Abstract
Aims
This prospective study aimed to characterize the population pharmacokinetics of intravenous oxycodone and to determine the minimum effective concentration (MEC) and minimum effective analgesic concentration (MEAC) of oxycodone for major open intra‐abdominal surgery.
Methods
In the pharmacokinetic study, patients were administered intravenous oxycodone (0.1 mg kg−1), and arterial blood was sampled at pre‐set intervals. In the analgesic‐potency study, patients were administered intravenous oxycodone (0.1 mg kg−1) 30 min before the end of the surgery, were placed in the postoperative anaesthesia care unit (PACU), and were asked to rate their pain every 10 min using a visual analogue scale (0 = no pain, 10 = most severe pain). On the first occasion that wound pain at rest and during compression was rated as ≥3 or ≥5, respectively, the first blood sample was obtained to determine the MEC. A second blood sample was obtained after titration with 2 mg of oxycodone to yield wound pain <3 at rest and <5 during wound compression, and MEAC was determined. MEC and MEAC were determined again in each patient.
Results
In the population pharmacokinetic study (n = 54), oxycodone plasma concentration over time was well described by a three‐compartment mammillary model. Lean body mass and age were significant covariates for the volume of distribution and metabolic clearance of the pharmacokinetic model of oxycodone, respectively. The analgesic‐potency study (n = 50) showed that the median (95% CI) MEC and MEAC were 31.5 (19.2–42.8) and 74.1 (29.2–128.3) ng ml−1 (first measurements) and 63.4 (15.6–120.1) and 76.1 (32.9–132.7) ng ml−1 (second measurements), respectively.
Conclusions
In major intra‐abdominal open surgery, the MEAC and analgesic potency of oxycodone were 75 ng ml−1 and 60 ng ml−1, respectively.
Keywords: analgesia, model, oxycodone, pharmacokinetic, potency
What is Already Known About This Subject
Oxycodone is a semisynthetic opioid analgesic widely used in the treatment of both acute and chronic pain.
The elimination of oxycodone is decreased with advancing age.
What This Study Adds
The pharmacokinetics of oxycodone were best described by a three‐compartment mammillary model.
The mean effective analgesic concentration and analgesic potency were 75 ng ml−1 and 60 ng ml−1, respectively.
Tables of Links
| TARGETS |
|---|
| GPCRs 2 http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5194 |
| Opioid receptors |
| LIGANDS |
|---|
| Oxycodone |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Recently, use of the opioid oxycodone has increased markedly. Indeed, in several countries, it has replaced morphine as a rescue analgesic 3. There are formulations for immediate and extended oral release, oral syrup and intravenous use. In 2013, intravenous oxycodone was approved for market by the Ministry of Food and Drug Safety (MFDS) of the Republic of Korea for moderate to severe pain, including postoperative intravenous patient‐controlled analgesia (IV PCA) 4. Currently, the most common opioid analgesic used for IV PCA in Korea is fentanyl, and the clinical experience with IV PCA with oxycodone in Korea has been extremely limited. The dosage regimen for postoperative pain relief with intravenous oxycodone that was approved by the MFDS is an IV loading bolus of 2 mg, followed by IV PCA consisting of demand boluses of 1 mg and no background infusion 4. However, this dosing strategy might not be suitable for all patients because several studies have shown that oxycodone pharmacokinetics vary markedly 5, 6, 7. Age is a particularly significant covariate for metabolic oxycodone clearance 5, 6. Hence, older patients might require lower and more carefully titrated doses to avoid harmful adverse events. Two studies have characterized the population pharmacokinetics of intravenous oxycodone, but both had some limitations 6, 8. One study was performed in children, while the other, by Saari et al., had a delayed initial sampling time and a relatively large number of healthy young volunteers, and calculated lean body mass (LBM) using the James formula 6, 8. Hence, it is necessary to perform a new population pharmacokinetic study to determine the inter‐individual variability in pharmacokinetic parameters in surgical patients who require IV PCA.
Earlier studies showed that laparoscopic cholecystectomy and cardiac surgery differ in terms of the minimum effective concentration (MEC) and minimum effective analgesic concentration (MEAC) by at least 20 ng ml−1 9, 10. Thus, laparoscopic cholecystectomy requires a smaller IV oxycodone dose to treat pain effectively and to induce analgesia than cardiac surgery. This surgical procedure‐related variability suggests that the dosage regimen of IV PCA with oxycodone might have to be adjusted for major intra‐abdominal surgeries, such as stomach, colorectal and hepatobiliary surgeries, which are the most common surgeries in Korea. To the best of our knowledge, the MEC and MEAC with oxycodone for major intra‐abdominal surgeries have not been evaluated.
The aims of this study were to characterize the population pharmacokinetics of IV oxycodone following a single IV bolus of 0.1 mg kg−1 in surgical patients and to determine the MEC and MEAC of intravenous oxycodone for major intra‐abdominal open surgeries such as stomach, colorectal and hepatobiliary surgeries.
Materials and Methods
Patient population
This study consisted of two clinical trials. Both clinical trials were approved by the IRB (Institutional Review Board) of AMC (Asan Medical Centre) (2014‐0600 for the pharmacokinetic study, 2014‐0601 for the analgesic‐potency study) and were registered on an international clinical trials registry platform (http://cris.nih.go.kr, KCT0001336 for the pharmacokinetic study; KCT0001340 for the analgesic‐potency study). Written informed consent was obtained from all of the patients. The patient groups in both clinical trials consisted of all consecutive patients who were scheduled to undergo elective stomach, colorectal or hepatobiliary surgery between August 2014 and March 2015 at AMC (a tertiary referral centre) and who had an American Society of Anesthesiologists Physical Status (ASA PS) of 1 or 2. The patients in the pharmacokinetic and analgesic‐potency study were enrolled in August 2014–March 2015 and August 2014–February 2015, respectively. Patients were excluded if they were allergic to oxycodone, had long‐term use of opioid medications, were pregnant, had a history of hepatic, cardiopulmonary or renal disease, and/or had a history of chronic pain. Moreover, in the analgesic‐potency study, patients undergoing laparoscopic surgery were excluded.
Study procedures
All of the patients fasted for 6–8 h prior to surgery. They were monitored routinely with conventional equipment in the operating theatre. Anaesthesia was induced and maintained with target effect site concentration‐controlled infusion of propofol and remifentanil (Asan Pump, version 2.1.3, Bionet Co., Ltd., Seoul, Republic of Korea) 11, 12. Tracheal intubation was performed after cisatracurium 0.2 mg kg−1 was administered. For frequent blood sampling, a 20‐gauge catheter was inserted into a radial artery. The target concentrations of propofol and remifentanil were adjusted to maintain bispectral index (BIS, Aspect 2000, Aspect Medical Systems, Inc., Newton, USA) values of less than 60 and stable haemodynamics (systolic blood pressure > 80 mm Hg; heart rate > 45 beats min−1), respectively. If necessary, ephedrine or atropine was administered to maintain stable haemodynamics.
Intervention for the pharmacokinetic study
Before skin incision, the patients were administered a 0.1 mg kg−1 intravenous bolus of oxycodone hydrochloride (Oxynorm®, 10 mg ml−1; Mundipharma Korea Ltd., Seoul, Republic of Korea). Arterial blood samples were obtained at pre‐set intervals thereafter (0, 2.5, 5, 10, 15, 20, 30, 45, and 60 min and 1.5, 2, 4, 6, 8, 10, 12, and 24 h) to measure the oxycodone hydrochloride concentration in the plasma.
Intervention for the analgesic‐potency study
At least 30 min before the anticipated end of surgery, the patients were administered a 0.1 mg kg−1 intravenous bolus of oxycodone, taken to the postoperative anaesthesia care unit (PACU), and assessed for pain every 10 min using a visual analogue scale (VAS) (0 = no pain; 10 = the most severe pain). Pain was measured at rest and when the wound areas were compressed with a force of 20 N (i.e., 2 kg of pressure imposed by three fingers on a 10 cm2 area). The wound compression was performed by nurses who were trained with an algometer (Commander Algometer, J Tech Medical Industries, Midvale, UT, USA) to apply this force consistently. On the first occasion that wound pain at rest and during compression was rated as ≥3 or ≥5, respectively, the first venous blood sample was obtained to determine the MEC of oxycodone 9. The patient was then administered IV oxycodone 2 mg (body weight < 80 kg) or 3 mg (>80 kg) every 10 min until the VAS assessments showed that the pain intensity had decreased to <3 at rest and <5 on wound compression. At this point, the second blood sample was obtained, and the MEAC of oxycodone was measured 9. This process for measuring MEC and MEAC was repeated again in each patient. During the study period in the PACU, heart rate, non‐invasive blood pressure, respiratory rate and adverse events were monitored and recorded every 10 min. The sedation level was assessed every 30 min using the Modified Observer's Assessment of Alertness/Sedation (MOAA/S) score.
Blood sample acquisition and assay
Blood samples were collected in ethylene‐diamine‐tetra‐acetic acid (EDTA) tubes and were centrifuged for 10 min at 1500 × g. The plasma was stored at −80°C until assay. The plasma concentration of oxycodone hydrochloride was determined using a method of fully validated liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS). The plasma samples (0.2 ml) were mixed with 0.02 ml of an internal standard (oxycodone‐d6) working solution, extracted with tert butyl methyl ether by vortex‐mixing for 2 min at high speed, centrifuged at 27 000 × g for 5 min, and evaporated under a stream of nitrogen at less than 45°C. The residues were dissolved in 0.1 ml of mobile phase and were transferred to autosampler vials, and 5 μl was injected into the LC–MS/MS. The LC–MS/MS system consisted of an Agilent 1200 series HPLC (Agilent Technologies, Palo Alto, CA, USA) coupled to an API4000 mass spectrometer (Applied Biosystems/MDS Sciex, Toronto, Canada). The separation was performed on a Shiseido MG3 μm (2.0 × 150 mm) column (Shiseido, Tokyo, Japan) using a mobile phase of acetonitrile–water–formic acid (40: 60: 0.1, v/v/v) at a flow rate of 0.2 ml min−1. The LC–MS/MS was an API 4000 (ABSciex, Foster City, CA, USA) that was operated in positive electrospray ionization mode with multiple reaction monitoring. The method was validated with regard to specificity, matrix effect, linearity, recovery, accuracy, precision and stability. The calibration curve was linear in the range of 0.2–1000 ng ml−1, and the coefficients of determination (R 2 values) were >0.9990. The intraday accuracy and precision (coefficient of variation, CV) of this essay were 93.00–97.65% and 1.27–4.81%, respectively. The intraday accuracy and precision (CV) were 97.25–98.67% and 4.33–6.37%, respectively.
Non‐compartmental analysis of oxycodone
Plasma concentration–time data were fit by noncompartmental methods to determine the AUC last (area under the curve from administration to the last measured concentration), AUC inf (area under the curve from administration to infinity), and λz (apparent terminal rate constant) using WinNonlin software, version 6.3 (Pharsight, a Certara Company, St. Louis, MO, USA).
Population pharmacokinetic analysis
A population pharmacokinetic analysis was performed with NONMEM VII level 3 (ICON Development Solutions, Ellicott City, MD, USA). A log‐normal model was used to estimate the inter‐individual random variabilities (IIV) of pharmacokinetic parameters, and diagonal matrices were applied to estimate the various distributions of η, where η represented the IIV. Combined additive and constant CV residual error models were applied to the model building. NONMEM computed the minimum objective function value (OFV), a statistical equivalent to the −2 log likelihood of the model. An α level of 0.05, which corresponds to a reduction in the OFV of 3.84 (Chi‐square distribution, degrees of freedom = 1, P < 0.05), was used to distinguish between hierarchical models 13. One‐, two‐, and three‐compartment disposition models with first‐order elimination were tested. The covariates that were analysed were age, sex (0 = male, 1 = female), weight, height, body surface area 14, body mass index, ideal body weight 15, lean body mass 16, systolic blood pressure, diastolic blood pressure, mean arterial pressure 17, heart rate, hourly fluid volume infused and hourly urine output during the study period, and blood loss during operation. Non‐parametric bootstrap analysis served to validate the models internally (fit4NM 3.7.9, http://www.fit4nm.org/download, last accessed 17 October 2011) 18. Predictive checks and random permutation tests were also performed using fit4NM 3.7.9 19, 20. Simulations were performed to characterize the effect of covariates on the oxycodone pharmacokinetics, using the estimated pharmacokinetic parameters of the final model.
Determination of analgesic potency using logistic regression
Every measured plasma oxycodone concentration was joined to 0 (MEC) or 1 (MEAC). The relationship between the probability of analgesia and the measured plasma oxycodone concentration was analysed using a sigmoid E max model:
| (1) |
where C p is the measured plasma oxycodone concentration, C p50 is the plasma concentration associated with a 50% probability of analgesia, and γ is the steepness of the concentration vs. response relation. The likelihood, L, of the observed response, R, is described by the following equation:
| (2) |
where Prob is the probability of analgesia. Model parameters were estimated using the option “LIKELIHOOD LAPLACE METHOD = conditional” in NONMEM. The IIV of C p50 and γ was modelled using a log‐normal model.
Simulation
Deterministic simulations that considered neither the inter‐individual nor the intra‐individual random variability were performed using Asan Pump software. The changes in oxycodone plasma concentration over time after a 0.1 mg kg−1 bolus of oxycodone were simulated in hypothetical patients whose weight and height were 65 kg and 165 cm, respectively. The predicted oxycodone concentration in the plasma over time after an intravenous bolus of 0.1 mg kg−1, followed by demand boluses of 1 mg every 15 min with or without background infusion of 1 mg h−1, was also simulated.
Safety
Safety profiles were evaluated on the basis of the incidence of adverse events, vital signs and clinical laboratory test results. In the analgesic‐potency study, the patients in the PACU were monitored in terms of heart rate, non‐invasive blood pressure and respiratory rate, which were recorded every 10 min. The sedation level was assessed every 30 min using the MOAA/S score.
Statistics
Statistical analysis was conducted using SigmaStat software, version 3.5 for Windows (Systat Software, Inc., Chicago, IL, USA). The data are expressed as the means (SDs) for normally distributed continuous variables, medians (25–75%) for non‐normally distributed continuous variables, and counts and percentages for categorical variables. A P‐value less than 0.05 was considered as statistically significant.
Results
Patient populations
A consort diagram of participants in the two sub‐studies and the characteristics of the two patient populations are shown in Figure 1 and Table 1, respectively.
Figure 1.
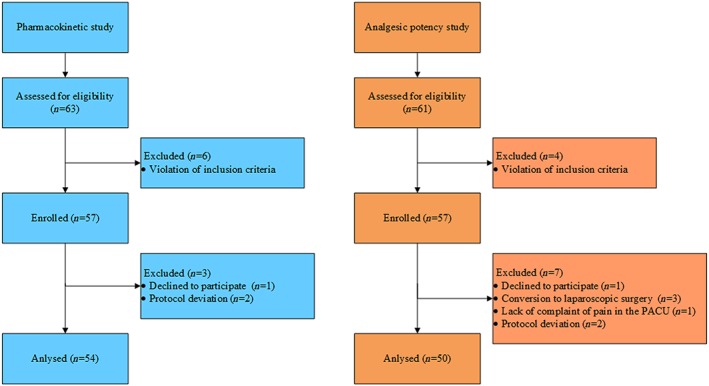
Consort diagram of participants in the two studies. For the pharmacokinetic study, a total of 63 patients were screened, and of these, six patients were excluded due to violations of the inclusion criteria. A total of 57 patients were enrolled in this study, and seven patients dropped out from the study because of withdrawal of consent before administration of oxycodone (n = 1) and protocol deviations (n = 2). Hence, 54 patients were included in the safety and pharmacokinetic analyses. For the analgesic potency study, 61 patients were screened, and of these, four patients were excluded due to violations of the inclusion criteria. A total of 57 patients were enrolled in this study, and five patients dropped out from the study because of conversion to laparoscopic surgery (n = 3), no complaint of pain at PACU (n = 1), and withdrawal of consent at PACU (n = 1). Additionally, two patients were excluded from the evaluation of MEC, MEAC and analgesic potency of oxycodone because of protocol deviations. Hence, 52 and 50 patients were included in the safety and the MEAC analyses, respectively. PACU: post‐anaesthesia care unit
Table 1.
Characteristics of the patient populations in the pharmacokinetic and analgesic‐potency studies
| Pharmacokinetics (n = 54) | Analgesic potency (n = 50) | |
|---|---|---|
| ASA PS 1/2 | 23/31 | 13/37 |
| Age, yr | 58 ± 11 | 58 ± 11 |
| Weight, kg | 65 ± 11 | 64 ± 9 |
| Male/Female | 34/20 | 32/18 |
| Height, cm | 164 ± 8 | 165 ± 8 |
| BSA, m 2 | 1.7 (1.6–1.9) | 1.7 ± 0.2 |
| LBM, kg | 47.2 ± 9.6 | 48.7 (39.0–54.0) |
| IBW, kg | 59.5 ± 7.4 | 62.3 (54.1–65.2) |
| BMI, kg m −2 | 24.0 ± 3.1 | 23.3 ± 2.8 |
| Operation | ||
| ST | 31 | 38 |
| CRS | 13 | 7 |
| HBP | 10 | 7 |
The data are expressed as mean ± SD, median (25–75%), or count as appropriate. ASA PS, American Society of Anesthesiologists Physical Status; BMI, body mass index; BSA, body surface area calculated using the Mosteller formula 14; CRS, colorectal surgery including right hemicolectomy, anterior resection, low anterior resection, and ileocecal resection; HBP, hepatobiliary surgery including extended cholecystectomy, left lobectomy, S5 segmentectomy, central bisegmentectomy, right anterior segmentectomy, left medial sectionectomy, partial hepatectomy, and pylorus‐preserving pancreaticoduodenectomy; IBW, ideal body weight calculated using the Robinson formula 15; LBM, lean body mass calculated using the Janmahasatian formula 24; ST, stomach surgery including distal or total gastrectomy
Non‐compartmental analysis
In total, 849 plasma concentration measurements from 54 patients were used to characterize the pharmacokinetics of oxycodone in patients undergoing major intra‐abdominal open surgery. The plasma concentration–time data are shown in Figure 2A. The mean (SD) AUC last and AUC inf were 11.6 (3.1) and 12.1 (3.3) min μg ml−1, respectively. The mean (SD) λz was 0.16 (0.04) h−1. In all of the subjects, at least 80% of the total area under the curve was covered by the measured concentrations (3.7% of AUC %Extra, percentage of the extrapolated area under the curve to the total area under the curve).
Figure 2.
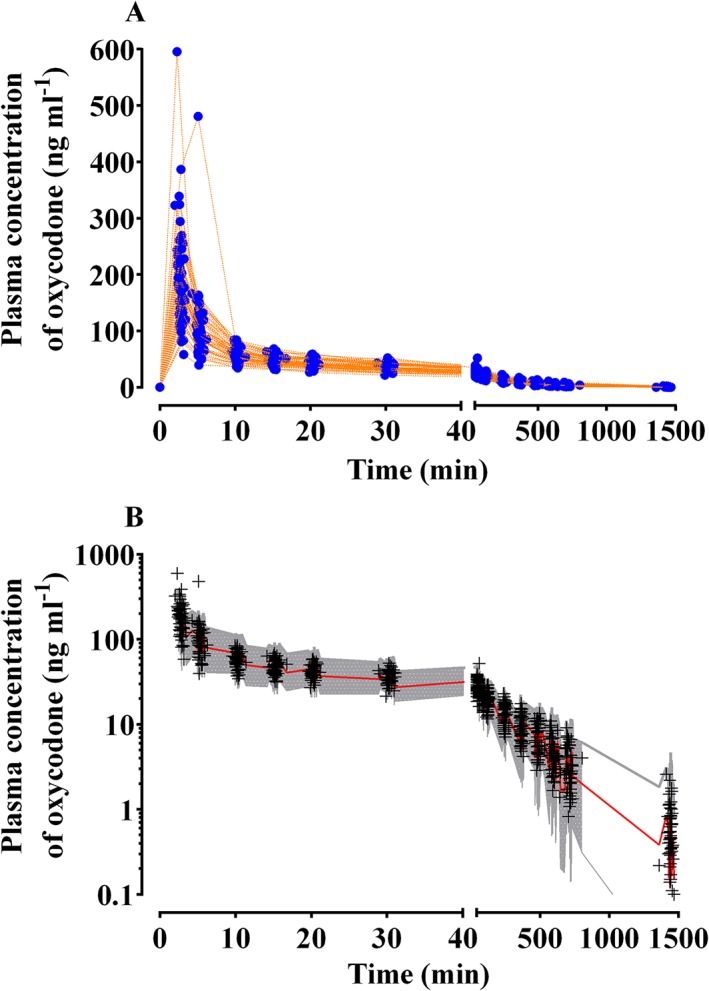
Measured plasma concentrations of oxycodone plotted against time after a single intravenous bolus of 0.1 mg kg−1 (A) and predictive checks of the final pharmacokinetic model for oxycodone (B) in the pharmacokinetic study. Blue closed circles: measured plasma concentration of oxycodone; orange dotted lines: individual time course of plasma concentration of oxycodone; +, observed plasma concentrations of oxycodone. The red solid line and shaded areas indicate the 50% prediction line and 95% prediction intervals, respectively
Population pharmacokinetics
A three‐compartment mammillary model best described the pharmacokinetics of oxycodone in surgical patients. LBM was a significant covariate for the central volume of distribution (V d) (equation (3)), and it resulted in improvement in the OFV (20.74, P < 0.001, df = 1), compared with the basic model (number of model parameters = 14). The δ value between the basic and covariate (LBM on V 1) models in the randomization test was 3.70.
| (3) |
LBM was also a significant covariate for the slow peripheral V d (equation (4)) and led to a further improvement in OFV (36.81, P < 0.001, df = 1) compared with the OFV of a pharmacokinetic model that included LBM as a covariate for the central V d only (number of model parameters = 15). The δ value between the previous covariate (LBM on V 1) and present covariate (LBM on V 1 and V 3) pharmacokinetic models was 2.45.
| (4) |
Age was a significant covariate for the metabolic clearance of oxycodone (equation (5)) and resulted in an improvement in OFV (4.69, P < 0.05, df = 1) compared with the OFV of a pharmacokinetic mode that included LBM as a covariate for the central and slow peripheral V ds (number of model parameters = 16). The δ value between the previous covariate (LBM on V 1 and V 3) and present covariate (LBM on V 1 and V 3, and age on Cl) pharmacokinetic models was 2.30.
| (5) |
LBM was also a significant covariate for the rapid peripheral V d (equation (6)) and led to further improvement in OFV (16.81, P < 0.001, df = 1), compared with the OFV of a pharmacokinetic model that included LBM as a covariate for the central and slow peripheral volumes of distributions and age as a covariate for metabolic clearance (number of model parameters = 17). The δ value between the covariate (LBM on V 1 and V 3, and age on Cl) and final pharmacokinetic (LBM on V 1, V 2 and V 3, and age on Cl) models was 1.91
| (6) |
Parameter estimates of the competing base and covariate pharmacokinetic models of oxycodone are described in the supplementary materials (Table 2). The results of the randomization test provided sufficient evidence to conclude that the effects of LBM on V 1, V 2 and V 3, and age on Cl, were statistically significant.
Table 2.
Parameter estimates (RSE, % CV) of competing basic and covariate pharmacokinetic models of oxycodone
| Model 1 | Model 2 | Model 3 a | Model 4 | Model 5 | Model 6 | Model 7 b | |
|---|---|---|---|---|---|---|---|
| Covariate | — | — | — | V 1: LBM | V 1, V 3: LBM | V 1, V 3: LBM Cl: age | V 1, V 2, V 3: LBM Cl: age |
| V 1 , (l) | 96.9 (3.7, 31.0) | 26.7 (16.0, 45.9) | 12.1 (8.3, 41.4) | 12.6 + 0.378 × (LBM‐47) (5.5, 23.7, 27.6) | 12.7 + 0.374 × (LBM‐47) (5.6, 26.4, 29.1) | 12.5 + 0.362 × (LBM‐47) (5.9, 23.1, 28.1) | 11.8 + 0.36 × (LBM‐47) (11.4, 31.4, 30.5) |
| V 2 , (l) | — | 129 (3.4, 17.9) | 29.7 (7.3, 29.5) | 29.2 (7.4, 34.1) | 29.9 (7.3, 30.0) | 29.7 (7.2, 29.9) | 29.3 + 0.671 × (LBM‐47) (7.4, 41.3, 21.1) |
| V 3 , (l) | — | — | 120 (2.6, 19.8) | 119 (2.7, 19.1) | 120 + 1.73 × (LBM‐47) (2.1, 15.9, 12.8) | 121 + 1.72 × (LBM‐47) (2.1, 16.3, 12.9) | 121 + 1.48 × (LBM‐47) (2.7, 23.9, 12.9) |
| Cl, (l min −1 ) | 0.503 (3.5, 26.3) | 0.584 (10.3, 24.3) | 0.576 (3.5, 19.8) | 0.572 (3.6, 26.2) | 0.576 (3.5, 12.8) | 1.58‐(age/58)0.241 (1.2, 39.7, 25.0) | 1.58‐(age/58)0.203 (1.7, 40.2, 26.2) |
| Q 1 , (l min −1 ) | — | 3.43 (10.3, 40.99) | 2.69 (8.5, 49.1) | 2.61 (9.3, 49.7) | 2.72 (8.2, 48.9) | 2.68 (8.5, 48.6) | 2.52 (15.3, 48.1) |
| Q 2 , (l min −1 ) | — | — | 1.79 (6.3, 44.8) | 1.77 (6.5, 49.7) | 1.77 (6.4, 44.2) | 1.78 (6.3, 44.0) | 1.81 (11.4, 42.9) |
| OFV | 5570.2 | 3239.1 | 2618.1 | 2597.4 | 2560.6 | 2555.9 | 2539.4 |
| Number of parameters (p) | 6 | 10 | 14 | 15 | 16 | 17 | 18 |
| AIC | 5582.2 | 3259.1 | 2646.1 | 2627.4 | 2592.6 | 2589.9 | 2575.4 |
AIC, Akaike information criteria (−2LL + 2 × p); Cl, metabolic clearance (l min−1); CV, coefficient of variation; LBM, lean body mass; OFV, objective function value (−2 log likelihood, −2LL); RSE, relative standard error = SE/estimate × 100 (%); Q 1, inter‐compartmental clearance of rapid peripheral compartment (l/min); Q 2, inter‐compartmental clearance of slow peripheral compartment (l min−1);V 1, central volume of distribution (l); V 2, rapid peripheral volume of distribution (l); V 3, slow peripheral volume of distribution (l).
Selected basic model.
Selected final model
Table 3 represents the population pharmacokinetic parameter estimates and the results of nonparametric bootstrap replicates of the final pharmacokinetic model of oxycodone. Predictive checks of the final pharmacokinetic model are presented in Figure 2B. In total, 2.3% of the data were distributed outside of the 95% prediction intervals of the predictive check.
Table 3.
Population pharmacokinetic parameter estimates, inter‐individual variability, and median parameter values (2.5–97.5%) of the non‐parametric bootstrap replicates of the final pharmacokinetic model of oxycodone
| Parameters | Estimates (RSE, %) | CV (%) | Median (2.5–97.5%) |
|---|---|---|---|
| V 1 (l) = θ 1 + θ 2 × (LBM‐47) | |||
| θ 1 | 11.8 (11.4) | 30.5 | 13.5 (10.6–30.1) |
| θ 2 | 0.36 (31.4) | 0.393 (0.094–0.769) | |
| V 2 (l) = θ 3 + θ 4 × (LBM‐47) | |||
| θ 3 | 29.3 (7.3) | 21.1 | 31.0 (25.7–132) |
| θ 4 | 0.671 (41.3) | 0.733 (0.101–2.07) | |
| V 3 (l) = θ 5 + θ 6 × (LBM‐47) | |||
| θ 5 | 121 (2.7) | 12.9 | 122 (113–150 000) |
| θ 6 | 1.48 (23.9) | 1.24 (0.0001–31.55) | |
| CL (l/min) = θ 7 ‐(age/58) θ8 | |||
| θ 7 | 1.58 (1.7) | 26.2 | 1.56 (0.09–1.61) |
| θ 8 | 0.203 (40.2) | 0.216 (0.0008–0.692) | |
| Q 1 (l min −1 ) | 2.52 (15.3) | 48.1 | 2.78 (2.02–3.81) |
| Q 2 (l min −1 ) | 1.81 (11.4) | 42.9 | 1.71 (0.37–2.04) |
| σ 1 | 0.185 (10.5) | — | 0.145 (0.001–0.362) |
| σ 2 | 0.081 (1.745) | — | 0.085 (0.057–0.154) |
A log‐normal distribution of inter‐individual random variability was assumed. Residual random variability was modelled using an additive (σ1) plus proportional (σ2) error model. Non‐parametric bootstrap analysis was repeated 2000 times. RSE, relative standard error = SE/mean × 100 (%). LBM, lean body mass calculated using the Janmahasatian formula 24.
MEC, MEAC and analgesic potency
Total doses of 8 (6–12) mg and 2 (2–4) mg of oxycodone were required to achieve the first and second MEAC, respectively. A total of 200 plasma concentration measurements from 50 patients was used to determine MEC and MEAC and to perform the logistic regression analysis. At the first onset of pain (the first MEC), the median plasma concentration of oxycodone was 31.5 ng ml−1 (95% CI: 19.2–42.8 ng ml−1). At the first pain relief (the first MEAC), the median plasma concentration was 74.1 ng ml−1 (29.2–128.3 ng ml−1). The second MEC and MEAC were 63.4 (15.6–120.1) and 76.1 (32.9–132.7) ng ml−1, respectively (Figure 3). The relationship between the probability of analgesia and the measured plasma oxycodone concentration is shown in Figure 4. The C p50 (the measured plasma oxycodone concentration that was associated with a 50% probability of analgesia) estimate (SE) was 59.9 (2.40) ng ml−1. The γ estimate (SE) and the inter‐individual variability presented as %CV were 3.73 (0.729) and 182%, respectively.
Figure 3.
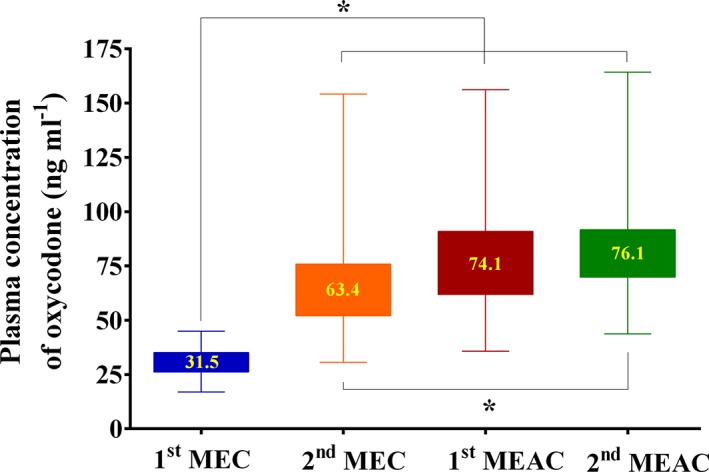
Median values of minimum effective concentration (MEC) and minimum effective analgesic concentration (MEAC) in the analgesic potency study. Asterisk: 2.5–97.5 percentiles. *P < 0.05. Numbers within asterisks indicate median MEC or MEAC
Figure 4.
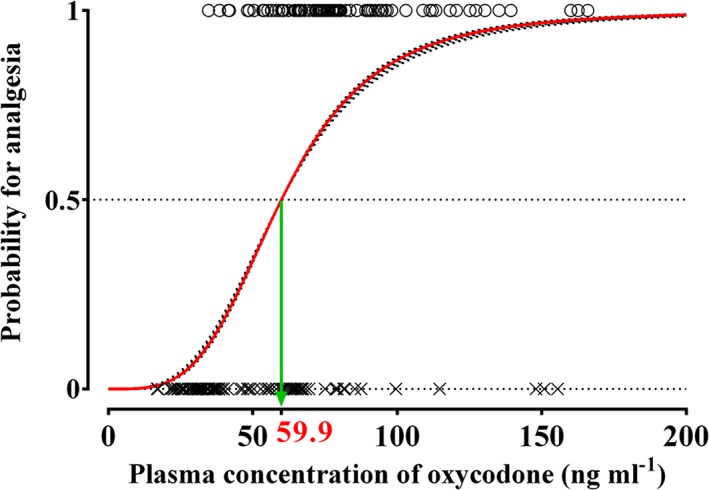
Predicted probability for analgesia plotted against plasma concentrations of oxycodone in the analgesic potency study. X: plasma concentration of oxycodone at MEC (minimum effective concentration); O: plasma concentration of oxycodone at MEAC (minimum effective analgesic concentration). Red solid line indicates population prediction, and black dotted lines indicate individual prediction. The estimate of measured plasma oxycodone concentration associated with a 50% probability of analgesia (C p50) was 59.9 ng ml−1
Simulation
The predicted oxycodone concentration in the plasma over time after an intravenous bolus and on continuous infusion using IV PCA are shown in Figure 5. This simulation showed that when the oxycodone loading dose was the dose approved by the MFDS (2 mg), it generated plasma oxycodone concentrations over time after surgery that were less than the MEC (Figure 4A). In contrast, when a 0.1 mg kg−1 bolus of oxycodone (6.5 mg for a 65 kg person) was administered as the loading dose, it generated concentrations that were higher than the MEC for 30 min after the end of surgery (Figure 4B). In another simulation, an intravenous oxycodone loading dose of 0.1 mg kg−1 was administered at the end of surgery, and intravenous PCA with or without 1 mg h−1 background infusion was started 5 min later. During the immediate postoperative period, the MEAC was most rapidly attained when both the higher loading dose (0.1 mg ml−1) and background infusion were used (Figure 4C and 4D).
Figure 5.
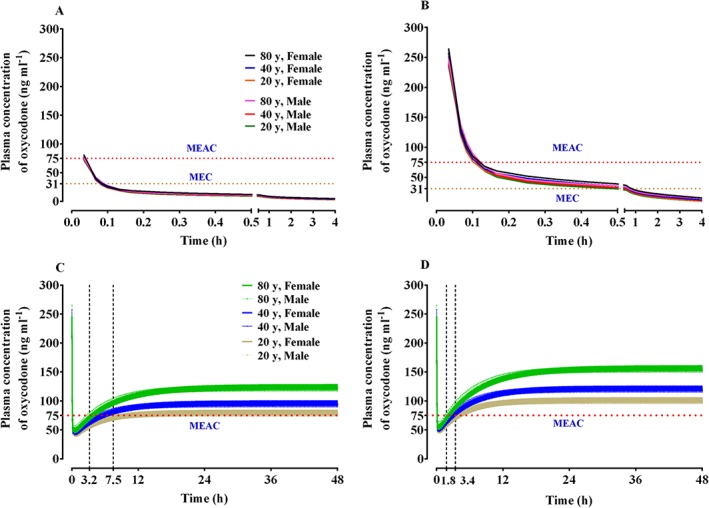
Predicted concentration of oxycodone in the plasma over time after an intravenous bolus of 2 mg (A) and 0.1 mg kg−1 (B) and the predicted concentration of oxycodone in the plasma over time after an intravenous bolus of 0.1 mg kg−1, followed by demand boluses of 1 mg every 15 min without background infusion (C) and with background infusion of 1 mg h−1 (D). The body weights and heights of all individuals were 65 kg and 165 cm, respectively. The demand bolus, background infusion rate, and lock‐out time of postoperative intravenous patient‐controlled analgesia (IV PCA) were set at 1 mg, 1 mg h−1, and 15 min, respectively. MEAC: minimum effective analgesic concentration, MEC: minimum effective concentration
Safety analysis
In the pharmacokinetic study, there were six adverse events in five patients during the study period. All were mild (n = 4) or moderate (n = 2) and were not caused by oxycodone. All of the adverse events resolved completely without sequelae. In one patient, the adverse event was generalized oedema that occurred after intravenous administration of 10 mg metoclopramide; it resolved after intravenous administration of 4 mg chlorpheniramine maleate. In two other patients, the adverse events were transient hyperthermia (38.5°C) and hypertension (175/93 mm Hg), which occurred on postoperative days 1 and 2, respectively; both events resolved spontaneously. In the fourth patient, the adverse event was nausea and vomiting, which occurred after administration of intravenous prophylactic antibiotics; the event resolved spontaneously after the patient emerged from anaesthesia. In the fifth patient, the adverse event was an increase in plasma creatinine level from 1.23 to 4.19 mg dl−1; this event arose after a magnetic resonance imaging scan.
In the analgesic‐potency study, the median (25–75%) systolic blood pressure, heart rate, respiration rate and MOAA/S in the PACU were 141 (130–156) mm Hg, 80 (71–89) beats/min, 16 (13–19) breaths min−1, and 5 (5–5), respectively. Three adverse events in three patients were reported during the study period. All were mild and resolved completely without sequelae. One event was definitely caused by oxycodone, the second was possibly caused by oxycodone, and the third was not caused by oxycodone. In one patient, the adverse event was chest tightness, which occurred 50 min after the last administration of intravenous oxycodone; the symptom resolved after intravenous administration of naloxone 60 μg. In the second patient, the adverse event was transient hypotension (68/55 mm Hg) in the PACU; it resolved after fluid administration and a change in position from supine to the Trendelenburg position. The third adverse event was urticaria on the face and trunk, which occurred after administration of intravenous prophylactic antibiotics; this event resolved spontaneously.
Discussion
In the present study, the pharmacokinetics of oxycodone were best described using a three‐compartment mammillary model. This finding differed from the population pharmacokinetic study of Saari et al., who found that a two‐compartment model described well the plasma concentrations of oxycodone 6. This disparity could be explained by differences in the initial sampling time. In the study by Saari et al., the data were pooled from four studies 6. In nearly half (47%) of the individuals in their study, the first blood sample was drawn only 15 min after the administration of intravenous oxycodone 0.1 mg kg−1, which resulted in relatively low maximal plasma concentrations of oxycodone (approximately 100 ng ml−1) 6. In contrast, in our study, blood was obtained 2.5, 5, 10, and 15 min after the intravenous administration of a 0.1 mg kg−1 bolus of oxycodone. If the first blood sample is not obtained rapidly, it is not possible to identify the rapid distribution phase, during which there is an initial striking decline in oxycodone concentration. This fact explains why a two‐compartment model described well the concentration–time data of Saari et al.
There was also another drawback in the oxycodone pharmacokinetic study of Saari et al. 6. They calculated LBM using the James equation 21. The James equation can yield incorrect results: when LBM calculated using the James equation is plotted against body weight, an inverted parabolic function is created. In other words, when LBM is measured using the James equation, the values start to decrease as the actual body weight increases beyond a certain body weight 22, 23. Thus, the LBM of obese patients will be underestimated. Janmahasatian et al. developed new equations that yielded adjusted fat‐free mass (which is almost equivalent to LBM) for a broad range of body weights (41–216 kg) and BMIs (17–70 kg/m2) 24. In the present study, LBM, determined using the Janmahasatian formula, was used to build the covariate models. We showed that as LBM increased, the volume of distribution increased. This finding was consistent with the observations of Saari et al. 6, who found that LBM was a significant covariate in the central volume of distribution. We also showed that the elimination of oxycodone decreased with advancing age. A previous oxycodone pharmacokinetic study with non‐compartmental methods also observed that there was an age‐dependent decrease in the metabolic clearance of oxycodone 5.
The present study showed that the oxycodone MEAC was reached more rapidly when a higher loading dose was used together with IV PCA with background infusion 25. However, even with this dosage regimen, rescue analgesics might be required to relieve pain for at least 2 h after the end of surgery. The time to 90% steady state concentration was shorter with the higher loading dose of oxycodone (0.1 mg kg−1, 6.5 mg) compared to when the loading dose was 2 mg; it was not affected by background infusion of 1 ml/h. The IV PCA regimen with background infusion achieved a higher steady state concentration regardless of the loading dose. Because LBM and age were significant covariates for the metabolic clearance of oxycodone (see Table 2), steady state concentrations tended to rise as age increased and LBM decreased. The steady state concentration for the dosage regimens in this simulation was approximately two to three times higher than the MEAC of oxycodone.
The second MEC values of oxycodone were nearly two‐fold higher than the first MEC values (31.5 vs. 63.4 ng ml−1), the first and second MEAC values were similar (74.1 vs. 76.1 ng ml−1), and the MEAC value was higher than the corresponding MEC value in each of the patients. These findings were in accordance with the findings of a previous study 9. These patterns could reflect the different levels of alertness at the first and second MECs and MEACs. The samples used to obtain the first and second MEC values were obtained 7.7 (4.9–12.7) and 64.8 (53.4–90.7) min after arriving in the PACU, respectively, while the samples used to determine the first and second MEAC values were obtained 54.5 ± 21.8 and 88.0 ± 28.0 min after arriving in the PACU, respectively. In general, the patients were more alert at the time that they were discharged from the PACU than at the time of arrival. Thus, patients might feel pain more severely at the point of the second MEC than at the point of the first MEC, which would explain why the second MEC was higher than the first MEC. In contrast, the patients were likely to be fully awake at both the first and second MEACs, which explains why the first and second MEACs were similar. Notably, our MEC and MEAC values were higher than those reported previously for patients after cardiac surgery (6–12 and 15–25 ng ml−1, respectively) 10 and for patients after laparoscopic cholecystectomy (11–57 and 14–91 ng ml−1, respectively) 9. Both the type of surgery and perioperative care likely were largely responsible for these differences. The MEC and MEAC for the cardiac patients might have been lower than our values because these patients were infused with fentanyl at a rate of 0.1 μg kg−1 min−1 for approximately 275–285 min; the fentanyl infusion was only discontinued at the end of the operation. Moreover, for sedation, propofol infusion of 4 mg kg−1 h−1 was started after the patients arrived in the intensive care unit (ICU). In addition, the patients received 1 g paracetamol during the first 2 h in the ICU, followed by the same dose at 8‐h intervals. Furthermore, pain intensity was only assessed after extubation. The long infusion of fentanyl in particular could have lowered the MEC and MEAC of oxycodone because fentanyl has a prolonged context‐sensitive half‐life. In contrast, the MEC and MEAC for the patients who underwent laparoscopic cholecystectomy were lower than our MEC and MEAC values likely because laparoscopic cholecystectomy results in less pain than open abdominal surgery 26.
There was a recent study to evaluate the MEC and MEAC of oxycodone in Finnish patients undergoing laparoscopic cholecystectomy 27. The median MEC and MEAC values in patients receiving an intravenous 10 mg dose of dexketoprofen 15 min before the end of surgery were higher than those of our study. This discrepancy might be due to the enrolment of different ethnic groups. Stamer et al. reported that the CYP2D6 genotype had an impact on oxycodone metabolism in postoperative patients 28. There are pronounced interethnic differences in the CYP2D6 allele distribution. Distributions of CYP2D6 phenotype classes predicted from genotypes between European subjects and East Asian subjects showed differences 3. The frequency of occurrence of the poor metabolizer phenotype was higher in Europeans 3.
Because pain is a complex sensation, the nociceptive stimulus and pain assessment scale should be standardized in analgesic studies 9, 29. In this study, a VAS was used to assess pain at rest and with wound compression. The noxious stimulus was compression of the wound site with a standard 20 N force over a 10 cm2 area. A previous study showed that wound compression with a standard pressure was a more feasible method for evaluating postoperative pain in the PACU than all other methods except for asking the patient to roll over in the bed 30.
There are several issues to be considered as limitations of this study. First, this population pharmacokinetic study was conducted in surgical patients experiencing pain. To exclude various factors affecting blood concentrations of opioids, including surgical stress, anaesthetics, fluid volume and blood loss, it would be appropriate to conduct pharmacokinetic studies of opioids in healthy volunteers. However, intravenous bolus administration of opioid can cause muscle rigidity. It is well known that the incidence of opioid‐induced rigidity is related to the dose and the rate of administration 31. In a clinical situation, a 0.1 mg kg−1 intravenous bolus of oxycodone is not used to relieve postoperative pain because it is likely to induce respiratory depression and muscle rigidity. In general, surgical patients receive a 2–5 mg intravenous bolus of oxycodone at a time. When muscle rigidity occurs, the volunteer could be awake and unable to move or breathe spontaneously, which might be an unethical practice. Patients who receive muscle relaxants do not experience muscle rigidity under general anaesthesia. Additionally, as mentioned above, intravenous oxycodone was approved for market by the Ministry of Food and Drug Safety of the Republic of Korea for moderate to severe pain, including postoperative intravenous patient‐controlled analgesia. The results from these studies could be used to determine a suitable dosing strategy for postoperative pain management using patient‐controlled analgesia with oxycodone. Hence, it would be appropriate to perform pharmacokinetic studies in surgical patients who require IV PCA. In several studies, the population pharmacokinetics of opioids have been evaluated in surgical patients 6, 17, 32, 33.
Second, physiological response to surgical insult and interactions related to concomitant medications were not considered in this study of the population pharmacokinetics of oxycodone. Surgical stress induces a series of hormonal and metabolic changes 34. However, it is very difficult to quantify the physiological response to surgical insult as a single surrogate measurement explaining inter‐individual variability of pharmacokinetic parameters. Additionally, concomitant medication including propofol, remifentanil and muscle relaxants can directly and indirectly influence the pharmacokinetics of oxycodone. In fact, pharmacokinetic differences between patients and healthy volunteers were observed with propofol 35. Because propofol is formulated in a lipid vehicle, propofol infused during surgery can influence the distribution of oxycodone. In an experimental study, the elimination clearance and rapid and slow distribution clearance of alfentanil were decreased in the presence of propofol 36. To the best of our knowledge, there have been no studies of pharmacokinetic interactions between oxycodone and propofol in humans. Although cardiovascular effects induced by concomitant medication have been observed in experimental studies 37, 38, these effects might not affect the metabolism of oxycodone. Because the hepatic extraction ratio of oxycodone is not high 39, oxycodone clearance depends on hepatic enzyme capacity rather than hepatic blood flow, which might explain why blood pressure was not a significant covariate on clearance. Hepatic function, evaluated by laboratory tests and abdominal computed tomography, was normal in the patients enrolled in this study.
Third, the concentrations of oxycodone metabolites were not measured. Oxycodone is primarily metabolized via CYP3A4/3A5 and to a lesser extent via CYP2D6 3, 40. Drug interactions modulating CYP3A and CYP2D6 activities have major effects on oxycodone analgesic efficacy 7. However, little is known thus far about pharmacokinetic interactions between oxycodone and its metabolites. The concentrations of oxycodone metabolites were also not considered for the pharmacokinetics of oxycodone in previous studies 6, 8.
In conclusion, the time course of plasma oxycodone concentration was described well by the three‐compartment mammillary model. LBM and age were significant covariates for the volume of distribution and metabolic clearance, respectively, in the final pharmacokinetic model of oxycodone. The MEAC and analgesic potency of oxycodone in major intra‐abdominal open surgeries were approximately 75 and 60 ng ml−1, respectively.
Competing Interests
All of the authors completed the Unified Competing Interest form. No author has had any financial relationships over the previous 3 years with any organizations that might have an interest in this submitted work, and no author has any other relationships or has engaged in any activities that could appear to have influenced the submitted work. This study was sponsored by Mundipharma Pte Ltd., Seoul, Republic of Korea.
We are grateful to Ae‐Kyung Hwang, B.S ., and Hyun‐Jung Park (Clinical Research Center, Asan Medical Center, Seoul, Republic of Korea) for measuring the plasma concentrations of oxycodone.
Contributors
B.M.C. and G.J.N. designed the study; Y.H.L., S.M.A. and B.M.C. collected the data; S.H.L., E.K.L. and B.M.C. performed the data analysis and interpretation. All of the authors contributed to the writing of the manuscript, provided critical revisions, and approved the final version. G.J.N. was the principal investigator.
Choi, B. ‐M. , Lee, Y. ‐H. , An, S. ‐M. , Lee, S. ‐H. , Lee, E. ‐K. , and Noh, G. ‐J. (2017) Population pharmacokinetics and analgesic potency of oxycodone. Br J Clin Pharmacol, 83: 314–325. doi: 10.1111/bcp.13101.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Söderberg Löfdal KC, Andersson ML, Gustafsson LL. Cytochrome p450‐mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs 2013; 73: 533–543. [DOI] [PubMed] [Google Scholar]
- 4. Jung KW, Kang HW, Park CH, Choi BH, Bang JY, Lee SH, et al. Comparison of the analgesic effect of patient‐controlled oxycodone and fentanyl for pain management in patients undergoing colorectal surgery. Clin Exp Pharmacol Physiol 2016; 43: 745–752. [DOI] [PubMed] [Google Scholar]
- 5. Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Neuvonen M, Neuvonen PJ, et al. Elimination of intravenous oxycodone in the elderly: a pharmacokinetic study in postoperative orthopaedic patients of different age groups. Drugs Aging 2011; 28: 41–50. [DOI] [PubMed] [Google Scholar]
- 6. Saari TI, Ihmsen H, Neuvonen PJ, Olkkola KT, Schwilden H. Oxycodone clearance is markedly reduced with advancing age: a population pharmacokinetic study. Br J Anaesth 2012; 108: 491–498. [DOI] [PubMed] [Google Scholar]
- 7. Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, et al. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol 2010; 160: 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El‐Tahtawy A, Kokki H, Reidenberg BE. Population pharmacokinetics of oxycodone in children 6 months to 7 years old. J Clin Pharmacol 2006; 46: 433–442. [DOI] [PubMed] [Google Scholar]
- 9. Kokki M, Broms S, Eskelinen M, Rasanen I, Ojanpera I, Kokki H. Analgesic concentrations of oxycodone – a prospective clinical PK/PD study in patients with laparoscopic cholecystectomy. Basic Clin Pharmacol Toxicol 2012; 110: 469–475. [DOI] [PubMed] [Google Scholar]
- 10. Pesonen A, Suojaranta‐Ylinen R, Hammarén E, Tarkkila P, Seppälä T, Rosenberg PH. Comparison of effects and plasma concentrations of opioids between elderly and middle‐aged patients after cardiac surgery. Acta Anaesthesiol Scand 2009; 53: 101–108. [DOI] [PubMed] [Google Scholar]
- 11. Struys MM, De Smet T, Depoorter B, Versichelen LF, Mortier EP, Dumortier FJ, et al. Comparison of plasma compartment versus two methods for effect compartment‐controlled target‐controlled infusion for propofol. Anesthesiology 2000; 92: 399–406. [DOI] [PubMed] [Google Scholar]
- 12. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 1997; 86: 10–23. [DOI] [PubMed] [Google Scholar]
- 13. Beal S, Sheiner L. NONMEM User's Guides. Part V. Introductory Guide. San Francisco: NONMEM Project Group, University of California, 1994.
- 14. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med 1987; 317: 1098. [DOI] [PubMed] [Google Scholar]
- 15. Robinson JD, Lupkiewicz SM, Palenik L, Lopez LM, Ariet M. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm 1983; 40: 1016–1019. [PubMed] [Google Scholar]
- 16. Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol 1981; 11: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin SJ, Jung JY, Noh MH, Lee SH, Lee EK, Choi BM, et al. The population pharmacokinetics of fentanyl in patients undergoing living‐donor liver transplantation. Clin Pharmacol Ther 2011; 90: 423–431. [DOI] [PubMed] [Google Scholar]
- 18. Kern SE, Xie G, White JL, Egan TD. A response surface analysis of propofol‐remifentanil pharmacodynamic interaction in volunteers. Anesthesiology 2004; 100: 1373–1381. [DOI] [PubMed] [Google Scholar]
- 19. Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther 2007; 82: 17–20. [DOI] [PubMed] [Google Scholar]
- 20. Wahlby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 2001; 28: 231–252. [DOI] [PubMed] [Google Scholar]
- 21. James WPT, Waterlow JC. UK Department of Health and Social Security/Medical Research Council Group on Obesity Research. Research on obesity: a report of the DHSS/MRC Group. London: Her Majesty's Stationery Office, 1976.
- 22. La Colla L, Albertin A, La Colla G, Porta A, Aldegheri G, Di Candia D, et al. Predictive performance of the ‘Minto’ remifentanil pharmacokinetic parameter set in morbidly obese patients ensuing from a new method for calculating lean body mass. Clin Pharmacokinet 2010; 49: 131–139. [DOI] [PubMed] [Google Scholar]
- 23. Tahari AK, Chien D, Azadi JR, Wahl RL. Optimum lean body formulation for correction of standardized uptake value in PET imaging. J Nucl Med 2014; 55: 1481–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet 2005; 44: 1051–1065. [DOI] [PubMed] [Google Scholar]
- 25. Choi BM. A new therapeutic option for postoperative pain management with oxycodone HCI injection. Korean J Anesthesiol 2016; 69: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg 2000; 87: 273–284. [DOI] [PubMed] [Google Scholar]
- 27. Piirainen A, Kokki H, Immonen S, Eskelinen M, Häkkinen MR, Hautajärvi H, et al. A dose‐finding study of dexketoprofen in patients undergoing laparoscopic cholecystectomy: a randomized clinical trial on effects on the analgesic concentration of oxycodone. Drugs R D 2015; 15: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stamer UM, Zhang L, Book M, Lehmann LE, Stuber F, Musshoff F. CYP2D6 genotype dependent oxycodone metabolism in postoperative patients. PLoS One 2013; 8: e60239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myles PS, Power I. Clinical update: postoperative analgesia. Lancet 2007; 369: 810–812. [DOI] [PubMed] [Google Scholar]
- 30. Tiippana E, Bachmann M, Kalso E, Pere P. Effect of paracetamol and coxib with or without dexamethasone after laparoscopic cholecystectomy. Acta Anaesthesiol Scand 2008; 52: 673–680. [DOI] [PubMed] [Google Scholar]
- 31. Bennett JA, Abrams JT, Van Riper DF, Horrow JC. Difficult or impossible ventilation after sufentanil‐induced anesthesia is caused primarily by vocal cord closure. Anesthesiology 1997; 87: 1070–1074. [DOI] [PubMed] [Google Scholar]
- 32. Franken LG, Masman AD, de Winter BC, Koch BC, Baar FP, Tibboel D, et al. Pharmacokinetics of morphine, morphine‐3‐glucuronide and morphine‐6‐glucuronide in terminally ill adult patients. Clin Pharmacokinet 2016; 55: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hudson RJ, Thomson IR, Burgess PM, Rosenbloom M. Alfentanil pharmacokinetics in patients undergoing abdominal aortic surgery. Can J Anaesth 1991; 38: 61–67. [DOI] [PubMed] [Google Scholar]
- 34. Kehlet H. The modifying effect of anesthetic technique on the metabolic and endocrine responses to anesthesia and surgery. Acta Anaesthesiol Belg 1988; 39: 143–146. [PubMed] [Google Scholar]
- 35. Eleveld DJ, Proost JH, Cortinez LI, Absalom AR, Struys MM. A general purpose pharmacokinetic model for propofol. Anesth Analg 2014; 118: 1221–1237. [DOI] [PubMed] [Google Scholar]
- 36. Vuyk J. Clinical interpretation of pharmacokinetic and pharmacodynamic propofol‐opioid interactions. Acta Anaesthesiol Belg 2001; 52: 445–451. [PubMed] [Google Scholar]
- 37. Runciman WB, Mather LE, Selby DG. Cardiovascular effects of propofol and of thiopentone anaesthesia in the sheep. Br J Anaesth 1990; 65: 353–359. [DOI] [PubMed] [Google Scholar]
- 38. Akine A, Suzuka H, Hayashida Y, Kato Y. Effects of ketamine and propofol on autonomic cardiovascular function in chronically instrumented rats. Auton Neurosci 2001; 87: 201–208. [DOI] [PubMed] [Google Scholar]
- 39. Tallgren M, Olkkola KT, Seppala T, Hockerstedt K, Lindgren L. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther 1997; 61: 655–661. [DOI] [PubMed] [Google Scholar]
- 40. Lalovic B, Kharasch E, Hoffer C, Risler L, Liu‐Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 2006; 79: 461–479. [DOI] [PubMed] [Google Scholar]


