Abstract
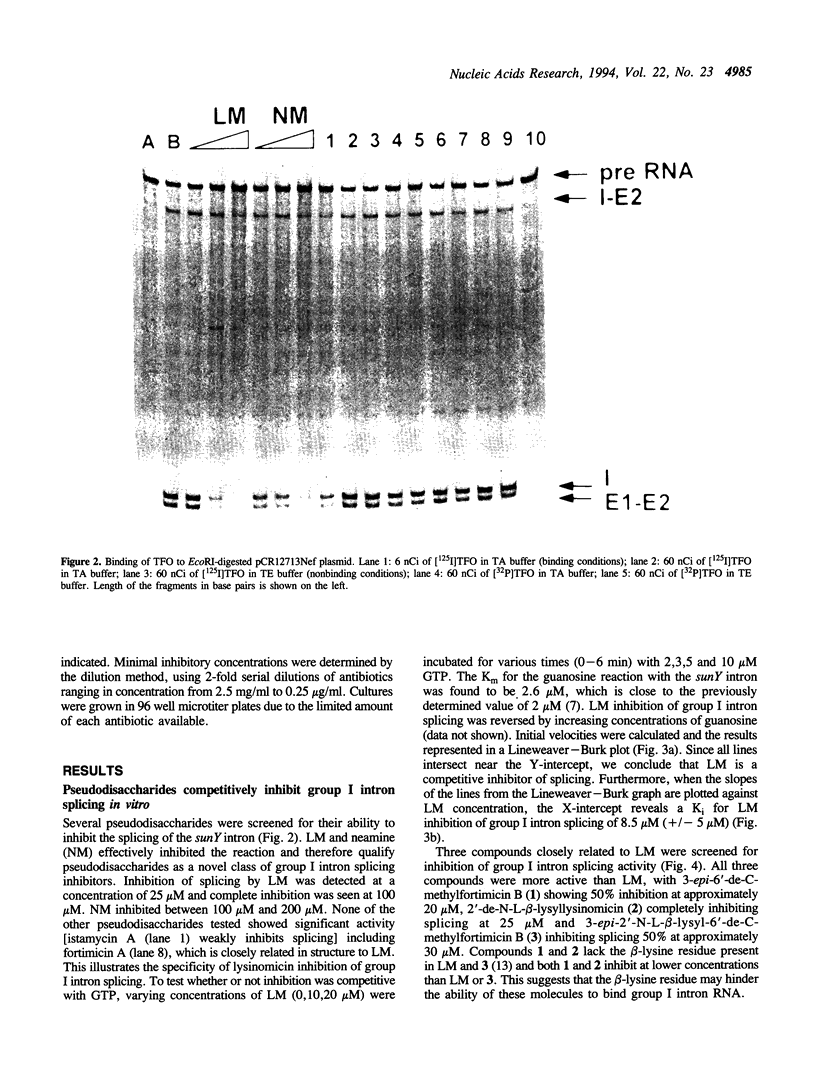
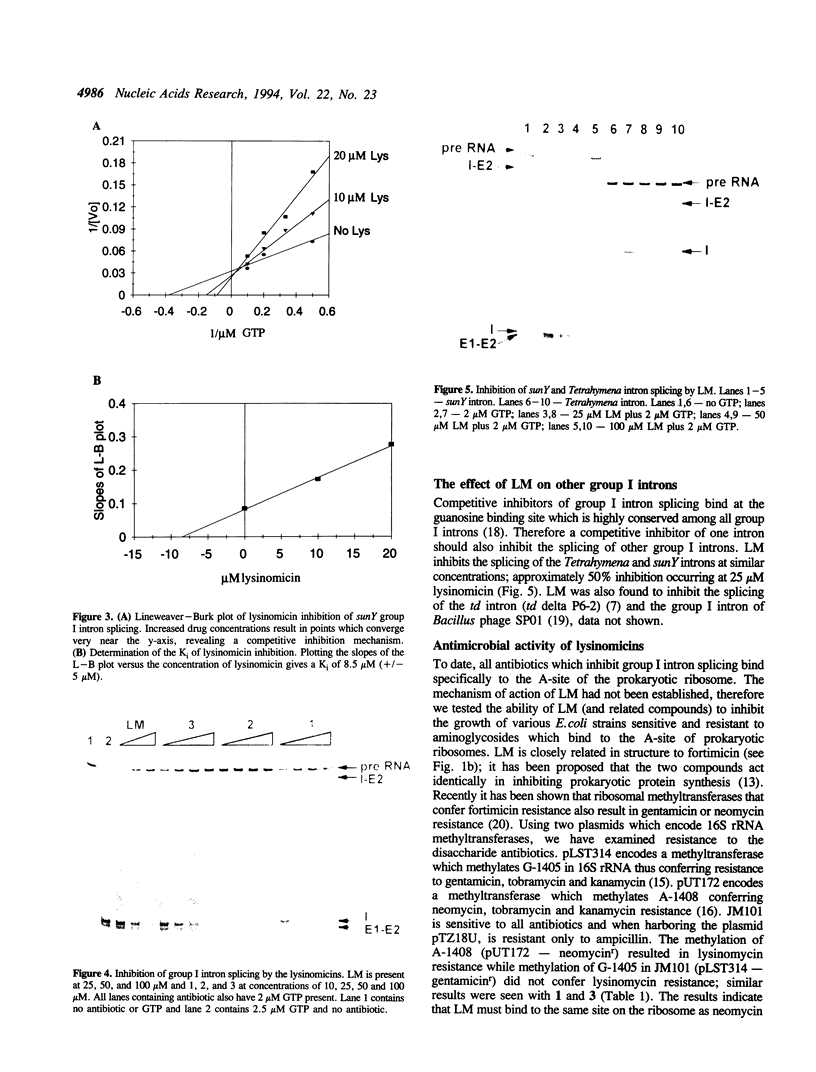
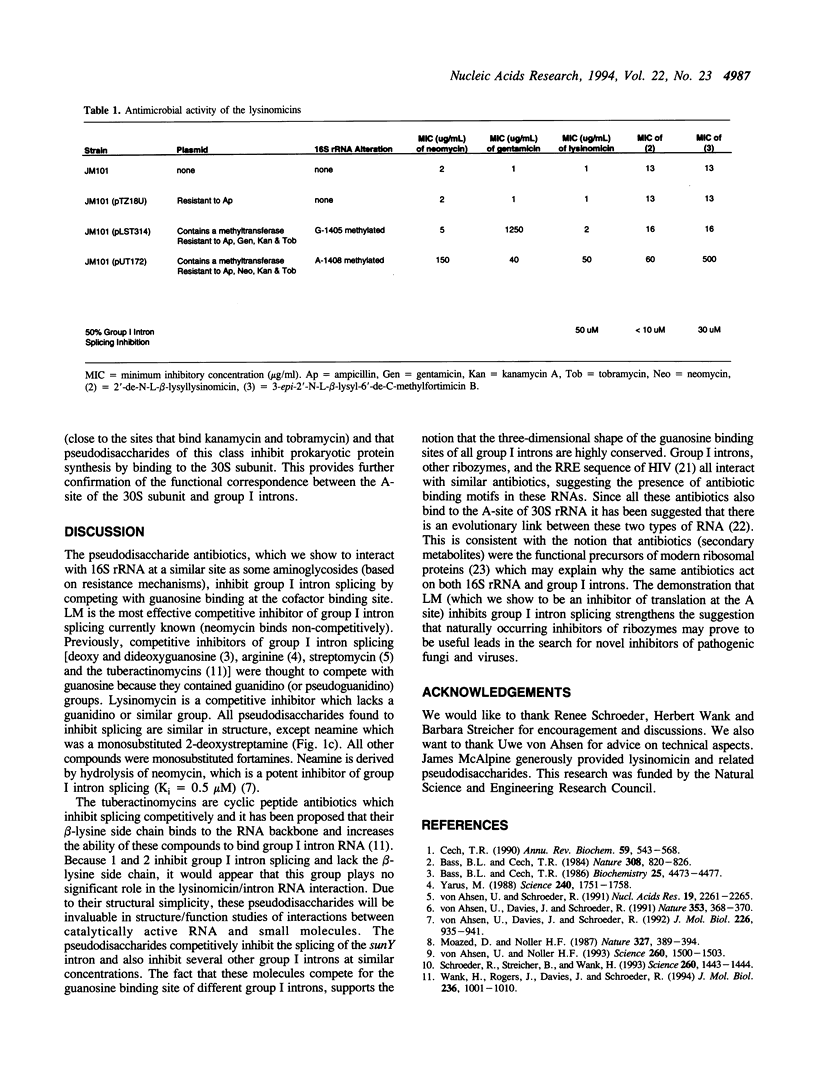
Lysinomicin, a naturally-occurring pseudodisaccharide, inhibits translation in prokaryotes. We report that lysinomicin (and three related compounds) are able to inhibit the self-splicing of group I introns, thus identifying pseudodisaccharides as a novel class of group I intron splicing inhibitors. Lysinomicin inhibited the self-splicing of the sunY intron of phage T4 with a Ki of 8.5 microM (+/- 5 microM) and was active against other group I introns. Inhibition was found to be competitive with the substrate guanosine, unlike aminoglycoside antibiotics, which act non-competitively to inhibit the splicing of group I introns. Competitive inhibitors of group I intron splicing known to date all contain a guanidino group that was thought to be required for inhibition; lysinomicin lacks a guanidino group.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. L., Cech T. R. Ribozyme inhibitors: deoxyguanosine and dideoxyguanosine are competitive inhibitors of self-splicing of the Tetrahymena ribosomal ribonucleic acid precursor. Biochemistry. 1986 Aug 12;25(16):4473–4477. doi: 10.1021/bi00364a001. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Cech T. R. Specific interaction between the self-splicing RNA of Tetrahymena and its guanosine substrate: implications for biological catalysis by RNA. 1984 Apr 26-May 2Nature. 308(5962):820–826. doi: 10.1038/308820a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Davies J. What are antibiotics? Archaic functions for modern activities. Mol Microbiol. 1990 Aug;4(8):1227–1232. doi: 10.1111/j.1365-2958.1990.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Goodrich-Blair H., Scarlato V., Gott J. M., Xu M. Q., Shub D. A. A self-splicing group I intron in the DNA polymerase gene of Bacillus subtilis bacteriophage SPO1. Cell. 1990 Oct 19;63(2):417–424. doi: 10.1016/0092-8674(90)90174-d. [DOI] [PubMed] [Google Scholar]
- Holmes D. J., Cundliffe E. Analysis of a ribosomal RNA methylase gene from Streptomyces tenebrarius which confers resistance to gentamicin. Mol Gen Genet. 1991 Oct;229(2):229–237. doi: 10.1007/BF00272160. [DOI] [PubMed] [Google Scholar]
- Holmes D. J., Drocourt D., Tiraby G., Cundliffe E. Cloning of an aminoglycoside-resistance-encoding gene, kamC, from Saccharopolyspora hirsuta: comparison with kamB from Streptomyces tenebrarius. Gene. 1991 Jun 15;102(1):19–26. doi: 10.1016/0378-1119(91)90532-g. [DOI] [PubMed] [Google Scholar]
- Kurath P., Rosenbrook W., Jr, Dunnigan D. A., McAlpine J. B., Egan R. S., Stanaszek R. S., Cirovic M., Mueller S. L., Washburn W. H. Lysinomicin, a new aminoglycoside antibiotic. II. Structure and stereochemistry. J Antibiot (Tokyo) 1984 Oct;37(10):1130–1143. doi: 10.7164/antibiotics.37.1130. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Moreau N., Jaxel C., Le Goffic F. Comparison of fortimicins with other aminoglycosides and effects on bacterial ribosome and protein synthesis. Antimicrob Agents Chemother. 1984 Dec;26(6):857–862. doi: 10.1128/aac.26.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Hasegawa M. Analysis of the self-defense gene (fmrO) of a fortimicin A (astromicin) producer, Micromonospora olivasterospora: comparison with other aminoglycoside-resistance-encoding genes. Gene. 1993 May 15;127(1):63–69. doi: 10.1016/0378-1119(93)90617-c. [DOI] [PubMed] [Google Scholar]
- Schroeder R., Streicher B., Wank H. Splice-site selection and decoding: are they related? Science. 1993 Jun 4;260(5113):1443–1444. doi: 10.1126/science.8502988. [DOI] [PubMed] [Google Scholar]
- Schroeder R., von Ahsen U., Belfort M. Effects of mutations of the bulged nucleotide in the conserved P7 pairing element of the phage T4 td intron on ribozyme function. Biochemistry. 1991 Apr 2;30(13):3295–3303. doi: 10.1021/bi00227a018. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Mizuno S. Studies on the cross-resistance of Mycobacterium tuberculosis, strain H37Rv, to aminoglycoside- and peptide-antibiotics. Microbiol Immunol. 1980;24(9):777–787. doi: 10.1111/j.1348-0421.1980.tb02883.x. [DOI] [PubMed] [Google Scholar]
- Wank H., Rogers J., Davies J., Schroeder R. Peptide antibiotics of the tuberactinomycin family as inhibitors of group I intron RNA splicing. J Mol Biol. 1994 Mar 4;236(4):1001–1010. doi: 10.1016/0022-2836(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Yarus M. A specific amino acid binding site composed of RNA. Science. 1988 Jun 24;240(4860):1751–1758. doi: 10.1126/science.3381099. [DOI] [PubMed] [Google Scholar]
- Zapp M. L., Stern S., Green M. R. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993 Sep 24;74(6):969–978. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Davies J., Schroeder R. Antibiotic inhibition of group I ribozyme function. Nature. 1991 Sep 26;353(6342):368–370. doi: 10.1038/353368a0. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Davies J., Schroeder R. Non-competitive inhibition of group I intron RNA self-splicing by aminoglycoside antibiotics. J Mol Biol. 1992 Aug 20;226(4):935–941. doi: 10.1016/0022-2836(92)91043-o. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Noller H. F. Footprinting the sites of interaction of antibiotics with catalytic group I intron RNA. Science. 1993 Jun 4;260(5113):1500–1503. doi: 10.1126/science.8502993. [DOI] [PubMed] [Google Scholar]
- von Ahsen U., Schroeder R. Streptomycin inhibits splicing of group I introns by competition with the guanosine substrate. Nucleic Acids Res. 1991 May 11;19(9):2261–2265. doi: 10.1093/nar/19.9.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]