Abstract
Aims
Current trends in pharmacovigilance systems are veering towards patient involvement in spontaneous reporting of adverse drug reactions (ADRs). The aim of the current systematic review was to identify what is known and what remains unknown with respect to patient reporting to pharmacovigilance systems.
Methods
A systematic literature search was conducted in PubMed, CINAHL, Journals@Ovid and the Cochrane Library. Studies were included if they contained: (i) reviews about patient reporting; (ii) evaluation of patient reports to national or supranational pharmacovigilance authorities; (iii) a comparison between patient and healthcare professional (HCP) reports submitted to pharmacovigilance authorities; and (iv) surveys of patient experiences, opinions and awareness about reporting ADRs. The methodological quality of the studies was assessed according to principles of Grading of Recommendations, Assessment, Development and Evaluations (GRADE).
Results
A total of thirty four studies were included. Five of the studies were reviews (two of which systematic reviews), fourteen retrospective observational studies, nine surveys and six applied mixed research methods. Patient reporting has the advantages of bringing novel information about ADRs. It provides a more detailed description of ADRs, and reports about different drugs and system organ classes when compared with HCP reporting. In addition, patients describe the severity and impact of ADRs on daily life, complementing information derived from HCPs. Patient reporting is relatively rare in most countries.
Conclusions
Patient reporting adds new information, and perspective about ADRs in a way otherwise unavailable. This can contribute to better decision‐making processes in regulatory activities. The present review identified gaps in knowledge that should be addressed to improve our understanding of the full potential and drawbacks of patient reporting.
Keywords: adverse drug reaction reporting systems, drug monitoring, drug‐related side effects and adverse reactions, patients, pharmacovigilance
What is Already Known about this Subject
The reporting of suspected adverse drug reactions (ADRs) in clinical practice is the backbone of postmarket surveillance for early detection of new, rare and serious ADRs.
The potential of patient reporting has been described in the literature as a noteworthy source of new information about drug safety, particularly the safety profiles of individual medicines.
A growing number of countries are involving patients in the direct reporting of ADRs (e.g. European Union countries since 2012) but little is known about what patient reporting adds to the pharmacovigilance system.
What this Study Adds
The present study summarizes current evidence on patient reporting to authority‐based pharmacovigilance systems systematically, pulling together evidence from different countries and settings.
The majority of the evidence comes from countries in Europe with well‐established patient reporting systems.
Patients report mainly on ADRs and drugs affecting the central nervous system, general disorders and administration site conditions, and this has helped to strengthen ADR signal detection. They also play an important role in providing a perspective on the experience and impact of ADRs on daily life.
Several gaps in knowledge were identified. The study also identified that there is a general trend in the evidence towards the positive impact of patient reporting. Further research is needed to improve our understanding of the value and problems in patient reporting to pharmacovigilance authorities.
Introduction
Pharmacovigilance plays a key role in assessing, monitoring and preventing adverse drug reactions (ADRs) 1. ADRs have a high clinical, social and economic cost as they can result in risk to life and having to stop taking an effective drug therapy, and a requirement for additional medical interventions and use of health services, with long hospitalizations 2. Although randomized clinical trials are considered the gold standard for the evaluation of the efficacy and safety of drugs 3, the design of such trials includes small and homogeneous populations monitored for short periods, making it difficult to detect many drug‐related reactions 4. Thus, the detection and reporting of suspected ADRs in clinical practice are the backbone of postmarket surveillance 5.
Current pharmacovigilance systems have been able to identify many major safety issues, even though their functions and methods leave considerable room for improvement 5. These systems comprise, among other mechanisms, spontaneous reporting (SR) 6. The main purpose of the SR system (SRS) is the early detection of new, rare and serious ADRs 7 and it has the advantage of covering the entire population in a cost‐effective way 8. The SRS has weaknesses, of which the most important is under‐reporting; it has been estimated that only 6% of all ADRs are reported 9. Under‐reporting delays the detection and identification of safety problems, making it more difficult for health authorities to take action and preserve public health.
The formal inclusion of patients in SR is part of the present trend to foster a more proactive pharmacovigilance system 10. Having more information available about ADRs through medicine users can possibly tackle under‐reporting and identify new risks in a given subgroup of patients 8. Although historically restricted to healthcare professionals (HCPs), recent years have brought a worldwide trend in allowing patients directly to report their accounts of ADRs to national pharmacovigilance authorities. Organizations such as the World Health Organization (WHO) and the European Union (EU) acknowledge the value of direct patient reporting 6. In the EU, the pharmacovigilance system underwent a major reform in 2012. Among the major changes were the expansion of the definition of ADRs, the harmonization of several risk‐based postmarketing surveillance methods and the introduction of the legal right for individual citizens to report suspected ADRs directly to the authorities 11, 12. In the EU, there were 48 782 patient reports in 2015, representing an increase of 30% on 2014 13. In spite of these extensions to patient involvement in SR, the real benefits of incorporating patient reporting in pharmacovigilance systems are still being debated 12. Some questions have been raised regarding the quality of documentation in patient reports, or the ‘noise’ that patients can produce when reporting already well‐known ADRs 14.
The aim of the present systematic review was to identify what is known and what remains to be discovered regarding the utility of patient reporting, and to summarize the views and opinions of patients as reporters. The review identified which aspects of the ADR information that patients provide are observational and which are subjective, and how this can be used to strengthen current pharmacovigilance systems.
Methods
Systematic literature search
A systematic literature search was conducted on the databases PubMed, CINAHL, Journals@Ovid and the Cochrane Library. The following combined text and medical subject headings (MeSH) terms were used: pharmacovigilance, direct patient report, patient adverse drug reaction reporting, consumer reporting and general public reporting. The complete search string used for the search was: ((adverse drug reaction OR adverse drug reactions OR side effect OR side effects OR adverse outcome OR adverse outcomes OR adverse event OR adverse events)) AND (patient OR patients OR consumer OR consumers OR general public)) AND (report OR reports OR spontaneous OR spontaneous report OR monitoring OR intensive monitoring OR direct report OR spontaneous reports)) AND (pharmacovigilance OR pharmacovigilance system).
The search was performed in January 2015, and was not limited to any type of study design or publication date (Figure 1). The language for the search was limited to English. The studies were selected on the basis of their title and abstract. A manual search was also performed; the reference list of key articles identified during the selection process was searched manually to detect further eligible studies not previously found. To complement the information, an internet search was conducted using Google Scholar and the general search engine Google, using the same terms as used in the literature search in the electronic scientific databases.
Figure 1.
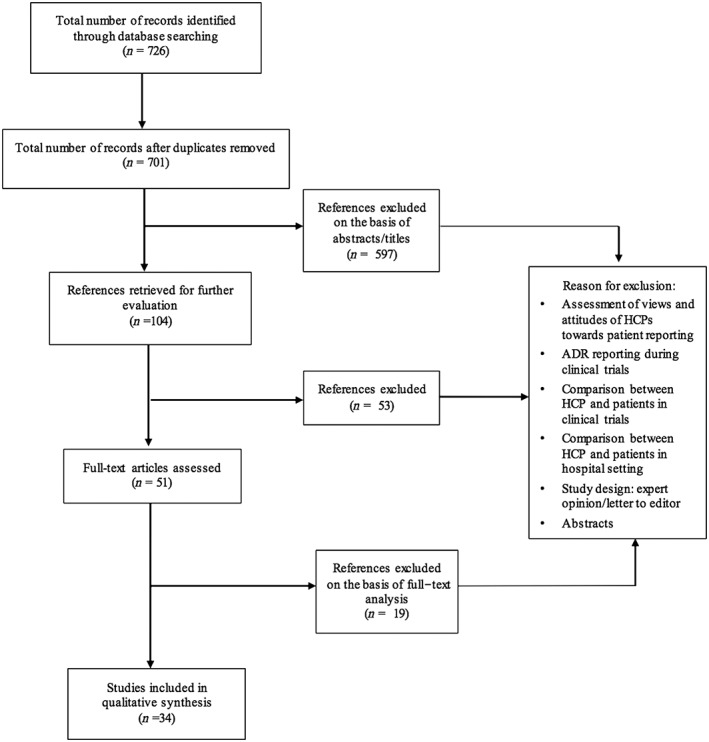
Flow diagram of study selection
Study selection and data abstraction
Studies were selected if they included: (i) reviews about patient reporting; (ii) an evaluation of patient reports in national or supranational official pharmacovigilance authorities; (iii) a comparison between patient and HCP reports submitted to pharmacovigilance authorities; and iv) surveys of patient experiences, opinions and awareness about reporting ADRs to pharmacovigilance authorities.
Patient reporting was defined as reports about adverse drug reactions submitted directly by patients or relatives to a pharmacovigilance authority, by means of passive or active surveillance methods. Exclusion criteria were as follows: studies in which patients or the public reported to pharmaceutical companies, patient organizations or another authority not related to pharmacovigilance; cases where patients reported ADRs to a hospital for purposes other than reporting to a pharmacovigilance authority; or general public surveys. Abstracts without full‐text studies were excluded. There was no limitation on whether the study concerned only one specific drug or pharmaceutical formulation, such as a vaccine.
In short, the following ‘PICO’ was applied in the present study: Patients (people taking any form of medication, or their relatives); Intervention (patients or relatives reporting to a pharmacovigilance authority); Comparison (comparison with HCPs); and Outcomes (new information; new ADRs; type of reaction reported).
An electronic matrix was developed in Microsoft Excel prior to the full‐text review, with predetermined characteristics. The articles were assessed independently by two reviewers (P.I. and M.A.) based on their titles and abstracts, according to the inclusion and exclusion criteria. Studies that fulfilled the inclusion criteria were retrieved for full assessment. The articles were then read carefully, and checked for content. A full‐text analysis of the articles was made, and the information was condensed according to categories into an Excel file. This was done by one reviewer (P.I.). While conducting the full‐text analysis, the information presented in Table 1 was extracted, with a description of study characteristics, methods and key findings. After conducting the analysis, the results on the advantages and limitations/drawbacks brought by patient reporting were organized in Table 2, and a summary of the contributions to pharmacovigilance through patient reporting emerging from the studies was extracted into Table 3. Disagreements were resolved through discussion and consensus. The tables were reviewed by the other researchers (A.C. and M.A.) for accuracy and completeness. Disagreements were solved by consensus.
Table 1.
Description and results of the studies included in the systematic review (n = 34)
| Authors, country of origin of study | Year of study | Country/region being studied | Objective of the study | Study design | Type of drug or drug class | Study population/reporting method | Data collection | Results |
|---|---|---|---|---|---|---|---|---|
| Reviews (n = 5) | ||||||||
| Blenkinsopp et al. UK 30 | 2007 | Denmark, Netherlands, Sweden, USA, Canada, Australia | To synthesize data from studies, in order to identify potential benefits and drawbacks of patient reporting | Systematic review | General | Patients, HCPs, others | 8 studies describing patient ADR reporting | Evidence indicates that patient reporting has more potential than drawbacks. Further evidence is needed. |
| Herxheimer et al. UK 28 | 2010 | The Netherlands, Denmark, Italy, Sweden, Belgium, UK, Norway, USA, Finland, France, Germany, Ireland, Portugal, Spain | To assess the impact of direct patient reporting and evaluate the experience of countries who have introduced it | Interviews and literature review | NA | Patients | Interview with 15 regulatory agencies in European countries about practice and experience with patient reporting, and literature review (2 articles) on direct patient reporting of ADRs | Patient participation allows for faster accumulation of knowledge, gives more detail and shows the effect of ADRs on daily life. Patient reporting should be encouraged, and resources should be placed in achieving this |
| Avery et al. UK 29, a | 2011 | Argentina, Armenia, Australia, Belgium, Botswana, Brazil, Burkina Faso, Canada, Democratic Republic of Congo, Cote d'Ivoire, Czech Republic, Denmark, Ghana, Guatemala, Iceland, Iran, Ireland, Italy, Kenya, Madagascar, Mexico, Moldova, Myanmar, Namibia, the Netherlands, New Zealand, Norway, Oman, Philippines, Saudi Arabia, Sierra Leone, Slovakia, South Africa, Sri Lanka, Saint Lucia, Sweden, Switzerland, Tanzania, Thailand, Togo, UK, Uruguay, USA, Zambia, Zanzibar, Zimbabwe | To review the published literature on patient and HCP reporting systems in different settings | Literature review and survey | NA | Patients | Survey to identify which countries allowed for patient reporting, and systematic review to identify comparative studies of patient and HCP reports | 46 countries were identified as having patient reporting by 2010. A number of studies of patient reporting of suspected ADRs were identified |
| Inch et al. UK 19 | 2012 | The Netherlands, UK, Denmark, USA, Canada | To identify comparative studies of patient and HCP ADR reports to pharmacovigilance authorities | Systematic review | General | Patients and HCPs | 3 comparative studies from Denmark, the Netherlands and UK | Despite an increasing number of countries accepting ADR reports from patients, few comparative studies were identified. Patient reports have both similarities and differences when compared with HCPs. Further investigation is required |
| Härmark et al. 31 | 2015 | The Netherlands, Sweden | To compare the experience, methods and outcomes of direct patient reporting in the Netherlands and Sweden | Literature review | General | Patients | Literature review of the experiences of 2 European countries with patient reporting of ADRs | Patient reporting increases the number of reported ADRs, and provides a new perspective of the experiences in a way not otherwise available |
| Observational studies (n = 14) | ||||||||
| de Langen et al. The Netherlands 16 | 2008 | The Netherlands | To study the first 3 years of experience with patient reporting in daily practice. Patient reports were compared with reports from HCPs | Retrospective data collection | General | Patients and HCPs | The number of patient reports received (n = 2522), age and gender of the reporters, characteristics of the most frequently reported drugs and characteristics of the ADRs (most frequently reported ADRs, seriousness, outcome) in a 3‐year period (April 2004–April 2007) were compared between patient reports and reports from HCPs | There are valuable differences between ADR reports from patients from HCPs. The similarities between patient reports and those from HCPs for most frequently reported ADRs and most frequently reported drugs are striking |
| Aagaard et al. Denmark 17 | 2009 | Denmark | To compare ADRs reported by patients with those reported from other sources, in terms of their type, seriousness and the suspected medicines involved | Retrospective data collection | General | Patients and HCPs | The number of ADRs reported in Denmark from 2004 to 2006 was analysed in terms of category of reporter, seriousness, category of ADR by system organ class and the anatomical therapeutic chemical classification system. ADR reports from consumers were compared with reports from other sources (physicians, pharmacists, lawyers, pharmaceutical companies and other healthcare professionals) (n = 544) | Compared with other sources, patients reported different categories of ADRs for different types of medicine. Consumers should be actively included in systematic drug surveillance systems |
| van Hunsel et al. The Netherlands 32 | 2009 | The Netherlands | To compare ADR reports from patients and HCPs after the broadcast of a Dutch television consumer programme about the benefits and risks of statins | Retrospective data collection | HMG‐CoA inhibitors | Patients and HCPs | A quantitative and qualitative analysis was performed on the reports sent by patients (n = 265) and HCPs (n = 111) after media exposition about statins on a Dutch television broadcast (n = 376). These reports were received by the Dutch pharmacovigilance centre between March 2007 and August 2007. The reports were compared with those from HCPs for the same period | Media attention affects drug use and ADR reporting by patients. Patient reports can provide additional information, making them a useful source of information next to HCP reports. Patients' concerns about ADRs should be recognized in reports to national pharmacovigilance centres |
| van Hunsel et al. The Netherlands 33 | 2010 | The Netherlands | To study the influence of media attention about statins and ADRs on the level of disproportionality, expressed as the reporting odds ratio (ROR) for statins based on patients' reports | Retrospective data collection | General | Patients | Patient reports about statins, before (April 2003–April 2007, n = 2484) and after (March 2007–June 2007, n = 604) the broadcast of a consumer programme about statins, were compared. The type of reported ADRs associated with statins before and after the broadcast was compared both on the level of system organ class (SOC) and preferred terms. | Media attention does not necessarily influence the relative reporting by patients expressed as RORs in the national ADR database. At the SOC level, the relative reporting increased only in musculoskeletal and connective tissue disorders. For myalgia and arthralgia, there was a proportional increase in reporting within the statin class but not for the other ADRs that were explicitly mentioned in the television programme about statins |
| McLernon et al. UK 18 | 2010 | UK | To compare patient characteristics, suspect drugs and ADR reports by patients with those reported by HCPs using the Yellow Card Scheme (YCS) | Retrospective data collection | General | Patients and HCPs | Participants were patients reporting to the national regulatory agency either by themselves, a representative or an HCPS, as having one or more suspected ADRs between October 2005 and September 2007 in the UK (n = 5180). The main outcome measures were ADRs and time taken to report | While patients report more suspected ADRs to more suspect drugs than HPCs, the latter tend to report more serious reactions that result in hospitalization, are life threatening or cause death. Further research is required to investigate the extent to which the extra information from patient reporters contributes to signal identification when assessing drug safety |
| Durrieu et al. France 34 | 2012 | France | To compare the characteristics of patient and HCP ADR reports in order to assess the qualitative and quantitative contribution of patient reporting to the French Pharmacovigilance System | Retrospective data collection | Pandemic influenza vaccines | Patients and HCPs | All spontaneous ADRs (n = 4746) registered in the French Pharmacovigilance Database from 21 October 2009 to 15 June 2010, in which either of the most frequently administered pandemic vaccines was involved, were analysed. Among the reports received during the study period, 1006 (21.2%) originated from patients | No major qualitative difference between patient and HCP reports were found. ADR profiles reported by patients appeared to be consistent with those from professionals. Further investigations are necessary to assess the quality of notifications coming from patients |
| Danish Health and Medicines Agency, 2013, Denmark 20 | 2013 | Denmark | To compare ADRs reported by patients to the Danish pharmacovigilance database between 2003 and 2011 with other reporters | Retrospective data collection | General | Patients and HCPs Spontaneous reporting | ADR reports submitted by patients (n = 4631) were compared with those submitted by other reporters (n = 20 269) between 2003 and 2011 | The contribution of patients is significant. Patient reports complement HCP reports |
| Aagaard L et al. Denmark 22 | 2013 | European Union, Norway, Liechtenstein, Iceland | To characterize ADRs reported by European patients for central nervous system medications | Retrospective data collection | Central nervous system medications (ATC group N) | Patients. Spontaneous reporting | ADRs submitted by European patients and listed in the EudraVigilance database concerning central nervous system medications between 2007 and 2011 (n = 7434 patients) | The majority of ADRs from central nervous system medications reported by consumers that were identified from the EudraVigilance database were serious. The value of consumer reports in pharmacovigilance still remains unclear |
| Aagaard et al. Denmark 21 | 2013 | European Union, Norway, Liechtenstein, Iceland | To characterize patient‐reported ADRs for phosphodiesterase type 5 (PDE5) inhibitors | Retrospective data collection | PDE5 inhibitors | Patients. Spontaneous reporting | ADR reports submitted between 2007 and 2011 by adults and collected from the EudraVigilance database concerning erectile dysfunction medicines. ADRs were classified according to type, seriousness and age (n = 7437) | Relatively few ADRs for PDE5 inhibitors were reported by consumers, and only a few were serious |
| Hazell et al. UK 25 | 2013 | UK | To investigate the relative contribution of patient reporting to signal detection through disproportionality analysis | Retrospective data collection | General | Patients and HCPs Spontaneous reporting | Data were analysed from all reports submitted directly to the UK pharmacovigilance centre between October 2005 and September 2007. Three datasets of drug–ADR pairs were created: one for patient reports, one for HCP reports and one for all reports combined. The proportional reporting ratio method was used to identify signals of disproportionate reporting (SDRs) in each dataset. The number of SDRs identified from patient and HCP reports were compared, as well as the type of ADR and suspect drug involved | Results suggest that patient reporting may provide a positive complementary contribution to that of HCPs. Patient reporting may make an important contribution to drug safety by identifying different SDRs not identified from HCP reports alone |
| Härmark et al. The Netherlands 35 | 2013 | The Netherlands | To compare the diabetes population participating in a web‐based non‐interventional observational cohort with an external diabetes reference population on characteristics that may influence the patient's susceptibility for ADRs | Retrospective data collection | NA | Patients. Intensive monitoring | Patients participating in a specific postmarketing surveillance system (n = 2828) in the Netherlands were compared with others in the Dutch diabetes patient registry (n = 11 852). Comparisons were made regarding age, gender, body mass index and polypharmacy, as well as diabetes medication used and disease/treatment duration | Diabetes patients participating in a web‐based intensive monitoring system differ from a reference population. The observed differences might lead to an underestimation of ADRs, but it is not clear whether this would also influence the type or time course of the reported ADRs |
| Aagaard L et al. Denmark 23 | 2014 | European Union, Norway, Liechtenstein, Iceland | To characterize ADRs in children reported by patients in Europe between 2007 and 2011 | Retrospective data collection | General | Paediatric population Spontaneous reporting | ADRs (n = 670 ADRs, n = 240 reports) reported by consumers to the European ADR database EudraVigilance for individuals from birth to 17 years of age between 2007 and 2011. Data were characterized with respect to the age and gender of the child, type of ADR (SOC and preferred term), seriousness and suspected medicines | Few pediatric ADR reports were found in EudraVigilance that had been reported by consumers. Many ADRs were serious, and fatal cases were reported; however, nonserious reports were also present. The findings indicate that consumer reports may be of value in paediatric ADR signal detection |
| Li et al. China 36 | 2014 | Shanghai (China) | To evaluate ADRs in children reported to the spontaneous reporting system (SRS) of Shanghai in 2009 | Retrospective data collection | Paediatric population. Spontaneous reporting | ADR reports submitted to the Shanghai SRS in 2009 for individuals aged from birth up to and including 17 years (n = 97) were included. Data were analysed with respect to age, gender, category of ADR, the severity of reports and the type of reporter | Consumers were more likely to report new ADRs, although they appear to contribute a relatively small percentage of total reports (2.52%). Patients would take a more active role in reporting ADRs. More research is needed in order to achieve a better understanding of the characteristics of ADRs in the paediatric population of China | |
| Rolfes et al. The Netherlands 27 | 2015 | The Netherlands | To explore the differences between ADR reports from patients and HCPs, and to explore possible correlation between reported elements of information | Retrospective data collection | Patients and HCPs. Spontaneous reporting | The study compared information between ADR reports by patients (n = 100) and HCPs (n = 100). Reports were scored according to objective elements (e.g. start date of the ADR) or subjective elements (e.g. the impact or severity of the ADR) | This study shows the differences in reported information between the ADR reports of patients and HCPs. Patient reports are more focused on patient‐related information and the impact of the reported ADRs, whereas reports from HCPs provide more clinically related information | |
| Surveys (n = 8) | ||||||||
| van Hunsel et al. The Netherlands 37 | 2010 | The Netherlands | To quantify the reasons and opinions of patients who reported ADRs in the Netherlands to a pharmacovigilance centre | Web‐based questionnaire | NA | Patients | A web‐based questionnaire was sent to 1370 patients who had previously reported an ADR to a pharmacovigilance centre, giving rise to 1005 responses | The main motives for patients for reporting ADRs to a pharmacovigilance centre were the severity of the ADR and their need to share experiences. The high level of response to the questionnaire shows that patients are involved when it comes to ADRs and that they are also willing to share their motivations for and opinions about the reporting of ADRs to a pharmacovigilance centre |
| Krska et al. UK 39 | 2011 | UK | To determine how patients report ADRs to the UK YCS | Qualitative data collection from questionnaires and interview | NA | Patients | A qualitative analysis of data from three sources: responses to open questions in postal questionnaires sent to all reporters during March 2008–January 2009 (n = 1362); telephone interviews with a purposive sample of these reporters (n = 27); and the free‐text field from completed Yellow Card reporting forms submitted during October 2005–September 2007 (n = 230) | Most patient reporters feel able to identify suspected ADRs adequately and describe processes of assessing causality that mirror those in standard algorithms designed for use by HCPs. These findings should help to reduce concerns among HCPs about the ability of patients to identify suspected ADRs when reporting to authorities |
| Anderson et al. UK 38 | 2011 | UK | To explore the opinions of patients reporting ADRs to the UK YCS on the importance of the scheme | Postal questionnaires and telephone interview | NA | Patients | Patients who submitted ADRs to the UK pharmacovigilance authority received a postal questionnaire to answer (n = 1238) | Direct patient reporting through the YCS is viewed as important by those who have used the scheme, in order to provide the patient experience for the benefit of pharmacovigilance, as an independent perspective from those of HCPs |
| van Hunsel et al. The Netherlands 42 | 2012 | Australia, Canada, Denmark, The Netherlands, New Zealand, Norway, Malaysia, Philippines, Sweden, UK, USA | To review the methods of patient reporting of ADRs in 11 countries worldwide and to compare different aspects of their experiences | Telephone and e‐mail survey combined with qualitative data collection | NA | Patients | A survey based on telephone interviews, e‐mail discussions and field visits of existing practices in consumer and patient reporting of ADRs was performed in the second half of 2010 | Although there are some differences in the way various countries handle patient reports of ADRs, the importance of giving the public the opportunity to report and the additional scientific value of the collected data are widely recognized by the countries who participated in this survey |
| Arnott et al. UK 40 | 2013 | UK | To investigate parents' views and experiences of direct reporting of a suspected ADR in their child | Semi‐structure interview | NA | Paediatric population (parents) | Two sample groups of parents with (n = 17) and without (n = 27) previous experience of submitting a spontaneous report (n = 44) to the YCS. The interviews were audio‐recorded | Promoting wider participation in pharmacovigilance schemes will depend on raising public awareness. Additionally, there is a need to empower lay people to submit reports and to reassure them about the value of their reports |
| Margraff et al. France 26 | 2014 | Algeria, Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, China, Colombia, Croatia, Cuba, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, India, Ireland, Israel, Italy, Japan, Kenya, Latvia, Lithuania, Luxembourg, Malta, Mexico, Morocco, Netherlands, New Zealand, Nigeria, Norway, Peru, Poland, Portugal, Romania, Russia, Slovakia, Slovenia, South Africa, Spain, Sweden, Switzerland, UK, USA | The modalities and contributions to drug safety of patient ADR reporting systems in 50 countries were reviewed and analysed | Quantitative data collection | NA | Patients | The means made available by National Health Competent Authorities (n = 50) for patients to report drug side effects were compared through a literature review and questionnaire | Direct patient reporting systems exist in 44 countries and represent 9% of total reports, the rest coming from HCPs. Most of the surveyed countries have implemented a patient ADR reporting system. It seems that an online reporting form increases the rate of reporting. Currently, many different forms exist worldwide; these should be harmonized by considering the strengths and weaknesses of all existing forms. To increase the number of reports, each country should promote ADR reporting systems |
| Rolfes et al. The Netherlands 41 | 2014 | The Netherlands | To give an overview of patients' and HCPs’ views about patient reporting | Semi‐structured personal interview | NA | Patients, HCPs, others | 16 semi‐structured interviews with patients (n = 3), HCPs (n = 6) and pharmacovigilance officers in the Netherlands (n = 7) were conducted. All interviews were audio‐taped and transcribed verbatim. Content analysis was used on the data | Patients reported that the severity of ADRs and their impact on daily life were important subjects. In the interviews with HCPs, either reporters or assessors, the focus was mainly on causality. The correctness of the given information was considered by ADR assessors to be very important. Regarding patient reporting, the overall view was positive. As HCPs and patients have different views regarding ADR reporting, in daily practice it is important to receive reports from both groups to assess the true nature of the ADR |
| Yamamoto et al. Japan 43 | 2015 | Japan | To determine whether web‐based patient ADR reporting would work in Japan, and to describe the characteristics of the patient reporters, and clarify patient views and experiences of reporting | Questionnaire | NA | Patients | Patients who submitted online ADR reports (n = 94) were contacted to respond to an ADR reporting questionnaire. Subjects with multiple responses were excluded from analysis. The questionnaire consisted of both closed and open questions | Online patient ADR reporting was received with a forward‐looking, positive approach. To facilitate smoother web‐based reporting experiences in future, some improvements may be required in online ADR reporting forms, particularly with regard to respondent feedback |
| Other study designs (n = 7) | ||||||||
| van Hunsel et al. The Netherlands 24 | 2011 | The Netherlands | To determine the contribution of patients' ADR reports to signal detection, through a study of the signals sent to the Dutch Medicines Evaluation Board | Case–control design | General | Patients and HCPs Spontaneous reporting | The number of patient reports which contributed to a signal has increased from 0 reports in 2003 to 31 reports in 2008 (9% of total). Since 2005, patient reports have triggered particular associations to be selected as a signal. In 2007, 28% of all trigger reports were reported by a patient. The case–control analysis showed that patient reports were equally present in the reports used in signal formation (cases) as in the controls (reports not contributing to a signal) | The proportion of patient reports contributing to generate signals was equal to the proportion of patient reports in the database. Patients’ reports of ADRs can provide a valuable contribution to the detection of signals in addition to HCP reports. In the Netherlands, direct patient reports have added to the signals of ADRs sent to the Medicines Evaluation Board |
| Härmark et al. The Netherlands 46 | 2011 | The Netherlands | To gain insight into the safety and user profile of pregabalin, reported by patients via a web‐based intensive monitoring system based in the Netherlands | Observational prospective data collection | Anticonvulsants (pregabalin) | Patients. Intensive monitoring | First‐time users of pregabalin were identified (n = 1373) through the first prescription signal in intensive monitoring participating pharmacies between 1 August 2006 and 31 January 2008. Patient demographics and information about drug use and ADRs were collected through electronic questionnaires sent 2 weeks, 6 weeks, 3 months and 6 months after the start of pregabalin administration. ADRs were quantified and signal detection was performed on a case‐by‐case basis | This study indicated that pregabalin is a relatively safe drug. Less than 1% of patients experienced a serious ADR while using the drug. The most frequently reported ADRs correspond to the reactions most frequently reported during clinical trials. A web‐based intensive monitoring system can contribute to greater knowledge about a reaction. It can also detect signals worthy of further investigation |
| Härmark et al. The Netherlands 44 | 2011 | The Netherlands | To demonstrate how a web‐based intensive monitoring system using the patient as a source of information can be used to gather longitudinal safety data for a drug. In this study, pregabalin was used as an example | Observational prospective data collection | Anticonvulsants (pregabalin) | Patients. Intensive monitoring | First‐time users of pregabalin (n = 1373) were approached in Dutch pharmacies between 1 August 2006 and 31 January 2008. After online registration, patients received questionnaires by email 2 weeks, 6 weeks, 3 months and 6 months after the start of the drug use. Data on patient characteristics, drug use and ADRs were collected and analysed | With web‐based intensive monitoring, it is possible to study the time course of ADRs. This method can be a valuable addition to pharmacovigilance because it can generate other types of information as compared with spontaneous reporting and other intensive monitoring methodologies |
| Härmark et al. The Netherlands 45 | 2011 | The Netherlands | To evaluate how patients can be a source of information about ADRs in an intensive monitoring programme targeting vaccines | Observational prospective data collection | Pandemic influenza vaccines | Patients. Intensive monitoring | A cohort of patient aged 60 years and older with a risk‐elevating medical condition recommended for vaccination in general practice were eligible for participation (n = 3569). After receipt of the first pandemic vaccine, the patient could sign up for study participation online. Within 1 week, 3 weeks and 3 months after the first immunization, questions were asked about demographics and health, immunizations, injection site reactions and labelled reactions, as well as other possible new ADRs | The web‐based intensive monitoring system among patients immunized in general practice revealed ADRs due to pandemic vaccination in one‐third of participants. There were no unexpected serious adverse events in this population. This advanced methodology can be further developed to monitor real‐time use |
| Avery et al. UK 29, a | 2011 | UK | To evaluate the pharmacovigilance impact of patient reporting of ADRs by analysing reports of suspected ADRs from the UK YCS and comparing reports from patients and HCPs. To elicit the views and experiences of patients and the public about patient reporting of ADRs | Qualitative and quantitative analysis, questionnaire survey | General | Patients and HCPs | Patients and HCPs submitting spontaneous reports between October 2005 and September 2007 (n = 5180)a This involved: (1) descriptive analysis of Yellow Card reports; (2) signal generation analysis of Yellow Card reports; (3) qualitative analysis of Yellow Card reports; (4) questionnaire survey of patients reporting on Yellow Cards; (5) qualitative analysis of telephone interviews with patient reporters to the scheme; (6) qualitative analysis of focus groups and usability testing of the patient YCS; and (7) national omnibus telephone survey of public awareness of the YCS | Patient reporting of suspected ADRs has the potential to add value to pharmacovigilance by reporting types of drugs and reactions different from those reported by HCPs; generating new potential signals; and describing suspected ADRs in enough detail to provide useful information on likely causality and impact on patients' lives. These findings suggest that further promotion of patient reporting to the YCS is justified, along with improvements to existing reporting systems |
| Härmark et al. The Netherlands 47 | 2013 | The Netherlands | To gain insight into the user and safety profile of duloxetine in daily practice, reported by patients via a web‐based intensive monitoring system during their first 6 months of use in the Netherlands | Observational prospective data collection | Serotonin selective reuptake inhibitors (duloxetine) | Patients. Intensive monitoring. | First‐time users of duloxetine were identified (n = 333) through the first dispensing signal in the pharmacy. Patient demographics and information about drug use and ADRs were collected through electronic questionnaires sent 2 weeks, 6 weeks, 3 months and 6 months after the start of duloxetine administration. ADRs were quantified and signal detection was performed on a case‐by‐case basis | Web‐based intensive monitoring in an observational prospective cohort study mirroring the use and ADRs of duloxetine in daily practice. This study indicated that duloxetine is a relatively safe drug, as used by patients for 6 months in daily practice. Patients contributed to the identification of new signals. Intensive monitoring of patients is an important tool for collecting more information about ADRs |
| Oosterhuis et al. The Netherlands 48 | 2014 | The Netherlands | To gain insight into the safety and use of varenicline in daily practice | Observational prospective data collection | Nicotinic receptor agonist (varenicline) | Patients. Intensive monitoring | The study population was defined as first‐time users recruited through participating pharmacies between 1 December 2008 and 31 March 2012. Patients could sign up for the study on a dedicated website. Web‐based questionnaires were sent after 1 week, 2 weeks, 6 weeks, 3 months and 4 months after patients started to use varenicline. Questions were asked about drug use and ADRs. Information about the ADR, its seriousness and the action taken when experiencing an ADR was gathered | This study gave an insight into latency time and action taken with varenicline when ADRs occur during treatment with this agent in daily practice. It confirms the ADR pattern detected prior to the marketing of the drug |
Total n = 34. ADR, adverse drug reaction; HCP, healthcare professional; HMG‐coA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; NA, not available; ROR, reporting odds ratio
Number of patients.
This study aggregates several other studies.
Table 2.
Evidence on the advantages and limitations of patient reporting identified from the studies included in the systematic review (n = 34)
| Gathered evidence | Number of studies N [references] | Advantages | Limitations/drawbacks |
|---|---|---|---|
| Patients confirm or add new information, and help to identify safety signals | 19 17, 18, 21, 22, 23, 25, 27, 29, 30, 31, 32, 33, 34, 36, 45, 46, 47, 48 | • Information is accumulated in a faster way • Complements information from HCPs | • SR is not the only method to collect information
• Lack of further studies concerning other methods • New information is not superfluous or of low quality; however, the volume is still low |
| Patients report different ADRs when compared with HCPs | 15 16, 17, 18, 19, 20, 25, 26, 27, 29, 30, 31, 32, 33, 36 | • Patients report ADRs affecting different body systems • Nervous system disorders, general disorders and administration site disorders are the biggest groups | • Limited information yet • Level of detail that patients provide needs to be better assessed |
| Patients identify new ADRs | 11 16, 17, 18, 20, 24, 25, 29, 31, 32, 36 | • Patients provide more detailed information • Makes the system more robust | • Awareness must be raised so that patients can become more engaged |
| Patients report the impact of ADRs in daily life | 11 27, 29, 31, 32, 33, 37, 40, 41, 43, 48 | • Patients report more subjective factors
• Patients report similar concerns to HCPs • Patient views help in the assessment of the nature of ADRs |
• Media attention does not seem to affect reporting
• Some patients question the validity of their judgement on ADRs • Questions regarding conflict with HCPs |
| Patients have diverse reasons to report | 7 29, 35, 37, 38, 39, 42, 43 | • Patients express the need to share experiences, and have different view from HCPs,
• Patients feel capable of reporting ADRs • Altruistic attitude |
• Differences between patients who participate and the rest of the population • There is need to raise awareness |
| The possibility of patient reporting is increasing worldwide | 5 26, 29, 30, 32, 42 | • More information about drugs/ADRs in the system • Patients approve direct reporting | • Participation is low
• Maturity and sophistication of the system have an impact on reporting rates • The system must be improved • So far, there has been no strain on pharmacovigilance resources |
| Patients report serious ADRs | 4 21, 22, 23, 29 | • Patients report same proportion of serious ADRs as HCPs | • Differences in the interpretation of the meaning of seriousness by patients • Some studies suggest lesser severity of reactions reported by patients |
| Patients help to detect or strengthen safety signals | 4 23, 24, 25, 29 | • Patients provide valuable contribution to signal detection • Patients have identified different signals compared with HCPs | • More research needs to be done |
ADR, adverse drug reaction; HCP, healthcare professional; SR, spontaneous reporting
Table 3.
Evidence on the contribution of patients to pharmacovigilance through reporting ADRs to authorities
| Summarized evidence of the studies | Results and comments | References |
|---|---|---|
| Clinical evidence | ||
| 1. Most frequently reported SOC | a. Nervous system disorders
b. General disorders and administration site conditions c. Musculoskeletal and connective tissue disorders d. Psychiatric disorders e. Gastrointestinal disorders |
17, 20, 22, 29, 34, 44, 45 |
| 2. Most frequently reported medicines | a. Pregabalinb
b. Simvastatin c. Sex hormones (drosperinone and oestrogen) d. Serotonin‐selective reuptake inhibitors (duloxetinea, citalopram) e. Influenza vaccines |
18, 20, 29, 34, 44, 47 |
| 3. Most frequently reported ADRs (PT level) | a. Nausea
b. Headache c. Dizziness d. Somnolence e. Fatigue |
16, 19, 20, 44, 46, 48 |
| 4. Signal detection | a. Amenorrhoea, shock‐like paraesthesias and micturition associated with serotonin‐selective reuptake inhibitors
b. Weight loss, inflammation of the eye, change in sense of taste c. Pathological gambling associated with gabapentin d. Ear ache, thirst, stomach discomfort, associated with influenza vaccine f. Patchy baldness, dry skin, food allergy, associated with papillomavirus vaccine |
16, 20, 47 |
| 5. Gender | a. Female reporters represent around 60% of all reports | 17, 19, 20, 23, 44, 48 |
| Subjective evidence | ||
| 1. Difference in reported information compared with HCPs | a. Patients report on impact of ADR on daily life b. Add more information on medication, personal characteristics | 16, 29, 41 |
| 2. Seriousness | a. Patients report more disability than HCPs b. Definition of ‘seriousness’ might differ between patients and HCPs | 27, 38, 39 |
| 3. Reasons to report | a. Altruism seems to be the main reason to report b. Wanting to have an independent voice from HCPs seems important | 37, 38, 40, 41 |
ADR, adverse drug reaction; PT level, preferred term of the Medical Dictionary for Regulatory Activities; SOC, system organ class
Most of the evidence was gathered through intensive monitoring
Quality assessment
The process of conducting the systematic review followed the steps recommended by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist 15. Two authors (P.I. and M.A.) read and carefully assessed each retrieved study. The methodological quality of the studies was assessed according to principles of Grading of Recommendations, Assessment, Development and Evaluations (GRADE), and the studies were organized to four categories on the basis of the methodology applied (reviews, observational studies, surveys, other study designs). Due to a lack of homogeneity among the studies, a meta‐analysis of the data was not possible, but a qualitative analysis was conducted. This systematic review of published studies did not require ethics approval.
Results
Of the identified 721 studies, 34 full‐text studies were included in the qualitative analysis (Figure 1). The identification, screening and evaluation of eligibility of articles for final inclusion are presented in Figure 1.
Of the identified 34 studies, 5 were literature reviews, 14 observational studies and 8 surveys, and 6 studies applied mixed methods (Table 1). Most of the studies (30 out of 34) came from European countries, particularly the Netherlands (n = 15), United Kingdom (UK) (n = 10) and Denmark (n = 5).
Summary of evidence on advantages and limitations of patient reporting
Combining the evidence on the advantages and limitations of direct patient reporting, it was shown that patients report different ADRs when compared with HCPs, and identify novel ADRs 16, 17, 18, 19, 20 (Table 2). Patients provide new insights into ADRs by bringing information on different body systems affected by medicines, such as the central nervous system 16, 17, 19 (Table 3). Patients can be very important in identifying ADRs in specific populations, or types of medicine 17, 21, 22, 23. The information provided by patient reports can be significant for ADR signal detection 24, 25. One important characteristic of patient reports is the subjective description of ADRs.
Some of the barriers were also identified. Despite the fact that many countries allow patient reporting to take place, the reporting rate and awareness are still low 26. It was pointed out that awareness should be raised so that patients can become more engaged 26. The new information provided by patients seemed to be valuable and of high quality 27. None of the studies provided insight on what is being done to increase participation and awareness.
Descriptive findings from the studies
Reviews
The search strategy identified five reviews 19, 28, 29, 30, 31, of which two were systematic reviews 19, 30. The studies synthesized data to identify the evidence on the potential of direct patient reporting 28, 29, 30, 31, and to compare the differences between patient and HCP reports 19, 30. All the reviews concluded that patient reporting was valuable, with the differences between patient and HCP reports adding to the knowledge about ADRs 19, 29, 30, 31. Most of the reviews (three out of five) stated the need for further evidence on the benefits and drawbacks of patient reporting 19, 29, 30. As the reviews were published between 2006 and 2015, they included data from different periods and different countries, reflecting the different maturation states of patient involvement in ADR reporting 19, 28, 29, 30.
Observational studies
All of the 14 observational studies 16, 17, 18, 20, 21, 22, 23, 25, 27, 33, 34, 35, 36, 37 were retrospective, and most commonly (8 out of 14 studies) had the aim of comparing patient and HCP ADR reports 16, 17, 18, 20, 25, 27, 33, 35. There were five studies from Denmark 17, 20, 21, 22, 23, five from the Netherlands 16, 27, 32, 33, 35, two from the UK 18, 25, one from France 34 and one from China 36. Two of the Danish studies focused on analysing the Danish experience with patient ADR reporting 17, 20, and three studies performed an analysis of the ADRs presented in the European ADR database EudraVigilance 21, 22, 23. The majority of the studies coming from the Netherlands 35 and the UK 18, 25 compared the differences between ADR reports from patients and HCPs. Two studies were concerned with media exposition of statins 32, 33, and one with the patient contribution to ADR signal detection 25. Both UK studies analyzed the UK's national pharmacovigilance database but looked at different outcomes 18, 25.
Overall, these studies found that patient reporting is valuable, with no major qualitative differences between patient and HCP reports 20, 34. Patients provided well‐documented, consistent information 17, 43, reporting different categories of ADRs for different types of medicines when compared with HCPs. Patients provided more consistent information, a greater number of categories of ADRs and a greater range of medicines 16, 17, 20, 22, 23, 25, 34. This contribution can provide a positive, complementary input to that of HCPs for safety signal generation 25. Patients were found to report more often than HCPs on the impact of ADRs on daily life, making a more detailed description of reactions 27. A common feature of almost all of the studies from Denmark was the identification that most of the ADRs reported by patients related to central nervous system medication 17, 20, 22, 23. Four out of the 14 studies referred to the need for further research to clarify the value and characteristics of patient reporting 18, 22, 34, 36.
Some of the studies found differences between patient and HCP reporting 18, 32, 33, 35, 36. For example, UK patients seemed to report more ADRs with a lesser degree of severity 18. In a study from the Netherlands, differences were found in the level of participation of the population in a web‐based intensive monitoring programme, which might have led to an underestimation of ADRs 35. A study from China investigated ADRs in a paediatric population, concluding that patients are more likely than HCP to report new ADRs, although patient participation was low 36. Two studies from the Netherlands focused on the impact of media exposition on the reporting of ADRs for a specific class of drugs (statins) 32, 33. In general, media attention led to a peak in patient reports received by the pharmacovigilance authority, without affecting the overall reporting rate by patients.
Surveys
Eight of the identified studies were surveys, carried out using different methods, such as web‐based 26, 38 or postal 38, 39 questionnaires, and group 40, 41 or personal 38, 39, 42, 43 interviews. Six studies were from either the Netherlands 37, 41, 42 or the UK 38, 39, 40. Most of the studies from the UK 38, 39, 40 and one from the Netherlands 37 provided an overview of patients' views and experiences with ADR reporting. These studies showed that patients consider the severity of the ADR and the need to share experiences, such as the impact of ADRs on their daily life, as the main motives to report 37, 41 (Table 3). Patients perceived the possibility of reporting ADRs directly as important 38, and were able to identify ADRs in a manner that mirrored HCPs 39. Overall, patients' views were positive 38, 41, 43. However, studies identified a need to empower people to report, and provide feedback on their reports 40, 43.
Two studies, one from the Netherlands 42 and the other from France 26, aimed to study the countries in which patient reporting was available. It was found to be available in 44 countries worldwide in 2012 26. Although there were some differences in the way that different countries dealt with these reports, patient reporting was recognized as valuable 26, 42. The availability of the reporting form online seemed to increase the reporting rate 26. However, the content of the form was different for different countries, and should be harmonized 26.
Mixed methods
Almost all of the studies 24, 29, 44, 45, 46, 47, 48, were from the Netherlands 44, 45, 46, 47, 48. They explored the value of a web‐based intensive monitoring system using patients as an information source. These studies focused on specific drugs, such as pregabalin 44, 46 and duloxetine 47. The studies concluded that this type of intensive monitoring can gain insight about the daily use of the medicine and its safety profile 44, 46, 47, 48. It can generate other types of information when compared with SR, and provide more information about reactions, including the quantification of latency 44, 46, 48. One of the Dutch studies focused on the contribution that patient reports provide to ADR signal generation 24. It concluded that patients provide a valuable and considerable contribution to the detection of safety signals, in addition to that of HCPs 24. This has also been corroborated in another study 47. A UK study aimed to provide a general evaluation of the impact of patient reporting on the UK pharmacovigilance system. It concluded that patient reporting has the potential to add value to the pharmacovigilance system by providing reports with novel information about different types of drug and ADRs, thus complementing HCP reports and generating new safety signals. Reports by patients were found to describe ADRs in sufficient detail 29.
Discussion
The evidence gathered in the present systematic review confirmed the view that patient reporting makes a positive contribution to the general knowledge about ADRs. Most of the studies showed this, although in a general way. Thus, there was a lack of evidence on any of the possible drawbacks of patient reporting. These include the identification and accuracy of reported symptoms, the seriousness of the reactions and costs to the system 11, 49. No study elaborated on the prevalence of female reporters. Furthermore, there are limitations to what patients can add – e.g. it would be more difficult for a patient to report on abnormal blood results or renal clearance. These topics should be researched further. Current evidence is biased, in that the experiences of patient reporting came primarily from the Netherlands, UK and Denmark 16, 17, 19, 29. This also applied to the evidence on the clinical value of ADRs identified by patients. There was limited information about the type of reactions that patients identify in other countries, both in Europe and in the rest of the world. There was a lack of data coming from such countries as the USA and Australia, or from the WHO Uppsala Monitoring Centre, representing various countries from different continents. Further research is needed to improve our understanding of the value of, and problems in, patient reporting to pharmacovigilance authorities.
The present review gathered evidence that patients add new clinical and subjective information on ADRs 19, 28, 40. Patient reports are not meant to replace the reports coming from HCPs, but to provide an additional source of information 7. Patients report different ADRs and medicines when compared with HCPs 21, 22, 23. Reports coming from patients have also allowed for the identification of new ADRs, and led to the strengthening of safety signals 18, 25. This contribution is considered important by pharmacovigilance authorities as they allow for a faster accumulation of knowledge about ADRs 42.
An important aspect of patient reports is that they describe the ADRs with more detail and subjective factors than HCPs 27, 32. Patients can provide first‐hand information about medicine use and experiences, such as the way an ADR affects their daily lives 38, 40. However, the studies did not provide data about the number of patient reports that need to be followed up, in order to be confirmed medically. Although all such reports are treated in the same way in the EU 50, there is scant information about how the rest of the world treats patient reports. HCP reports are often incomplete and the quality of the language can be a barrier in the description of the ADR 7, 51. The quality of the reports should be researched further to evaluate the input provided by patients, especially in special groups with a strong drug therapy burden.
Despite the possibility of patient reporting increasing worldwide 26, awareness is still low 26. The UK has had patient involvement in SR since 2005, but only 8.5% of patients are aware of the possibility of reporting 29. The length of time since the introduction of direct patient reporting seems to play a part in this – e.g. the countries that introduced patient reporting earlier, such as the Netherlands, Denmark and the UK, have a higher reporting rate 16, 17, 29. By contrast, countries such as Portugal, Malta and Hungary, which introduced this facility more recently, show low levels of patient reporting 19, 26. Although not the aim of the present review, it is not clear how HCP reporting is evolving in the countries that have introduced patient reporting. Raising patient and HCP awareness of ADR reporting should be treated as a priority by national regulatory agencies, especially in countries with a low reporting rate. Patients who report have different characteristics to those who do not 35, 38; therefore, further research should focus on identifying the characteristics of patient reporters, in particular the psychological aspects that help to explain the desire to participate.
Existing pharmacovigilance systems have proven to be useful in identifying patient safety issues, although there is scope for optimizing and improving. SR has known limitations on data collection and reporting. The inclusion of an active form of vigilance seems to play an important role. Web‐based sources are becoming increasingly important, and this method can collect more information about certain drugs 53. The fact that this form of stimulated reporting targets specific drugs or patient populations can play a role in providing more safety data in a shorter period of time. However, only the Netherlands has experience of this type of reporting. The majority of studies have shown that patients provide mostly confirmatory information on the safety profile of drugs 17, 19. Further research on this topic is needed.
The drug regulatory pathway is evolving, adding more transparency in its decision‐making process and taking into account the voice of patients 14. There is also a growing interest in whether social media can capture patient‐generated safety information. The present review did not identify any studies that provided social media‐generated safety surveillance information. This type of tool could play a role in pharmacovigilance in the future 53, 54.
Strengths and limitations
The present systematic review collected an updated and comprehensive set of information about the direct patient reporting of ADRs. One of the strengths of the studies included was that they summarized current evidence on the contribution of patient reporting in different settings and countries, as well as patients’ opinions and views. The range of existing studies indicated that there are several gaps in knowledge related to patient reporting. Limitations to the study included the heterogeneity of methods used in the published studies, hindering comparisons between them. This poses challenges when examining studies, practices and countries. Another limitation was the geographical origin of the articles, as most came from only three countries in Europe.
The review analysed only published studies concerning patient reports to pharmacovigilance authorities. Although there is extensive literature on patients reporting in hospital settings or to consumer organizations, the use of this information remains limited. The inclusion of information submitted only to pharmacovigilance authorities allowed us to make comparisons with HCP‐reported ADRs. The review did not include any unpublished data, such as abstracts without the full text, to avoid the inclusion of incomplete data or methodological issues with the selected studies.
Conclusions
Patient reports add new information and perspective about ADRs in a way otherwise unavailable. This new information can lead to the strengthening of safety signals and increase the knowledge about ADRs. There are differences between patient and HCP reports. This is seen as positive, as the information provided by patients complements that coming from HCPs. The subjective information that patients bring to the system can be used to strengthen the current pharmacovigilance system with more evidence about the impact of ADRs on patients' daily lives. However, the present review identified gaps in knowledge that should be addressed in future research in order to understand better the full potential and problems of patient reporting.
Competing Interests
All authors have completed the conflict of interest disclosure form and declare no financial relationships with any organizations that might have an interest in the submitted work. There are no other relationships or activities that could appear to have influenced the submitted work. The authors had no support from any organization for the submitted work.
Inácio, P. , Cavaco, A. , and Airaksinen, M. (2017) The value of patient reporting to the pharmacovigilance system: a systematic review. Br J Clin Pharmacol, 83: 227–246. doi: 10.1111/bcp.13098.
References
- 1. Lopes P, Nunes T, Campos D, Furlong LI, Bauer‐Mehren A, Sanz F, et al. Gathering and exploring scientific knowledge in pharmacovigilance. PLoS One 2013; 8: e83016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta‐analysis of prospective studies. JAMA 1998; 279: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 3. Silverman SL. From randomized controlled trials to observational studies. Am J Med 2009; 122: 114–120. [DOI] [PubMed] [Google Scholar]
- 4. Alves C, Macedo AF, Marques FB. Sources of information used by regulatory agencies on the generation of drug safety alerts. Eur J Clin Pharmacol 2013; 69: 2083–2094. [DOI] [PubMed] [Google Scholar]
- 5. Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf 2013; 36: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Härmark L, van Grootheest AC. Pharmacovigilance: methods, recent developments and future perspectives. Eur J Clin Pharmacol 2008; 64: 743–752. [DOI] [PubMed] [Google Scholar]
- 7. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf 2006; 29: 385–396. [DOI] [PubMed] [Google Scholar]
- 8. Meyboom RH, Egberts AC, Gribnau FW, Hekster YA. Pharmacovigilance in perspective. Drug Saf 1999; 21: 429–447. [DOI] [PubMed] [Google Scholar]
- 9. Biagi C, Montanaro N, Buccellato E, Roberto G, Vaccheri A, Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia‐Romagna region). Eur J Clin Pharmacol 2013; 69: 237–244. [DOI] [PubMed] [Google Scholar]
- 10. Scurti V, Romero M, Tognoni G. A plea for a more epidemiological and patient‐oriented pharmacovigilance. Eur J Clin Pharmacol 2012; 68: 11–19. [DOI] [PubMed] [Google Scholar]
- 11. Borg JJ, Aislaitner G, Pirozynski M, Mifsud S. Strengthening and rationalizing pharmacovigilance in the EU: where is Europe heading to? A review of the new EU legislation on pharmacovigilance. Drug Saf 2011; 34: 187–197. [DOI] [PubMed] [Google Scholar]
- 12. Steurbaut S, Hanssens Y. Pharmacovigilance: empowering healthcare professionals and patients. Int J Clin Pharm 2014; 36: 859–862. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency . Annual Report 2015[online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Annual_report/2016/05/WC500206482.pdf (last accessed 19 May 2016).
- 14. van Grootheest K, de Graaf L, de Jong‐van den Berg LT. Consumer adverse drug reaction reporting: a new step in pharmacovigilance? Drug Saf 2003; 26: 211–217. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Langen J, van Hunsel F, Passier A, de Jong‐van den Berg L, van Grootheest K. Adverse drug reaction reporting by patients in the Netherlands: three years of experience. Drug Saf 2008; 31: 515–524. [DOI] [PubMed] [Google Scholar]
- 17. Aagaard L, Nielsen LH, Hansen EH. Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish adverse drug reaction database from 2004 to 2006. Drug Saf 2009; 32: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 18. McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L, et al. Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf 2010; 33: 775–788. [DOI] [PubMed] [Google Scholar]
- 19. Inch J, Watson MC, Anakwe‐Umeh S. Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Drug Saf 2012; 35: 807–818. [DOI] [PubMed] [Google Scholar]
- 20. Danish Health Agency [online]. Available at http://laegemiddelstyrelsen.dk/en/publications/2015/adverse‐drug‐reaction‐reports‐adrs‐from‐consumers‐may‐improve‐patient‐safety (last accessed 16 January 2015).
- 21. Aagaard L, Hansen EH. Side effects reported by European consumers for medications for erectile dysfunction. J Res Pharm Pract 2013; 2: 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aagaard L, Hansen EH. Adverse drug reactions reported by consumers for nervous system medications in Europe 2007 to 2011. BMC Pharmacol Toxicol 2013; 14: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aagaard L, Hansen EH. Adverse drug reactions in children reported by European consumers from 2007 to 2011. Int J Clin Pharm 2014; 36: 295–302. [DOI] [PubMed] [Google Scholar]
- 24. van Hunsel F, Talsma A, van Puijenbroek E, de Jong‐van den Berg L, van Grootheest K. The proportion of patient reports of suspected ADRs to signal detection in the Netherlands: case–control study. Pharmacoepidemiol Drug Saf 2011; 20: 286–291. [DOI] [PubMed] [Google Scholar]
- 25. Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ. How do patients contribute to signal detection?: A retrospective analysis of spontaneous reporting of adverse drug reactions in the UK's Yellow Card Scheme. Drug Saf 2013; 36: 199–206. [DOI] [PubMed] [Google Scholar]
- 26. Margraff F, Bertram D. Adverse drug reaction reporting by patients: an overview of fifty countries. Drug Saf 2014; 37: 409–419. [DOI] [PubMed] [Google Scholar]
- 27. Rolfes L, van Hunsel F, Wilkes S, van Grootheest K, van Puijenbroek E. Adverse drug reaction reports of patients and healthcare professionals‐differences in reported information. Pharmacoepidemiol Drug Saf 2015; 24: 152–158. [DOI] [PubMed] [Google Scholar]
- 28. Health Action International . Direct patient reporting of adverse drug reactions: a fifteen‐country survey and literature review [online]. Available at http://consumers.cochrane.org/sites/consumers.cochrane.org/files/uploads/10%20May%202010%20Report%20Direct%20Patient%20Reporting%20of%20ADRs.pdf (last accessed 16 January 2015).
- 29. Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford PC, et al. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 2011; 15: 1–234. [DOI] [PubMed] [Google Scholar]
- 30. Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol 2007; 63: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Härmark L, van Hunsel F, Grundmark B. ADR Reporting by the general public: lessons learnt from the Dutch and Swedish systems. Drug Saf 2015; 38: 337–347. [DOI] [PubMed] [Google Scholar]
- 32. van Hunsel F, Passier A, van Grootheest K. Comparing patients' and healthcare professionals' ADR reports after media attention: the broadcast of a Dutch television programme about the benefits and risks of statins as an example. Br J Clin Pharmacol 2009; 67: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Hunsel F, van Puijenbroek E, de Jong‐van den Berg L, van Grootheest K. Media attention and the influence on the reporting odds ratio in disproportionality analysis: an example of patient reporting of statins. Pharmacoepidemiol Drug Saf 2010; 19: 26–32. [DOI] [PubMed] [Google Scholar]
- 34. Durrieu G, Palmaro A, Pourcel L, Caillet C, Faucher A, Jacquet A, et al. First French experience of ADR reporting by patients after a mass immunization campaign with Influenza A (H1N1) pandemic vaccines: a comparison of reports submitted by patients and healthcare professionals. Drug Saf 2012; 35: 845–854. [DOI] [PubMed] [Google Scholar]
- 35. Härmark L, Alberts S, van Puijenbroek E, Denig P, van Grootheest K. Representativeness of diabetes patients participating in a web‐based adverse drug reaction monitoring system. Pharmacoepidemiol Drug Saf 2013; 22: 250–255. [DOI] [PubMed] [Google Scholar]
- 36. Li H, Guo XJ, Ye XF, Jiang H, Du WM, Xu JF, et al. Adverse drug reactions of spontaneous reports in Shanghai pediatric population. PLoS One 2014; 9: e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K. Motives for reporting adverse drug reactions by patient‐reporters in the Netherlands. Eur J Clin Pharmacol 2010; 66: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson C, Krska J, Murphy E, Avery A, Yellow Card Study C. The importance of direct patient reporting of suspected adverse drug reactions: a patient perspective. Br J Clin Pharmacol 2011; 72: 806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krska J, Anderson C, Murphy E, Avery AJ. How patient reporters identify adverse drug reactions: a qualitative study of reporting via the UK Yellow Card Scheme. Drug Saf 2011; 34: 429–436. [DOI] [PubMed] [Google Scholar]
- 40. Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol 2013; 75: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rolfes L, Wilkes S, van Hunsel F, van Puijenbroek E, van Grootheest K. Important information regarding reporting of adverse drug reactions: a qualitative study. Int J Pharm Pract 2014; 22: 231–233. [DOI] [PubMed] [Google Scholar]
- 42. van Hunsel F, Härmark L, Pal S, Olsson S, van Grootheest K. Experiences with adverse drug reaction reporting by patients: an 11‐country survey. Drug Saf 2012; 35: 45–60. [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto M, Kubota K, Okazaki M, Dobashi A, Hashiguchi M, Doi H, et al. Patients’ views and experiences in online reporting adverse drug reactions: findings of a national pilot study in Japan. Patient Prefer Adherence 2015; 9: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Härmark L, Puijenbroek E, Grootheest K. Longitudinal monitoring of the safety of drugs by using a web‐based system: the case of pregabalin. Pharmacoepidemiol Drug Saf 2011; 20: 591–597. [DOI] [PubMed] [Google Scholar]
- 45. Härmark L, van Hunsel F, Hak E, van Grootheest K. Monitoring the safety of influenza A (H1N1) vaccine using web‐based intensive monitoring. Vaccine 2011; 29: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 46. Härmark L, van Puijenbroek E, Straus S, van Grootheest K. Intensive monitoring of pregabalin: results from an observational, web‐based, prospective cohort study in the Netherlands using patients as a source of information. Drug Saf 2011; 34: 221–231. [DOI] [PubMed] [Google Scholar]
- 47. Härmark L, van Puijenbroek E, van Grootheest K. Intensive monitoring of duloxetine: results of a web‐based intensive monitoring study. Eur J Clin Pharmacol 2013; 69: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oosterhuis I, Härmark L, van Puijenbroek E. Experiences with the use of varenicline in daily practice in the Netherlands: a prospective, observational cohort study. Drug Saf 2014; 37: 449–457. [DOI] [PubMed] [Google Scholar]
- 49. Chaipichit N, Krska J, Pratipanawatr T, Uchaipichat V, Jarernsiripornkul N. A qualitative study to explore how patients identify and assess symptoms as adverse drug reactions. Eur J Clin Pharmacol 2014; 70: 607–615. [DOI] [PubMed] [Google Scholar]
- 50. European Medicines Agency . Guidelines on good pharmacovigilance practices (GVP). Module VI – Management and reporting of adverse drug reactions to medicinal products (Rev 1) [online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500172402.pdf (last accessed 19 May 2016).
- 51. Inacio P, Airaksinen M, Cavaco A. Language does not come ‘in boxes’: assessing discrepancies between adverse drug reactions spontaneous reporting and MedDRA(R) codes in European Portuguese. Res Social Adm Pharm 2015; 11: 664–674. [DOI] [PubMed] [Google Scholar]
- 52. Banerjee AK, Ingate S. Web‐based patient‐reported outcomes in drug safety and risk management: challenges and opportunities? Drug Saf 2012; 35: 437–446. [DOI] [PubMed] [Google Scholar]
- 53. Coloma PM, Becker B, Sturkenboom MC, van Mulligen EM, Kors JA. Evaluating social media networks in medicines safety surveillance: two case studies. Drug Saf 2015; 38: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Topaz M, Lai K, Dhopeshwarkar N, Seger DL, Sa'adon R, Goss F, et al. Clinicians' reports in electronic health records versus patients' concerns in social media: a pilot study of adverse drug reactions of aspirin and atorvastatin. Drug Saf 2016; 39: 241–250. [DOI] [PubMed] [Google Scholar]


