrar GJ, Kenna PF, Humphries P. An experimental platform for systemic drug delivery to the retina. Proc Natl Acad Sci U S A. 2009;106:17817–17822. doi: 10.1073/pnas.0908561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012;31:89–119. doi: 10.1016/j.preteyeres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Carquin M, D'Auria L, Pollet H, Bongarzone ER, Tyteca D. Recent progress on lipid lateral heterogeneity in plasma membranes: From rafts to submicrometric domains. Prog Lipid Res. 2015;62:1–24. doi: 10.1016/j.plipres.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella RJ, Neely AR, Sexton P. Multiple forms of 22 kDa caveolin-1 alpha present in bovine lens cells could reflect variable palmitoylation. Exp Eye Res. 2006;82:229–235. doi: 10.1016/j.exer.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Chang JY, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR, Overby DR. Role of nitric oxide in murine conventional outflow physiology. Am J Physiol Cell Physiol. 2015;309:C205–214. doi: 10.1152/ajpcell.00347.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Feng D, Nagy JA, Sciuto TE, Dvorak AM, Dvorak HF. Vascular permeability and pathological angiogenesis in caveolin-1-null mice. Am J Pathol. 2009;175:1768–1776. doi: 10.2353/ajpath.2009.090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Gomez GA, Howes MT, Lo HP, McMahon KA, Rae JA, Schieber NL, Hill MM, Gaus K, Yap AS, Parton RG. Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 2014;12:e1001832. doi: 10.1371/journal.pbio.1001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Klein AP, Klein BE, Lee KE, Truitt B, Klein R, Iyengar SK, Duggal P. Exome array analysis identifies CAV1/CAV2 as a susceptibility locus for intraocular pressure. Invest Ophthalmol Vis Sci. 2015;56:544–551. doi: 10.1167/iovs.14-15204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jump DB, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:18–26. doi: 10.1167/iovs.06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Whiting C, Borza C, Hu W, Mont S, Bulus N, Zhang MZ, Harris RC, Zent R, Pozzi A. Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression. Mol Cell Biol. 2010;30:3048–3058. doi: 10.1128/MCB.00892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Moiseyev G, Wu BX, Ma JX, Crouch RK. Visual cycle retinoid processing proteins are present in HEK293S cells. Vision Res. 2003;43:3037–3044. doi: 10.1016/j.visres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cheng JP, Mendoza-Topaz C, Howard G, Chadwick J, Shvets E, Cowburn AS, Dunmore BJ, Crosby A, Morrell NW, Nichols BJ. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J Cell Biol. 2015;211:53–61. doi: 10.1083/jcb.201504042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Nichols BJ. Caveolae: One Function or Many? Trends Cell Biol. 2016;26:177–189. doi: 10.1016/j.tcb.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Chidlow JH, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2010;36:931–934. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers I, Kim Y, Farkas RH, Gunatilaka TL, Hackam AS, Campochiaro PA, Finnemann SC, Zack DJ. Changes in retinal pigment epithelial gene expression induced by rod outer segment uptake. Invest Ophthalmol Vis Sci. 2004;45:2098–2106. doi: 10.1167/iovs.03-0863. [DOI] [PubMed] [Google Scholar]
- Clark R, Nosie A, Walker T, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013;12:194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira de Souza A, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- Collins BM, Davis MJ, Hancock JF, Parton RG. Structure-based reassessment of the caveolin signaling model: do caveolae regulate signaling through caveolin-protein interactions? Dev Cell. 2012;23:11–20. doi: 10.1016/j.devcel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley SC, Albert AD. Assessment of bovine rod outer segment disk membrane heterogeneity utilizing flow cytometry. Exp Eye Res. 2011;92:20–27. doi: 10.1016/j.exer.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997a;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997b;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010;51:451–467. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Fitzgerald JB, Matveev AV, McClellan ME, Elliott MH. Functional activity of photoreceptor cyclic nucleotide-gated channels is dependent on the integrity of cholesterol- and sphingolipid-enriched membrane domains. Biochemistry. 2008;47:3677–3687. doi: 10.1021/bi7019645. [DOI] [PubMed] [Google Scholar]
- Doonan F, Wallace DM, O'Driscoll C, Cotter TG. Rosiglitazone acts as a neuroprotectant in retinal cells via up-regulation of sestrin-1 and SOD-2. J Neurochem. 2009;109:631–643. doi: 10.1111/j.1471-4159.2009.05995.x. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Franzini-Armstrong C. The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J Physiol. 1975;250:513–539. doi: 10.1113/jphysiol.1975.sp011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Del Pozo MA. Caveolae - mechanosensitive membrane invaginations linked to actin filaments. J Cell Sci. 2015;128:2747–2758. doi: 10.1242/jcs.153940. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Ashpole NE, Gu X, Herrnberger L, McClellan ME, Griffith GL, Reagan AM, Boyce TM, Tanito M, Tamm ER, Stamer WD. Caveolin-1 modulates intraocular pressure: implications for caveolae mechanoprotection in glaucoma. Sci Rep. 2016 doi: 10.1038/srep37127. Under review. [DOI] [PMC free article] [PubMed]
- Elliott MH, Fliesler SJ, Ghalayini AJ. Cholesterol-dependent association of caveolin-1 with the transducin alpha subunit in bovine photoreceptor rod outer segments: disruption by cyclodextrin and guanosine 5'-O-(3-thiotriphosphate) Biochemistry. 2003;42:7892–7903. doi: 10.1021/bi027162n. [DOI] [PubMed] [Google Scholar]
- Elliott MH, Nash ZA, Takemori N, Fliesler SJ, McClellan ME, Naash MI. Differential distribution of proteins and lipids in detergent-resistant and detergent-soluble domains in rod outer segment plasma membranes and disks. J Neurochem. 2008;104:336–352. doi: 10.1111/j.1471-4159.2007.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Wang DY, Lam DS, Pang CP. Gene mapping for primary open angle glaucoma. Clin Biochem. 2006;39:249–258. doi: 10.1016/j.clinbiochem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Fang PK, Solomon KR, Zhuang L, Qi M, McKee M, Freeman MR, Yelick PC. Caveolin-1alpha and -1beta perform nonredundant roles in early vertebrate development. Am J Pathol. 2006;169:2209–2222. doi: 10.2353/ajpath.2006.060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB. VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci. 1999a;40:157–167. [PubMed] [Google Scholar]
- Feng Y, Venema VJ, Venema RC, Tsai N, Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999b;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Forbes A, Wadehra M, Mareninov S, Morales S, Shimazaki K, Gordon LK, Braun J. The tetraspan protein EMP2 regulates expression of caveolin-1. J Biol Chem. 2007;282:26542–26551. doi: 10.1074/jbc.M702117200. [DOI] [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335:41–47. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- Fu Y, Moore XL, Lee MK, Fernández-Rojo MA, Parat MO, Parton RG, Meikle PJ, Sviridov D, Chin-Dusting JP. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Arterioscler Thromb Vasc Biol. 2012;32:e117–125. doi: 10.1161/ATVBAHA.112.254151. [DOI] [PubMed] [Google Scholar]
- Gabella G. Quantitative morphological study of smooth muscle cells of the guinea-pig taenia coli. Cell Tissue Res. 1976;170:161–186. doi: 10.1007/BF00224297. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- García-Cardeña G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Archer DB. Does unidirectional vesicular transport occur in retinal vessels? Br J Ophthalmol. 1986a;70:249–254. doi: 10.1136/bjo.70.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner TA, Archer DB. Endocytosis in the retinal and choroidal microcirculation. Br J Ophthalmol. 1986b;70:361–372. doi: 10.1136/bjo.70.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986;102:1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovingo M, Nolan M, McCarty R, Pang IH, Clark AF, Beverley RM, Schwartz S, Stamer WD, Walker L, Grybauskas A, Skuran K, Kuprys PV, Yue BY, Knepper PA. sCD44 overexpression increases intraocular pressure and aqueous outflow resistance. Mol Vis. 2013;19:2151–2164. [PMC free article] [PubMed] [Google Scholar]
- Glenney JR, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108:2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Epstein DL, Borrás T. Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest Ophthalmol Vis Sci. 2000;41:3678–3693. [PubMed] [Google Scholar]
- Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, Iwakiri Y, Groszmann R, Claffey KP, Cheng YC, Sessa WC. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Gu X, Fliesler SJ, Zhao YY, Stallcup WB, Cohen AW, Elliott MH. Loss of caveolin-1 causes blood-retinal barrier breakdown, venous enlargement, and mural cell alteration. Am J Pathol. 2014a;184:541–555. doi: 10.1016/j.ajpath.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Reagan A, Yen A, Bhatti F, Cohen AW, Elliott MH. Spatial and temporal localization of caveolin-1 protein in the developing retina. Adv Exp Med Biol. 2014b;801:15–21. doi: 10.1007/978-1-4614-3209-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck SM, Dietter J, Kramer RL, Hofmaier F, Zipplies JK, Amann B, Feuchtinger A, Deeg CA, Ueffing M. Deciphering membrane-associated molecular processes in target tissue of autoimmune uveitis by label-free quantitative mass spectrometry. Mol Cell Proteomics. 2010;9:2292–2305. doi: 10.1074/mcp.M110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001;20:595–624. doi: 10.1016/s1350-9462(01)00005-2. [DOI] [PubMed] [Google Scholar]
- Head BP, Hu Y, Finley JC, Saldana MD, Bonds JA, Miyanohara A, Niesman IR, Ali SS, Murray F, Insel PA, Roth DM, Patel HH, Patel PM. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. J Biol Chem. 2011;286:33310–33321. doi: 10.1074/jbc.M111.255976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Peart JN, Panneerselvam M, Yokoyama T, Pearn ML, Niesman IR, Bonds JA, Schilling JM, Miyanohara A, Headrick J, Ali SS, Roth DM, Patel PM, Patel HH. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS One. 2010;5:e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A, Ohashi K, Shibata R, Sono-Romanelli S, Walsh K, Ouchi N. Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arterioscler Thromb Vasc Biol. 2010;30:46–53. doi: 10.1161/ATVBAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Daud NH, Aung CS, Loo D, Martin S, Murphy S, Black DM, Barry R, Simpson F, Liu L, Pilch PF, Hancock JF, Parat MO, Parton RG. Co-regulation of cell polarization and migration by caveolar proteins PTRF/Cavin-1 and caveolin-1. PLoS One. 2012;7:e43041. doi: 10.1371/journal.pone.0043041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- Hofman P, Blaauwgeers HG, Tolentino MJ, Adamis AP, Nunes Cardozo BJ, Vrensen GF, Schlingemann RO. VEGF-A induced hyperpermeability of blood-retinal barrier endothelium in vivo is predominantly associated with pinocytotic vesicular transport and not with formation of fenestrations. Vascular endothelial growth factor-A. Curr Eye Res. 2000;21:637–645. [PubMed] [Google Scholar]
- Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci. 1994;35:2948–2961. [PubMed] [Google Scholar]
- Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ljubimova JY, Inoue S, Konda B, Patil R, Ding H, Espinoza A, Wawrowsky KA, Patil C, Ljubimov AV, Black KL. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS One. 2010;5:e10108. doi: 10.1371/journal.pone.0010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Wang W, Zhou M, Zhang X. Association of single-nucleotide polymorphism rs4236601 near caveolin 1 and 2 with primary open-angle glaucoma: a meta-analysis. Clin Experiment Ophthalmol. 2014;42:515–521. doi: 10.1111/ceo.12201. [DOI] [PubMed] [Google Scholar]
- Hudson N, Powner MB, Sarker MH, Burgoyne T, Campbell M, Ockrim ZK, Martinelli R, Futter CE, Grant MB, Fraser PA, Shima DT, Greenwood J, Turowski P. Differential apicobasal VEGF signaling at vascular blood-neural barriers. Dev Cell. 2014;30:541–552. doi: 10.1016/j.devcel.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, Vitart V, Nag A, Hewitt AW, Hohn R, Venturini C, Mirshahi A, Ramdas WD, Thorleifsson G, Vithana E, Khor CC, Stefansson AB, Liao J, Haines JL, Amin N, Wang YX, Wild PS, Ozel AB, Li JZ, Fleck BW, Zeller T, Staffieri SE, Teo YY, Cuellar-Partida G, Luo X, Allingham RR, Richards JE, Senft A, Karssen LC, Zheng Y, Bellenguez C, Xu L, Iglesias AI, Wilson JF, Kang JH, van Leeuwen EM, Jonsson V, Thorsteinsdottir U, Despriet DD, Ennis S, Moroi SE, Martin NG, Jansonius NM, Yazar S, Tai ES, Amouyel P, Kirwan J, van Koolwijk LM, Hauser MA, Jonasson F, Leo P, Loomis SJ, Fogarty R, Rivadeneira F, Kearns L, Lackner KJ, de Jong PT, Simpson CL, Pennell CE, Oostra BA, Uitterlinden AG, Saw SM, Lotery AJ, Bailey-Wilson JE, Hofman A, Vingerling JR, Maubaret C, Pfeiffer N, Wolfs RC, Lemij HG, Young TL, Pasquale LR, Delcourt C, Spector TD, Klaver CC, Small KS, Burdon KP, Stefansson K, Wong TY, Group BG, Viswanathan A, Mackey DA, Craig JE, Wiggs JL, van Duijn CM, Hammond CJ, Aung T Consortium N, Wellcome Trust Case Control C. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46:1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Tanaka K, Taniguchi Y. Disruption of blood-retinal barrier in experimental diabetic rats: an electron microscopic study. Exp Eye Res. 1980;30:401–410. doi: 10.1016/0014-4835(80)90055-x. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Janssen SF, Gorgels TG, Ramdas WD, Klaver CC, van Duijn CM, Jansonius NM, Bergen AA. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res. 2013;37:31–67. doi: 10.1016/j.preteyeres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Jiang G, Ke Y, Sun D, Wang Y, Kaplan HJ, Shao H. Regulatory role of TLR ligands on the activation of autoreactive T cells by retinal astrocytes. Invest Ophthalmol Vis Sci. 2009;50:4769–4776. doi: 10.1167/iovs.08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G, Minshall RD, Malik AB, Hu G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J Immunol. 2013;191:6191–6199. doi: 10.4049/jimmunol.1300873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Lee SJ, Minshall RD, Choi AM. Caveolin-1: a critical regulator of lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300:L151–160. doi: 10.1152/ajplung.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Chan D, Read AT, Christensen C, Sit A, Ethier CR. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43:2950–2955. [PubMed] [Google Scholar]
- Joshi B, Bastiani M, Strugnell SS, Boscher C, Parton RG, Nabi IR. Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation. J Cell Biol. 2012;199:425–435. doi: 10.1083/jcb.201207089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi S, Yamazaki A, Usukura J. Localization of caveolin-1 in photoreceptor synaptic ribbons. Invest Ophthalmol Vis Sci. 2001;42:850–852. [PubMed] [Google Scholar]
- Kamada C, Mukai R, Kondo A, Sato S, Terao J. Effect of quercetin and its metabolite on caveolin-1 expression induced by oxidized LDL and lysophosphatidylcholine in endothelial cells. J Clin Biochem Nutr. 2016;58:193–201. doi: 10.3164/jcbn.16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Loomis SJ, Yaspan BL, Bailey JC, Weinreb RN, Lee RK, Lichter PR, Budenz DL, Liu Y, Realini T, Gaasterland D, Gaasterland T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Forman JP, Buys ES, Kraft P, Zhang K, Allingham RR, Pericak-Vance MA, Richards JE, Hauser MA, Haines JL, Wiggs JL, Pasquale LR. Vascular tone pathway polymorphisms in relation to primary open-angle glaucoma. Eye (Lond) 2014;28:662–671. doi: 10.1038/eye.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Hutcheon AE, Zieske JD. Transforming growth factor-β:3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med. 2011;5:e228–238. doi: 10.1002/term.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Kleinfeld AM, Hoover RL, Klausner RD. The concept of lipid domains in membranes. J Cell Biol. 1982;94:1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast B, Schori C, Grimm C. Hypoxic preconditioning protects photoreceptors against light damage independently of hypoxia inducible transcription factors in rods. Exp Eye Res. 2016;146:60–71. doi: 10.1016/j.exer.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim K, Heo DW, Kim JS, Park CK, Kim CS, Kang C. Expression-associated polymorphisms of CAV1-CAV2 affect intraocular pressure and high-tension glaucoma risk. Mol Vis. 2015;21:548–554. [PMC free article] [PubMed] [Google Scholar]
- Kindzelskii AL, Elner VM, Elner SG, Yang D, Hughes BA, Petty HR. Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments. J Gen Physiol. 2004;124:139–149. doi: 10.1085/jgp.200409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Ryan M, Marchant JK, Henrich S, John SW. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014;12:e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009;89:4–15. doi: 10.1016/j.exer.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, Welge-Lüssen UC. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008;49:1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- Kraehling JR, Hao Z, Lee MY, Vinyard D, Velazquez H, Liu X, Stan RV, Brudvig G, Sessa WC. Uncoupling Caveolae from Intracellular Signaling In Vivo. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.307767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MH, Wang K, Roos B, Stone EM, Kwon YH, Alward WL, Mullins RF, Fingert JH. Chromosome 7q31 POAG locus: ocular expression of caveolins and lack of association with POAG in a US cohort. Mol Vis. 2011;17:430–435. [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Shamsuddin N. Retinal Muller glia initiate innate response to infectious stimuli via toll-like receptor signaling. PLoS One. 2012;7:e29830. doi: 10.1371/journal.pone.0029830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Birdsall NJ, Metcalfe JC, Toon PA, Warren GB. Clusters in lipid bilayers and the interpretation of thermal effects in biological membranes. Biochemistry. 1974;13:3699–3705. doi: 10.1021/bi00715a013. [DOI] [PubMed] [Google Scholar]
- Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzalez R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti MP. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- Lei Y, Song M, Wu J, Xing C, Sun X. eNOS Activity in CAV1 Knockout Mouse Eyes. Invest Ophthalmol Vis Sci. 2016;57:2805–2813. doi: 10.1167/iovs.15-18841. [DOI] [PubMed] [Google Scholar]
- Li J, Scherl A, Medina F, Frank PG, Kitsis RN, Tanowitz HB, Sotgia F, Lisanti MP. Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle. 2005;4:1599–1607. doi: 10.4161/cc.4.11.2117. [DOI] [PubMed] [Google Scholar]
- Li P, Xu X, Zheng Z, Zhu B, Shi Y, Liu K. Protective effects of rosiglitazone on retinal neuronal damage in diabetic rats. Curr Eye Res. 2011;36:673–679. doi: 10.3109/02713683.2011.572220. [DOI] [PubMed] [Google Scholar]
- Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996a;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996b;271:3863–3868. [PubMed] [Google Scholar]
- Li X, Gu X, Boyce TM, Zheng M, Reagan AM, Qi H, Mandal N, Cohen AW, Callegan MC, Carr DJ, Elliott MH. Caveolin-1 increases proinflammatory chemoattractants and blood-retinal barrier breakdown but decreases leukocyte recruitment in inflammation. Invest Ophthalmol Vis Sci. 2014;55:6224–6234. doi: 10.1167/iovs.14-14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, McClellan ME, Tanito M, Garteiser P, Towner R, Bissig D, Berkowitz BA, Fliesler SJ, Woodruff ML, Fain GL, Birch DG, Khan MS, Ash JD, Elliott MH. Loss of caveolin-1 impairs retinal function due to disturbance of subretinal microenvironment. J Biol Chem. 2012;287:16424–16434. doi: 10.1074/jbc.M112.353763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ying C, Zuo X, Yi H, Yi W, Meng Y, Ikeda K, Ye X, Yamori Y, Sun X. Green tea polyphenols down-regulate caveolin-1 expression via ERK1/2 and p38MAPK in endothelial cells. J Nutr Biochem. 2009;20:1021–1027. doi: 10.1016/j.jnutbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Lin D, Lobell S, Jewell A, Takemoto DJ. Differential phosphorylation of connexin46 and connexin50 by H2O2 activation of protein kinase Cgamma. Mol Vis. 2004;10:688–695. [PubMed] [Google Scholar]
- Lin D, Zhou J, Zelenka PS, Takemoto DJ. Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci. 2003;44:5259–5268. doi: 10.1167/iovs.03-0296. [DOI] [PubMed] [Google Scholar]
- Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849–2856. doi: 10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994a;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994b;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–1344. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li WP, Machleidt T, Anderson RG. Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat Cell Biol. 1999;1:369–375. doi: 10.1038/14067. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hauser MA, Akafo SK, Qin X, Miura S, Gibson JR, Wheeler J, Gaasterland DE, Challa P, Herndon LW, Ritch R, Moroi SE, Pasquale LR, Girkin CA, Budenz DL, Wiggs JL, Richards JE, Ashley-Koch AE, Allingham RR Glaucoma I.C.o.A.A.R.i. Investigation of known genetic risk factors for primary open angle glaucoma in two populations of African ancestry. Invest Ophthalmol Vis Sci. 2013;54:6248–6254. doi: 10.1167/iovs.13-12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaverias G, Vazquez-Carrera M, Sanchez RM, Noe V, Ciudad CJ, Laguna JC, Alegret M. Rosiglitazone upregulates caveolin-1 expression in THP-1 cells through a PPAR-dependent mechanism. J Lipid Res. 2004;45:2015–2024. doi: 10.1194/jlr.M400049-JLR200. [DOI] [PubMed] [Google Scholar]
- Lo HP, Nixon SJ, Hall TE, Cowling BS, Ferguson C, Morgan GP, Schieber NL, Fernandez-Rojo MA, Bastiani M, Floetenmeyer M, Martel N, Laporte J, Pilch PF, Parton RG. The caveolin-cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. J Cell Biol. 2015;210:833–849. doi: 10.1083/jcb.201501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WK, Zhou CJ, Reddan J. Identification of caveolae and their signature proteins caveolin 1 and 2 in the lens. Exp Eye Res. 2004;79:487–498. doi: 10.1016/j.exer.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Loomis SJ, Kang JH, Weinreb RN, Yaspan BL, Cooke Bailey JN, Gaasterland D, Gaasterland T, Lee RK, Lichter PR, Budenz DL, Liu Y, Realini T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Kraft P, Zhang K, Allingham RR, Pericak-Vance MA, Richards JE, Hauser MA, Haines JL, Pasquale LR, Wiggs JL. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology. 2014;121:508–516. doi: 10.1016/j.ophtha.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv XJ, Li YY, Zhang YJ, Mao M, Qian GS. Over-expression of caveolin-1 aggravate LPS-induced inflammatory response in AT-1 cells via up-regulation of cPLA2/p38 MAPK. Inflamm Res. 2010;59:531–541. doi: 10.1007/s00011-010-0157-9. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Schilling JM, Cui W, Egawa J, Niesman IR, Kellerhals SE, Staples MC, Busija AR, Risbrough VB, Posadas E, Grogman GC, Chang JW, Roth DM, Patel PM, Patel HH, Head BP. Neuron-Targeted Caveolin-1 Improves Molecular Signaling, Plasticity, and Behavior Dependent on the Hippocampus in Adult and Aged Mice. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Invest Ophthalmol Vis Sci. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- McKay TB, Lyon D, Sarker-Nag A, Priyadarsini S, Asara JM, Karamichos D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci Rep. 2015;5:9003. doi: 10.1038/srep09003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Milici AJ, Watrous NE, Stukenbrok H, Palade GE. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987;105:2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 2010;176:2344–2351. doi: 10.2353/ajpath.2010.091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L405–413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- Moon H, Lee CS, Inder KL, Sharma S, Choi E, Black DM, Lê Cao KA, Winterford C, Coward JI, Ling MT, Craik DJ, Parton RG, Russell PJ, Hill MM, BioResource APC. PTRF/cavin-1 neutralizes non-caveolar caveolin-1 microdomains in prostate cancer. Oncogene. 2014;33:3561–3570. doi: 10.1038/onc.2013.315. [DOI] [PubMed] [Google Scholar]
- Mora RC, Bonilha VL, Shin BC, Hu J, Cohen-Gould L, Bok D, Rodriguez-Boulan E. Bipolar assembly of caveolae in retinal pigment epithelium. Am J Physiol Cell Physiol. 2006;290:C832–843. doi: 10.1152/ajpcell.00405.2005. [DOI] [PubMed] [Google Scholar]
- Morais C, Ebrahem Q, Anand-Apte B, Parat MO. Altered angiogenesis in caveolin-1 gene-deficient mice is restored by ablation of endothelial nitric oxide synthase. Am J Pathol. 2012;180:1702–1714. doi: 10.1016/j.ajpath.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Zhou X, Rajaiya J, Chodosh J. Ultrastructure of adenovirus keratitis. Invest Ophthalmol Vis Sci. 2015;56:472–477. doi: 10.1167/iovs.14-15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Muranaka K, Yanagi Y, Tamaki Y, Usui T, Kubota N, Iriyama A, Terauchi Y, Kadowaki T, Araie M. Effects of peroxisome proliferator-activated receptor gamma and its ligand on blood-retinal barrier in a streptozotocin-induced diabetic model. Invest Ophthalmol Vis Sci. 2006;47:4547–4552. doi: 10.1167/iovs.05-1432. [DOI] [PubMed] [Google Scholar]
- Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Hata Y, Ishibashi T, Kim S, Hsueh WA, Law RE, Hinton DR. Response of experimental retinal neovascularization to thiazolidinediones. Arch Ophthalmol. 2001;119:709–717. doi: 10.1001/archopht.119.5.709. [DOI] [PubMed] [Google Scholar]
- Murata T, He S, Hangai M, Ishibashi T, Xi XP, Kim S, Hsueh WA, Ryan SJ, Law RE, Hinton DR. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:2309–2317. [PubMed] [Google Scholar]
- Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Balasubramanian N, Slepak VZ. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr Biol. 2002;12:421–425. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Grebe R, Bhutto IA, Edwards M, McLeod DS, Lutty GA. Albumen Transport to Bruch's Membrane and RPE by Choriocapillaris Caveolae. Invest Ophthalmol Vis Sci. 2016;57:2213–2224. doi: 10.1167/iovs.15-17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano L. The differentiation of white adipose cells. An electron microscope study. J Cell Biol. 1963;18:663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar ZD, Hill MM, Parton RG, Francois M, Parat MO. Non-caveolar caveolin-1 expression in prostate cancer cells promotes lymphangiogenesis. Oncoscience. 2015;2:635–645. doi: 10.18632/oncoscience.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassoy P, Lamaze C. Stressing caveolae new role in cell mechanics. Trends Cell Biol. 2012;22:381–389. doi: 10.1016/j.tcb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA. Genome-wide analysis of Müller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6:e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Litman BJ. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: effects on receptor activation. J Biol Chem. 2002;277:20139–20145. doi: 10.1074/jbc.M200594200. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Carter A, Wegner J, Ferguson C, Floetenmeyer M, Riches J, Key B, Westerfield M, Parton RG. Caveolin-1 is required for lateral line neuromast and notochord development. J Cell Sci. 2007;120:2151–2161. doi: 10.1242/jcs.003830. [DOI] [PubMed] [Google Scholar]
- Normando EM, Davis BM, De Groef L, Nizari S, Turner LA, Ravindran N, Pahlitzsch M, Brenton J, Malaguarnera G, Guo L, Somavarapu S, Cordeiro MF. The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson's disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain. Acta Neuropathol Commun. 2016;4:86. doi: 10.1186/s40478-016-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- Omri S, Behar-Cohen F, de Kozak Y, Sennlaub F, Verissimo LM, Jonet L, Savoldelli M, Omri B, Crisanti P. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCζ in the Goto Kakizaki rat model. Am J Pathol. 2011;179:942–953. doi: 10.1016/j.ajpath.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opreanu M, Tikhonenko M, Bozack S, Lydic TA, Reid GE, McSorley KM, Sochacki A, Perez GI, Esselman WJ, Kern T, Kolesnick R, Grant MB, Busik JV. The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes. 2011;60:2370–2378. doi: 10.2337/db10-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortegren U, Karlsson M, Blazic N, Blomqvist M, Nystrom FH, Gustavsson J, Fredman P, Strålfors P. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. Eur J Biochem. 2004;271:2028–2036. doi: 10.1111/j.1432-1033.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, Dang Q, Kim JH, Ethier CR, Stamer WD, Fredberg JJ, Johnson M. Altered mechanobiology of Schlemm's canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, Scott K, Rozsa F, Pawar H, Musch DC, Lichter PR, Gaasterland D, Branham K, Gilbert J, Garnai SJ, Chen W, Othman M, Heckenlively J, Swaroop A, Abecasis G, Friedman DS, Zack D, Ashley-Koch A, Ulmer M, Kang JH, Liu Y, Yaspan BL, Haines J, Allingham RR, Hauser MA, Pasquale L, Wiggs J, Richards JE, Li JZ Consortium N. Genome-wide association study and meta-analysis of intraocular pressure. Hum Genet. 2014;133:41–57. doi: 10.1007/s00439-013-1349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424–1424. [Google Scholar]
- Palade GE. Blood capillaries of the heart and other organs. Circulation. 1961;24:368–388. doi: 10.1161/01.cir.24.2.368. [DOI] [PubMed] [Google Scholar]
- Palade GE, Bruns RR. Structural modulations of plasmalemmal vesicles. J Cell Biol. 1968;37:633–649. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Jung KI, Na KS, Park SH, Park CK. Visual field characteristics in normal-tension glaucoma patients with autonomic dysfunction and abnormal peripheral microcirculation. Am J Ophthalmol. 2012;154:466–475. e461. doi: 10.1016/j.ajo.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol. 2016;27:94–101. doi: 10.1097/ICU.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino RM. Restriction to endogenous plasma proteins by a fenestrated capillary endothelium: an ultrastructural immunocytochemical study of the choriocapillary endothelium. Am J Anat. 1985;172:279–289. doi: 10.1002/aja.1001720403. [DOI] [PubMed] [Google Scholar]
- Pino RM, Essner E. Permeability of rat choriocapillaris to hemeproteins. Restriction of tracers by a fenestrated endothelium. J Histochem Cytochem. 1981;29:281–290. doi: 10.1177/29.2.7252121. [DOI] [PubMed] [Google Scholar]
- Predescu D, Horvat R, Predescu S, Palade GE. Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc Natl Acad Sci U S A. 1994;91:3014–3018. doi: 10.1073/pnas.91.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L, Brightman MW. The sarcolemma of Aplysia smooth muscle in freeze-fracture preparations. Tissue Cell. 1976;8:241–258. [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;25(Suppl):27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- Raviola G, Butler JM. Unidirectional vesicular transport mechanism in retinal vessels. Invest Ophthalmol Vis Sci. 1983;24:1465–1474. [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan A, Gu X, Hauck SM, Ash JD, Cao G, Thompson TC, Elliott MH. Retinal Caveolin-1 Modulates Neuroprotective Signaling. Adv Exp Med Biol. 2016;854:411–418. doi: 10.1007/978-3-319-17121-0_54. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy T, Rittenberg A, Dwyer M, D'Ortona S, Pier G, Gadjeva M. Homotrimeric macrophage migration inhibitory factor (MIF) drives inflammatory responses in the corneal epithelium by promoting caveolin-rich platform assembly in response to infection. J Biol Chem. 2013;288:8269–8278. doi: 10.1074/jbc.M112.351064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim JH, Kim JH, Yeo EJ, Kim JC, Park SC. Caveolin-1 as a novel indicator of wound-healing capacity in aged human corneal epithelium. Mol Med. 2010;16:527–534. doi: 10.2119/molmed.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe B, Rosengren BI, Carlsson O, Venturoli D. Transendothelial transport: the vesicle controversy. J Vasc Res. 2002;39:375–390. doi: 10.1159/000064521. [DOI] [PubMed] [Google Scholar]
- Rodriguez NE, Gaur U, Wilson ME. Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cell Microbiol. 2006;8:1106–1120. doi: 10.1111/j.1462-5822.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Muller glial cells. J Comp Neurol. 2008;509:225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Stadler MB, Cepko CL. Gene expression changes within Muller glial cells in retinitis pigmentosa. Mol Vis. 2012;18:1197–1214. [PMC free article] [PubMed] [Google Scholar]
- Rong SS, Chen LJ, Leung CK, Matsushita K, Jia L, Miki A, Chiang SW, Tam PO, Hashida N, Young AL, Tsujikawa M, Zhang M, Wang N, Nishida K, Pang CP. Ethnic specific association of the CAV1/CAV2 locus with primary open-angle glaucoma. Sci Rep. 2016;6:27837. doi: 10.1038/srep27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren BI, Rippe A, Rippe C, Venturoli D, Sward K, Rippe B. Transvascular protein transport in mice lacking endothelial caveolae. Am J Physiol Heart Circ Physiol. 2006;291:H1371–1377. doi: 10.1152/ajpheart.01364.2005. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rujoi M, Jin J, Borchman D, Tang D, Yappert MC. Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Invest Ophthalmol Vis Sci. 2003;44:1634–1642. doi: 10.1167/iovs.02-0786. [DOI] [PubMed] [Google Scholar]
- Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–148. doi: 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- Sabah JR, Schultz BD, Brown ZW, Nguyen AT, Reddan J, Takemoto LJ. Transcytotic passage of albumin through lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:1237–1244. doi: 10.1167/iovs.06-0620. [DOI] [PubMed] [Google Scholar]
- Sagaties MJ, Raviola G, Schaeffer S, Miller C. The structural basis of the inner blood-retina barrier in the eye of Macaca mulatta. Invest Ophthalmol Vis Sci. 1987;28:2000–2014. [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yaghoobzadeh Y, Hu J, Ljubimova JY, Black KL, Castro MG, Ljubimov AV. Adenovirus-driven overexpression of proteinases in organ-cultured normal human corneas leads to diabetic-like changes. Brain Res Bull. 2010;81:262–272. doi: 10.1016/j.brainresbull.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci U S A. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling JM, Roth DM, Patel HH. Caveolins in cardioprotection - translatability and mechanisms. Br J Pharmacol. 2015;172:2114–2125. doi: 10.1111/bph.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann RO, Hofman P, Anderson L, Troost D, van der Gaag R. Vascular expression of endothelial antigen PAL-E indicates absence of blood-ocular barriers in the normal eye. Ophthalmic Res. 1997;29:130–138. doi: 10.1159/000268007. [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, Hofman P, Vrensen GF, Blaauwgeers HG. Increased expression of endothelial antigen PAL-E in human diabetic retinopathy correlates with microvascular leakage. Diabetologia. 1999;42:596–602. doi: 10.1007/s001250051200. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Senin II, Hoppner-Heitmann D, Polkovnikova OO, Churumova VA, Tikhomirova NK, Philippov PP, Koch KW. Recoverin and rhodopsin kinase activity in detergent-resistant membrane rafts from rod outer segments. J Biol Chem. 2004;279:48647–48653. doi: 10.1074/jbc.M402516200. [DOI] [PubMed] [Google Scholar]
- Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada Y, Yoshiyama W, Liu H, Hayashi F. Light- and guanosine 5'-3-O-(thio)triphosphate-sensitive localization of a G protein and its effector on detergent-resistant membrane rafts in rod photoreceptor outer segments. J Biol Chem. 2001;276:20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- Sethna S, Chamakkala T, Gu X, Thompson TC, Cao G, Elliott MH, Finnemann SC. Regulation of Phagolysosomal Digestion by Caveolin-1 of the Retinal Pigment Epithelium is Essential for Vision. J Biol Chem. 2016;291:6494–6506. doi: 10.1074/jbc.M115.687004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton PS, Neely AR, Cenedella RJ. Distribution of caveolin-1 in bovine lens and redistribution in cultured bovine lens epithelial cells upon confluence. Exp Eye Res. 2004;78:75–82. doi: 10.1016/j.exer.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Sharma S, Singh M, Sharma PL. Ameliorative effect of daidzein: a caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J Exp Biol. 2012;50:28–34. [PubMed] [Google Scholar]
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Shvets E, Bitsikas V, Howard G, Hansen CG, Nichols BJ. Dynamic caveolae exclude bulk membrane proteins and are required for sorting of excess glycosphingolipids. Nat Commun. 2015;6:6867. doi: 10.1038/ncomms7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Weiler JM, Boujaoude M, Woodman OL. Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17beta-estradiol. J Pharmacol Exp Ther. 2004;310:135–140. doi: 10.1124/jpet.103.063255. [DOI] [PubMed] [Google Scholar]
- Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, Ghisdal P, Grégoire V, Dessy C, Balligand JL, Feron O. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res. 2004;95:154–161. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- Sorensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639–2647. doi: 10.1097/01.asn.0000033277.32822.23. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- Sparks J, Slobodkin G, Matar M, Congo R, Ulkoski D, Rea-Ramsey A, Pence C, Rice J, McClure D, Polach KJ, Brunhoeber E, Wilkinson L, Wallace K, Anwer K, Fewell JG. Versatile cationic lipids for siRNA delivery. J Control Release. 2012;158:269–276. doi: 10.1016/j.jconrel.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Braakman ST, Zhou EH, Ethier CR, Fredberg JJ, Overby DR, Johnson M. Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction. Prog Retin Eye Res. 2015;44:86–98. doi: 10.1016/j.preteyeres.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary CM, Tsutsumi YM, Patel PM, Head BP, Patel HH, Roth DM. Caveolins: targeting pro-survival signaling in the heart and brain. Front Physiol. 2012;3:393. doi: 10.3389/fphys.2012.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA, Tuor UI. Blood-eye barriers in the rat: correlation of ultrastructure with function. J Comp Neurol. 1994;340:566–576. doi: 10.1002/cne.903400409. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Bhaduri T, McMullen CB, Gardiner TA, Archer DB. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell Biol Res Commun. 2000a;3:380–388. doi: 10.1006/mcbr.2000.0243. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Burke GA, Chen F, McMullen CB, Vlassara H. Advanced glycation end-product receptor interactions on microvascular cells occur within caveolin-rich membrane domains. FASEB J. 2000b;14:2390–2392. doi: 10.1096/fj.00-0289fje. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Surgucheva I, Surguchov A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol Vis. 2011;17:2878–2888. [PMC free article] [PubMed] [Google Scholar]
- Tahir SA, Kurosaka S, Tanimoto R, Goltsov AA, Park S, Thompson TC. Serum caveolin-1, a biomarker of drug response and therapeutic target in prostate cancer models. Cancer Biol Ther. 2013;14:117–126. doi: 10.4161/cbt.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, Goltsov A, Ittmann M, Morrisett JD, Thompson TC. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–3885. [PubMed] [Google Scholar]
- Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Braunger BM, Fuchshofer R. Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways. Prog Mol Biol Transl Sci. 2015;134:301–314. doi: 10.1016/bs.pmbts.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Tencer L, Burgermeister E, Ebert MP, Liscovitch M. Rosiglitazone induces caveolin-1 by PPARgamma-dependent and PPRE-independent mechanisms: the role of EGF receptor signaling and its effect on cancer cell drug resistance. Anticancer Res. 2008;28:895–906. [PubMed] [Google Scholar]
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tian XF, Xia XB, Xu HZ, Xiong SQ, Jiang J. Caveolin-1 expression regulates blood-retinal barrier permeability and retinal neovascularization in oxygen-induced retinopathy. Clin Exp Ophthalmol. 2012;40:e58–66. doi: 10.1111/j.1442-9071.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- Tsai TH, Chen SF, Huang TY, Tzeng CF, Chiang AS, Kou YR, Lee TS, Shyue SK. Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll-like receptor 4-Myd88 signaling in caveolin-1-deleted macrophages and mice. Shock. 2011;35:92–99. doi: 10.1097/SHK.0b013e3181ea45ca. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Le Y, Chollangi S, Muller W, Ash J. Preconditioning-induced protection of photoreceptors requires activation of the signal-transducing receptor gp130 in photoreceptors. Proc Natl Acad Sci U S A. 2009;106:21389–21394. doi: 10.1073/pnas.0906156106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koolwijk LM, Ramdas WD, Ikram MK, Jansonius NM, Pasutto F, Hysi PG, Macgregor S, Janssen SF, Hewitt AW, Viswanathan AC, ten Brink JB, Hosseini SM, Amin N, Despriet DD, Willemse-Assink JJ, Kramer R, Rivadeneira F, Struchalin M, Aulchenko YS, Weisschuh N, Zenkel M, Mardin CY, Gramer E, Welge-Lussen U, Montgomery GW, Carbonaro F, Young TL, Group DER, Bellenguez C, McGuffin P, Foster PJ, Topouzis F, Mitchell P, Wang JJ, Wong TY, Czudowska MA, Hofman A, Uitterlinden AG, Wolfs RC, de Jong PT, Oostra BA, Paterson AD, Mackey DA, Bergen AA, Reis A, Hammond CJ, Vingerling JR, Lemij HG, Klaver CC, van Duijn CM Wellcome Trust Case Control C. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8:e1002611. doi: 10.1371/journal.pgen.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang X, Zhang L, He F, Zhang G, Jamrich M, Wensel TG. Activation-dependent hindrance of photoreceptor G protein diffusion by lipid microdomains. J Biol Chem. 2008;283:30015–30024. doi: 10.1074/jbc.M803953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wiggs JL. Common and rare genetic risk factors for glaucoma. Cold Spring Harb Perspect Med. 2014;4:a017244. doi: 10.1101/cshperspect.a017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- Way M, Parton RG. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1996;378:108–112. doi: 10.1016/0014-5793(96)82884-5. [DOI] [PubMed] [Google Scholar]
- Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Kang JH, Yaspan BL, Mirel DB, Laurie C, Crenshaw A, Brodeur W, Gogarten S, Olson LM, Abdrabou W, DelBono E, Loomis S, Haines JL, Pasquale LR Consortium G. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20:4707–4713. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Carmichael TR, Allingham RR, Hauser M, Ramsay M. The genetics of POAG in black South Africans: a candidate gene association study. Sci Rep. 2015;5:8378. doi: 10.1038/srep08378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska-Kruk J, Klaassen I, Vogels IM, Magno AL, Lai CM, Van Noorden CJ, Schlingemann RO, Rakoczy EP. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res. 2014;122:123–131. doi: 10.1016/j.exer.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Wisniewska-Kruk J, van der Wijk AE, van Veen HA, Gorgels TG, Vogels IM, Versteeg D, Van Noorden CJ, Schlingemann RO, Klaassen I. Plasmalemma Vesicle-Associated Protein Has a Key Role in Blood-Retinal Barrier Loss. Am J Pathol. 2016;186:1044–1054. doi: 10.1016/j.ajpath.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004;44:155–163. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- Woodman SE, Ashton AW, Schubert W, Lee H, Williams TM, Medina FA, Wyckoff JB, Combs TP, Lisanti MP. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003;162:2059–2068. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle PL. Cholesterol modulation of (Na+ + K+)-ATPase ATP hydrolyzing activity in the human erythrocyte. Biochim Biophys Acta. 1983;727:39–44. doi: 10.1016/0005-2736(83)90366-8. [DOI] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf MA, Zhou X, Mukherjee S, Chintakuntlawar AV, Lee JY, Ramke M, Chodosh J, Rajaiya J. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One. 2013;8:e77462. doi: 10.1371/journal.pone.0077462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Gu X, Crabb JS, Yue X, Shadrach K, Hollyfield JG, Crabb JW. Quantitative proteomics: comparison of the macular Bruch membrane/choroid complex from age-related macular degeneration and normal eyes. Mol Cell Proteomics. 2010;9:1031–1046. doi: 10.1074/mcp.M900523-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]