Abstract
In this study we examined the suitability of the 3H-imidazo[4,5-b]pyridine ring system in developing novel anticancer and anti-inflammatory agents incorporating a diaryl pharmacophore. Eight 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives retrieved from our in-house database were evaluated for their cytotoxic activity against nine cancer cell lines. The results indicated that the compounds showed moderate cytotoxic activity against MCF-7, MDA-MB-468, K562 and SaOS2 cells, with K562 being the most sensitive among the four cancer cell lines. The eight 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives were also evaluated for their COX-1 and COX-2 inhibitory activity in vitro. The results showed that compound 3f exhibited 2-fold selectivity with IC50 values of 9.2 and 21.8 µmol/L against COX-2 and COX-1, respectively. Molecular docking studies on the most active compound 3f revealed a binding mode similar to that of celecoxib in the active site of the COX-2 enzyme.
KEY WORDS: 3H-Imidazo[4,5-b]pyri-dine; Cytotoxicity; MTT assay; COX inhibitors; Docking studies
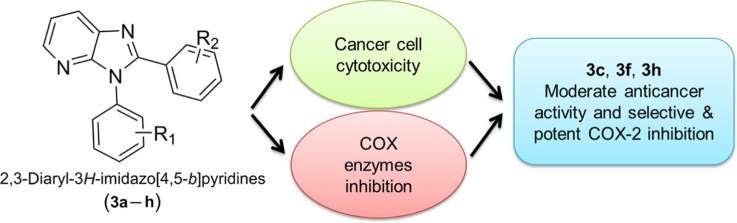
Graphical abstract
This research investigates the anticancer and anti-inflammatory activities of eight novel 2,3-diaryl-substituted 3H-imidazo[4,5-b]pyridine derivatives. The compounds showed moderate cytotoxicity and potent selective COX-2 inhibition.
1. Introduction
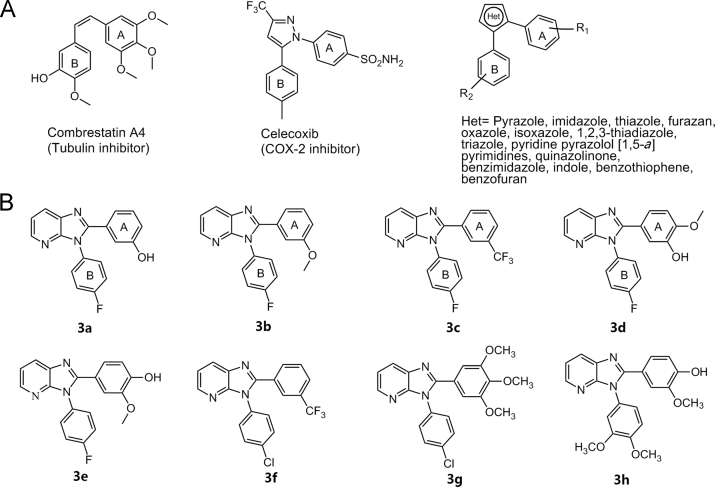
Heterocycles with a vicinal diaryl substitution are an important pharmacophore in medicinal chemistry and are key building blocks for a variety of compounds showing anticancer and anti-inflammatory activities1, 2. The cytotoxic properties of these compounds are mainly attributed to tubulin inhibition whereas their anti-inflammatory activity is predominantly elicited through the inhibition of cyclooxygenase (COX) enzymes (Fig. 1A)1, 3. Both activities require the diaryl pharmacophore with a cis-olefin configuration4. Hence, several aromatic heterocyclic rings including five-membered (pyrazole5, Imidazole6, thiazole5, furazan (1,2,5-oxadiazole)7, oxazole6, isoxazole8, 1,2,3-thiadiazole9, triazole10), six-membered (pyridine) and fused bicyclic rings, like pyrazolo[1,5-a]pyrimidines11, quinazolinone, benzimidazole, indole12, benzothiophene7 and benzofuran7, have been investigated as a heterocyclic core in stabilizing the cis-olefin orientation of the two aryl rings (Fig. 1A). 3H-imidazo[4,5-b]pyridine represents an interesting class of heterocyclic core for the design of diaryl derivatives with anticancer and anti-inflammatory activities. Motivated by the above observations, the present research strives to examine the utility of 3H-imidazo[4,5-b]pyridine as a heterocyclic bridge for the design of novel 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives with anticancer and anti-inflammatory activities. Eight compounds with either OH, OCH3 or both, or CF3 in aryl ring A and halo (F and Cl) or OCH3 substitution in aryl ring B, were retrieved from our in-house database (Fig. 1B) and tested for anticancer activity against a panel of nine cancer cell lines (breast, prostate, colon, blood and bone cancer) and two non-cancerous cell lines. The eight compounds were also evaluated for their inhibitory on COX-1 and COX-2 enzymes. To the best our knowledge, the cytotoxic and anti-inflammatory potential of this class of compounds have not been reported in literature.
Figure 1.
(A) Structures of some biologically active molecules with diaryl pharmacophore and different heterocyclic scaffolds employed for stabilizing the ‘cis’ diaryl configuration in literature; (B) Structures of molecules 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives evaluated in this study.
2. Results and discussion
2.1. Chemistry
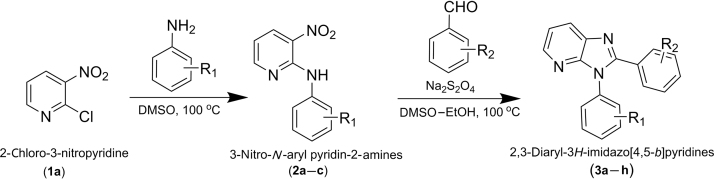
Diaryl substituted 3H-imidazo[4,5-b]pyridine derivatives were previously synthesized in our laboratory using a one pot synthetic protocol from the corresponding N-aryl nitropyridines as shown in Scheme 112. The requisite 3-nitro-N-aryl pyridin-2-amines (2a–c) were prepared in situ by heating a mixture of 2-chloro-3-nitropyridine (1a) and substituted anilines in DMSO at 100 oC. Subsequent treatment of the DMSO solution of the resulting 3-nitro-N-phenylpyridin-2-amine with aromatic aldehydes and sodium dithionite at 80 °C afforded the titled compounds in moderate to good yields. The structures assigned to the compounds were supported by the data obtained from IR, 1H NMR and mass spectral studies.
Scheme 1.
Synthesis of 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives (3a–3h).
2.2. Cytotoxicity studies
The cytotoxic activity of eight diaryl substituted 3H-imidazo[4,5-b]pyridine derivatives was evaluated against nine cancer cell lines and two non-cancerous cell lines using the MTT assay. For many of the cell lines, the concentration of the compounds had to be over 100 μmol/L for any cell death to occur. For the prostate cancer cell line DU-145, lung cancer cell line A549, and breast cancer cell line MDAMB-231, none of the eight compounds showed any effect as in Table 1. Minimal cell death was seen in the pancreatic cancer cell line PANC-1, with compounds 3g and 3h having an IC50 of 83.54 and 57.69 μmol/L, respectively. Only compound 3h had an effect on the colon cancer cell line HCT-15 with an IC50 of 66.92 μmol/L. The cancer cell lines that had the most cell growth inhibition were MCF-7, MDA-MB-468, K562 and SaOS2. The compounds showed maximal growth inhibitory potency against K562 with IC50 values ranging from 42 to 57 μmol/L. In the case of MCF-7 and SaOS2, growth inhibition was observed at concentration ranging from 44 to 72 μmol/L and 52.5 to 71.5 μmol/L, respectively. Three compounds 3b, 3e and 3g showed modest inhibition of MDA-MB-468 cell growth with IC50 values of 66.46, 78.82 and 69.34 μmol/L, respectively. The mouse fibroblast cell line KB-31 also had effects with IC50 values of 3b of 74.96 μmol/L, 3e of 95.58 μmol/L, 3g of 64.36 μmol/L and 3h of 70.91 μmol/L. The normal cell line used was HEK 293 and only one of the compounds in the series, 3h, caused cell death with an IC50 of 80 μmol/L.
Table 1.
Cytotoxic activity of 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives against various cell lines (cancerous and non-cancerous).
| Compd. | IC50 (µmol/L) |
|||||
|---|---|---|---|---|---|---|
| Breast |
Prostate | Pancreas | ||||
| MCF-7 | MDAMB-231 | MDAMB-468 | DU-145 | PANC-1 | ||
| 3a | 61.5 | >100 | >100 | >100 | >100 | |
| 3b | 69 | >100 | 66.46 | >100 | >100 | |
| 3c | 44 | >100 | >100 | >100 | >100 | |
| 3d | 72 | >100 | >100 | >100 | >100 | |
| 3e | 60 | >100 | 78.82 | >100 | >100 | |
| 3f | 49.9 | >100 | >100 | >100 | >100 | |
| 3g | 56 | >100 | 69.34 | >100 | 83.54 | |
| 3h | 53 | >100 | >100 | >100 | 57.69 | |
| Compd. | IC50 (µmol/L ) |
|||||
| Blood | Bone | Colon | Lung | Normal | Mouse fibroblast | |
| K562 | SaoS2 | HCT15 | A549 | HEK293 | KB-31 | |
| 3a | 49 | 64 | >100 | >100 | >100 | >100 |
| 3b | 46 | 67 | >100 | >100 | >100 | 74.96 |
| 3c | 42 | 52.5 | >100 | >100 | >100 | >100 |
| 3d | 57 | 71.5 | >100 | >100 | >100 | >100 |
| 3e | 53 | 62.5 | >100 | >100 | >100 | 95.58 |
| 3f | 43 | 53.5 | >100 | >100 | >100 | >100 |
| 3g | 47 | 55 | >100 | >100 | >100 | 64.36 |
| 3h | 54.5 | 59 | 66.92 | >100 | 80 | 70.91 |
2.3. COX inhibition studies
In addition to cytotoxic activity, the eight compounds were also evaluated for COX-1 and COX-2 inhibitory activity as diaryl pharmacophore is also an integral component of several COX inhibitors. The COX inhibition as given by IC50 values in Table 2 clearly demonstrate the effectiveness of all 3H-imidazo[4,5-b]pyridine derivatives against both COX-1 and COX-2 enzymes. The compounds inhibited COX-1 with IC50 values ranging from 10 to 43 μmol/L, whereas COX-2 inhibition was observed in concentration ranging from 9.2 to 279.3 μmol/L. Most notably, structural variations in both aryl rings appeared to affect both the potency and selectivity of these analogues against these enzymes. This is a desirable trait especially for the development of clinically useful anti-inflammatory agents targeting the COX-2 enzyme. Compound 3f with a 4-chloro substitution in the N-phenyl ring and a 3-CF3 substitution in the 2-phenyl ring was found to be the most active (COX-2, IC50 = 9.2 μmol/L) in the series. Compounds 3c that had 4-fluoro and 3-CF3 substitutions on the N-phenyl and 2-phenyl rings, respectively and compound 3h that possessed 3,4-dimethoxyl and 3-OCH3, 4-OH substituents in the same positions exhibited the modest COX-2 inhibitory activity (IC50 ≈ 15 μmol/L).
Table 2.
COX inhibition data of 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives.
| Compd. | IC50 (µmol/L) |
Selectivity ratio | |
|---|---|---|---|
| COX-1 | COX-2 | COX-2/COX-1 | |
| 3a | 10.0±0.01 | 35.9±2.0 | 3.59 |
| 3b | 15.0±0.13 | NA | NA |
| 3c | 36.0±1.4 | 15.1±0.9 | 0.42 |
| 3d | 42.7±2.5 | 25.6±1.0 | 0.60 |
| 3e | 16.8±0.1 | 279.3±7.2 | 16.58 |
| 3f | 21.8±1.1 | 9.2±0.02 | 0.42 |
| 3g | 19.1±0.3 | 22.4±2.5 | 1.17 |
| 3h | 18.5±0.00 | 15.4±3.1 | 0.83 |
| Indomethacin | 0.0067±0.00 | 0.048±0.00 | 7.16 |
| Celecoxib | 15±0.6 | 0.042±0.00 | 0.0028 |
NA: not achieved at the tested concentrations.
2.4. Docking studies
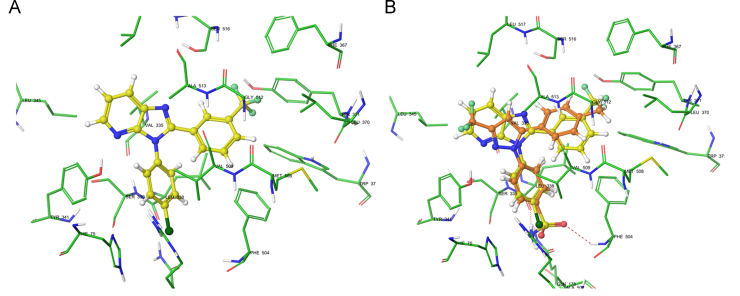
Molecular docking studies were proposed for compound 3f in order to rationalize its potency and selectivity against COX-2. Prior to docking studies with compound 3f, the glide docking protocol was validated by evaluating the ability to reproduce the binding conformation of the co-crystallized ligand celecoxib. The docking protocol followed was found reliable as the root mean square deviation (RMSD) between the predicted conformation and the observed X-ray crystallographic conformation of the celecoxib (PDB ID: 3LN1) was found to be 0.33 Å. The binding mode predicted for 3f in the docking studies is shown in Fig. 2A. The binding mode observed for 3f is very similar to the one observed for the co-crystallized celecoxib (Fig. 2B). The docked structures of 3f and celecoxib were oriented so that the N-phenyl ring of 3f overlapped with that of 4-sulfamoylphenyl ring in celecoxib in the selectivity pocket of COX-2 and the 3H-imidazo[4,5-b]pyridine ring overlapped with the trifluoromethyl substituted pyrazolyl ring in celecoxib. Furthermore, the chlorine atom at the p-position of the N-phenyl ring is favorably placed in the selectivity pocket facilitating electrostatic interactions with Arg499 and Phe504. However, the 2-phenyl ring in 3f is more planar in comparison to the second phenyl ring in celecoxib, resulting in a partial overlap of both these rings. This deviation in the orientation of the 2-phenyl ring might serve to accommodate the 3-trifluoromethyl substitution in the hydrophobic pocket formed by Phe367, Leu370, Trp373 and Met508. Nevertheless, the binding parallels observed between celecoxib and 3f may rationalize the inhibitory potency shown by the compound against COX-2 enzyme.
Figure 2.
(A) XP-Glide predicted binding mode of 3f in the active site of COX-2 (PDB ID: 3LN1); (B) Overlay of docked conformation of 3f on docked conformation of celecoxib (yellow) in the active site of COX-2. Important amino acids are depicted as sticks with the atoms colored as carbon (green), hydrogen (white), nitrogen (blue), oxygen (red), sulfur (yellow), whereas the ligands are shown with the same color scheme as above except for carbon atoms are represented in yellow for 3f and orange for celecoxib. Fluorine atoms in 3f are represented in light green and chlorine atom is represented in dark green. Red dotted lines represent hydrogen bonding.
3. Conclusions
The present study is an attempt to examine the suitability of 3H-imidazo[4,5-b]pyridine as a heterocyclic core for the development of anticancer and anti-inflammatory molecules incorporating a diaryl pharmacophore. Eight 1,2-diaryl-3H-imidazo[4,5-b]pyridines retrieved from our in-house database were evaluated for their cytotoxic activity against nine cancer cell lines and COX (1 and 2) inhibitory activity in vitro. The compounds showed moderate cytotoxicity against MCF-7, MDA-MB-468, K562, and SaOS2 cells with K562 being the most sensitive among the four cancer cell lines. The compounds also exerted inhibitory activity against both the COX-1 and COX-2 enzymes. Most interesting was compounds 3c, 3f and 3h, which showed potent (IC50 < 20 μmol/L) and selective COX-2 inhibition. The docking study of the most active analogue 3f into the COX-2 active site supported the in vitro potency shown by the compound and also revealed the nature of the interactions between the molecule and amino acid residues in the active site of COX-2. Taken together, these promising findings established 1,2-diaryl-3H-imidazo[4,5-b]pyridines as a promising scaffold in developing anticancer and anti-inflammatory agents.
4. Experimental
4.1. Chemistry
All chemicals and solvents used in this study were purchased locally from commercial suppliers and were used without further purification. Melting points were determined on a Veego Digital Melting Point apparatus in open capillaries and have not been corrected. The 1H NMR spectra were recorded on in DMSO-d6 on a Bruker Avance II 400 MHz spectrometer. Chemical shifts were recorded in parts per million (ppm) and were reported relative to tetramethylsilane (TMS). The infrared (IR) spectra were recorded on a Schimadzu IR Prestige-21 FTIR spectrophotometer in KBr. Mass spectra were recorded on an Applied Biosystem QTRAP 3200 MS/MS system in ESI mode.
4.1.1. Synthesis of 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives (3a–3h)
The synthesis of 2,3-diaryl-3H-imidazo[4,5-b]pyridine derivatives (3a–3h) was performed according to a reported literature procedure13.
3-(3-(4-Fluorophenyl)-3H-imidazo[4,5-b]pyridin-2-yl)phenol (3a): Yield 44%; m.p. 235–236 oC; FT-IR (KBr) υ (cm−1): 3184 (O-H), 3059 (aromatic C-H), 1589 (C=N), 1388 (C-N), 1224 (C-F), 1159 (C-O); 1H NMR (400 MHz, DMSO-d6) δ 9.35 (s, 1H, ArOH), 8.26 (d, 1H, J=4.72 Hz, ArH), 8.05 (d, 1H, J=8.0 Hz, ArH), 7.38–7.35 (m, 2H, ArH), 7.29–7.26 (m, 1H, ArH), 7.18 (t, 2H, J=8.52 Hz, ArH), 7.08 (t, 1H, J=7.92 Hz, ArH), 7.03 (s, 1H, ArH), 6.88 (d, 1H, J=7.72 Hz, ArH), 6.84 (d, 1H, J=4.12 Hz, ArH); MS-API: [M+H]+ 306 (Calcd. 305.10).
3-(4-Fluorophenyl)-2-(3-methoxyphenyl)-3H-imidazo[4,5-b]pyridine (3b): Yield 57%; m.p. 236–237 oC; FT-IR (KBr) υ (cm−1): 3053 (C-H aromatic), 2955 (methyl C-H), 1593 (C=N), 1381 (C-N), 1230 (C-F), 1251, 1033 (C-O-C); 1H NMR (400 MHz, DMSO-d6): δ 8.39 (d, 1H, J=4.8 Hz, ArH), 8.16 (d, 1H, J=8.04 Hz,ArH), 7.39–7.36 (m, 2H, ArH), 7.33–7.30 (m, 1H, ArH), 7.26–7.18 (m, 4H, ArH), 7.125–7.09 (m, 1H, ArH), 6.97–6.95 (m, 1H, ArH), 3.74 (s, 3H, -OCH3); MS-API: [M+H]+ 320 (Calcd. 319.11).
3-(4-Fluorophenyl)-2-(3-(trifluoromethyl)phenyl)-3H-imidazo[4,5-b]pyridine (3c): Yield 26%; m.p. 185–187 oC; FT-IR (KBr) υ (cm−1): 3068 (aromatic C-H), 1599 (C=N), 1383 (C-N), 1228 (C-F,CF3), 1185 (C-F); 1H NMR (400 MHz, DMSO-d6): δ 8.43 (d, 1H, J=4.76 Hz, ArH), 8.19 (d, 1H, J=9.2 Hz, ArH), 7.96 (s, 1H, ArH), 7.72–7.66 (m, 2H, ArH), 7.47 (t, 1H, J=7.92 Hz, ArH), 7.39–7.34 (m, 3H, ArH), 7.27–7.22 (m, 1H, ArH); MS-API: [M+H]+ 358 (Calcd. 357.1).
4-(3-(4-Fluorophenyl)-3H-imidazo[4,5-b]pyridin-2-yl)-2-methoxyphenol (3d): Yield 44%; m.p. 244–246 oC; FT-IR (KBr) υ (cm−1): 3063 (O-H), 2972 (C-H),1585 (C=N), 1386 (C-N), 1228 (C-F), 1249, 1024 (C-O-C); 1H NMR (400 MHz, DMSO-d6): δ 9.047 (s, 1H, ArOH), 8.233 (d, 1H, J=9.44 Hz, ArH), 8.0 (d, 1H, J=7.9 Hz, ArH), 7.36–7.33 (m, 2H, ArH), 7.24–7.16 (m, 3H, ArH), 7.09 (s, 1H, ArH), 6.9 (d, 1H, J=8.28 Hz, ArH), 6.73 (d, 1H, J=8.24 Hz, ArH), 3.66 (s, 3H, OCH3); MS-API: [M+H]+ 336 (Calcd. 335.11).
5-(3-(4-Fluorophenyl)-3H-imidazo[4,5-b]pyridin-2-yl)-2-methoxyphenol (3e): Yield 85%; m.p. 190–192 oC; FT-IR (KBr) υ (cm−1): 3117 (O-H), 2964 (methyl C-H), 1606 (C=N), 1363 (C-N), 1228 (C-F), 1253, 1026 (C-O-C); 1H NMR (400 MHz, DMSO-d6): δ 8.97 (s, 1H, ArOH), 8.28 (d, 1H, J=4.8 Hz, ArH), 8.06 (d, 1H, J=7.96 Hz, ArH), 7.43–7.40 (m, 2H, ArH), 7.31–7.24 (m, 3H, ArH), 7.16 (s, 1H, ArH), 7.091 (s, 1H, ArH), 6.95 (d, 1H, J=8.16 Hz, ArH), 6.82 (d, 1H, J=8.48 Hz, ArH), 3.86 (s, 3H, OCH3); MS-API: [M+H]+ 336 (Calcd. 335.11).
3-(4-Chlorophenyl)-2-(3-(trifluoromethyl)phenyl)-3H-imidazo[4,5-b]pyridine (3f): Yield 68%; m.p. 220–223 oC; FT-IR (KBr) υ (cm−1): 3045 (aromatic C-H), 1591 (C=N), 1383 (C-N), 1329 (C-F, CF3), 1095 (C-Cl); 1H NMR (400 MHz, DMSO-d6): δ 8.43 (d, 1H, J=4.8 Hz, ArH), 8.18 (d, 1H, J=8.0 Hz, ArH), 8.02 (s, 1H, ArH), 7.67 (t, 2H, J=8.12 Hz, ArH), 7.54–7.45 (m, 3H, ArH), 7.37–7.32 (m, 3H, ArH); MS-API: [M+H]+ 374 (Calcd. 373.06).
3-(4-Chlorophenyl)-2-(3,4,5-trimethoxyphenyl)-3H-imidazo[4,5-b]pyridine (3g): Yield 88%; m.p. 193–195 oC; FT-IR (KBr) υ (cm−1): 3024 (aromatic C-H), 2962 (methyl C-H), 1587 (C=N), 1373 (C-N), 1240, 1010 (C-O-C), 1089 (C-Cl); 1H NMR (400 MHz, DMSO-d6): δ 8.31 (d, 1H, J=4.88 Hz, ArH), 8.07 (d, 1H, J=8.0 Hz, ArH), 7.47–7.44 (m, 2H, ArH), 7.32–7.29 (m, 2H, ArH), 7.26–7.23 (m, 1H, ArH), 6.75 (s, 2H, ArH), 3.81 (s, 3H, OCH3), 3.63 (s, 6H, OCH3); MS-API: [M+H]+ 396 (Calcd. 395.1).
5-(3-(3,4-Dimethoxyphenyl)-3H-imidazo[4,5-b]pyridin-2-yl)-2-methoxyphenol (3h): Yield 52%; m.p. 193–195 oC; FT-IR (KBr) υ (cm−1): 3200 (O-H), 3066 (C-H aromatic), 2945 (methyl C-H), 1597 (C=N), 1384 (C-N), 1240, 1018 (C-O-C); 1H NMR (400 MHz, DMSO-d6): δ 8.35 (d, 1H, J=4.84Hz, ArH), 8.11 (d, 1H, J=8.0 Hz, ArH), 7.29–7.26 (m, 3H, ArH), 6.99 (s, 2H, ArH), 6.84 (s, 1H, ArH), 6.79 (d, 1H, J = 8.52 Hz, ArH) 3.95 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 3.8 (s, 3H, OCH3); MS-API: [M+H]+ 378 (Calcd. 377.14).
4.2. Pharmacology
4.2.1. Cytotoxicity studies
Lung cancer cell line A549, colon carcinoma cell line HCT-15, breast carcinoma cell lines MDA-MB-231 and MDA-MB-468, prostatic cancer cell line DU-145, pancreatic cancer cell line PANC-1, human embryonic kidney cells HEK293, and mouse fibroblast KB-31 were grown as adherent monolayers in flasks with Dulbecco׳s modified Eagle׳s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a humidified incubator containing 5% CO2 at 37 oC. The human cancerous cell lines MCF-7 (breast cancer), SaOS-2 (osteosarcoma), and K562 (myelogenous leukemia) were maintained in Eagle׳s minimal essential medium, McCoy׳s 5a medium and RPMI-1640, respectively, and supplemented with NaHCO3, sodium pyruvate, 10% fetal calf serum, and 1% penicillin and streptomycin. These cells were grown at 37 oC, 5% CO2 in a humidified air.
The MTT assay was used to determine the cytotoxicity of the compounds to the following cells: HCT-15, MCF-7, MDA-MB-231, MDA-MB-468, SaOS-2, K562, DU-145, PANC-1, HEK293, and KB-31. Briefly, the cells used in this study were harvested with trypsin and suspended at a final concentration of 5×103 cells in each well. Cells were seeded (180 µL/well) into 96-well multiplates. Different concentrations of each compound in the series (20 µL) were added. After 72 h of incubation, 20 µL of the MTT solution (4 mg/mL) was added to each well, and the plates were incubated for 4 h. This allowed viable cells to convert the yellow-colored MTT into dark-blue formazan crystals. Subsequently, the medium was discarded, and 100 µL of DMSO was added into each well to dissolve the formazan crystals. The absorbance was determined at 570 nm with a DTX 880 multimode detector. The mean ± SD concentrations were calculated from 2 to 3 experiments performed in triplicate. The IC50 values were calculated from the cell survival percentages.
4.2.2. Assays for COX-1 and COX-2 inhibition
COX-1 has been isolated from Ram seminal vesicles. Recombinant human COX-2 has been expressed in an insect cell expression system. These enzymes have been purified by employing conventional chromatographic techniques. Enzymatic activities of COX-1 and COX-2 were measured according to the method13 with slight modifications using a chromogenic assay based on the oxidation of N,N,N׳,N׳,-tetramethyl-p-phenylene diamine (TMPD) during the reduction of PGG2 to PGH214, 15. Briefly, the assay mixture containing Tris HCl buffer (100 mmol/L, pH 8.0), hematin (15 mmol/L), EDTA (3 mmol/L), enzyme (100 mg COX-1 or COX-2) and the test compound were pre-incubated at 25 °C for 1 min and then the reaction was initiated by the addition of arachidonic acid and TMPD, in total volume of 1 mL. The enzyme activity was determined by estimating the velocity of TMPD oxidation for the first 25 s of the reaction by following the increase in absorbance at 603 nm. A low rate of non-enzymatic oxidation observed in the absence of COX-1 and COX-2 was subtracted from the experimental value while calculating the percent inhibition.
4.3. Molecular docking studies
The docking analysis of most active molecule was performed using Maestro (version 9.2; Schrödinger, LLC: New York, NY, USA, 2012). The molecules were sketched in 3D format using build panel and LigPrep module was used to produce low-energy conformers. The structural coordinates of COX-216 was obtained from protein data bank. The protein was prepared by giving preliminary treatment like adding hydrogen atoms, adding missing residues, refining the loop with prime and finally minimized using the OPLS-2005 force field. Grid for molecular docking was generated with bound co-crystallized ligand (celecoxib). The molecules were docked using Glide (version 5.7; Schrödinger, LLC: New York, NY, 2012) in the extra-precision mode, with up to three poses saved. Ligands were kept flexible by producing the ring conformations and by penalizing non-polar amide bond conformations, whereas the receptor was kept rigid throughout the docking studies. All other parameters of the Glide module were maintained at their default values. The lowest energy conformation was selected for the prediction of ligand interactions with the active sites of COX-2.
Acknowledgments
The authors gratefully acknowledge the Sophisticated Analytical Instrumentation Facility, Indian Institute of Technology, Delhi, India for the NMR spectral analysis of the compounds used in this study. The authors Tarun Batra and Deepak Chowrasia are the recipients of GPAT scholarship offered by All India Council of Technical Education, New Delhi, India. We thank Ms. Charisse Montgomery, University of Toledo for editing this manuscript in the present form.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Piyush Trivedi, Email: piyush.trivedi@rgtu.net.
Amit K. Tiwari, Email: amit.tiwari@utoledo.edu.
References
- 1.Smith D.A. Metabolism, pharmacokinetics and toxicity of functional groups: impact of the building blocks of medicinal chemistry in ADMET. Royal Society of Chemistry; Cambridge: 2010. [Google Scholar]
- 2.Koyama Nobuhiro, Sato Hirofumi, Tomoda Hiroshi. Discovery of new hazimycin congeners from Kitasatospora sp. P07101. Acta Pharm Sin B. 2015;6:564–568. doi: 10.1016/j.apsb.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manivannan E., Chaturvedi S.C. Analogue-based design, synthesis and molecular docking analysis of 2,3-diaryl quinazolinones as non-ulcerogenic anti-inflammatory agents. Bioorg Med Chem. 2011;19:4520–4528. doi: 10.1016/j.bmc.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Romagnoli R., Baraldi P.G., Cruz-Lopez O., Lopez Cara C., Carrion M.D., Brancale A. Synthesis and antitumor activity of 1,5-disubstituted 1,2,4-triazoles as cis-restricted combretastatin analogues. J Med Chem. 2010;53:4248–4258. doi: 10.1021/jm100245q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohsumi K., Hatanaka T., Fujita K., Nakagawa R., Fukuda Y., Nihei Y. Syntheses and antitumor activity of cis-restricted combretastatins: 5-membered heterocyclic analogues. Bioorg Med Chem Lett. 1998;8:3153–3158. doi: 10.1016/s0960-894x(98)00579-4. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Woods K.W., Li Q., Barr K.J., McCroskey R.W., Hannick S.M. Potent, orally active heterocycle-based combretastatin A–4 analogues: synthesis, structure−activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J Med Chem. 2002;45:1697–1711. doi: 10.1021/jm010523x. [DOI] [PubMed] [Google Scholar]
- 7.Tron G.C., Pagliai F., Del Grosso E., Genazzani A.A., Sorba G. Synthesis and cytotoxic evaluation of combretafurazans. J Med Chem. 2005;48:3260–3268. doi: 10.1021/jm049096o. [DOI] [PubMed] [Google Scholar]
- 8.Kaffy J., Pontikis R., Carrez D., Croisy A., Monneret C., Florent J.C. Isoxazole-type derivatives related to combretastatin A–4, synthesis and biological evaluation. Bioorg Med Chem. 2006;14:4067–4077. doi: 10.1016/j.bmc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Wu M.J., Sun Q.M., Yang C.H., Chen D.D., Ding J., Chen Y. Synthesis and activity of combretastatin A–4 analogues: 1,2,3-thiadiazoles as potent antitumor agents. Bioorg Med Chem Lett. 2007;17:869–873. doi: 10.1016/j.bmcl.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 10.Odlo K., Hentzen J., dit Chabert J.F., Ducki S., Gani OABSM Sylte I. 1,5-Disubstituted 1,2,3-triazoles as cis-restricted analogues of combretastatin A–4: synthesis, molecular modeling and evaluation as cytotoxic agents and inhibitors of tubulin. Bioorg Med Chem. 2008;16:4829–4838. doi: 10.1016/j.bmc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 11.Almansa C., de Arriba A.F., Cavalcanti F.L., Gómez L.A., Miralles A., Merlos M. Synthesis and SAR of a new series of COX-2-selective inhibitors: pyrazolo[1,5-a]pyrimidines. J Med Chem. 2001;44:350–361. doi: 10.1021/jm0009383. [DOI] [PubMed] [Google Scholar]
- 12.Yang D.L., Fokas D., Li J.Z., Yu L.B., Baldino C.M. A versatile method for the synthesis of benzimidazoles from o-nitroanilines and aldehydes in one step via a reductive cyclization. Synthesis. 2005;2005:47–56. [Google Scholar]
- 13.Copeland R.A., Williams J.M., Giannaras J., Nurnberg S., Covington M., Pinto D. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc Natl Acad Sci U S A. 1994;91:11202–11206. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan R.W., Paxton J., Kuehl F.A., Jr. Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976;251:7329–7335. [PubMed] [Google Scholar]
- 15.Pagels W.R., Sachs R.J., Marnett L.J., Dewitt D.L., Day J.S., Smith W.L. Immunochemical evidence for the involvement of prostaglandin H synthase in hydroperoxide-dependent oxidations by ram seminal vesicle microsomes. J Biol Chem. 1983;258:6517–6523. [PubMed] [Google Scholar]
- 16.Wang J.L., Limburg D., Graneto M.J., Springer J., Hamper J.R.B., Liao S.B. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett. 2010;20:7159–7163. doi: 10.1016/j.bmcl.2010.07.054. [DOI] [PubMed] [Google Scholar]