Abstract
Object
Glioblastoma multiforme (GBM) is an aggressive brain cancer with median survival of less than two years with current treatment. GBM exhibits extensive intra-tumor and inter-patient heterogeneity, suggesting that successful therapies should exert broad anti-cancer activities. Therefore, the natural non-toxic pleiotropic agent, resveratrol, was studied for anti-tumorigenic effects against GBM.
Methods
Resveratrol’s effects on cell proliferation, sphere-forming ability, and invasion were tested using multiple patient-derived GBM stem-like cell (GSC) lines and established U87 glioma cells, and changes in oncogenic AKT and tumor suppressive p53 were analyzed. Resveratrol was also tested in vivo against U87 glioma flank xenografts using multiple delivery methods, including direct tumor injection. Finally, resveratrol was delivered directly to brain tissue to determine toxicity and achievable drug concentrations in the brain parenchyma.
Results
Resveratrol significantly inhibited proliferation in U87 glioma and multiple patient-derived GSC lines, demonstrating similar inhibitory concentrations across these phenotypically heterogeneous lines. Resveratrol also inhibited the sphere-forming ability of GSCs, suggesting anti-stem cell effects. Additionally, resveratrol blocked U87 glioma and GSC invasion in an in vitro Matrigel transwell assay at doses similar to those mediating anti-proliferative effects. In U87 glioma cells and GSCs, resveratrol reduced AKT phosphorylation and induced p53 expression and activation that led to transcription of downstream p53 target genes. Resveratrol administration via oral gavage or ad libitum in the water supply significantly suppressed GBM xenograft growth; intra-tumor or peri-tumor resveratrol injection further suppressed growth and approximating tumor regression. Intracranial resveratrol injection resulted in 100-fold higher local drug concentration compared to intravenous delivery, and with no apparent toxicity.
Conclusions
Resveratrol potently inhibited GBM and GBM stem-like cell growth and infiltration, acting partially via AKT deactivation and p53 induction, and suppressed glioblastoma growth in vivo. The ability of resveratrol to modulate AKT and p53, as well as reportedly many other anti-tumorigenic pathways, is attractive for therapy against a genetically heterogeneous tumor such as GBM. Although resveratrol exhibits low bioavailability when administered orally or intravenously, novel delivery methods such as direct injection (i.e. convection enhanced delivery) could potentially be used to achieve and maintain therapeutic doses in brain. Resveratrol’s non-toxic nature and broad anti-GBM effects make it a compelling candidate to supplement current GBM therapies.
Keywords: brain tumor, cancer stem-like cells, glioblastoma multiforme, natural products, resveratrol
INTRODUCTION
An estimated 22,000 new primary malignant brain tumors are diagnosed in the United States each year, accounting for 1.5% of all cancer-related deaths 3. The most prevalent and aggressive malignant brain tumor is glioblastoma multiforme (GBM, also called grade IV astrocytoma), a cancer of the supporting glial cells, which accounts for greater than 14,000 cases 3. GBMs confer a median survival of less than two years due to rapid recurrence despite maximal surgery, temozolomide chemotherapy and radiation 47. The subset of glioblastoma stem-like cells (GSCs) and/or other therapeutically resistant GBM cells are hypothesized to drive tumor recurrence 12,46. Therefore, therapeutic GSC targeting will be critical for improving clinical outcomes.
Glioblastomas harbor diverse genetic abnormalities, including dysregulation of receptor tyrosine kinases (PDGFRα, EGFR), tumor suppressors (NF1, p53), and cell cycle regulatory (p16, Rb) pathways 11. Recent efforts have also identified involvement of epigenetic 35 and metabolic (IDH1/2) 38,58 mutations in GBM. Altogether, GBMs are characterized by a complex somatic genetic landscape and redundancy in oncogenic and tumor suppressor pathways 11. This complexity suggests that specifically targeted molecular agents will likely be ineffective for improving GBM patient outcomes, with many such agents failing to show benefit in clinical trials 5.
New therapies delivering broad anti-cancer effects would be desirable to effectively treat GBM. The natural plant-derived compound, resveratrol (3,4′,5-trihydroxy-trans-stilbene), exerts multiple anti-carcinogenic effects on tumor cell growth, inflammation, apoptosis, angiogenesis, invasion/metastasis, sensitization to radiation and chemotherapies 4, and inhibits growth and invasion of glioma cell lines 19,24,25,30. Resveratrol crosses the blood-brain barrier 56, although precise intracranial concentrations have not been established, and then exhibits pleiotropic molecular effects to alter many different signaling pathways important for GBM proliferation (i.e., nuclear factor-kB, Rb-E2F, p53, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase pathways) 1,4. Some of these pathways are also important for GSC maintenance, such as Akt 17,20, JAK/STAT 59, and Nanog 43. Importantly, resveratrol is non-toxic and exhibits virtually no serious adverse effects at doses up to 5 g in humans 10, and could potentially be used as a non-toxic supplement to current adjuvant temozolomide chemo-radiation therapy. To date, suboptimal bioavailability due to resveratrol’s poor aqueous solubility and rapid metabolism to inactive products have hampered development of resveratrol-based therapies 8,39,44,55,57. However, novel delivery systems applicable to resveratrol including nanoparticle formulations 36,41 or neurosurgical methodology such as convection enhanced delivery (CED)53 are close to practical clinical applications, and could potentially deliver therapeutically relevant doses of resveratrol.
In this study, we analyzed the anti-tumorigenic effects of resveratrol against GBM and GSC cells in vitro and in vivo. Resveratrol inhibited proliferation of GBM cell lines and sphere-forming self-renewal of GSCs, and blocked invasion of both GBM and highly invasive GSC cells in vitro. Resveratrol altered multiple oncogenic and tumor suppressor pathways, including deactivating oncogenic Akt while stimulating p53 and associated tumor suppressor gene network. Finally, resveratrol inhibited in vivo GBM xenografts growth through multiple routes of administration: daily oral administration, ad libitum water intake, or direct intra- and peri-tumoral injection. Preliminary pharmacokinetic studies in normal brain demonstrated 100-fold increase in local resveratrol concentration using intracranial compared to intravenous delivery, without any observed toxicity. Altogether, resveratrol demonstrated broad anti-GBM activity, and as a non-toxic compound could be a valuable adjuvant therapy to improve GBM patient outcomes.
MATERIALS AND METHODS
Cell culture
Glioblastoma stem-like cells (GSCs) were derived directly from patient specimens obtained from the operating room, under a protocol approved by the University of Wisconsin – Madison Institutional Review Board (IRB). Glioblastoma patient specimens were collected anonymously as per IRB protocol, so verifying cell lines compared to original patient tumor was not possible. Each cell line was rigorously validated for self-renewal by neurosphere formation, multipotency, and tumor initiation (below) before experiments were performed. Establishing of cell cultures came from cryopreservation of cell cultures ranging from passages 15–22. Cells used for experiments ranged from passage 20–25. The U87 glioma cell line was purchased from the American Type Culture Collection (ATCC) and used within 6 months of receipt without additional testing or authentication.
GSCs were cultured under marker neutral conditions in serum-free stem cell medium as previously described 15,60. Briefly, tumor tissue was collected directly from the operating room, weighed, coarsely minced with a microdissection scissors, and enzymatically dissociated (Accutase, Millipore Corporation) to single cells at 37 °C for 20–30 min. The cell slurry was passed through a 40 μm cell strainer and red blood cells eliminated using a lysis buffer (Red Blood Cell Lysing Buffer, Sigma-Aldrich Co.). The final single cell suspension was plated as suspension culture at approximately 200,000 cells/ml in stem cell medium (70% DMEM-high glucose, 30% Ham’s F12, 1×B27 supplement, 5 μg/ml heparin, 1% antibiotics and 20 ng/ml each EGF and bFGF) 15,60. Cultures were passaged approximately every 10–21 days by tissue chopping 2× at 200 μm. Patient-specific GSC lines were isolated from primary GBM (lines 22, 33, and 44) or recurrent GBM (line 12.1). We have previously shown that these GSC lines display multi-lineage potential and self-renewal in cell culture, and efficiently (as few as 100 cells) initiate GBM orthotopically in mice that exhibit hallmarks of human GBM including infiltration into normal brain parenchyma 15,60. Specifically, the 44 GSC line is highly infiltrative in orthotopic xenografts60 and representative of more difficult treatment conditions in patients; therefore, resveratrol was tested against this patient-derived GSC line in most experiments. The U87 glioma cell line was maintained in growth medium (DMEM, 10% fetal bovine serum (FBS), and 1% antibiotics) and passaged when indicated using standard trypsin digestion (Life Technologies). The cells were maintained at 37°C with 5% CO2.
Proliferation assay
U87 glioma cells were plated in 96-well tissue culture plates at 750 cells/well. The 44-GSCs were plated on laminin coated 96-well tissue culture plates at 20,000 cells/well. Different dosages of resveratrol were added after overnight recovery, and viability monitored at multiple time-points using cell titer blue (Promega Corporation) according to the manufacturer’s instructions. Media was changed every other day with fresh aliquots of resveratrol. Fluorescence was measured at excitation/emission wavelengths of 560/590 nm respectively using a fluorescence plate reader (Synergy Molecular Devices) 51.
GSC sphere-forming assay
Plating densities resulting in visually near-clonogenic sphere growth for all GSC and NSC lines were optimized first. For experiments, spheres were enzymatically dissociated to single cells and incubated in 96-well plates at optimal density (500–1000 cells) in stem cell medium. After recovery overnight, resveratrol or vehicle control (0.1% DMSO) was added, establishing a dose-response curve. Upon formation of ≈200 μm diameter spheres in control groups (2–4 weeks), the total number of spheres was manually counted within each of the culture wells.
Invasion assays
Transwell inserts (8 μm pore polycarbonate Transwell permeable support, Millipore Corporation) were coated on the upper surface with 200 μg/ml of Matrigel at room temperature followed by 5 μg/ml of fibronectin (Sigma-Aldrich Co.) at 4°C overnight on the lower surface, as previously described 9. Actively growing GSCs or U87 glioma cells were treated with Accutase or trypsin, respectively, washed, and re-suspended at 1×106 cells/ml in serum-free medium containing DMSO or resveratrol of desired dosages. 1×105 cells in 100 μl were carefully placed in the upper chamber. The upper and lower chambers contained the same media as the cell suspensions. After 4 hr incubation at 37°C, cells from the upper chamber were wiped clean and cells of the lower chamber fixed with 4% paraformaldehyde. 4′,6-diamidino-2-phenylindole (DAPI) (Life Technologies) diluted to 10 μg/ml in PBS was then used to stain cell nuclei. Images were taken using a fluorescent microscope (Evos FL, Thermo Fisher Scientific Inc.). Nuclei were automatically counted using ImageJ software (Wayne Ras-band, NIH, rsbweb freeware) across multiple microscopic fields per experimental condition.
Western blot analysis
GSCs or U87 glioma cells were lysed with RIPA buffer supplemented with protease and phosphatase inhibitors and subsequently mixed with SDS-containing solution. Equal amounts of protein were loaded onto 10% polyacrylamide gels, transferred to polyvinylidene fluoride (PVDF) membrane and stained with antibodies. Primary antibodies toward the following antigens were used: phosphorylated (ser473) Akt (clone D9E), pan-AKT (clone C67E7), phosphorylated (ser15) p53 (clone 16G8), p53 (clone 7F5) (all from Cell Signaling Technology, Inc.), and GAPDH (Bio-Rad, Inc). Horseradish peroxidase-conjugated species-specific secondary antibodies (Jackson Immuno Research) were then used to label primary antibodies, followed by detection via a chemiluminescent substrate (ECL kit, GE Healthcare), as described previously 9,50.
Quantitative real-time PCR
After 24-hrs of resveratrol treatment, U87 cells and GSCs were collected and total RNA isolated using Trizol (Life Technologies). Following extraction with chloroform and addition of an equal volume of ethanol to the aqueous phase, samples were loaded onto RNeasy columns (Qiagen) and RNA was purified according to the manufacturer’s instructions. First strand cDNA was synthesized with random hexamers using the Superscript III First Strand Synthesis System (Life Technologies). Specific primers were designed using Primer-BLAST (NCBI) and purchased from Integrated DNA Technologies. Quantitative real-time PCR was performed with SYBR-Green supermix (Bio-Rad) on an iCycler (Bio-Rad) according to the manufacturer’s instructions. Relative expression levels and ranges were calculated according to the comparative method (ABI Prism 7700 Sequence Detection System: Relative Quantitation of Gene Expression. User bulletin 2: revision B. PE Applied Biosystems; 2001).
Tumor xenograft assays
Resveratrol formulations: 125 μl of ethanol was added to 125 mg of resveratrol, then dissolved in 5 ml of a 10% P104 aqueous solution, and filtered through a 0.2 μm syringe filter to obtain a clear solution (for intra-tumor injection). DMSO (4 ml) was added to 100 mg of resveratrol (for peri-tumor injection). While DMSO is a common vehicle used for intraperitoneal, peri-tumor and other routes of drug delivery, in our experience direct intra-tumor injections of DMSO can result in the formation of scabs surrounding the injection site and more lethargic animals. The difference observed between intra- and peri-tumor injections may be due to the extensive vasculature associated with the tumor. We find however that the problem associated with intra-tumor drug delivery is alleviated by using ethanol and P104 as vehicle instead of DMSO.
The Animal Care and Use Committee at the University of Wisconsin-Madison approved all animal experiments. All animal caretakers and laboratory staff followed standards set by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). 5–6 week-old female BALB/c Nude mice were purchased from Charles River Laboratories. Animals were housed in a pathogen-free isolation facility on a 12-hour light-dark cycle, with food and water provided ad libitum. Mice were each given a dorsal subcutaneous injection of 5 × 106 U87 glioma cells suspended in 500 μl of 1:1 culture medium and basement membrane matrix suspension (Matrigel, Becton Dickinson). Tumors were allowed to grow to approximately 200 mm3 before the animals were randomized into eight groups. Group 1 (10 animals) received water containing 0.1 mg/ml resveratrol ad libitum for 18 days, while group 2 (10 animals) received water ad libitum for the same period; group 3 (10 mice) received 50 mg/kg of resveratrol in 100 μl Neobee M5 oil daily by oral gavage for 18 days, while group 4 (10 mice) received Neobee M5 oil alone. Group 5 (3 mice) received 5 injections of 200 μl of 5 mg resveratrol in P104 (BASF) over a period of 2 weeks into the tumor (intra-tumor), while group 6 (3 mice) received 5 injections of P104 vehicle alone in a similar fashion; group 7 (3 mice) received 5 injections of 200 μl of resveratrol (5 mg) in DMSO into the tissue adjacent to the tumor (peri-tumor) over a period of 2 weeks, while group 8 (3 mice) received 5 injections of DMSO alone over the same period.
Tumor size was measured twice weekly with calipers in three dimensions (length, width, and height), and the volume was determined by multiplying the three values. One day following the last dose, mice were euthanized and their tumors harvested, and a final tumor measurement taken in three dimensions using calipers. Animal protocols required the sacrifice of all mice upon the observation of symptomatic/moribund control group animals, and therefore long-term follow-up of treated animals could not performed in this study. After euthanasia, the tumors were fixed in 10% neutral buffered formalin, and processed for histology. Five-micrometer sections were cut that encompassed the full circumference of the tumor and then stained with hematoxylin and eosin (H&E). The outline of the tumor in each section was traced from a microscopically digitized image, and the areas of viable- and nonviable-appearing tumor were measured using ImageJ software.
Means and standard deviations of the tumor volume in each group were calculated. Statistical differences between each treatment group and their respective control group were determined using an unpaired two-tailed t-test. P values less than 0.05 were considered significant.
Resveratrol brain pharmacokinetic analysis
Resveratrol was dissolved in PBS at 100 μg/ml which is approximately maximum solubility.
For intravenous delivery, the tails of ICR outbred mice were first submerged in warm (30–35°C) water to stimulate vein dilation, and then 100 μl resveratrol solution was slowly injected into the tail vein.
For intracranial delivery, using a Hamilton syringe, 10 μl of the resveratrol solution was stereotactically injected into the right striatum of anesthetized ICR outbred mice at 1 μl/min at the following coordinates referenced from bregma: 0 mm antero-posterior, +2.5 mm medio-lateral, and −3.5 mm dorso-ventral 15,60.
In initial experiments, mice were observed for 1 hour for toxic effects. After verifying no toxic effects, an additional cohort of mice were euthanized at 5, 30, and 60 mins (3 mice per time point) for a pharmacokinetic analysis. Mice were euthanized at each time point by exsanguination and perfused with PBS. The brain was then rapidly removed, weighed, and frozen in liquid nitrogen.
For pharmacokinetic analysis, brains were extracted with 3 volumes of PBS using 30 strokes of a dounce (A pestle) homogenizer. Fifty-five μl of each extract was then transferred to a 500 μl amber microcentrifuge tube. To construct calibration curves, extracts from untreated brains were spiked with 2.2 μl of serial dilutions of resveratrol dissolved in 100% acetonitrile. Extracts from treated and untreated brains were then transferred onto Ostro plates (Waters Corporation), triturated, and incubated ~2 min with occasional shaking. Additionally, 150 μl of acetonitrile/1% acetic acid was spiked with an internal standard, Naproxen D3. Resulting samples were run through a CSH-C18 column (Thermo Fisher Scientific Inc.), transferred to Waters vials, and analyzed by LC/MS/MS (Agilent 1100 LC-MSD quadrupole SL). Data were analyzed compared to internal standards to obtain mass concentrations (μg/g) of resveratrol in whole brain. For intravenous delivery, resveratrol whole brain molar concentration (μM) was approximated using a value for brain density of 1.081 g/ml 7. For intracranial delivery, local brain molar concentrations were approximated from whole brain total drug mass using CED parameters reported in the literature. A volume distribution (Vd) to volume injected (Vi) ratio of 5.23±1.67 32 was used to calculate the molar concentration of resveratrol at infusion site. This Vd/Vi ratio is an experimentally-derived value for CED using a 1 μl/min flow rate into non-human primate brain 32.
RESULTS
Resveratrol inhibits glioma cell and GSC proliferation in vitro
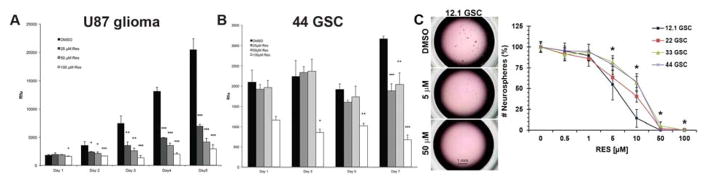
Resveratrol’s anti-proliferative effects on GBM were investigated by determining dose response growth curves in cell culture. Administration of resveratrol significantly inhibited U87 glioma cell proliferation compared to vehicle control (Figure 1A), with measured IC50 = 20 μM on day 5 of drug exposure. In parallel experiments, resveratrol also inhibited GSC proliferation (Figure 1B). Similar effects were observed for GSC self-renewal and proliferation using a sphere-forming assay. Compared to DMSO vehicle control, addition of resveratrol (doses from 5–50 μM) resulted in greater than 50% inhibition of sphere formation in four independent patient-derived GSC lines (12.1, 22, 33, 44) (Figure 1C).
Figure 1.
Resveratrol inhibits the growth of GBM cells and GSCs. (A) Decreased U87 glioma cell and (B) 44-GSC proliferation upon resveratrol treatment compared to vehicle control (mean±S.D.; *p≤0.05; **p≤0.01; ***p≤0.001 using Student’s two-tailed t-Test, n=3 in each of 3 independent experiments). (C) Likewise, as little as 5 μM resveratrol inhibited sphere formation in multiple patient-derived GSC lines compared to the vehicle control (mean±S.D.; *p≤0.05 compared to 0 μM RES by ANOVA followed by post-hoc Tukey test, n = 3 in each of 3 independent experiments). RES, resveratrol.
Resveratrol inhibits GBM cell migration in vitro
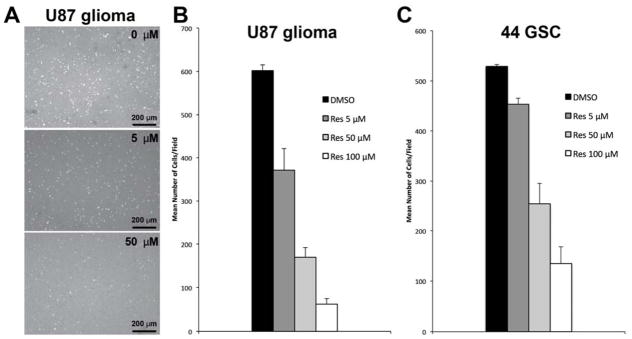
Tumor cell infiltration into normal brain parenchyma is a hallmark feature of GBM, preventing complete surgical extirpation and limiting the effectiveness of adjuvant radiation and chemotherapy 31. Resveratrol inhibition of GBM cell invasion was tested using a transwell assay with Matrigel substrate. Four hours of exposure to as little as 5μM resveratrol significantly decreased U87 glioma cell invasion compared to the DMSO control (Figure 2A and 2B; p<0.05, n=3 in each of 3 independent experiments), with greater than 75% inhibition at 50 μM and higher resveratrol doses (Figure 2B). Implantation of the highly invasive 44-GSC line results in orthotopic tumor xenografts throughout most of the brain parenchyma 60. Notably, 44-GSCs were similarly inhibited by resveratrol in the above Matrigel invasion assay, as compared to vehicle controls (Figure 2C; p<0.05, n=3 in each of three independent experiments).
Figure 2.
Resveratrol inhibits tumor cell invasion in a Matrigel transwell assay. (A) U87 glioma cell invasion as a function of RES concentration; DAPI nuclear stain: white. (B): U87 glioma cell line invasion; mean±S.D.; p<0.05, n=3 in each of 3 independent experiments. (C) 44 GSC invasion; mean±S.D.; p<0.05, n=3 in each of 3 independent experiments). Res, resveratrol.
Resveratrol affects AKT and p53 signaling pathways
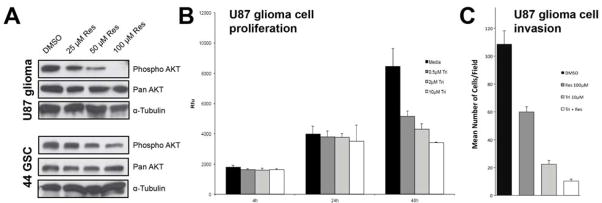
Resveratrol has pleiotropic effects that alter many cellular signaling pathways 1, including core oncogenic pathways such as AKT and p53 mechanisms involved in GBM growth, survival, and invasion 49. After resveratrol or DMSO vehicle treatment, U87 glioma cells and 44-GSCs were processed for Western analysis. AKT activation, tested via ser473 phosphorylation, was reduced in a dose-dependent manner in U87 glioma cells treated with resveratrol (Figure 3A, top panel). Resveratrol treatment also decreased AKT phosphorylation in 44-GSCs (Figure 3A, bottom panel). There were no discernable changes in total AKT protein levels.
Figure 3.
Resveratrol decreases AKT activity in GBM cells and GSCs. (A) Top panel: Resveratrol treatment resulted in reduced AKT phosphorylation (ser473) in U87 glioma cells and the 44 GSC cell line (bottom panel), 48 hrs post-treatment, while minimally affecting total AKT protein. (B) Triciribine, a specific AKT inhibitor, did not affect U87 glioma cell growth up to 24 hrs post-treatment, although (C) it significantly suppressed migration as early as 4 hrs after treatment, compared to vehicle controls (mean±S.D.; p<0.05, n=3 in each of 3 independent experiments), and demonstrated synergistic anti-invasion effects with resveratrol (mean±S.D.; p<0.05, n=3 in each of 3 independent experiments). Res: resveratrol; Tri: triciribine.
To further test the involvement of AKT in GBM cell invasion, U87 glioma cells were incubated with triciribine, an AKT inhibitor, and a transwell migration assay with Matrigel was performed. Triciribine exposure up to 24 hr did not change U87 glioma cell viability (Figure 3B), but inhibited U87 glioma cell invasion by greater than 80% within 4 hr of treatment (Figure 3C). The combination of resveratrol and triciribine inhibited cell invasion to a larger degree than either compound alone (Figure 3C).
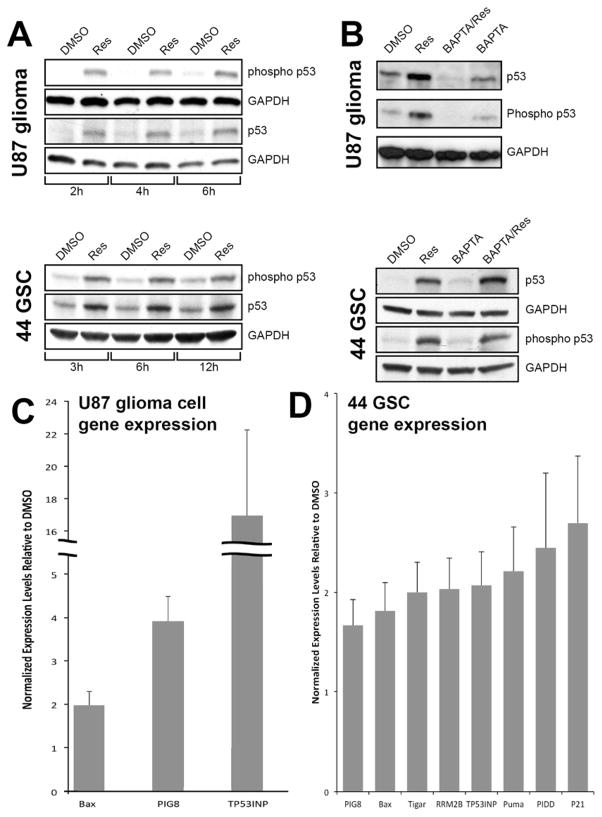
By regulating expression of diverse downstream target genes, p53 can induce cell cycle arrest and apoptosis, contributing to the inhibition of glioma cell growth as shown in Figure 1. To test this possibility, U87 glioma cells and 44-GSCs were treated with resveratrol and Western analysis performed using antibodies specific to p53 and phosphorylated (ser15) p53. Compared to vehicle controls, resveratrol treatment increased p53 expression and concomitantly, the levels of phosphorylated p53 in U87 glioma cells (Figure 4A, top panel) and GSCs (Figure 4A, bottom panel). Resveratrol induction of p53 in U87 glioma cells was dependent upon calcium (Figure 4B, top panel). Pre-incubation with BAPTA (a cell-permeable calcium chelator) blocked resveratrol-induced p53 expression and phosphorylation. This finding is consistent with previous reports that intracellular calcium increased after exposure of tumor cells to resveratrol 42. However, the resveratrol-induced increase of p53 expression in 44-GSCs was independent of calcium (Figure 4B, bottom panel). To further verify that resveratrol induces the expression and phosphorylation of p53, quantitative PCR was employed to measure changes in expression of p53-dependent downstream genes. As shown in Figure 4C, resveratrol treatment of U87 glioma cells led to increased expression of Bax, Pig8 and TP53INP. Increased expression of these and additional p53-dependent target genes were observed in 44-GSCs treated with resveratrol (Figure 4D).
Figure 4.
Resveratrol activates p53 and p53 pathway genes. (A) Administration of 100 μM resveratrol to U87 glioma cells (top panel) or 44-GSCs (bottom panel) increased expression and phosphorylation of p53 tumor suppressor protein. (B) P53 activation was reduced by co-administration of a calcium chelator (BAPTA) in U87 glioma cells (top panel) but not in 44-GSCs (bottom panel). Resveratrol activation of p53 in U87 cells (C) and GSCs (D) resulted in expression of multiple downstream genes of p53 suppressor network, compared to vehicle control and detected by quantitative PCR.
Resveratrol reduces tumor growth in vivo
The anti-tumorigenic effects of resveratrol were tested in vivo using a U87 glioma xenograft model. Tumors cells implanted in the dorsal flanks of immune-deficient mice formed tumor xenografts of approximately 200 mm3 after 14–21 days. Different routes of resveratrol administration were tested to measure therapeutic efficacy and detect any toxicity. As dictated by the animal protocol, when control animals became symptomatic or moribund, all animals were sacrificed and treated animals therefore could not be followed for long-term survival.
In the first experiment, resveratrol was administered ad libitum through the drinking water supply. Resveratrol has limited solubility of approximately 100 μg/ml in aqueous solutions. Mice utilized in this study consume about 5 ml of fluid/day, and thereby attained a daily intake of approximately 500 μg of resveratrol or a dose equivalent to 25 mg/kg/day. In contrast to dosing via oral gavage, ad libitum consumption of water that contains resveratrol occurs over the full 24 hours of each day. Administration of resveratrol in the water supply resulted in significantly smaller average tumor volumes in resveratrol-treated animals compared to xenografts in control animals (p = 0.01). Significantly reduced tumor volumes were observed with resveratrol administration compared to vehicle-treated animals as early as 7 days, and continued until sacrifice at 18 days (Figure 5A). The average tumor volume in the resveratrol group at the end of the experiment was 916 ± 94 mm3 versus 1,502 ± 173 mm3 for the control group, or approximately a 40% inhibition of tumor growth at 18 days post-treatment (Figure 5A).
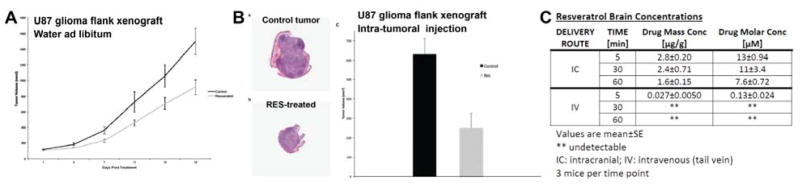
Figure 5.
Resveratrol inhibits U87 xenograft growth. (A) Resveratrol administered in water ad libitum to mice harboring U87 flank xenografts resulted in significantly reduced tumor growth compared to vehicle-treated controls (p<0.05, mean±S.D.; n=10 mice per group). Similar results were obtained with intragastric delivery of resveratrol; see Results. (B) Direct intra-tumoral injection of resveratrol (b, H&E) significantly decreased tumor volume in mice harboring U87 flank xenografts, compared to vehicle controls (a) (c: p<0.05, mean±S.D.; n=3 mice per group). (C) Brain concentrations of resveratrol were measured 5, 30, and 60 mins after either intravenous (IV) tail vein injection or intracranial (IC) delivery. Resveratrol molar concentrations [μM] are whole brain values for intravenous delivery and localized concentration for intracranial infusion, calculated as described in Materials and Methods.
In the second delivery route, a single daily delivery of resveratrol by oral gavage was approximately 1 mg/mouse, equivalent to a 50 mg/kg/day dose or roughly twice the amount of resveratrol delivered via the drinking water regimen in the above experiment. With the oral gavage treatment protocol, the average tumor volume of animals treated with resveratrol also was significantly smaller compared to tumors in the control group (p = .04). The average tumor volume in the treatment group was 896 ± 167 mm3 compared to the control group 1,435 ± 182 mm3, or a 39% inhibition of tumor growth by day 18 post-treatment.
The bioavailability of resveratrol can be increased by direct injection, thus avoiding limited uptake in the stomach and gastrointestinal tract as well as rapid metabolism in the gut and liver 51. As shown in Figure 5B (panels a–c), the intra-tumor injection of resveratrol decreased tumor volume by 60% compared to tumors injected with vehicle alone (p = 0.03). Peri-tumor injection of resveratrol had a comparable effect, decreasing tumor volume by 56% compared to the vehicle control. Subsequent histological analysis of the tumors obtained at necropsy revealed approximately 25% of the tumor volume consisted of necrotic cells (Figure 5B, panels a & b). There was no statistically significant difference between necrotic areas in tumors obtained from control or resveratrol-treated mice (p= 0.29). Even at these higher doses of resveratrol attained by direct injection, there were no adverse effects to vital organs observed upon histological examination.
The ability to deliver resveratrol to the normal brain parenchyma was also explored using multiple delivery mechanisms. After intravenous injection, resveratrol was detectable in brain tissue after 5 min (0.027±0.0050 μg/g, ≈0.13±0.024 μM; Figure 5C), but undetectable at 30 and 60 min. Using intracranial delivery however, local resveratrol concentrations were increased approximately 100-fold after 5 mins (2.8±0.20 μg/g, ≈13±0.94 μM; Figure 5C), and remained detectable up to 60 mins (30 mins: 2.4±0.71 μg/g, ≈11±3.4 μM; 60 mins: 1.6±0.15 μg/g, ≈7.6±0.72 μM; Figure 5C). No adverse side-effects were observed up to one hour following intracranial delivery.
DISCUSSION
There is considerable interest in using the non-toxic, plant polyphenol resveratrol as a potential cancer therapeutic agent 19,21,41. It exhibits anti-tumorigenic activity against a broad range of cancers by modulating diverse oncogenic and tumor suppressor pathways, including proliferation, apoptosis, autophagy, migration, angiogenesis, and inflammation 1. Resveratrol has also been demonstrated in other cancers to activate the intrinsic apoptotic pathway 51, an ER stress response 37, and a family of calcium-dependent cysteine proteases 42, thereby causing tumor cell death and improving cancer control 28.
In this study, we demonstrate that resveratrol induces widespread anti-GBM and anti-GSC effects by deactivating oncogenic AKT and activating the tumor suppressor p53 gene network, and inhibiting GBM hallmark features of cell proliferation and infiltration. Resveratrol’s broad anti-GBM effects are very desirable due to an extensive landscape of tumor genetic aberrations 11 and a large number of mutated or abnormal cancer pathways resulting in tumor heterogeneity 11,49, as well as intrinsic and rapidly acquired resistances of GBM cells and GBM stem-like cells 6,12,15,29. Additionally, median survival is poor despite the current clinical treatment of maximal safe surgical resection followed by adjuvant radiation and temozolomide chemotherapy 47, a relatively toxic regimen for patients. Resveratrol was demonstrably shown to be non-toxic, with even up to 5 g doses showing no adverse side effects in humans 10; therefore it is a good candidate to supplement current treatments. Lenz and colleagues recently reported in vitro studies showing resveratrol’s synergy with temozolomide, the current clinical GBM chemotherapy, to cause mitotic catastrophe in tumor cells 18. In addition, the ability of resveratrol to target and reduce the therapeutically resistant GSC subpopulation, shown in this study and by other groups 43,59, could also improve GBM outcomes 28. Taken together, there is a compelling evidence for combining resveratrol with standard GBM treatments.
Resveratrol inhibition of GBM cell and GSC growth was first demonstrated against the U87 glioma cell line and 4 independent patient-derived GSC lines isolated via marker-neutral stem cell culture 15,60. Initial viability assays suggested that resveratrol may affect GSCs less than established U87 cells, not surprising as GSCs are notoriously resistant to many commonly used therapies 6,29. However, the effect of resveratrol on cell viability, as shown in Figure 1, is a composite of anti-proliferative and pro-apoptotic events. As can be seen by comparing the data in Figures 1A & B, the GSCs have a much slower growth rate, therefore the anti-proliferative effects of resveratrol are not as evident until day 7, much later than that observed for the faster growing U87 glioma cells. In contrast to these findings, in the longer sphere-forming assay (2–4 weeks) compared to the proliferation assay (5–7 days), the GSCs demonstrated similar drug sensitivity, which suggests anti-stem cell effects of resveratrol that may help with clinical long-term control of glioblastoma. That resveratrol has less of an impact on the levels of AKT phosphorylation in GSCs (Figure 3) during the shorter time line of the experiment correlates with the results obtained in the viability assays comparing GSCs and U87 cells. Additionally, some differences in resveratrol sensitivity were observed among GSC lines (Figure 1C), likely due to inter-tumor heterogeneity of patient-derived GSCs as has been described 54,60. Interestingly, the resveratrol-sensitive GSC lines in this study show increased stem cell marker expression, result in less invasive orthotopic xenografts, and are positive for oligodendroglial marker 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNP), compared to other GSC lines with more invasive orthotopic xenograft phenotypes and high GFAP protein expression 60. The “proneural” class 22,40 shares attributes with the CNP+ GSC class, such as increased stem cell gene/protein expression and less invasive orthotopic xenograft growth. Therefore, resveratrol might be used to selectively target the proneural GBM subtype that is known to be relatively resistant to current treatments 27,52. Although less sensitive to resveratrol’s anti-proliferative effects than the 12.1- and 22-GSC lines, the 44-GSC line was sensitive to the anti-invasive effects of resveratrol (Figure 2), demonstrating resveratrol’s utility to broadly inhibit multiple tumorigenic processes in this molecularly heterogeneous cancer.
The specific molecular mechanism(s) underlying resveratrol’s anti-GBM activities is still being elucidated. In this study, we demonstrated inactivation of AKT and activation of the p53 tumor suppressor pathway. Resveratrol-mediated decrease in AKT protein and inactivation has been demonstrated in glioma cell lines, resulting in decreased proliferation, increased apoptosis, and decreased migration 24,25. In this study, resveratrol decreased AKT phosphorylation at ser473 in both GBM cells and GSCs while minimally affecting total AKT protein levels. The AKT oncogene specifically regulates infiltration into normal brain parenchyma 33 and promotes maintenance of the GSC sub-population 17,20. It is important to note that pan-AKT protein assays were done in this study, and individual AKT isoforms were not tested. Some AKT isoform-specific effects have been reported in glioblastoma 13,34.
Our results showing resveratrol activation of the p53 tumor suppression network is consistent with other studies 43. The U87 glioma cell line used in this study genetically exhibits wild-type p5318, while p53 genotyping of the 44 GSC line is ongoing. Wild-type p53 is not always crucial to elicit a resveratrol response as some p53 mutant lines (U138) also display resveratrol sensitivity18, owing to multifaceted anti-proliferative and pro-apoptotic effects of resveratrol, while others do not (LN-18)45. Serine-15 phosphorylation of p53 leads to decreased affinity for the negative regulator Mdm2, and increases p53 protein stability. In addition, it promotes nuclear retention of p53 and promotes recruitment of transcriptional co-activators of p53 target genes. We demonstrated that resveratrol activates transcription of several key downstream p53 target genes such as TP53INP1, an alternatively spliced gene that encodes two nuclear protein isoforms (TP53INPα and TP53INP1β). When overexpressed, both isoforms induce cell cycle arrest in G1 and enhance p53-mediated apoptosis. Another p53-activated gene is EI24/PIG8, a novel ER-localized Bcl-2-binding protein that may contribute to apoptosis by modulating the activity and/or function of Bcl-2 in the ER. EI24/PIG8 may serve to prevent tumor spreading, consistent with its suspected role as a tumor suppressor. Bax is a pro-apoptotic member of the Bcl-2 family thought to activate the mitochondrial voltage-dependent anion channel that is also activated by p53. Other studies have shown that resveratrol activates the mitochondrial or intrinsic apoptotic pathway leading to cytochrome c release and the eventual activation of caspase-3 42,51.
Sato et al. (2013) demonstrated resveratrol-induced p53 activation results in proteosomal degradation of Nanog, a transcription factor that maintains stem-like identity, and loss of stemness in GSCs 43. Other groups have shown that resveratrol regulation of STAT3 and related genes suppresses tumorigenicity and induces GSC differentiation 59. Resveratrol has also repeatedly been demonstrated to alter organism and cellular metabolism, acting as a mimetic of calorie restriction 14. Dysregulated metabolic function in GBM, such as in lipogenesis 23 or through isocitrate dehydrogenase (IDH) mutation 38,58, is now established in GBM tumorigenesis. Although not tested in this study, resveratrol correction of abnormal metabolism in GBM cells and GSCs could be another potential mechanism of its anti-tumorigenic effect. Overall, resveratrol exerts pleiotropic molecular effects against cancer 1, suggesting a general upstream molecular mechanism with broad downstream effects that could be advantageous in cancers like GBM with an extensive mutational and abnormal genetic landscape 11. Altogether, we have demonstrated resveratrol’s anti-tumorigenic modulation of AKT and p53 in glioblastoma, in agreement with other groups; however, the extensive repertoire of resveratrol’s anti-tumorigenic cellular and molecular mechanisms was not tested in this study and such additional mechanisms also likely contribute to the observed anti-GBM effects observed. Although resveratrol has repeatedly demonstrated positive anti-tumorigenic effects across a broad range of cancer types, its translation to clinical use has been challenging due to pharmacologic and pharmacokinetic difficulties 48. In mouse xenografts, resveratrol significantly suppressed GBM growth via oral administration and could ablate tumors while sparing normal tissue via intra- and peri-tumoral injection. Resveratrol is rapidly metabolized in vivo to adducts that possess significantly less anti-cancer properties, and achieving efficacious intra-tumor concentrations of active resveratrol after oral administration is difficult 14. Similar to a previously reported study for neuroblastoma 51, oral administration of resveratrol significantly suppressed GBM growth in vivo (Figure 5), but did not cause tumor regression, presumably because intra-tumor concentrations were insufficient to activate apoptotic mechanisms in GBM cells. Direct injection of resveratrol, a method reported to increase resveratrol concentration in xenograft models 1000-fold 26, exhibited a stronger anti-tumor effect than oral delivery and almost completely halted tumor growth with no observed toxicity to surrounding normal tissue. To overcome biodistribution issues in humans, direct injection or convection-enhanced delivery could be used for therapeutic delivery to brain tumors 2,16. This is supported by our preliminary pharmacokinetic studies in normal brain (Figure 5C), showing direct delivery achieved 100-fold higher concentrations of local resveratrol with longer duration. In addition to the direct injection of resveratrol as a therapeutic agent, or its combination with temozolomide, we are pursuing development of novel resveratrol-based analogs that remain safe and effective, designed with new features to improve solubility, achieve more efficient and effective intravenous delivery, reduce first-pass metabolism and enhance bioavailability.
CONCLUSIONS
In conclusion, we have demonstrated widespread inhibitory effects of resveratrol against GBM cells and glioblastoma stem-like cells, as well as in vivo growth suppression of GBM through multiple administration routes. Resveratrol’s non-toxic profile and broad anti-GBM effects strongly support its further development as an adjuvant therapy to improve patient outcomes, especially through its direct delivery.
Acknowledgments
Financial and material support: This research was supported by NIH grants R21CA161704, P30 EY016665, T32GM007507, UL1RR025011, NCI HHSN261201000130C, and P30CA014520. We also appreciate support from the Wisconsin Partnership Program core grant to the Center for Stem Cell and Regenerative Medicine, the University of Wisconsin (Graduate School, School of Medicine and Public Health and Dept. of Neurological Surgery), Research to Prevent Blindness, and the Retina Research Foundation (RRF). JSK and PAC were partially supported by NIH R01NS75995, R01CA158800 grants, the Headrush Brain Tumor Research Professorship award and the Roger Loff Memorial Fund for GBM Research. ASP is the Kathryn and Latimer Murfee RRF Chair.
Footnotes
Portions of this work were presented at the 2014 Society for Neuro-oncology Annual Meeting (November 13–16, Miami, FL, USA) and the 2015 AANS Annual Meeting 2015 (May 2–6, Washington, DC, USA).
DISCLOSURE: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
AUTHORS’ CONTRIBUTIONS: PAC helped design and performed experiments, analyzed data, and drafted the manuscript. SB helped design and performed experiments, analyzed data, and edited the manuscript. AE performed GSC sphere-formation experiments. SRD performed animal studies. BAT and MBY performed immunoblotting assays. PRG helped design and performed experiments, analyzed data, and edited the manuscript. ASP and JSK conceived, designed and planned this study, supervised and provided support, and edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 2.Alrfaei BM, Vemuganti R, Kuo JS. microRNA-100 targets SMRT/NCOR2, reduces proliferation, and improves survival in glioblastoma animal models. PLoS One. 2013;8:e80865. doi: 10.1371/journal.pone.0080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts and Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 4.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–312. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 7.Barber TW, Brockway JA, Higgins LS. The density of tissues in and about the head. Acta Neurol Scand. 1970;46:85–92. doi: 10.1111/j.1600-0404.1970.tb05606.x. [DOI] [PubMed] [Google Scholar]
- 8.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Darjatmoko SR, Polans AS. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011;21:180–187. doi: 10.1097/CMR.0b013e3283456dfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 11.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin YR, Yuan X, Balk SP, Toker A. PTEN-deficient tumors depend on AKT2 for maintenance and survival. Cancer Discov. 2014;4:942–955. doi: 10.1158/2159-8290.CD-13-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, et al. Activation of Multiple ERBB Family Receptors Mediates Glioblastoma Cancer Stem-like Cell Resistance to EGFR-Targeted Inhibition. Neoplasia. 2012;14:420–428. doi: 10.1596/neo.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9:1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippi-Chiela EC, Thome MP, Bueno e Silva MM, Pelegrini AL, Ledur PF, Garicochea B, et al. Resveratrol abrogates the temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide-induced senescence in glioma cells. BMC Cancer. 2013;13:147. doi: 10.1186/1471-2407-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagliano N, Aldini G, Colombo G, Rossi R, Colombo R, Gioia M, et al. The potential of resveratrol against human gliomas. Anticancer Drugs. 2010;21:140–150. doi: 10.1097/CAD.0b013e32833498f1. [DOI] [PubMed] [Google Scholar]
- 20.Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386–393. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gescher A, Steward WP, Brown K. Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann N Y Acad Sci. 2013;1290:12–20. doi: 10.1111/nyas.12205. [DOI] [PubMed] [Google Scholar]
- 22.Gunther HS, Schmidt NO, Phillips HS, Kemming D, Kharbanda S, Soriano R, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27:2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 23.Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009;2:ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009;8:25–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Shang X, Wu H, Huang G, Wang Y, Al-Holou S, et al. Combination treatment with resveratrol and sulforaphane induces apoptosis in human U251 glioma cells. Neurochem Res. 2010;35:152–161. doi: 10.1007/s11064-009-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenealey JD, Subramanian L, Van Ginkel PR, Darjatmoko S, Lindstrom MJ, Somoza V, et al. Resveratrol Metabolites Do Not Elicit Early Pro-apoptotic Mechanisms in Neuroblastoma Cells. J Agric Food Chem. 2011;59:4979–4986. doi: 10.1021/jf104901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim TM, Huang W, Park R, Park PJ, Johnson MD. A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs. Cancer Res. 2011;71:3387–3399. doi: 10.1158/0008-5472.CAN-10-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kma L. Synergistic effect of resveratrol and radiotherapy in control of cancers. Asian Pac J Cancer Prev. 2013;14:6197–6208. doi: 10.7314/apjcp.2013.14.11.6197. [DOI] [PubMed] [Google Scholar]
- 29.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu KH, Chen YW, Tsai PH, Tsai ML, Lee YY, Chiang CY, et al. Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv Syst. 2009;25:543–550. doi: 10.1007/s00381-009-0826-6. [DOI] [PubMed] [Google Scholar]
- 31.Mangiola A, de Bonis P, Maira G, Balducci M, Sica G, Lama G, et al. Invasive tumor cells and prognosis in a selected population of patients with glioblastoma multiforme. Cancer. 2008;113:841–846. doi: 10.1002/cncr.23624. [DOI] [PubMed] [Google Scholar]
- 32.Miranpuri G, Hinchman A, Wang A, Schomberg D, Kubota K, Brady M, et al. Convection Enhanced Delivery: A Comparison of infusion characteristics in ex vivo and in vivo non-human primate brain tissue. Ann Neurosci. 2013;20:108–114. doi: 10.5214/ans.0972.7531.200306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina JR, Hayashi Y, Stephens C, Georgescu MM. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–463. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mure H, Matsuzaki K, Kitazato KT, Mizobuchi Y, Kuwayama K, Kageji T, et al. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Oncol. 2010;12:221–232. doi: 10.1093/neuonc/nop026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert Opin Drug Deliv. 2014;11:1285–1298. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Woo KJ, Lee JT, Lim JH, Lee TJ, Kim SH, et al. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–1273. [PubMed] [Google Scholar]
- 38.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Pistollato F, Bremer-Hoffmann S, Basso G, Cano SS, Elio I, Vergara MM, et al. Targeting Glioblastoma with the Use of Phytocompounds and Nanoparticles. Target Oncol. 2015 doi: 10.1007/s11523-015-0378-5. [DOI] [PubMed] [Google Scholar]
- 42.Sareen D, Darjatmoko SR, Albert DM, Polans AS. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 43.Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Suzuki K, et al. Resveratrol promotes proteasome-dependent degradation of Nanog via p53 activation and induces differentiation of glioma stem cells. Stem Cell Res. 2013;11:601–610. doi: 10.1016/j.scr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 45.Shu XH, Li H, Sun XX, Wang Q, Sun Z, Wu ML, et al. Metabolic patterns and biotransformation activities of resveratrol in human glioblastoma cells: relevance with therapeutic efficacies. PLoS One. 2011;6:e27484. doi: 10.1371/journal.pone.0027484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 47.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 48.Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR. Resveratrol: challenges in translation to the clinic--a critical discussion. Clin Cancer Res. 2010;16:5942–5948. doi: 10.1158/1078-0432.CCR-10-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.TCGA: Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Ginkel PR, Darjatmoko SR, Sareen D, Subramanian L, Bhattacharya S, Lindstrom MJ, et al. Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2008;49:1299–1306. doi: 10.1167/iovs.07-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Ginkel PR, Sareen D, Subramanian L, Walker Q, Darjatmoko SR, Lindstrom MJ, et al. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res. 2007;13:5162–5169. doi: 10.1158/1078-0432.CCR-07-0347. [DOI] [PubMed] [Google Scholar]
- 52.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogelbaum MA, Aghi MK. Convection-enhanced delivery for the treatment of glioblastoma. Neuro Oncol. 2015;17(Suppl 2):ii3–ii8. doi: 10.1093/neuonc/nou354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wakimoto H, Mohapatra G, Kanai R, Curry WT, Jr, Yip S, Nitta M, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14:132–144. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 57.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 58.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang YP, Chang YL, Huang PI, Chiou GY, Tseng LM, Chiou SH, et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J Cell Physiol. 2012;227:976–993. doi: 10.1002/jcp.22806. [DOI] [PubMed] [Google Scholar]
- 60.Zorniak M, Clark PA, Leeper HE, Tipping MD, Francis DM, Kozak KR, et al. Differential expression of 2′,3′-cyclic-nucleotide 3′-phosphodiesterase and neural lineage markers correlate with glioblastoma xenograft infiltration and patient survival. Clin Cancer Res. 2012;18:3628–3636. doi: 10.1158/1078-0432.CCR-12-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]