Abstract
Objective
Standard predictors do not fully explain variations in the frequency and timing of heart failure (HF) adverse events (AEs). Psychological stress can trigger acute cardiovascular (CV) events, but it is not known whether stress can precipitate AEs in HF patients. We investigated prospective associations of psychological stress with AEs in patients with HF.
Methods
144 HF patients (77% male; 57.5±11.5, range 23–87 years, LVEF≤40%) were longitudinally evaluated for psychological stress (Perceived Stress Scale; PSS) and AEs (CV hospitalizations/death) at 2-week intervals for 3 months, and at 9-month follow- up.
Results
42 patients (29.2%) had at least one CV hospitalization and 9 (6.3%) died. Patients reporting high average perceived stress across study measurements had a higher likelihood of AEs during the study period compared to those with lower stress (OR=1.10, 95% CI=1.04, 1.17). In contrast to average levels, increases in stress did not predict AEs (p=.96). Perceived stress was elevated after a CV hospitalization (B=2.70, SE=0.93, p=.004) suggesting that CV hospitalizations increase stress. Subsequent analysis indicated that (24 of 38; 63%) of patients showed a stress increase following hospitalization. However, a prospective association between stress and AEs was present when accounting for prior hospitalizations (B=2.43, SE=1.23, p=.05).
Conclusions
Sustained levels of perceived stress are associated with increased risk of AEs, and increased distress following hospitalization occurs in many, but not all, HF patients. Patients with chronically high stress may be an important target group for HF interventions aimed at reducing hospitalizations.
Keywords: Psychological stress, heart failure, hospitalization, post-hospitalization syndrome
INTRODUCTION
Heart failure (HF) is characterized by high mortality, frequent hospitalizations, and impaired quality of life. Rehospitalization rates in the U.S. are estimated to be 20–35% within 30 days after discharge.[1,2] Despite significant advances in treatment, hospitalizations cause substantial physical and psychological burden for HF patients.[3,4] Interventions for reducing HF exacerbations, adverse events (AEs), and hospitalizations have focused on preventing and treating known biomedical and behavioral precipitating factors,[5,6] including hemodynamic, renal, and pulmonary dysfunction, myocardial ischemia and infarction, and poor medication adherence.[7,8] Chronic psychosocial risk factors such as depression and low social support also predict HF clinical outcomes in prospective studies.[9,13] However, the effects of increases in psychological precipitating factors, such as perceived stress[11,12] on short-term hospitalizations and AEs in HF are not well-understood.
Psychological stress can adversely affect important predictors of HF-related AEs, including hemodynamic and neuroendocrine function,[13] medication and diet adherence, and self-care.[10,14] Most research on stress and HF is limited to retrospective studies,[15] or to laboratory studies that have shown that mental stress can induce myocardial ischemia in HF patients with coronary artery disease.[16,17]
Studies examining whether short-term increases in psychological stress are associated with adverse outcomes such as hospitalization in HF patients are rare.[4,15,18] Therefore, it is not known whether chronic stress levels and/or shorter-term increases in psychological stress can precipitate AEs in HF patients. These questions would have implications for choosing appropriate preventive targets.
In addition, a post-hospitalization syndrome may result from the stressful experience of a hospitalization,[4] suggesting that the physiological and psychological consequences of being hospitalized may heighten risk of subsequent hospitalizations and AEs.[14] Evidence for the prevalence of stress as part of a post-hospital syndrome is limited,[18] and it is not known whether short-term increases in psychological stress predict subsequent hospitalizations or whether chronically stressed HF patients are more susceptible to AEs.
The purpose of the present study is to determine whether psychological stress is a predictor of CV hospitalizations or death in HF patients, and to assess the frequency and magnitude of post-hospitalization effects on patient distress. To test these relationships, we used a prospective study design involving multiple assessments of stress and AEs (CV hospitalization or death) over time.
METHODS
In a prospective clinical cohort study (the BETRHEART Study), 144 patients (mean age 57.5±11.5, range 23–87 years, 77% male) with a primary diagnosis of systolic HF were recruited at the University of Maryland Medical Center (UMMC). Inclusion criteria were: LVEF≤ 40% assessed by echocardiography, age≥18 years, and symptomatic HF (NYHA class II–IV) for >3 months. Exclusion criteria were: significant valve disorder as primary diagnosis or thyroid dysfunction as primary etiologies, myocarditis or alcohol abuse in past 6 months, current LV assist device, active cancer treatment, nursing home residence, and significant cognitive impairment. The study was approved by Institutional Review Boards of the UMMC and the Uniformed Services University. All participants provided written informed consent.
Procedure
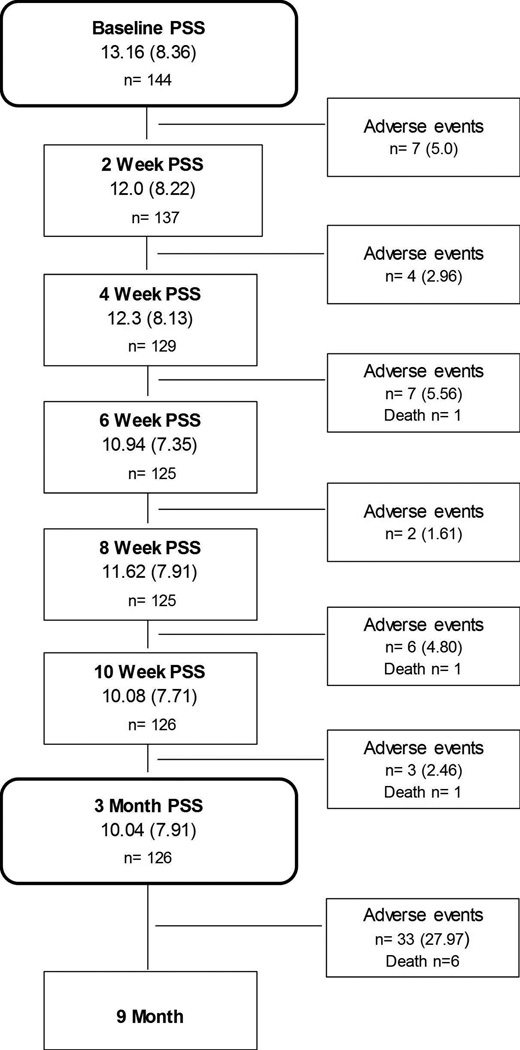
The study design is illustrated in Figure 1. All patients attended a baseline clinic assessment at the HF clinic at UMMC, where detailed clinical assessments were carried out by the clinical care team. In addition, sociodemographic, psychosocial and behavioral variables were measured via clinical interviews and questionnaires.
Figure 1.
Mean (SD) and sample size of patients with PSS data at each time point, and number (%) of patients experiencing at least one adverse event before the subsequent assessment.
Values are means (standard deviation) or N (%). PSS=Perceived Stress Scale. Adverse events=number of patients experiencing 1 or more cardiovascular hospitalizations or death from any cause before the subsequent assessment. 144 patients had complete data on the PSS at baseline, and 140 patients had complete data on Adverse Events.
Following baseline assessment, all patients were scheduled for biweekly telephone interviews between baseline and a 3-month follow-up clinic visit (intervening weeks 2, 4, 6, 8, and 10). During interviews, patients were administered the Perceived Stress Scale (PSS)[19] to quantify psychological stress in the previous 2 weeks. Hospitalization events occurring in the previous 2 weeks were determined from patient reports and subsequently verified through systematic review of medical records. Deaths were recorded from household member reports, and verified in medical records.
At 3 months following baseline (week 12), all patients attended a clinic assessment, during which patients repeated the same clinical assessments as at baseline, and hospitalization events recorded and subsequently verified.
Six months after the 3 month assessment (9-month follow-up), patients were again interviewed via telephone for AEs that occurred since last contact, and hospital records verified. If the patient had died, spouse or next of kin was interviewed regarding date and cause of death.
Study Measures
Perceived Stress
The 10-item PSS[19] measures generalized perception of stress over the previous two weeks. This standardized instrument was developed to assess the extent to which an individual perceives events or situations as stressful or out of control (e.g., during the past two weeks: “how often did you feel that you were unable to control the important things in your life”? and “how often have you felt confident about your ability to handle your personal problems”?). Reliability and validity of the PSS is well-established[20] and it has been used widely to quantify stress in epidemiological and clinical research.[12,21] PSS scores range from 0 to 40 with higher scores indicating greater perceived stress. Scale internal reliability (Cronbach’s α) ranges from 0.85 to 0.92.
Adverse Events (AEs)
Adverse events were defined as hospitalizations for CV causes or death. CV causes included: HF specific (diagnosis of fluid overload or pump failure), angina, myocardial infarction, ischemia or new-onset arrhythmia. Hospitalization causes were expert-adjudicated by a cardiologist on the research team and confirmed via medical records. Death from any cause was used as an endpoint. An “adverse event” variable was computed by determining, at each assessment, the presence of CV hospitalizations (or death) events occurring between that assessment and the subsequent assessment. For each assessment, each patient was assigned a “0” (for no AE before next assessments) or “1” (for >1 AEs before the next assessment).
Data Analysis
Data are presented as means (M) and standard deviations, or N and percentages, as appropriate. Linear mixed models (LMMs) analyses using SAS PROC MIXED (continuous outcomes) or PROC GLIMMIX (binary outcomes) were conducted. LMMs account for dependence between observations and allow for different numbers of participant observations.[22] A random (subject-specific) intercept was employed. Parameter estimates are reported as B (SE) or as OR (95% CI); t values with associated p values test the null hypotheses that the B value is equal to zero, or that the OR is equal to one, in the population. Time was entered as a continuous variable in all models.
In LMMs, stress was coded as “Mean PSS” (i.e., PSS scores aggregated over all available assessments for each subject), and “Deviation PSS” (the difference between the PSS score at each assessment and each subject’s “Mean PSS” score).[23] A significant coefficient for “Mean PSS” reflects a patient characteristic or “between subjects” effect that examines “who” is generally at risk of AEs. A significant coefficient for “Deviation PSS” reflects an effect of short-term changes in stress, i.e., a “within-subject” effect that captures “when” an individual is at risk of an AE.
To examine whether hospitalization results in increased post-hospitalization stress, LMM tested whether PSS scores were elevated at assessments occurring immediately following, compared to observations not immediately following, a CV hospitalization. We further examined whether stress was associated with AEs independent of the influence of prior recent hospitalizations. In this analysis, PSS scores of the patient subgroup without any AEs in the study (“No event patients”) were compared to PSS scores of patients who had AEs, using only assessments before their initial study event (“Event patients - before first event”). Associations between baseline only PSS scores (Baseline PSS) and subsequent AEs are also presented to determine whether serial stress assessments improve AE prediction compared to a baseline only assessment. Cox Proportional Hazards survival analysis was also used to examine the relationship between Baseline PSS and time to first AE; parameter estimates are presented as Hazard Ratios (HR) and 95% Confidence Intervals.
Covariates
Due to the relatively small sample size, the covariates were limited to demographic variables, indices of disease severity, and a limited number of risk factors chosen because of their possible association with stress or the study outcome. The following covariates, measured at baseline, and each with complete data, were used: age (years), sex, race, body mass index (BMI; in Kg/m2), smoking, New York Heart Association (NYHA) classification (II; III; and IV), LVEF(%), and hypertension history. Two additional variables had missing data and were not related to cardiac events in this sample: creatinine levels (mg/dL) (4 missing values), and household income (in USD: <15k; ≥15k-<30k; ≥30-<70k; and ≥70k) (1 missing value). In supplementary analyses not presented, we used multiple imputation of missing values and also included these variables as covariates. Results from these analyses did not differ from the analyses presented.
RESULTS
Sample demographic and clinical characteristics are presented in Table 1, as are baseline comparisons of patients who did and did not have AEs during the study. The study design and descriptive statistics for predictor and outcome variables between baseline and nine months are presented in Figure 1.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Patients Who Did and Did Not Experience Adverse Events During the Study
| Variable ↓ | All Patients | No Adverse Event patients |
Adverse Event patients |
p |
|---|---|---|---|---|
|
N=144 Mean ± SD or % |
n=93 Mean ± SD or % |
n=47 Mean ± SD or % |
||
| Age (yrs) | 57.51 ± 11.52 | 57.88 ± 10.34 | 56.47 ± 13.29 | .53 |
| Sex (%) | .97 | |||
| Male | 77.1 | 76.3 | 76.6 | |
| Race (%) | .69 | |||
| Caucasian | 29.2 | 31.2 | 27.7 | |
| African-American | 70.1 | 67.7 | 72.3 | |
| American-Indian | 0.7 | 1.1 | 0.0 | |
| BMI (kg/m2) | 30.87 ± 7.50 | 31.4 ± 7.51 | 30.28 ± 7.46 | .40 |
| Baseline PSS (0–40) | 13.16 ± 8.36 | 12.32 ± 8.06 | 14.64 ± 8.34 | .11 |
| Ejection Fraction (%) | 23.10 ± 7.48 | 23.23 ± 7.39 | 22.83 ± 7.55 | .77 |
| Recent HF Hospitalization | .87 | |||
| Yes | 11.8 | 11.8 | 12.8 | |
| No | 88.2 | 88.2 | 87.2 | |
| Creatinine (mg/dL) | 1.38 ± 0.71 | 1.34 ± 0.74 | 1.43 ± 0.56 | .44 |
| Smoking (%) | .56 | |||
| Yes | 27.1 | 28.0 | 23.4 | |
| Hypertension History (%) | .98 | |||
| Yes | 79.2 | 78.5 | 78.7 | |
| NYHA Class (%) | .01 | |||
| II | 54.9 | 62.4 | 40.4 | |
| III and IV | 45.1 | 37.6 | 59.6 | |
| Employment status (%) | .76 | |||
| Full-time | 14.6 | 15.2 | 12.8 | |
| Part-time | 7.6 | 8.7 | 6.4 | |
| Disabled | 51.4 | 52.2 | 53.2 | |
| Unemployed | 4.9 | 5.4 | 2.1 | |
| Retired | 21.5 | 18.5 | 25.5 | |
| Income USD (%) | .92 | |||
| <15k | 34.3 | 33.7 | 34.0 | |
| ≥15 – <30k | 26.6 | 25.0 | 29.8 | |
| ≥30 – <70k | 30.1 | 32.6 | 27.7 | |
| ≥70k | 9.0 | 8.7 | 8.5 | |
Note: Adverse Event patients experienced at least 1 adverse event (hospitalization for cardiovascular reasons or death) during the study period. p-values derive from t-tests (continuous variables) or Chi Square tests (categorical variables). Data on adverse events were not available for 4 participants. Recent HF hospitalization: Yes = HF hospitalization ≤ 30 days before enrollment; No = HF hospitalization > 30 days before enrollment or no report of a prior HF hospitalization.
BMI=Body Mass Index; PSS=Perceived Stress Scale; NYHA=New York Heart Association; USD = US Dollars. N=144 except for Employment status (n=143), Creatinine (n=140), and Income (n=143).
During the study, 9 patients died and 42 (29.2%) experienced at least one CV hospitalization; 4 of these subsequently died, and 5 (3.5%) died without a previous hospitalization. 4 of these subsequently died, and 5 (3.5%) died without a previous hospitalization. There were a total of 62 events in the 51 patients with at least one event.
Sustained high stress, short-term changes in stress, and adverse events
The odds of experiencing an AE were greater in patients with higher (vs. lower) Mean PSS scores (unadjusted OR=1.11, 95% CI=1.04, 1.18 per unit PSS; adjusted OR=1.10, 95% CI=1.04, 1.17). This result means that individuals with higher Mean PSS scores have higher odds of AEs during the study period. In contrast to Mean PSS relationships, there was no association between Deviation PSS and AEs (unadjusted OR=1.00, 95% CI=0.94, 1.07, p=0.95; adjusted OR=1.00, 95% CI=0.94, 1.06, p=0.96), indicating that short-term increases in psychological stress were not associated with increased risk of subsequent AEs. In addition, the Baseline only PSS score was not associated with AEs (unadjusted B=0.04, SE=0.03, p=0.11; adjusted B=0.04, SE=0.03, p=0.14). Using Cox Proportional Hazards regression, Baseline PSS was similarly not associated with time to AE in either an unadjusted (HR=1.029, 95% CI=0.993, 1.065, p=.11) or adjusted analysis (HR=1.034, 95% CI=0.996, 1.073, p=.10).
The effect of Mean PSS was not attributable to depression[9,10] since the effect remained significant when controlling for baseline depression scores on the Beck Depression Inventory-II (BDI-II)[26] (adjusted OR=1.14, 95% CI=1.04, 1.25). Baseline BDI-II was also not associated with AEs (p=.29).
Psychological stress following CV hospitalizations
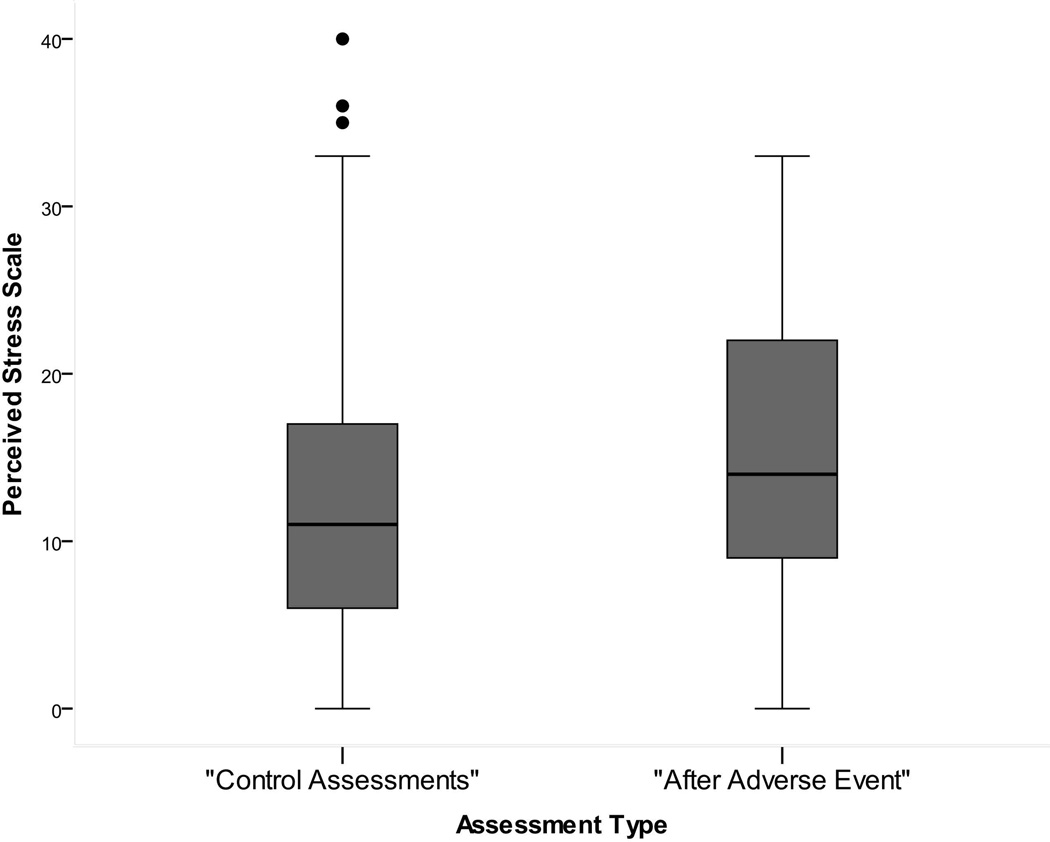
To determine whether experiencing a CV hospitalization increased stress (post-hospitalization stress effect), we conducted an analysis using only those patients who experienced at least one CV hospitalization during the study. PSS scores measured at assessments occurring immediately after one (or more) CV hospitalizations (“After Adverse Event”) were compared to PSS scores at assessments not preceded by a hospitalization (“Control Assessments”). PSS scores after adverse events were significantly higher compared to control assessments (M=15.47±8.14 vs. M=12.34±8.12; unadjusted B=2.72, SE=0.94, p=0.004; adjusted B=2.70, SE=0.93, p=0.004) (Figure 2). This indicates that stress was significantly higher following a CV hospitalization (when controlling for time and other covariates). A closer examination of these results indicated that this effect was not universal with 24 of 38 patients (63.2%) who were hospitalized reporting a “positive” post-hospitalization effect (higher stress at assessments following a CV hospitalization vs. control assessments).
Figure 2.
Association of prior hospitalizations for HF-specific or CV causes with subsequent stress levels.
Box and whisker plot of PSS scores from patients who experienced at least one hospitalization. Scores are aggregated over all assessments that followed Baseline. “After Adverse Event” =PSS scores measured at assessments that occurred after a hospitalization for CV causes (n=53). “Control Assessments” = PSS scores measured at assessments that were not preceded by a hospitalization for CV causes (n=231). Boxes and internal lines represent 25th and 75th percentiles and median, and whiskers show range and outliers.
Prospective association of psychological stress with AEs in patients without prior hospitalizations
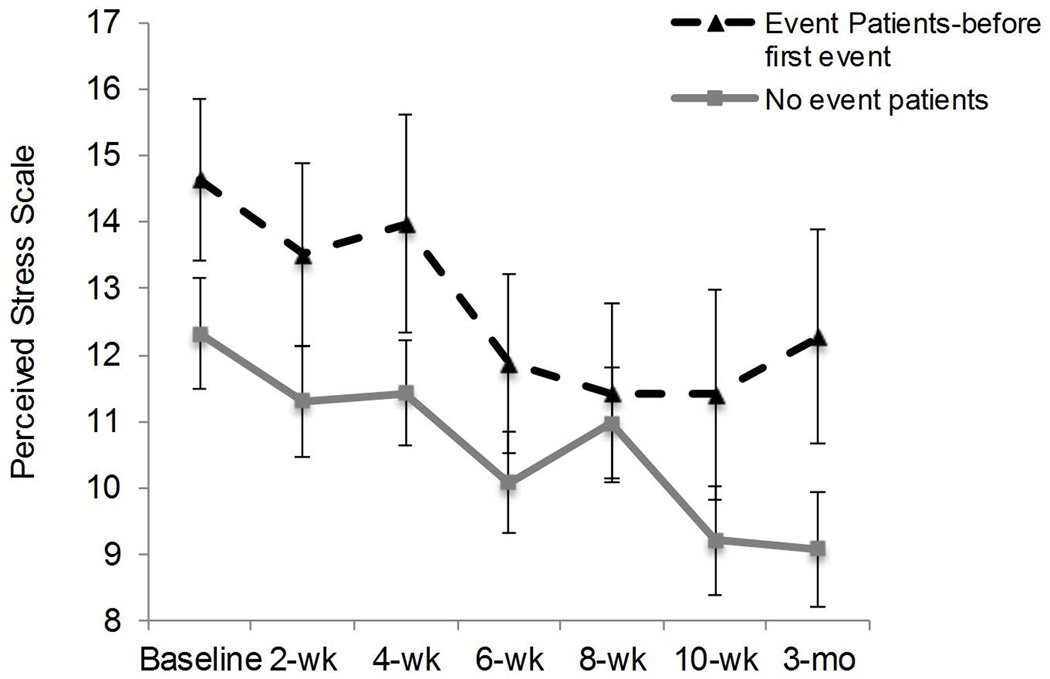
To determine whether the association between higher mean perceived stress (Mean PSS) and AEs was prospective and not a result of recent prior hospitalization, analyses were conducted using only PSS scores measured prior to a patient having any AE in the study. Among patients with at least one AE, we selected all their PSS scores before their first AE in the study (“Event patients, before a first event”). These scores were then compared with PSS scores of those who had no AEs in the study (“No event patients”) (Figure 3). PSS scores of “Event patients, before first event” were significantly higher than scores of “No event patients” (M=12.95±8.42 vs. M=10.65±7.73: unadjusted B=2.51, SE=1.21, p=.04; adjusted B=2.43, SE=1.23, p=0.05). Figure 3 also indicates that PSS scores declined over time (B=−0.47, SE=0.11, p=.0001).
Figure 3.
Prospective association of perceived stress with cardiovascular hospitalization or death in the study.
Data are PSS scores aggregated over all assessment time points that occurred prior to a patient’s first adverse event in the study. Each assessment point between baseline and 3 months only includes patients who have not yet had an event at that assessment point. PSS score following a hospitalization at any point in the study are excluded from this analysis.
“Event Patients-before first event” = PSS scores of 47 patients who experienced subsequent adverse events during the study (n=238 assessments). “No Event Patients” = PSS scores of 93 patients who had no adverse events during the study (n=615 assessments).
Additional evidence indicated that prospective results for higher stress were unlikely to be attributable to hospitalizations that occurred prior to study enrollment. Table 1 shows that “Adverse event patients” were not more likely to have been hospitalized within the 30 days before study enrollment compared to “No adverse event patients”, and PSS scores remained higher in Adverse event patients after adjusting for presence of a recent (30 day) CV hospitalization (adjusted B=2.52, SE=1.21, p=0.04).
DISCUSSION
This study demonstrates that sustained high levels of perceived stress are associated with increased risk of cardiac hospitalization or death in HF patients. In contrast, increases in PSS measured on a bi-weekly basis were not associated with exacerbations in the weeks immediately following each assessment. Therefore, measurement of psychological stress predicts which patients are at risk of AEs (“who”), but not the timing or “when” clinical events will occur. Identification of individuals with sustained high stress levels may help identify those HF patients at risk of subsequent AEs. Other acute biomedical or behavioral factors (e.g., increases in blood pressure and fluid overload, comorbidities, poor medication adherence, etc.) may be more important for predicting the timing of acute exacerbations.[7,8,24]
We also observed a post-hospitalization stress effect, with stress levels significantly elevated at assessments in the two weeks following a CV hospitalization. This is consistent with a post-hospitalization syndrome,[4] indicating that the experience of hospitalization adversely affects risk factors and heightens likelihood for subsequent hospitalization.[18] History of HF hospitalization is a known predictor of subsequent hospitalizations and adverse outcomes,[14] and the present findings suggest that this may be partially explained by the increase in psychological stress following hospitalization. HF patients with elevated perceived stress during emergency room admission are at great risk of 30 day rehospitalization,[18] and individuals with high stress after hospitalization may therefore be at increased risk of subsequent AEs. However, prior studies have not been able to determine whether increased stress reflects a chronic patient characteristic, or is associated with a specific hospitalization experience.[4] In the present study, not all individuals showed an increase in stress levels post-hospitalization. More than 1/3 (37%) of patients did not show this effect, and this may reflect chronic patient characteristics or differences in treatment during hospitalization. This issue has implications for whether preventive interventions should be targeted at modifying the hospitalization experience or reducing patient distress that may be more chronic in nature.
Despite the increase in stress following hospitalization, the predictive value of stress for future events in this study was not simply attributable to recent prior hospitalizations. A prospective relationship between perceived stress and subsequent AEs was present in patients without a recent prior hospitalization, and associations between stress and subsequent hospitalizations remained significant after controlling for recent hospitalizations prior to study enrollment. Perceived stress may be both a risk factor for future AEs as well as a consequence of hospitalization. HF is characterized by multiple exacerbations and hospitalizations over time, and a study design involving multiple longitudinal measurements allowed us to clarify acute and chronic associations between stress and AEs. This study design has been employed in research involving serial assessments of BNP,[25] and may also prove useful for investigating other risk factors for HF exacerbations, such as acute blood pressure elevations or medication non-compliance.
Several factors may account for the observation that average stress scores decreased between baseline and three months. Repeated contact and telephone conversation with study personnel may have reduced patients’ perceived stress, or attentive treatment because of study participation may have resulted in these patients’ stress level to decrease over time. Alternatively, a selection bias might have caused high stress patients to miss follow up assessments.
Pathways underlying observed associations of psychological stress with AEs may involve stress-induced increases in sympathetic, hemodynamic, pro-inflammatory and/or endocrine function that can contribute to worsening HF.[9,13,18, 27] Acute stress can also induce myocardial perfusion abnormalities and trigger malignant arrhythmias in HF patients.[17,26,27] Psychological stress has also important indirect effects on factors that increase risk of hospitalization, such as reduced ability to follow complex medication and lifestyle regimens,[11,24] impaired cognitive functioning, and reduced physical activity.[28]
Study Limitations
The present sample was majority African American, male, and had impaired LV function (systolic HF), which may limit the ability to generalize these findings to other populations. However, these data provide important information relevant to HF in minority populations and lower socioeconomic groups, who are known to develop markedly higher incident HF at early ages.[29]
Caution should also be exercised to not infer causality from observed associations of perceived stress and subsequent events. It is possible that factors such as an increase in symptoms or other physiological changes indicative of worsened clinical status may lead to heightened distress, or that those patients who are more distressed were generally sicker. This latter possibility is unlikely in this study since observed findings were present in multivariate analyses where HF risk factors were controlled. It is also possible that acute distress in the hours before the event may contribute to HF exacerbations, but this was not captured by our study design. If hospitalizations are triggered by a sudden worsening of the risk factor in the day or hours before the event, study designs that assess patients more frequently during daily life would be valuable.
Another limitation is the use of 8 covariates in relation to the 62 events in the study. This may have led to a lack of sufficient power in some of the analyses (e.g., the relationship of the Baseline PSS measurement to subsequent events) to detect effects should they exist.
Clinical Implications
Most interventions to reduce rehospitalizations include patient education and self-management strategies designed to increase adherence to complex medication regimens, making dietary changes, and assuring medical follow-up.[30] Stress is known to interfere with these behavioral changes. Behavioral interventions to reduce stress and enhance coping techniques may therefore enhance the efficacy of other multi-component interventions in reducing AEs. These data also suggest that evaluation of patient distress may help identify those individuals who are at increased risk of HF hospitalizations and AEs who may benefit most from preventive interventions. Evidence for a post-hospital syndrome[4,18] in a subgroup of patients also points to the need to identify individuals who may be vulnerable to these effects and also address aspects of hospitalization that may cause distress.[4] Taken together, these findings suggest psychological stress may be an important target when evaluating patients’ risk of short-term hospitalizations or death in HF.
Key Questions.
What is already known about this subject?
Psychosocial and behavioral variables (e.g., compliance with medications or diet, family support, etc.) may be important in heart failure exacerbations. However, little is known about the influence of psychological stress on heart failure exacerbations, including rehospitalizations or death.
What does this study add?
This study determined that perceived stress in heart failure patients is prospectively associated with short-term cardiac events in patients with systolic heart failure, and that there is a post-hospitalization increase in psychological distress. These findings suggest that stress may precipitate heart failure exacerbations, and that post-hospitalization stress may have further negative effects.
How might this impact clinical practice?
Assessment of distress in heart failure patients may help identify those at higher risk of short-term hospitalization. Reducing distress and addressing stressful aspects of hospitalization may help decrease future rehospitalizations and/or prevent heart failure exacerbations.
Acknowledgments
This work was supported by National Heart Lung and Blood Institute grant RO1-HL085730. The opinions and assertions expressed herein are those of the authors and are not to be construed as representing the views of Uniformed Services University or the US government.
Role of Sponsor: The National Heart Lung and Blood Institute had no role in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Abbreviation list
- AE
adverse event
- BMI
body mass index
- BDI
Beck depression inventory
- BNP
B-type natriuretic peptide
- CV
cardiovascular
- HF
heart failure
- LMM
linear mixed model
- LVEF
left ventricular ejection fraction
- NYHA
New York heart association
- PSS
Perceived Stress Scale
Footnotes
Conflict of Interest Disclosures: All authors report that there are not conflicts of interest.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights
References
- 1.Curtis LH, Greiner MA, Hammill BG, et al. Early and long-term outcomes of heart failure in elderly persons, 2001–2005. Archives of Internal Medicine. 2008;168:2481–2488. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. New England Journal of Medicine. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. European Heart Journal. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Archives of Internal Medicine. 2008;168:847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 8.Giamouzis G, Kalogeropoulos A, Georgiopoulou V, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. Journal of Cardiac Failure. 2011;17:54–75. doi: 10.1016/j.cardfail.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Kop WJ, Synowski SJ, Gottlieb SS. Depression in heart failure: biobehavioral mechanisms. Heart Failure Clinics. 2011;7:23–38. doi: 10.1016/j.hfc.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Wu JR, Lennie TA, Dekker RL, Biddle MJ, Moser DK. Medication adherence, depressive symptoms, and cardiac event-free survival in patients with heart failure. Journal of Cardiac Failure. 2013;19:317–324. doi: 10.1016/j.cardfail.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feenstra J, Grobbee DE, Jonkman FA, Hoes AW, Stricker BH. Prevention of relapse in patients with congestive heart failure: the role of precipitating factors. Heart. 1998;80:432–436. doi: 10.1136/hrt.80.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. American journal of Cardiology. 2012;110:1711–1716. doi: 10.1016/j.amjcard.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krantz DS, Sheps DS, Carney RM, Natelson BH. Effects of mental stress in patients with coronary artery disease: evidence and clinical implications. JAMA. 2000;283:1800–1802. doi: 10.1001/jama.283.14.1800. [DOI] [PubMed] [Google Scholar]
- 14.Bello NA, Claggett B, Desai AS, McMurray JJV, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circulation: Heart Failure. 2014;7:590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlman LV, Ferguson S, Bergum K, Isenberg EL, Hammarsten JF. Precipitation of congestive heart failure: social and emotional factors. Annals of Internal Medicine. 1971;75:1–7. doi: 10.7326/0003-4819-75-1-1. [DOI] [PubMed] [Google Scholar]
- 16.Akinboboye O, Krantz DS, Kop WJ, et al. Comparison of mental stress-induced myocardial ischemia in coronary artery disease patients with versus without left ventricular dysfunction. American Journal of Cardiology. 2005;95:322–326. doi: 10.1016/j.amjcard.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Wawrzyniak AJ, Dilsizian V, Krantz DS, et al. High Concordance Between Mental Stress-Induced and Adenosine-Induced Myocardial Ischemia Assessed Using SPECT in Heart Failure Patients: Hemodynamic and Biomarker Correlates. Journal of Nuclear Medicine. 2015 Oct;56(10):1527–1533. doi: 10.2967/jnumed.115.157990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edmondson D, Green P, Ye S, Halazun HJ, Davidson KW. Psychological stress and 30-day all-cause hospital readmission in acute coronary syndrome patients: an observational cohort study. PloS one. 2014;9:e91477. doi: 10.1371/journal.pone.0091477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 20.Cohen S, Janicki-Deverts D. Who's Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 20091. Journal of Applied Social Psychology. 2012;42:1320–1334. [Google Scholar]
- 21.Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long-term mortality and health status outcomes. Journal of the American College of Cardiology. 2012;60:1756–1763. doi: 10.1016/j.jacc.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers ND, Brincks AM, Ames AJ, Prado GJ, Penedo FJ, Benedict C. Multilevel modeling in psychosomatic medicine research. Psychosomatic Medicine. 2012;74:925–936. doi: 10.1097/PSY.0b013e3182736971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedeker D, Mermelstein RJ, Berbaum ML, Campbell RT. Modeling mood variation associated with smoking: An application of a heterogeneous mixed-effects model for analysis of ecological momentary assessment (EMA) data. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaya M, Phan A, Schwarz ER. Predictors of re-hospitalization in patients with chronic heart failure. World Journal of Cardiology. 2012;4:23–30. doi: 10.4330/wjc.v4.i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisel A, Barnard D, Jaski B, et al. Primary results of the HABIT Trial (heart failure assessment with BNP in the home) Journal of the American College of Cardiology. 2013;61:1726–1735. doi: 10.1016/j.jacc.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 27.Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. New England Journal of Medicine. 1988;318:1005–1012. doi: 10.1056/NEJM198804213181601. [DOI] [PubMed] [Google Scholar]
- 28.Rod NH, Gronbaek M, Schnohr P, Prescott E, Kristensen TS. Perceived stress as a risk factor for changes in health behaviour and cardiac risk profile: a longitudinal study. Journal of Internal Medicine. 2009;266:467–475. doi: 10.1111/j.1365-2796.2009.02124.x. [DOI] [PubMed] [Google Scholar]
- 29.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. New England Journal of Medicine. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]