SUMMARY
Obesity and obstructive sleep apnea (OSA) have a reciprocal relationship. Sleep disruptions characteristic of OSA may promote behavioral, metabolic, and/or hormonal changes favoring weight gain and/or difficulty losing weight. The regulation of energy balance (EB), i.e., the relationship between energy intake (EI) and energy expenditure (EE), is complex and multi-factorial, involving food intake, hormonal regulation of hunger/satiety/appetite, and EE via metabolism and physical activity (PA). The current systematic review describes the literature on how OSA affects EB-related parameters. OSA is associated with a hormonal profile characterized by abnormally high leptin and ghrelin levels, which may encourage excess EI. Data on actual measures of food intake are lacking, and not sufficient to make conclusions. Resting metabolic rate appears elevated in OSA vs. controls. Findings on PA are inconsistent, but may indicate a negative relationship with OSA severity that is modulated by daytime sleepiness and body weight. A speculative explanation for the positive EB in OSA is that the increased EE via metabolism induces an overcompensation in the drive for hunger/food intake, which is larger in magnitude than the rise in EI required to re-establish EB. Understanding how OSA affects EB-related parameters can help improve weight loss efforts in these patients.
Keywords: Sleep apnea, Obesity, Energy expenditure, Appetite-regulating hormones, Food intake
Introduction
Obesity is a well-established leading risk factor for obstructive sleep apnea (OSA), and OSA itself may promote further weight gain [1]. Although the reasons underlying this reciprocal relationship remain uncertain, evidence from observational and laboratory-based studies demonstrates a relationship between sleep and factors regulating body weight, such as food intake and physical activity (PA) [2]. Sleep disruptions characteristic of OSA are expected to be associated with behavioral, metabolic, and/or hormonal changes favoring weight gain and/or difficulty losing weight. Supporting evidence comes from the finding that newly diagnosed OSA patients have a history of weight gain vs. sex, age, and body mass index (BMI)-matched controls over the year prior to diagnosis [3]. Furthermore, compared to BMI-matched non-OSA individuals, OSA patients with visceral obesity had a smaller decrease in BMI and fat mass in response to a lifestyle intervention for weight loss [4].
When examining the effects of OSA on obesity, it is important to consider the factors regulating energy balance (EB). Body weight gain is expected as a consequence of excessively increased food intake and/or reduced PA [5]. Body weight stability is achieved when energy intake (EI) is equal to energy expenditure (EE). Thus, EB is the quantifiable relationship between the intake and output of energy from the body.
The current goal is to explore if OSA alters behavior, hormones, and/or metabolism to encourage an energy imbalance, such that EI is increased relative to EE, and to examine the functional implications of the disorder on EB regulation. This report will focus on investigations of outcomes like food intake, the hormonal regulation of hunger/appetite/satiety, PA levels, and energy metabolism in OSA patients, to determine if there is a dysregulation of EB-related parameters in these patients. A particular focus will be on methodological differences among studies, and how these may contribute to discrepancies.
Methods
Search strategy
A systematic literature review was conducted to identify manuscripts which investigated aspects of EB regulation, namely parameters related to either EE or EI, in OSA patients. The web-based literature search included PubMed/MEDLINE and Embase databases. Search terms were selected to reflect the condition and outcome parameters. For the condition, search terms included: sleep apnea OR sleep apnoea OR sleep disordered breathing OR CPAP (continuous positive airway pressure) OR positive airway pressure. For EI, search terms included: caloric intake; food intake; food preference; dietary quality; macronutrient; hunger; appetite; satiety; hunger hormone; appetite hormone; satiety hormone. For EE, search terms included: energy expenditure; thermogenesis; energy metabolism; physical activity. Terms were searched in all possible combinations using Boolean Logic operators. Additionally, a manual search of bibliographies of included articles was conducted to identify relevant references which may not have been found by the automated search. Obtained references were indexed and managed using EndNote X7 (Thompson Reuters, New York, NY).
Eligibility criteria
The following criteria were required for selection: 1) original research investigations; 2) conducted in humans; 3) conducted in adults; 4) include patients diagnosed with OSA of at least mild severity (apnea hypopnea index [AHI] ≥5 events/h) based on polysomnography (PSG). Studies were included if they were between-group comparisons of OSA patients vs. controls or if they included a group of OSA patients without a control group and examined them using regression analysis.
Studies were excluded if they were in diagnosed OSA patients but did not include a relevant EB-related parameter (i.e., food intake, hunger/appetite, EE, energy metabolism, PA), if they did not include a PSG-based diagnosis of OSA, and if they examined the effects of CPAP but did not include an examination of OSA vs. control at baseline.
To help reduce risk of source selection bias, there were no restrictions on date or country of origin of research/publication. Specified EB-related parameters could be secondary/minor outcomes within reports. Reviews, commentaries, editorials, letters to the editor, and case reports were not included. In addition to a “forward” (i.e., utilizing databases and search terms) search strategy, a “backward” or ancestry search strategy was utilized such that the reference list of all relevant reports was searched to include references missed by automatic search. Despite these efforts, having a single author conduct the literature search and data extraction might have unintentionally led to some degree of selection bias.
Data items
To be selected, studies must have included at least one relevant EB-related parameter within OSA patients. For EI, this included circulating hormones known to regulate hunger/satiety/appetite, and measures of food intake or habitual dietary patterns. For EE, this included measures from indirect calorimetry (IC), accelerometry, and questionnaires on PA levels.
Reviewing procedure and data extraction
Database searches were first conducted in December 2015. In May 2016, the search was conducted again to identify references published between December 2015 and May 2016. All obtained references were reviewed, and if retained, data extraction was conducted by a single author. The first level of review was title and abstract screening. Irrelevant references were removed. Potentially relevant studies were further assessed by obtaining and reading the full text and checking against the pre-specified eligibility criteria.
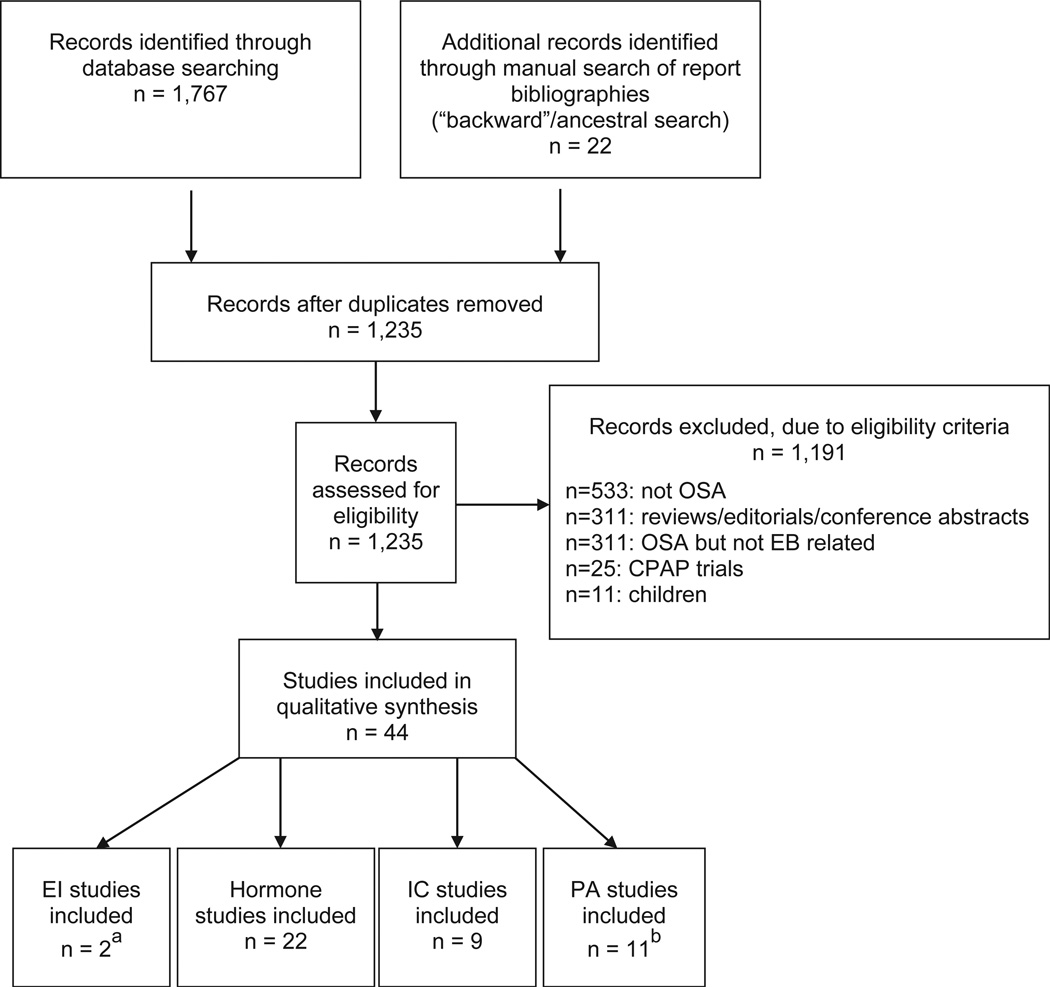
For each reference, the following variables were systematically extracted and entered into a summary table: 1) authors; 2) year of publication; 3) journal; 4) sample size; 5) criteria and cut-offs used to define OSA and control; 6) age and BMI; 7) study design; 8) outcomes; 9) time of assessment and if done under fasting conditions; 10) statistical approaches; 11) findings, including individual outcome variables (e.g., means, confidence intervals) and whether any confounder adjustments were added to statistical analyses. The principal summary measures were between-group differences in means for outcomes (OSA vs. controls), and regression results indicating relationships between OSA severity and EB-related outcome variables. A summary of the studies screened, assessed for eligibility, and included is presented in Fig. 1. Additionally, a Newcastle-Ottawa scale (NOS) score was assigned for case–control studies, [6]. NOS scores (up to nine stars, with increasing number for increasing quality of non-randomized studies) are indicated in Tables 1–3.
Fig. 1.
Flow chart for selection of references included in systematic review. CPAP: continuous positive sirway pressure; EB: energy balance; EI: energy intake; IC: indirect calorimetry; OSA: obstructive sleep apnea; PA: physical activity. a One study included in the EI category also contained data on PA. b One study included in the PA category also contained data on IC.
Table 1.
Summary of hormones regulating food intake in OSA patients.
| Author, year (reference#) [NOS rating] |
Participants | AHI cutoff, events/h |
Sample size | Age (SD), years |
BMI (SD), kg/m2 |
Design | Outcomes | Main findings |
|---|---|---|---|---|---|---|---|---|
| Leptin findings | ||||||||
| Barcelo et al., 2005 [10] [*******] |
OSA obese OSA nonobese Cx obese Cx nonobese |
≥20 ≥20 N/R N/R |
n = 23 n = 24 n = 19 n = 18 |
47 (9.6) 50 (9.8) 44 (13.1) 47 (4.2) |
34.9 (3.4) 25.9 (2.0) 33.6 (2.6) 25.5 (2.1) |
Btw-group comparison |
Leptin AM, fasted |
↑ Leptin in non-obese OSA vs. non-obese Cx (mean difference: −6.0 ng/ml; 95% CI: −12.2 to 0.1 ng/ml) ↔ Leptin in obese OSA vs. obese Cx (mean difference: −0.6 ng/ml; 95% CI: −6.5–5.3 ng/ml) |
| Basoglu et al., 2011 [11] [******] |
OSA Cx |
≥5 <5 |
n = 36 n = 34 |
50 (19.7) 49.7 (11.1) |
33.5 (5.7) 34.5 (2.9) |
Btw-group comparison |
Leptin fasted |
↑ Leptin in OSA vs. Cx (mean [SD]: 13.1 [1.7] vs. 9.0 [1.9] ng/ml) |
| Chihara et al., 2015 [20] [*******] |
OSA Cx |
≥15 <15 |
n = 39 n = 15 |
54.6 (12.4) 54.3 (14.3) |
26.5 (3.9) 26.2 (3.0) |
Btw-group comparison |
Leptin AM, fasted; PP |
↔ Leptin in OSA vs. Cx (mean [SD]: 7.1 [4.5] vs. 6.5 [3.8] ng/ml) |
| Harsch et al., 2003 [12] [*******] |
OSA Cx |
≥20 <5 |
n = 30 n = 30 |
52 (11.0) 51 (5.5) |
32.6 (5.5) 30.6 (3.3) |
Btw-group comparison |
Leptin AM, fasted |
↑ Leptin (adjusted for BMI and ESS) in OSA vs. Cx (mean [geometrical SEM]: 12.7 [10.7–14.9] vs. 4.4 [3.6–5.4] ng/ml) |
| Ip et al., 2000 [13] [*******] |
OSA Cx |
≥5 <5 |
n = 30 n = 30 |
43.6 (10.1) 41.9 (7.4) |
27.0 (2.9) 26.5 (2.1) |
Btw-group comparison |
Leptin AM, fasted |
↑ Leptin in OSA vs. Cx (mean [SD]: 9.2 [4.2] vs. 6.5 [3.8] ng/ml) |
| Kapsimalis et al., 2008 [14] [*******] |
severe OSA mild-mod OSA Cx |
≥30 5–29 <5 |
n = 26 n = 26 n = 15 |
55.3 (11.6) 50.5 (13.8) 47.0 (12.5) |
30.6 (3.4) 29.4 (3.8) 28.7 (4.3) |
Btw-group comparison |
Leptin AM, fasted |
↑ Leptin in OSA vs. Cx (mean [SD]: 21.6 [19.7] vs. 9.4 [6.5] IU/ml) |
| Ozturk et al., 2003 [15] [*******] |
severe OSA mild OSA Cx |
>20 5–20 <5 |
n = 8 n = 12 n = 12 |
47 (12) 49 (11) 45 (14) |
30.8 (2.7) 31.0 (3.7) 28.5 (4.5) |
Btw-group comparison |
Leptin AM, fasted |
↑ Leptin in severe OSA vs. mild OSA vs. Cx (mean [SD]: 21.2 [8.6] vs. 16.2 [5.2] vs. 10.6 (7.5) ng/ml) |
| Papaioannou et al., 2011 [16] [********] |
OSA Cx |
x̄ = 22.4 x̄ = 2.5 |
n = 68 n = 37 |
49 (9.9) 45 (10.2) |
30 (4.6) 28 (4.5) |
Btw-group comparison |
Leptin AM and PM, fasted |
↑ Leptin in OSA vs. Cx, PM (mean [IQR]: 9.6 [6.8, 15] vs. 7.9 [4, 12.9] ng/ml) |
| Patel et al. 2004 [22] [N/A] |
OSA | ≥5 | n = 138 | 44.2 (18.7) | 32.2 (8.5) | regression | Leptin AM and PM, fasted |
PM/AM leptin ratio (adjusted for age, sex, race) positively associated with AHI (linear trend p-value: 0.01) |
| Phillips et al., 2000 [17] [********] |
OSA Cx |
≥10 <10 |
n = 32 n = 32 |
43 (11.3) 38 (11.3) |
33 (5.7) 32 (5.7) |
Btw-group comparison |
Leptin PM, fasted |
↑ Leptin in OSA vs. Cx (mean [SEM]: 13.7 [1.3] vs. 9.2 [1.2] ng/ml) |
| Sanchez-de-la-Torre et al. 2011 [23] [*******] |
OSA, ESS ≥13 OSA, ESS <9 |
≥5 ≥5 |
n = 132 n = 132 |
50.15 (11.27) 50.72 (9.96) |
31.85 (5.68) 32.18 (4.84) |
Btw-group comparison |
Leptin AM, fasted |
↔ Leptin in OSA w/EDS vs. OSA without EDS (mean [SD]: 11.5 [10.6] vs. 10.1 [13.3] ng/ml) |
| Ulukavak Ciftci et al., 2005 [18] [*******] |
OSA Cx |
≥15 <5 |
n = 30 n = 22 |
matched | 32.1 (4.1) 31.0 (3.2) |
Btw-group comparison |
Leptin AM, fasted |
↑ Leptin in OSA vs. Cx (mean [SEM]: 29.42 [13.51] vs. 20.02 [13.45] ng/ml) |
| Ursavas et al., 2010 [21] [********] |
OSA Cx |
≥5 <5 |
n = 55 n = 15 |
51.1 (8.9) 48.4 (11.6) |
32.5 (6.7) 31.6 (7.0) |
Btw-group comparison |
Leptin AM, fasted |
↔ Leptin in OSA vs. Cx (mean [SEM]: 10.9 [2.7] vs. 9.4 [0.9] ng/ml) |
| Vgontzas et al., 2000 [19]a [********] |
OSA Cx obese Cx nonobese |
>20 <5 <5 |
n = 14 n = 11 n = 12 |
46.6 (11.2) 40.2 (7.3) 45.4 (9.7) |
38.4 (6.0) 36.2 (8.0) 26.0 (2.8) |
Btw-group comparison |
Leptin AM, fasted; PM |
↑ Leptin in OSA vs. Cx obese, AM (mean [SEM]: 24.67 [3.13] vs. 15.92 [2.60] ng/ml) ↔ Leptin in OSA vs. Cx obese, PM (mean [SEM]: 26.96 [3.39] vs. 17.12 [2.83] ng/ml) ↑ Leptin in OSA vs. Cx nonobese, AM (mean [SEM]: 24.67 [3.13] vs. 8.09 [1.60] ng/ml) and PM (mean [SEM]: 26.96 [3.39] vs. 7.86 [2.93] ng/ml) |
| Ghrelin findings | ||||||||
| Chihara et al., 2015 [20] [*******] |
OSA Cx |
≥15 <15 |
n = 39 n = 15 |
54.6 (12.4) 54.3 (14.3) |
26.5 (3.9) 26.2 (3.0) |
Btw-group comparison |
Ghrelin AM, fasted; PP |
↑ Ghrelin in OSA vs. Cx, AM and PP (mean [SD]: acyl: 11.4 [9.6] vs. 3.3 [1.8] fmol/ml; desacyl: 38.7 [20.9] vs. 19.5 [10.9] fmol/ml) |
| Harsch et al., 2003 [12] [*******] |
OSA Cx |
≥20 <5 |
n = 9 n = 9 |
54 (6) 49 (6) |
33.0 (4.2) 33.9 (3.9) |
Btw-group comparison |
Ghrelin AM, fasted |
↑ Ghrelin (adjusted for BMI, ESS) in OSA vs. Cx (mean [geometrical SEM]: 57.9 [46.0–72.9] vs. 10.8 [7.6–15.3] pg/ul) |
| Sanchez-de-la-Torre et al. 2011 [23] [*******] |
OSA, ESS ≥13 OSA, ESS <9 |
≥5 ≥5 |
n = 132 n = 132 |
50.15 (11.27) 50.72 (9.96) |
31.85 (5.68) 32.18 (4.84) |
Btw-group comparison |
Ghrelin AM, fasted |
↓ Ghrelin in OSA with EDS vs. OSA without EDS (mean [SD]: 6.3 [3.5] vs. 8.9 [5.7] ng/ml) |
| Takahashi et al., 2008 [24] [******] |
OSA Cx |
x̄ = 46.2 N/R |
n = 21 n = 13 |
52.5 (8.7) matched |
28.8 (3.8) 24.5 (3.0) |
Btw-group comparison |
Ghrelin AM, fasted |
↑ Ghrelin in OSA vs. Cx acylated (mean [SD]: 11.4 [5.86] vs. 7.19 [3.80] fmol/mL); desacyl (mean [SD]: 84.2 [50.6] vs. 48.3 [23.2] fmol/mL) |
| Ulukavak Ciftci 2005 [18] [*******] |
OSA Cx |
≥15 <5 |
n = 30 n = 22 |
matched | 32.1 (4.1) 31.0 (3.2) |
Btw-group comparison |
Ghrelin AM, fasted |
↔ Ghrelin in OSA vs. Cx (mean [SEM]: 130.8 [2] vs. 130.5 [2.4] pg/ml) |
| Ursavas et al., 2010 [21] [********] |
OSA Cx |
≥5 <5 |
n = 55 n = 15 |
51.1 (8.9) 48.4 (11.6) |
32.5 (6.7) 31.6 (7.0) |
Btw-group comparison |
Ghrelin AM, fasted |
↑ Ghrelin in OSA vs. Cx (mean [SEM]: 564 [44] vs. 403 [90] pg/mL) |
| Yang et al., 2013 [25] [********] |
OSA Cx |
≥5 <5 |
n = 25 n = 25 |
54.0 (7.0) 53.0 (7.0) |
27.39 (2.9) 26.27 (1.9) |
Btw-group comparison |
Ghrelin AM, fasted |
↔ Ghrelin in OSA vs. Cx (p > 0.05) |
| Orexin findings | ||||||||
| Aksu et al., 2009 [26] [******] |
OSA Cx |
≥15 N/R |
n = 41 n = 35 |
50.6 (8.4) 41.6 (9.9) |
30.1 (4.3) 28.3 (5.9) |
Btw-group comparison |
Orexin AM, fasted |
↓ Orexin in OSA vs. Cx (mean [SE]: 15.0 [4.6] vs. 31.4 [6.5] ng/ml) |
| Busquets et al., 2004 [27] [*****] |
OSA Cx |
x̄ = 54 N/R |
n = 27 n = 13 |
52 (10.4) 46 (7.2) |
31 (5.2) 24 (3.6) |
Btw-group comparison |
Orexin 12pm |
↓ Orexin in OSA vs. Cx (mean [SEM]: 9.4 [1.9] vs. 20.6 [4.5] pg/ml) |
| Igarashi et al., 2003 [30] [*******] |
OSA Cx |
≥5 <5 |
n = 30 n = 20 |
45.3 (13.7) 46.5 (13.0) |
28.6 (4.9) 26.4 (4.9) |
Btw-group comparison |
Orexin AM, fasted |
↑ Orexin in OSA vs. Cx (mean [SEM]: 36.3 [1.2] vs. 32.3 [1.3] pg/mL) |
| Nishijima et al., 2003 [28] [*******] |
OSA Cx |
≥5 <5 |
n = 156 n = 22 |
52.7 (13.7) 45.7 (12.7) |
28.3 (6.2) 27.3 (5.2) |
Btw-group comparison |
Orexin AM, fasted |
↓ Orexin in OSA vs. Cx (mean [SEM]: 4.4 [0.15] vs. 5.3 [0.45] pmol/l) |
| Sakurai et al., 2004 [29] [*******] |
OSA Cx |
≥5 <5 |
n = 14 n = 5 |
55.6 (2.9) 55.2 (6.0) |
29.0 (1.5) 25.2 (2.0) |
Btw-group comparison |
Orexin Afternoon, fasted |
↓ Orexin in OSA vs. Cx (mean [SEM]: 4.9 [0.8] vs. 12.3 [1.9] pmol/l) |
| Sanchez-de-la-Torre et al. 2011 [23] [*******] |
OSA, ESS ≥13 OSA, ESS <9 |
≥5 ≥5 |
n = 132 n = 132 |
50.15 (11.27) 50.72 (9.96) |
31.85 (5.68) 32.18 (4.84) |
Btw-group comparison |
Orexin AM, fasted |
↑ Orexin in OSA w/EDS vs. OSA without EDS (mean [SD]: 2.5 [0.3] vs. 1.6 [0.2] ng/ml) |
| NPY findings | ||||||||
| Barcelo et al., 2005 [10] [*******] |
OSA obese OSA nonobese Cx obese Cx nonobese |
≥20 ≥20 N/R N/R |
n = 23 n = 24 n = 19 n = 18 |
47 (9.6) 50 (9.8) 44 (13.1) 47 (4.2) |
34.9 (3.4) 25.9 (2.0) 33.6 (2.6) 25.5 (2.1) |
Btw-group comparison |
NPY AM, fasted |
↑ NPY in non-obese OSA vs. nonobese Cx (mean difference: −25.5 pmol/L; 95% CI: −41.2 to −9.8 pmol/L) ↑ NPY in obese OSA vs. obese Cx (mean difference: −20.4 pmol/L; 95% CI: −35.2 to −5.5 pmol/L) |
| Carlson et al., 1993 [31] [****] |
OSA Cx |
N/R N/R |
n = 11 n = 9 |
45.0 (10) 54.0 (12) |
30 (3.3) 25 (3) |
Btw-group comparison |
NPY, afternoon, non-fasted |
↔ NPY in OSA vs. Cx (p > 0.05) |
| Papaioannou et al., 2011 [16] [********] |
OSA Cx |
x̄ = 22.4 x̄ = 2.5 |
n = 68 n = 37 |
49 (9.9) 45 (10.2) |
30 (4.6) 28 (4.5) |
Btw-group comparison |
NPY AM and PM, fasted |
↑ NPY in OSA vs. Cx, PM (mean [SD]: 56.6 [52, 67] vs 51.1 [47.3, 61] pmol/L) |
| Sanchez-de-la-Torre et al. 2011 [23] [*******] |
OSA, ESS ≥13 OSA, ESS <9 |
≥5 ≥5 |
n = 132 n = 132 |
50.15 (11.27) 50.72 (9.96) |
31.85 (5.68) 32.18 (4.84) |
Btw-group comparison |
NPY AM, fasted |
↔ NPY in OSA w/EDS vs. OSA without EDS (mean [SD]: 0.9 [0.1] vs. 1.0 [0.1] ng/ml) |
AHI: apnea-hypopnea index; AM: morning; BMI: body mass index; CI: confidence interval; Cx: control; EDS: excessive daytime sleepiness; ESS: Epworth sleepiness scale; IQR: interquartile range; NPY: neuropeptide Y; N/R: not reported; NOS: Newcastle-Ottawa scale (up to nine stars); OSA: obstructive sleep apnea; PM: evening; PP: post-prandial; SD: standard deviation; SEM: standard error of the mean; ↑: significant increase; ↓: significant decrease; ↔: no difference.
leptin values here are reported as ng/ml, correcting a likely typo in the original article which indicated values as pg/ml.
Table 3.
Summary of physical activity levels in OSA patients.
| Author, year (reference#) [NOS rating] |
Participants | AHI cutoff, events/h |
Sample size |
Age (SD), years |
BMI (SD), kg/m2 |
Design | Outcomes | Main findings |
|---|---|---|---|---|---|---|---|---|
| Questionnaire-based assessment | ||||||||
| Basta et al., 2008 [53] [****] |
OSA, ESS >16 OSA, ESS 11–16 OSA, ESS <10 |
≥5 ≥5 ≥5 |
n = 193 n = 385 n = 528 |
~50.5 ~50.0 ~52.4 |
~37.8 ~38.6 ~36.8 |
Btw-group comparison |
PA Questionnaire | In men, ↑ exercise rate in OSA ESS <10 vs. OSA ESS 11–16 and OSA ESS >16 (regular exercise %: 43.7 vs. 36.9 vs. 30.3%); and in OSA ESS 11–16 vs. OSA ESS >16 (regular exercise %: 36.9 vs. 30.3%); |
| Beitler et al., 2004 [43] [*******] |
OSA Cx |
≥15 <15 |
n = 15 n = 19 |
47.9 (11.5) 34.3 (12.0) |
32.2 (7.8) 28.8 (6.5) |
Btw-group comparison |
PA Questionnaire | ↔ PA in OSA vs. Cx (median [IQR]: 2341 [1386, 4164] vs. 3936 [1260, 6492] MET-min/wk) |
| Hong et al. 2003 [50] [N/A] |
OSA | RDI ≥10 | n = 38 | 49.0 (8.9) | 30.1 (4.9) | regression | PA Questionnaire | PA negatively (r = −0.30, p = 0.07) associated with RDI |
| Lopes et al., 2008 [52] [****] |
Severe OSA Moderate OSA Mild OSA Cx |
≥30 15–29 5–14 <5 |
n = 488 n = 390 n = 506 n = 508 |
43.0 (13.0) 46.0 (13.0) 45.0 (12.0) 40.0 (12.0) |
31.5 (6.0) 29.5 (5.4) 28.0 (5.1) 26.4 (5.4) |
Btw-group comparison |
PA Questionnaire | ↓ Percentage of participants with severe OSA were physically active vs. Cx (20.14 vs. 31.03%), but BMI and ESS higher in severe OSA vs. Cx |
| Quan et al., 2007 [51] [******] |
No OSA to OSA | N/R | n = 5681 | ~64.5 | – | Btw-group comparison |
PA Questionnaire | ↓ Proportion of participants reporting moderate-to-vigorous PA in OSA vs. no OSA (RDI ≥10 [40 vs. 36%] or ≥15 [40 vs. 33%]) |
| Vasquez et al., 2008 [8] [******] |
No/mild OSA to severe OSA |
N/R | n = 304 | ~51.4 | ~32.36 | regression | PA Questionnaire | ↑ PA-related calories expended (adjusted for ESS, BMI, age) in RDI ≥50 events/h vs. RDI <50 events/h (coefficient: 224.6, 95% CI: 40.98, 408.18) |
| Accelerometry-based assessment | ||||||||
| Bamberga et al., 2015 [59] [********] |
OSA Cx |
≥15 N/R |
n = 107 n = 25 |
56.1 (3.9) 55.6 (4.1) |
35.2 (2.0) 35.0 (2.0) |
Btw-group comparison |
SenseWear Armband | ↑ SenseWear-derived EE in daytime in Cx vs OSA (mean [SD]: 1465.3 [176.5] vs. 1065.9 [131.4] kcal); ↑ SenseWear-derived EE in night-time in OSA vs. Cx (693.8 [69] vs. 556.1 [49.4] kcal) |
| Chasens et al. 2011 [55] [N/A] |
No OSA to OSA | x̄ = 21.7 | n = 37 | 49.5 (11.5) | 34.0 (7.4) | regression | SenseWear Armband | PA (steps counts; corrected for age, sex, BMI) negatively associated with AHI (beta: −64.25, p = 0.01) |
| Hasting et al., 2006 [54] [******] |
OSA Cx |
>15 N/R |
n = 22 n = 17 |
65.6 (10.5) 59.8 (12.5) |
29.4 (6.2) 28.5 (3.5) |
Btw-group comparison |
Wrist accelerometer | ↓ Daytime PA duration in OSA vs. Cx (15.2[1.2] vs. 16.3[1.0] h) |
| Mendelson et al. 2014 [57] [N/A] |
OSA and CVD risk | ≥10 | n = 95 | 63.3 (8.8) | 29.9 (4.9) | regression | SenseWear Armband | PA (daily METs) negatively associated with BMI (r = −0.28, p = 0.007) but not OSA severity |
| O’Driscoll et al., 2013 [58] [N/A] |
No OSA to OSA | x̄ = 24.8 | n = 50 | 45.5 (14.0) | 31.1 (6.4) | regression | SenseWear Armband | SenseWear-derived EE (kcal/h of sleep) positively associated with AHI |
| Verwimp et al., 2013 [56] [N/A] |
OSA, ESS >10 | >20 | n = 75 | 51.0 (10.0) | 36.0 (7.0) | regression | SenseWear Armband | PA (steps counts) negatively associated with REM sleep AHI (r = 0.54, p < 0.001) |
AHI: apnea-hypopnea index; BMI: body mass index; CVD: cardiovascular disease; Cx: control; EE: energy expenditure; ESS: Epworth sleepiness score; MET: metabolic equivalents; N/R: not reported; NOS: Newcastle-Ottawa scale (up to nine stars); OSA: obstructive sleep apnea; PA: physical activity; RDI: respiratory disturbance index; REM: rapid eye movement; SD: standard deviation; ↑: significant increase; ↓: significant decrease.
Results
Food intake and preference
Main findings
Food preference was studied in a group of patients undergoing screening for OSA, with the fiber-liking and fat-liking subscales of the Liking Scale completed in the morning following the diagnostic study [7]. In a hierarchical multiple regression model including sex and BMI, liking for high-fat food was associated with greater OSA severity, based on respiratory disturbance index (RDI) [7]. In terms of actual food intake, based on questionnaire, Vasquez et al. reported baseline findings from participants (n = 305) in the apnea positive pressure long-term efficacy study (APPLES) trial of responses to a Food frequency questionnaire [8]. After adjusting for age, BMI, and Epworth sleepiness scale (ESS) score, individuals with RDI ≥50 events/h consumed significantly more cholesterol per day than those with RDI <50 events/h, and consumption of protein, total fat, and saturated fatty acids was increased in women with RDI ≥50 events/h vs. RDI <50 events/h [8].
Summary/considerations
Findings suggest that OSA severity is associated with unhealthy dietary preference and choices, which might contribute to greater cardiovascular morbidity in patients, though possibly not also contributing to obesity. Food intake is essential to measure when considering EB regulation. Unfortunately, this has been one of the least studied components of EB within adult OSA patients. No studies to date have assessed actual/objective measures of food intake via a test meal or real-time tracking. Food choice and EI should be systematically studied under real-life and laboratory conditions in OSA patients.
Hormonal regulation of food intake
Main findings
Various circulating peptides and hormones play a role in regulating hunger, appetite, satiety, and food intake. These include hypothalamic factors (orexin, neuropeptide Y [NPY]), gut hormones (ghrelin), and adiposity signals (leptin) [9]. Within OSA, leptin, a satiety signal, has been the most extensively studied, and the hunger signals ghrelin, orexin, and NPY have also been investigated.
Findings on the hormonal regulation of food intake are summarized in Table 1. Most reports have been of significantly higher levels of plasma leptin within OSA vs. controls [10–19]. However, some have reported no differences in leptin between OSA and control [20,21]. Each of the aforementioned studies were between-group comparisons of OSA vs. controls, which matched for age and BMI [10–21]. Samples were uniformly collected under fasting conditions, in the morning [10–15,18,20,21], in both the morning and evening [16,19], or in the evening only [17]. It remains unclear why the two studies failed to detect between-group differences in leptin levels [20,21]. In looking at a cross-sectional distribution of leptin secretion across AHI quartiles of different OSA severity, leptin levels in both the morning and evening were positively correlated with AHI when adjusted for age-, sex-, and race, but not after adjustment for BMI [22]. The evening/morning leptin ratio remained significantly associated with AHI after correcting for age, sex, race, BMI, and waist-to-hip ratio [22]. Suggesting a role of obesity, significant differences in leptin levels were found between non-obese OSA and non-obese controls, but not between obese OSA and obese controls [10]. Leptin levels showed a trend (p = 0.07) to be increased in OSA patients with excessive daytime sleepiness (EDS; ESS ≥13) vs. OSA patients without EDS (ESS ≤9) [23].
Most studies which have investigated plasma ghrelin report significantly higher levels in OSA vs. controls [12,20,21,24]. However, no difference in ghrelin between OSA and control has also been reported by some [18,25]. These findings were from between-group comparisons of OSA vs. controls sampling under morning fasting conditions, which typically matched for age and BMI [12,18,20,21,25]. In the case which matched for age but not for BMI, significant between-group differences persisted after controlling for BMI in the analyses [24]. Chihara et al. additionally sampled ghrelin following a standardized breakfast and noted significantly increased postprandial ghrelin levels in patients vs. controls [20]. Plasma ghrelin levels were significantly lower in OSA with EDS vs. OSA without EDS [23].
Three studies focusing on plasma orexin report significantly lower levels in OSA vs. controls [26–29]. Conversely, one other study has reported significantly higher levels in patients vs. controls [30]. These were between-group comparisons of OSA vs. controls, some matched for both age and BMI [28–30], but others matched for only age [27] or BMI [26]. Orexin levels were significantly higher in OSA with EDS than without [23].
Two between-group studies reported significantly increased NPY levels in OSA vs. controls [10,16], and one reported no between-group difference [31]. The positive studies contained adequate age- and BMI matching, and were also conducted under fasting conditions in the morning [10] or evening [16]. The study showing no difference was matched for age but not BMI, and collected blood in the afternoon under non-fasted conditions [31].
Summary/considerations
The high amount of attention given to leptin in OSA is unsurprising, since leptin is involved in food intake and is also known to be affected by obesity. The evidence suggests that leptin levels are abnormally high in OSA, which, similar to the hyperleptinemia seen in obesity, may contribute to a “leptin resistance” within OSA. This, in turn, may predispose patients to further weight gain and help explain the reciprocal relationship between OSA and obesity [1,17]. Ghrelin levels also appear to be higher in OSA, consistent with a hormonal profile which would predispose to high EI. More work should be done to explore the effects of OSA on orexin, as this neuropeptide is known to be involved in the regulation of arousal/wakefulness and appetite. However, it should be noted that circulating orexin and NPY levels in plasma may not be representative of central levels and therefore a less clinically relevant outcome.
For these hormone studies, it is critical to control for BMI, time of sampling, and feeding state: leptin [32,33], orexin [34], and possibly ghrelin [35] show a dirurnal/circadian variation, and leptin levels are stimulated by food intake [36], whereas ghrelin levels decrease after a meal [37]. Although the data provide a potential mechanistic basis for a dysregulation of food intake within OSA, more studies are required to convincingly link the hormonal control of hunger and satiety to actual food intake in these patients.
Energy expenditure via indirect calorimetry
Main findings
Findings on EE via IC are summarized in Table 2. Resting metabolic rate (RMR) was investigated in 6 cross-sectional studies comparing values in OSA and controls, in the morning after an overnight fast [38–43]. In four of the six studies, in which the patients and controls were adequately matched for BMI and age [40–42], or just age [38], RMR was significantly higher in OSA vs. controls. In the remaining two [39,43], one of which did not match for age [43], no between-group difference in RMR was observed. Two additional studies utilized linear regression to assess the relationship between RMR and OSA severity [44,45]. Both found a significant positive association between RMR and increasing OSA severity, either defined with AHI [45] or RDI [44].
Table 2.
Summary of energy expenditure via indirect calorimetry in OSA patients.
| Author, year (reference#) [NOS rating] |
Participants | AHI cutoff, events/h |
Sample size |
Age (SD), years |
BMI (SD), kg/m2 |
Design | Outcomes | Main findings |
|---|---|---|---|---|---|---|---|---|
| Beitler et al., 2014 [43] [*******] |
OSA Cx |
≥15 <15 |
n = 15 n = 19 |
47.9 (11.5) 34.3 (12.0) |
32.2 (7.8) 28.8 (6.5) |
Btw-group comparison |
IC-hood for fasted RMR in AM |
↔ RMR between OSA and Cx (mean [SD]: 1650 [262] vs. 1604 [302] kcal/day) |
| de Jonge et al., 2012 [43] [N/A] |
No OSA to OSA |
x̄ RDI = 12.6 | n = 126 | 40.5 (6.9) | 38.6 (6.5) | regression | IC-hood for fasted RMR in AM |
RMR (corrected for FFM, age, sex) positively associated with RDI (r = 0.32, p = 0.002) |
| Fekete et al., 2015 [38] [*****] |
OSA Cx |
≥15a N/R |
n = 92 n = 19 |
45.3 (12.8) 50.8 (11.7) |
33.2 (4.7) 28.3 (3.1) |
Btw-group comparison |
IC-mask for pre-sleep and AM RMR |
↑ RMR in OSA vs. Cx pre-sleep (mean [SD]: 1959.8 [969.9] vs. 1380.7 [488.4] kcal) and AM (mean [SD]: 1754.1 [734.2] vs. 1308.8 [557.4] kcal) and after correcting for LBM pre-sleep (mean [SD]: 29.6 [12] vs. 22.9 [7.9] kcal/kg) and AM (mean [SD]: 26.4 [9.6] vs. 21.6 [9] kcal/kg) |
| Hins et al. 2006 [47] [N/A] |
OSA Cx |
N/R N/R |
n = 8 n = 86 |
51.4 (7.9) 40.2 (12.4) |
43.2 (7.5) 28.7 (5.3) |
regression | WRIC for 24-h EE and SMR; Cx data used as reference to determine predicted values in OSA |
Negative relationship between nocturnal desaturation and difference in predicted and measured 24-h EE (r = −0.74, p = 0.04) and SMR (r = −0.68, p = 0.08) |
| Kezirian et al. 2008 [45] [N/A] |
No OSA to OSA |
x̄ = 25.4 | n = 212 | 42.3 (12.6) | 28.3 (7.3) | regression | IC-mouthpiece for fasted RMR |
RMR (corrected for BMI, age, sex) positively associated with RDI (coefficient estimate [95% CI]: 2.69 [0.63 to 4.74], p = 0.01) |
| Lin et al., 2002 [39] [*******] |
OSA Cx |
RDI ≥20 N/R |
n = 25 n = 15 |
41.0 (6.0) 38.0 (5.0) |
29.8 (2.5) 28.4 (2.2) |
Btw-group comparison |
IC-hood for fasted RMR in AM, and SMR |
↔ RMR (corrected for weight) between OSA and Cx (mean [SD]: 0.81 [0.06] vs. 0.78 [0.05] kcal/kg/h) ↑ SMR (corrected for RMR) in OSA vs. Cx (mean [SD]: 1.02 [0.06] vs. 0.88 [0.05]) |
| Major et al., 2007 [46] [*******] |
OSA | N/R, x̄ = ~35.0 |
n = 24 | x̄ = ~49.0 | x̄ = ~32.8 | regression | WRIC for 24-h EE and SMR | 24-h EE (r = −0.46, p < 0.05) and SMR (corrected for body weight; r = −0.48, p < 0.05) negatively associated with total recording time with SaO2 <90% |
| Ryan et al., 1995 [40] [******] |
OSA Cx |
>30 <20 |
n = 14 n = 14 |
45.0 (10.0) 46.0 (8.0) |
39.8 (6.5) 34.2 (10.9) |
Btw-group comparison |
IC-hood for fasted RMR in AM |
↑ RMR in OSA vs. Cx (mean [SD]: 2140 [301] vs. 1813 [256] kcal/day), but not after correcting for LBM (mean [SD]: 26.6 [3.3] vs. 28.1 [3.6] kcal/kg) |
| Stenlof et al., 1996 [41] [*******] |
OSA Snoring Cx |
RDI >30 N/R |
n = 5 n = 6 |
46.0 (13.0) 50.0 (9.0) |
34.0 (7.0) 28.0 1.7) |
Btw-group comparison |
WRIC for 24-h EE, SMR, and fasted RMR in AM |
↑ 24-h EE (mean [SD]: 39.2 [3.0] vs. 33.9 [2.7] kcal/d/kg) and RMR (mean [SD]: 31.1 [2.7] vs. 25.4 [2.8] kcal/d/kg) but not SMR (mean [SD]: 32.4 [4.1] vs. 26.3 [1.9] kcal/d/kg), in OSA vs. Cx after correcting for FFM |
| Ucok et al., 2011 [42] [******] |
OSA Snoring Cx |
≥10 <10 |
n = 51 n = 47 |
48.0 (8.0) 49.7 (10.0) |
32.5 (5.5) 32.3 (6.1) |
Btw-group comparison |
IC-mask for fasted RMR in AM |
↑ RMR in OSA vs. Cx (mean [SD]: 1676.4 [600] vs. 1332.8 [285.2] kcal/day) |
AHI: apnea-hypopnea index; AM: morning; BMI: body mass index; CI: confidence interval; Cx: control; EE: energy expenditure; FFM: fat-free mass; IC: indirect calorimetry; LBM: lean-body mass; NOS: Newcastle-Ottawa scale (up to nine stars); OSA: obstructive sleep apnea; RDI: respiratory disturbance index; RMR: resting metabolic rate; SaO2: arterial oxygen saturation; SD: standard deviation; SMR: sleeping metabolic rate; WRIC: whole-room indirect calorimetry; ↑: significant increase; ↓: significant decrease; ↔: no difference.
Indicates inclusion was AHI ≥15 with no symptoms or ≥5 with symptoms (daytime sleepiness, poor sleep, snoring/gasping).
Although theoretically expected to be increased in OSA vs. controls, due to increased nocturnal arousals, findings on sleeping metabolic rate (SMR) are inconsistent [39,41,46]. Using a ventilated hood-IC system, Lin et al. reported a significantly increased SMR in patients vs. controls [39], whereas Stenlof et al., using a whole-room indirect calorimetry (WRIC), found no difference [41]. It should be noted that the latter study was small (n = 5 patients and n = 6 controls), and that the snoring control group was not completely without some degree of OSA [41]. Also using WRIC, a significant negative correlation was found for SMR and 24-h EE with OSA severity (total recording time with SaO2 <90%) [46]. These findings are inconsistent with Stenlof et al., the only other study to compare 24-h EE via WRIC, who observed significantly increased 24-h EE in patients vs. controls [41].
The effects of OSA on adaptive thermogenesis (i.e., the portion of EE which is considered non-obligatory thermogenesis, and which can be manifested as increases or decreases in EE after changes in body weight, food intake, hormones, or sympathetic activity) were investigated in OSA patients using WRIC [47]. In the investigation, non-OSA controls were first studied to determine reference values of predicted daily EE and sleeping EE based on different body weights. As a measure of possible adaptive thermogenesis, these reference values were used to calculate differences in the predicted and WRIC-measured EE values in OSA patients, which were then correlated with severity of nocturnal oxygen desaturation [47]. Within OSA patients, the severity of nocturnal hypoxia was negatively correlated with the difference between measured and predicted EE values [47]. These findings indicate that lower than predicted EE occurs in conjunction with worsened OSA severity, possibly as a function of intermittent hypoxia or increased sympathetic drive. The observed adaptive decrease in thermogenesis with worsened OSA severity may be a contributor to increased susceptibility to weight gain in these patients.
Summary/considerations
Based on studies utilizing IC, the gold-standard method to measure EE, OSA appears to result in increased RMR. Although the effects of OSA on SMR and 24-h EE are less consistent, this finding is important, since RMR represents the largest component of overall EE. These increases in RMR may seem paradoxical, since it can be assumed that increased EE would preclude the development of a positive EB. However, work from our group has shown that the added thermogenesis associated with increased wake time during short sleep is much smaller in magnitude than the induced increase in EI observed after sleep restriction [48]. A similar mechanism is potentially occurring within OSA [49], although this has not been directly tested. An alternate explanation points to an adaptive decrease in thermogenesis in OSA patients. This interpretation would suggest that despite similar or even increased EE compared to non-OSA controls, OSA patients may experience a lower than predicted relative EE that decreases as a function of the severity of their nocturnal desaturation.
Energy expenditure via physical activity
Main findings
Findings on PA levels are summarized in Table 3. In a between-group comparison matched for sex and BMI but not age, no difference in typical PA levels as determined by the International physical activity questionnaire was observed between moderate-to-severe OSA (AHI ≥15 events/h) and controls [43]. In a group of OSA patients (RDI ≥10 events/h) who completed the leisure time exercise questionnaire, regression analysis demonstrated a trend for a negative association between OSA severity and PA levels [50]. In the baseline data from the APPLES trial, the total adjusted daily EE based on the Arizona activity frequency questionnaire was significantly greater by ~224 kcal/d in participants with RDI ≥50 events/h vs. RDI <50 events/h, after adjusting for age, BMI and ESS [8].
Assembling data from several parent cohort studies as part of the sleep heart health study, it was observed that the proportion of individuals reporting moderate-to-vigorous PA was significantly lower in RDI ≥10 events/h or ≥15 events/h vs. controls [51]. However, the prevalence of obesity and EDS (ESS >10) was also increased in the OSA groups vs. control, which can confound results [51]. Similarly, in a large group of patients being evaluated for OSA (n = 1892), the percentage of participants who were considered as being physically active via self-report was significantly lower in severe OSA (AHI ≥30 events/h) vs. control. However, again, ESS and BMI were also significantly higher in the severe OSA cases vs. controls [52]. These latter findings may be partially explained by a study which stratified patients with OSA based on ESS (no EDS [ESS <10] vs. mild/moderate EDS [ESS 11–16] vs. severe EDS [ESS >16]), and found that in men self-reported regular exercise rate was highest in the no EDS group, intermediate in the mild/moderate EDS group, and lowest in the severe EDS group [53].
Several studies have shown a relationship between OSA and objectively monitored PA levels. In a between-group comparison of congestive heart failure patients with (AHI >15 events/h) and without OSA, daytime PA duration based on wrist-worn accelerometry was found to be significantly reduced in OSA [54]. Studies utilizing the SenseWear Armband accelerometer to quantify PA levels as step counts per day reported similar findings: increased OSA severity was associated with decreased objectively measured PA after controlling for age, sex, and ESS [55]. However, in another study also using the SenseWear, only AHI in rapid eye movement (REM) sleep was independently associated with number of steps after controlling for age and BMI [56]. Conversely, in a group of patients with AHI ≥10 events/h and high cardiovascular risk, PA levels were inversely related to BMI but not related to OSA severity [57], suggesting that obesity per se may play a larger role than OSA in influencing PA levels.
The SenseWear Armband can also quantify EE. Linear regression in a group of patients being evaluated for OSA revealed a significant positive association between AHI and SenseWear-derived EE per hour of sleep [58]. Also using the SenseWear, a recent study failed to observe a significant group difference in 24-h EE between patients with moderate-to-severe OSA and controls [59]. Importantly, however, there was a diurnal difference in EE such that controls had significantly higher daytime EE, whereas patients had significantly higher night-time EE [59]. Regression analysis in that study further revealed a significant positive relationship between OSA severity with night-time EE, and a significant negative relationship between OSA severity and daytime EE [59]. These findings are theoretically consistent with the IC-derived EE findings detailed above.
Summary/considerations
In the collected literature, PA levels were assessed either subjectively via questionnaire [8,43,50–53] or objectively via accelerometer [54–59]. Whereas objective monitoring may be preferable, the site of attachment of accelerometer devices, either at the upper arm, ankle, or wrist, may influence recorded activity levels. The transformation of activity counts to estimates of EE relies on algorithms that have their own inherent errors. These differences in methodology should be considered when accounting for discrepancies.
An inverse relationship appears to exist between PA levels and OSA severity, indicating that PA levels might be lower in OSA patients and those with more severe disordered breathing. This may contribute to obesity and cardiovascular risk within patients. However, evidence also suggests that both obesity and EDS are likely to be important modulators of PA within OSA. This is important to consider, since both of these conditions are very commonly observed within OSA patients.
Discussion
Based on a systematic review of the reported findings, some EB parameters are affected by OSA. A hormonal profile characterized by abnormally high circulating levels of leptin and ghrelin and to some extent NPY, may encourage excess EI in OSA, although data on food intake are lacking. Several studies observed that RMR, the largest component of EE, is significantly higher in OSA vs. controls, and that it shows a positive association with OSA severity. Although not entirely consistent, some reports indicate that OSA may be associated with abnormally high SMR and 24-h EE. In practical terms, an abnormally high EE, assuming constant EI, is expected to result in negative EB and weight loss. As the opposite (i.e., weight gain) is typically observed in OSA, a mechanism whereby the high EE may trigger a compensatory neuroendocrine adaptation to increase hunger and food intake beyond the requirements of EB may be occurring [49]. An adaptive decrease in thermogenesis or a reduction in objectively-recorded PA in OSA may exacerbate this.
As a disorder at the intersection between sleep and obesity, OSA itself and its associated disturbances in sleep quality/architecture seem to alter EB parameters in such a way as to induce further weight gain. Laboratory-based intervention studies have demonstrated the ways in which short sleep duration and/or disruptions in sleep quality and architecture can causally influence EB, particularly via enhanced hunger and food intake [2]. Experimental sleep curtailment is consistently observed to induce significant increases in EI [60–64]. With possibly more direct relevance to OSA, experimental sleep fragmentation, via repeated wake-up calls, was found to reduce levels of glucagon-like peptide 1 (a gut-derived hormone signaling satiety) and fullness scores relative to non-fragmented sleep [65]. Along these lines, reductions in REM sleep and slow wave sleep (SWS) have been reported in obese OSA patients vs. non-OSA obese individuals [66]. Since REM sleep is inversely related to hunger levels, and both REM sleep and SWS are inversely related to ad libitum intakes of fat and carbohydrate [67], the particular profile of sleep associated with OSA would indicate propensity towards positive EB via these mechanisms. It remains to be determined if the mechanisms driving increased EI after short/ disrupted sleep are the same as those possibly influencing hunger and appetite in individuals with OSA. For example, some of the hunger/satiety regulating hormones, such as leptin and ghrelin, affected in those with OSA are similarly altered by sleep restriction [68]. Beyond the hormonal/homeostatic control of hunger, researchers have become interested in how the hedonic control of food intake is influenced by sleep status. Specifically, recent work using functional magnetic resonance imaging indicates that after sleep restriction compared to an 8-hr sleep episode, brain regions involved in motivation, reward, and cognitive processing have enhanced activation in response to unhealthy vs. healthy food stimuli [69]. This presents an interesting and novel future research direction, which can help determine if a similar neural mechanism increases the motivational/rewarding aspects of food in individuals with OSA. As described, other EB-related behavioral, metabolic or hormonal effects of OSA may further enhance this state, favoring weight gain and/or difficulty losing weight.
Since treatment with CPAP is known to increase REM sleep and SWS and reduce arousals and respiratory disturbances [70], it might also be expected to induce weight loss. Yet in addition to not reducing weight, a recent meta-analysis of randomized controlled trials indicates that CPAP treatment actually promotes weight gain [71]. As described here, the regulation of EB in OSA is complex and multi-factorial, involving food intake, hormonal regulation of hunger/satiety/appetite, and EE via metabolism and free-living PA. CPAP may correct some of the OSA-related alterations in EB, like reducing abnormally high levels of leptin and ghrelin [12], although it may also reduce EE [41]. A recent investigation by Tachikawa and colleagues took an integrative approach of looking at energy metabolism via IC, objectively-recorded PA, hunger/satiety-regulating hormones, and dietary intake via questionnaire, to thoroughly characterize how CPAP treatment affects EB in OSA patients (n = 63, AHI >20 events/h) at baseline and after three mo of treatment [72]. They observed no change in leptin, ghrelin or PA levels after CPAP. However, they did report a reduction in basal metabolic rate after treatment, and that individuals who gained weight after CPAP had a significantly higher total EI at the post-treatment follow-up vs. non-weight gainers [72]. Finally, they reported that induced increases in EI, but not changes EE, predicted increases in BMI after CPAP which the authors suggest as an explanation for the observation of weight gain after treatment observed by some [72]. Based on the limited number of studies, many inconsistencies exist, and the full picture of EB regulation in response to CPAP is still incomplete, urging more experimental work.
In conclusion, EB regulation in OSA appears altered to encourage positive EB, and therefore, efforts should be made to reduce body weight in OSA patients and in patients treated with CPAP. Randomized controlled trials of prescribed PA and/or reduced caloric intake have demonstrated the effectiveness of intensive lifestyle interventions to induce weight loss-associated improvements in OSA severity [73–75]. Understanding how the components of EB are affected by OSA will allow clinicians to more effectively guide overall treatment approaches to optimize weight loss and symptom amelioration, and encourage cardiovascular health in OSA patients.
Practice points.
Obstructive sleep apnea may contribute to weight gain by promoting behavioral, metabolic, and/or hormonal changes that alter energy balance parameters. These are likely to include:
Abnormally high levels of circulating leptin (indicative of a “leptin resistance”) and ghrelin. These changes are expected to result in low satiety and high hunger, and therefore excess food intake.
Abnormally high energy metabolism, in particular resting metabolic rate, in sleep apnea and in association with worse sleep apnea severity.
A reduction in objectively monitored physical activity reported by some, that is associated with worsened apnea severity.
Research agenda.
Future studies should be conducted to further quantify how the disorder alters energy balance and its regulation. These include:
Objective measures of food intake under laboratory conditions and while free-living, via test meal and/or real-time tracking, respectively.
More work on hormones regulating hunger and satiety, especially in the context of behavioral food intake measurement.
Controlled experimental studies which investigate both energy expenditure and energy intake to allow for a quantification of energy balance within obstructive sleep apnea patients and in response to treatment with continuous positive airway pressure.
Acknowledgments
This work was funded in part by a Scientist Development Grant from the American Heart Association (15DG22680012). This funding agency had no role in the conduct of the review.
Glossary of terms
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- EB
energy balance
- EE
energy expenditure
- EI
energy intake
- EDS
excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- IC
indirect calorimetry
- NOS
Newcastle-Ottawa scale
- NPY
neuropeptide Y
- OSA
obstructive sleep apnea
- PA
physical activity
- PSG
polysomnographic
- RDI
respiratory disturbance index
- RMR
resting metabolic rate
- REM
rapid eye movement
- SMR
sleeping metabolic rate
- SWS
slow wave sleep
- WRIC
whole-room indirect calorimetry;
Footnotes
Conflicts of interest
The author declares no conflict of interest
References
* The most important references are denoted by an asterisk.
- 1.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St-Onge MP, Shechter A. Sleep disturbances, body fat distribution, food intake and/or energy expenditure: pathophysiological aspects. Hormone Mol Biol Clin Investig. 2014;17(1):29–37. doi: 10.1515/hmbci-2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips BG, Hisel TM, Kato M, Pesek CA, Dyken ME, Narkiewicz K, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17(9):1297–1300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 4.Borel AL, Leblanc X, Almeras N, Tremblay A, Bergeron J, Poirier P, et al. Sleep apnoea attenuates the effects of a lifestyle intervention programme in men with visceral obesity. Thorax. 2012;67(8):735–741. doi: 10.1136/thoraxjnl-2011-201001. [DOI] [PubMed] [Google Scholar]
- 5.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126(1):126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000 [Google Scholar]
- 7.Smith SS, Waight C, Doyle G, Rossa KR, Sullivan KA. Liking for high fat foods in patients with Obstructive Sleep Apnoea. Appetite. 2014;78:185–192. doi: 10.1016/j.appet.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the Apnea Positive Pressure Long-Term Efficacy Study (APPLES) J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2008;4(5):411–418. [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57(5):359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- 10.Barcelo A, Barbe F, Llompart E, de la Pena M, Duran-Cantolla J, Ladaria A, et al. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171(2):183–187. doi: 10.1164/rccm.200405-579OC. [DOI] [PubMed] [Google Scholar]
- 11.Basoglu OK, Sarac F, Sarac S, Uluer H, Yilmaz C. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thorac Med. 2011;6(3):120–125. doi: 10.4103/1817-1737.82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22(2):251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 13.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118(3):580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 14.Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186(4):209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 15.Ozturk L, Unal M, Tamer L, Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Archives Otolaryngol Head Neck Surg. 2003;129(5):538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 16.Papaioannou I, Patterson M, Twigg GL, Vazir A, Ghatei M, Morrell MJ, et al. Lack of association between impaired glucose tolerance and appetite regulating hormones in patients with obstructive sleep apnea. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2011;7(5):486–492b. doi: 10.5664/JCSM.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 18.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respir Int Rev Thorac Dis. 2005;72(4):395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 20.Chihara Y, Akamizu T, Azuma M, Murase K, Harada Y, Tanizawa K, et al. Among metabolic factors, significance of fasting and postprandial increases in acyl and desacyl ghrelin and the acyl/desacyl ratio in obstructive sleep apnea before and after treatment. J Clin Sleep Med. 2015;11(8):895–905. doi: 10.5664/jcsm.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ursavas A, Ilcol YO, Nalci N, Karadag M, Ege E. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: role of obesity. Ann Thorac Med. 2010;5(3):161–165. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SR, Palmer LJ, Larkin EK, Jenny NS, White DP, Redline S. Relationship between obstructive sleep apnea and diurnal leptin rhythms. Sleep. 2004;27(2):235–239. doi: 10.1093/sleep/27.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-de-la-Torre M, Barcelo A, Pierola J, Esquinas C, de la Pena M, Duran-Cantolla J, et al. Plasma levels of neuropeptides and metabolic hormones, and sleepiness in obstructive sleep apnea. Respir Med. 2011;105(12):1954–1960. doi: 10.1016/j.rmed.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Chin K, Akamizu T, Morita S, Sumi K, Oga T, et al. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology (Carlton, Vic) 2008;13(6):810–816. doi: 10.1111/j.1440-1843.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang D, Liu Z, Luo Q. Plasma ghrelin and pro-inflammatory markers in patients with obstructive sleep apnea and stable coronary heart disease. Med Sci Monit Int Med J Exp Clin Res. 2013;19:251–256. doi: 10.12659/MSM.883874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aksu K, Firat Guven S, Aksu F, Ciftci B, Ulukavak Ciftci T, Aksaray S, et al. Obstructive sleep apnoea, cigarette smoking and plasma orexin-A in a sleep clinic cohort. J Int Med Res. 2009;37(2):331–340. doi: 10.1177/147323000903700207. [DOI] [PubMed] [Google Scholar]
- 27.Busquets X, Barbe F, Barcelo A, de la Pena M, Sigritz N, Mayoralas LR, et al. Decreased plasma levels of orexin-A in sleep apnea. Respir Int Rev Thorac Dis. 2004;71(6):575–579. doi: 10.1159/000081757. [DOI] [PubMed] [Google Scholar]
- 28.Nishijima T, Sakurai S, Arihara Z, Takahashi K. Plasma orexin-A-like immunoreactivity in patients with sleep apnea hypopnea syndrome. Peptides. 2003;24(3):407–411. doi: 10.1016/s0196-9781(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai S, Nishijima T, Takahashi S, Yamauchi K, Arihara Z, Takahashi K. Clinical significance of daytime plasma orexin-A-like immunoreactivity concentrations in patients with obstructive sleep apnea hypopnea syndrome. Respir Int Rev Thorac Dis. 2004;71(4):380–384. doi: 10.1159/000079643. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi N, Tatsumi K, Nakamura A, Sakao S, Takiguchi Y, Nishikawa T, et al. Plasma orexin-A levels in obstructive sleep apnea-hypopnea syndrome. Chest. 2003;124(4):1381–1385. doi: 10.1378/chest.124.4.1381. [DOI] [PubMed] [Google Scholar]
- 31.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103(6):1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 32.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83(6):1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 34.Fenzl T, Flachskamm C, Rossbauer M, Deussing JM, Kimura M. Circadian rhythms of basal orexin levels in the hypothalamus are not influenced by an impaired corticotropin-releasing hormone receptor type 1 system. Behav Brain Res. 2009;203(1):143–145. doi: 10.1016/j.bbr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinolo Eur Fed Endocr Soc. 2005;152(6):845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- 36.Dallongeville J, Hecquet B, Lebel P, Edme JL, Le Fur C, Fruchart JC, et al. Short term response of circulating leptin to feeding and fasting in man: influence of circadian cycle. Int J Obes Relat Metab Disord. 1998;22(8):728–733. doi: 10.1038/sj.ijo.0800648. [DOI] [PubMed] [Google Scholar]
- 37.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 38.Fekete K, Boutou AK, Pitsiou G, Chavouzis N, Pataka A, Athanasiou I, et al. Resting energy expenditure in OSAS: the impact of a single CPAP application. Sleep Breath. 2016 Mar;20(1):121–128. doi: 10.1007/s11325-015-1194-y. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Chang KC, Lee KS. Effects of treatment by laser-assisted uvuloplasty on sleep energy expenditure in obstructive sleep apnea patients. Metabolism Clin Exp. 2002;51(5):622–627. doi: 10.1053/meta.2002.31969. [DOI] [PubMed] [Google Scholar]
- 40.Ryan CF, Love LL, Buckley PA. Energy expenditure in obstructive sleep apnea. Sleep. 1995;18(3):180–187. doi: 10.1093/sleep/18.3.180. [DOI] [PubMed] [Google Scholar]
- 41.Stenloöf K, Grunstein R, Hedner J, Sjoöstroöm L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol Endocrinol Metab. 1996;271(6 34e6):E1036–E1043. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 42.Ucok K, Aycicek A, Sezer M, Fidan F, Akgun L, Akkaya M, et al. Resting metabolic rate and anthropometric measurements in male sleep apnea patients. Intern Med (Tokyo, Japan) 2011;50(8):833–838. doi: 10.2169/internalmedicine.50.4779. [DOI] [PubMed] [Google Scholar]
- 43.Beitler JR, Awad KM, Bakker JP, Edwards BA, DeYoung P, Djonlagic I, et al. Obstructive sleep apnea is associated with impaired exercise capacity: a cross-sectional study. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2014;10(11):1199–1204. doi: 10.5664/jcsm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jonge L, Zhao X, Mattingly MS, Zuber SM, Piaggi P, Csako G, et al. Poor sleep quality and sleep apnea are associated with higher resting energy expenditure in obese individuals with short sleep duration. J Clin Endocrinol Metab. 2012;97(8):2881–2889. doi: 10.1210/jc.2011-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kezirian EJ, Kirisoglu CE, Riley RW, Chang E, Guilleminault C, Powell NB. Resting energy expenditure in adults with sleep disordered breathing. Archives Otolaryngol Head Neck Surg. 2008;134(12):1270–1275. doi: 10.1001/archotol.134.12.1270. [DOI] [PubMed] [Google Scholar]
- 46.Major GC, Series F, Tremblay A. Does the energy expenditure status in obstructive sleep apnea favour a positive energy balance? Clin Investig Med Med Clin Exp. 2007;30(6):E262–E268. doi: 10.25011/cim.v30i6.2955. [DOI] [PubMed] [Google Scholar]
- 47.Hins J, Series F, Almeras N, Tremblay A. Relationship between severity of nocturnal desaturation and adaptive thermogenesis: preliminary data of apneic patients tested in a whole-body indirect calorimetry chamber. Int J Obes (2005) 2006;30(3):574–577. doi: 10.1038/sj.ijo.0803159. [DOI] [PubMed] [Google Scholar]
- 48.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013 Dec;98(6):1433–1439. doi: 10.3945/ajcn.113.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97(6):1792–1801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35(7):1088–1092. doi: 10.1249/01.MSS.0000074566.94791.24. [DOI] [PubMed] [Google Scholar]
- 51.Quan SF, O’Connor GT, Quan JS, Redline S, Resnick HE, Shahar E, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath Schlaf Atmung. 2007;11(3):149–157. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 52.Lopes C, Esteves AM, Bittencourt LR, Tufik S, Mello MT. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Braz J Med Biol Res. 2008;41(10):908–913. doi: 10.1590/s0100-879x2008005000036. [DOI] [PubMed] [Google Scholar]
- 53.Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler E, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2008;4(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 54.Hastings PC, Vazir A, O’Driscoll DM, Morrell MJ, Simonds AK. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27(4):748–755. doi: 10.1183/09031936.06.00063005. [DOI] [PubMed] [Google Scholar]
- 55.Chasens ER, Sereika SM, Houze MP, Strollo PJ. Subjective and objective appraisal of activity in adults with obstructive sleep apnea. J Aging Res. 2011;2011:751819. doi: 10.4061/2011/751819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verwimp J, Ameye L, Bruyneel M. Correlation between sleep parameters, physical activity and quality of life in somnolent moderate to severe obstructive sleep apnea adult patients. Sleep Breath Schlaf Atmung. 2013;17(3):1039–1046. doi: 10.1007/s11325-012-0796-x. [DOI] [PubMed] [Google Scholar]
- 57.Mendelson M, Tamisier R, Laplaud D, Dias-Domingos S, Baguet JP, Moreau L, et al. Low physical activity is a determinant for elevated blood pressure in high cardiovascular risk obstructive sleep apnea. Respir Care. 2014;59(8):1218–1227. doi: 10.4187/respcare.02948. [DOI] [PubMed] [Google Scholar]
- 58.O’Driscoll DM, Turton AR, Copland JM, Strauss BJ, Hamilton GS. Energy expenditure in obstructive sleep apnea: validation of a multiple physiological sensor for determination of sleep and wake. Sleep Breath Schlaf Atmung. 2013;17(1):139–146. doi: 10.1007/s11325-012-0662-x. [DOI] [PubMed] [Google Scholar]
- 59.Bamberga M, Rizzi M, Gadaleta F, Grechi A, Baiardini R, Fanfulla F. Relationship between energy expenditure, physical activity and weight loss during CPAP treatment in obese OSA subjects. Respir Med. 2015;109(4):540–545. doi: 10.1016/j.rmed.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91(6):1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 61.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake and meal timing in healthy adults. Sleep. 2013;36(7):981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 66.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154(15):1705–1711. [PubMed] [Google Scholar]
- 67.Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R883–R889. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shechter A, Grandner MA, St-Onge MP. The role of sleep in the control of food intake. Am J Lifestyle Med. 2014;8(6):371–374. doi: 10.1177/1559827614545315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2014 Mar;38(3):411–416. doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma A, Radtke RA, VanLandingham KE, King JH, Husain AM. Slow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep quality. Sleep Med. 2001;2(3):215–223. doi: 10.1016/s1389-9457(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 71.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70(3):258–264. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]
- 72.Tachikawa R, Ikeda K, Minami T, Matsumoto T, Hamada S, Murase K, et al. Changes in energy metabolism after continuous positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 2016 Mar 1; doi: 10.1164/rccm.201511-2314OC. http://dx.doi.org/10.1164/rccm.201511-2314OC. [DOI] [PubMed] [Google Scholar]
- 73.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Archives Intern Med. 2009;169(17):1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuomilehto HP, Seppa JM, Partinen MM, Peltonen M, Gylling H, Tuomilehto JO, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(4):320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 75.Johansson K, Neovius M, Lagerros YT, Harlid R, Rossner S, Granath F, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4–b09. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]