Abstract
In order to develop agents with superior chemopreventive and chemotherapeutic properties against hepatocellular carcinomas, mitochondria-targeted hydroxycinnamic acids (MitoHCAs) were synthesized by conjugation with a triphenylphosphonium cation. These synthetic compounds were evaluated for their antioxidant activities in hepatic mitochondria, including against OH∙− and ROO∙− induced lipid peroxidation. H2O2 production was decreased significantly by increasing glutathione peroxidase and catalase activities. In addition, cell proliferation data from three cell lines (HepG2, L02 and WI38) indicated that the MitoHCAs were selective for cancer cells. Interestingly, the MitoHCAs both with or without Ca2+ triggered mitochondrial dysfunction by inducing mitochondrial swelling, collapsing the mitochondrial membrane potential and causing cytochrome c release. In particular, an inhibitor of the mitochondrial permeability transition pore (mPTP), cyclosporin A, attenuated mitochondrial damage and cell apoptosis, indicating that mPTP may be involved in the antiproliferative activity of MitoHCAs. Further studies focused on structural optimization of these compounds are onging.
Key words: Mitochondrial dysfunction, Hepatocellular carcinomas, Hydroxycinnamic acids, Antiproliferative activities, Mitochondrial permeability transition pore
Graphical abstract
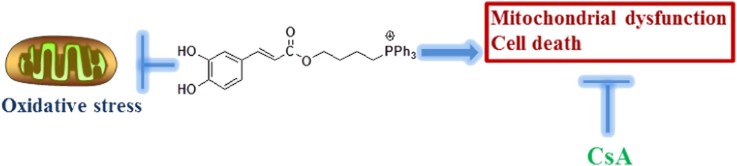
Mitochondria-targeted hydroxycinnamic acids were synthesized and evaluated for antioxidant and antiproliferative activities. The most active compound, MitoCaA, significantly induced apoptosis and mitochondrial dysfunction. The protective effects of cyclosporin A (CsA) indicated the involvement of mitochondrial permeability transition in cell death.
1. Introduction
The liver is responsible for detoxifying noxious metabolites and requires substantial amounts of adenosine triphosphate (ATP) from mitochondria to fulfill this biological process. Prolonged exposure of the liver to detrimental xenobiotics causes oxidative damage to mitochondria1, 2. The resulting defective mitochondria and decreased ATP synthesis may lead to the induction of hepatocellular carcinomas3.
Hepatocellular carcinomas continue to be a major health concern and are ranked as the fifth most common cancer worldwide. In China, high incidence rates have accounted for 55% of the world׳s cases4, 5. Typical treatments for hepatocellular carcinomas have included surgery, radiotherapy and chemotherapy, but current cure rates are not satisfactory6. Currently, mitochondria have been recognized as potential targets in cancer cells, as these organelles are a reservoir for proteins that promote apoptotic death when transported into the cytosol7. These findings underscore the importance of discovering mitochondria-targeted drugs that would protect healthy mitochondria from oxidative damage or selectively induce apoptosis in hepatocellular carcinomas, while simultaneously affecting as few healthy cells as possible.
Hence, mitochondria-targeted drugs have been developed using drug delivery carriers, such as the triphenylphosphonium (TPP) cation, mitochondria-targeted peptides, nanoparticles, cell penetrating peptides, etc8. The TPP cation allows for mitochondrial delivery via the electrical gradient across the mitochondrial inner membrane and has been developed by Murphy et al.9. Further, as a versatile therapeutic carrier, TPP-targeted drugs may exhibit selectivity towards tumor cells because cancer cells maintain a higher mitochondrial potential than non-malignant cells10. Additionally, because cancer mitochondria are structurally and functionally different from their normal counterparts, cancer cells display extensive metabolic reprogramming and are more susceptible to mitochondrial perturbations7. Therefore, mitochondria-targeted drugs have been synthesized based on active molecules, such as 1,4-naphthoquinone11, resveratrol12, coenzyme Q13, etc.
In recent years natural products have generated significant interest as chemopreventive and chemotherapeutic agents14, 15. Hydroxycinnamic acids (HCAs) and their derivatives are commonly isolated from Chinese medicinal herbs16, 17, 18 and are found in some plant-derived food products19. Epidemiological studies have reported that coffee drinkers demonstrate a significant reduction in the risk for hepatocellular carcinomas when compared to non-drinkers20. Recently, caffeic acid was reported to protect rat liver from reperfusion injury via regulation of the Sirt3-mitochondrial respiratory chain pathway21 and against tert-butyl hydroperoxide-induced oxidative hepatic damage22. Additionally, high doses of HCAs and their derivatives induced growth arrest and apoptosis in a variety of tumor cells by triggering either mitochondrial dysfunction or the mitochondria-dependent apoptotic pathway23, 24, 25. Despite the undoubted protective effects or anticancer efficacy, clinical trials have failed to establish a link between treatment with HCAs and cancer reduction, although this may be due to insufficient dosages to specific tissues26, 27.
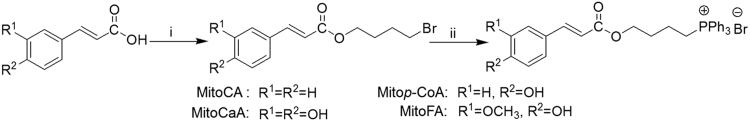
In this study, mitochondria-targeted HCA derivatives (MitoHCAs) were synthesized (Scheme 1), where p-coumaric (p-CoA), caffeic (CaA), ferulic (FA) and cinnamic acid (CA) were conjugated to the TPP cation, consequently resulting in Mitop-CoA, MitoCaA, MitoFA and MitoCA, respectively. The synthesized MitoHCAs protected mitochondria from lipid peroxidation and decreased mitochondrial H2O2 levels by regulating antioxidant enzymes. In addition, they displayed high toxicity against human hepatoma HepG2 cells, but low toxicity toward two healthy cell lines—human hepatic L02 and diploid human fibroblasts WI38 cells. Further, MitoHCAs, both with and without Ca2+, caused mitochondrial permeability transition pore (mPTP) opening, thereby collapsing the mitochondrial membrane potential (MMP) and causing cytochrome c (cyt c) release in isolated hepatic mitochondria, all of which initiate the apoptotic pathway28. Finally, cyclosporin A (CsA) attenuated MitoCaA-induced apoptosis and mitochondria damage.
Scheme 1.
Synthesis of MitoHCAs. Reagents and conditions: (i) Br(CH2)4Br, Et3N, Me2CO, 50 °C, 4 h (55%–65%); (ii) PPh3, toluene, reflux, 36 h (70%–85%).
2. Results and discussion
2.1. Synthesis
Synthesis of the MitoHCAs was performed as previously described (Scheme 1)12, 29. The MitoHCAs were obtained in good yields (>70%) and were characterized by 1H and 13C NMR spectroscopy and HR-MS mass spectrometry (Supplementary Figs. S1–S4). The purity was >95%, as determined by HPLC analysis (Supplementary Fig. S5).
2.2. Inhibition of mitochondrial lipid peroxidation
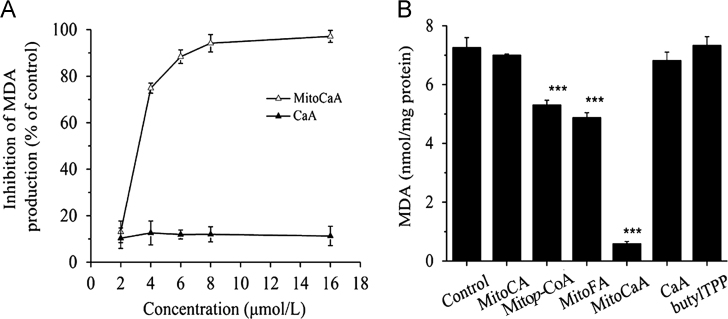
As HCAs are excellent antioxidants, the synthesized MitoHCAs could act as novel inhibitors of mitochondrial lipid peroxidation. MitoCaA exhibited a dose-dependent suppression of lipid peroxidation causing full inhibition at ~6 μmol/L, while the parent compound CaA had no effect on lipid peroxidation induced by FeCl2/ascorbate, which generates OH∙ radicals in situ30 (Fig. 1A). The inhibition potency of the synthesized MitoHCAs was measured: Mitop-CoA<MitoFA<MitoCaA (Fig. 1B). MitoCA and the control compounds (phosphonium salts butylTPP and CaA) were all ineffective at 6 μmol/L. Additionally, similar results were observed when mitochondria were treated with ROO∙ radicals and MitoHCAs (Supplementary Fig. S3). Overall, these data suggest that the inhibitory potency of HCAs against mitochondrial lipid peroxidation was improved by addition of the TPP carrier.
Figure 1.
Inhibition of lipid peroxidation in the energized hepatic mitochondria (1 mg protein/mL) induced by FeCl2/ascorbate supplementation. (A) In the presence of the various concentrations of MitoCaA and CaA. (B) In the presence of MitoHCAs, CaA and butylTPP (6 μmol/L). The values represent mean±SEM of three independent experiments. ***P<0.001 vs. the control groups.
2.3. Reduction of mitochondrial hydrogen peroxide levels and possible mechanisms
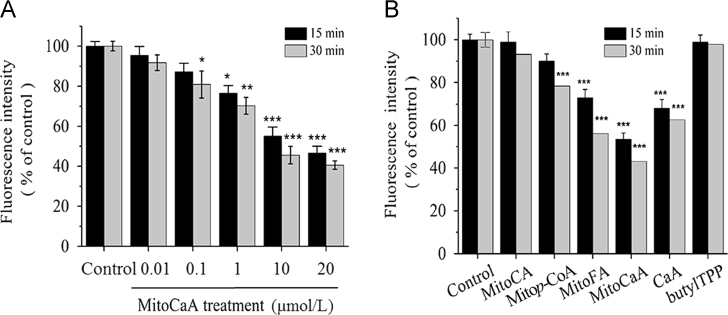
Supplementation with MitoHCAs protected liver mitochondrial lipids from exogenous ROS attack. In order to characterize this protection further, the effect of MitoHCAs on endogenous H2O2 generation in isolated hepatic mitochondria was investigated. H2O2 levels were measured by relative 2′,7′-di-chlorodihydrofluorescein diacetate (DCFDA) fluorescence. Incubation of mitochondria with MitoCaA significantly decreased H2O2 levels in both a concentration- and time-dependent manner (Fig. 2A). In order of increasing effect on H2O2 concentration was Mitop-CoA<CaA<MitoFA<MitoCaA. MitoCA and butylTPP did not affect the endogenous levels of H2O2 (Fig. 2B).
Figure 2.
Inhibition of H2O2 production in energized hepatic mitochondria (1 mg protein/mL). (A) In the presence of the various concentrations of MitoCaA. (B) In the presence of MitoHCAs, CaA and butylTPP (10 μmol/L). The fluorescence of the DCFDA probe was monitored by excitation at 485 nm and recording the emission at 521 nm. The results were expressed as a percentage of the control. The values represent mean±SEM of three independent experiments. ***P<0.001, **P<0.01, *P<0.05 vs. the control groups.
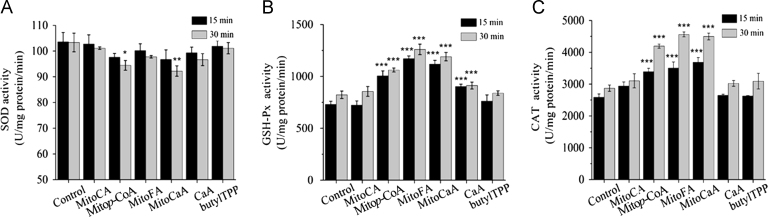
In order to further investigate the possible mechanisms by which MitoHCAs decreased mitochondrial H2O2 concentration, these compounds were assayed for their ability to directly scavenge H2O2, decrease production, or regulate antioxidant enzymes, for example, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT). No detectable changes in DCFDA fluorescence were noted after addition of MitoHCAs to H2O2, indicating that these compounds do not act as radical scavengers (results not shown). In addition, the MitoHCAs did not alter production in mitochondria as measured by dihydroethidium (DHE) probe fluorescence. Although MitoCA and butylTPP did not alter the activities of the antioxidant enzymes, MitoCaA, MitoFA and Mitop-CoA significantly increased both GSH-Px and CAT activities (Fig. 3). Interestingly, both Mitop-CoA and MitoCaA demonstrated ≤10% inhibition of mitochondrial SOD after 30 min, whereas MitoFA did not, which is consistent with previous observations that phenolic compounds do not inhibit SOD activity31. Together, these results suggest that MitoHCAs stimulate GSH-Px and CAT activites, thereby decreasing endogenous mitochondrial H2O2 levels.
Figure 3.
Impact of MitoHCAs on (A) SOD, (B) GSH-Px and (C) CAT activities. Energized hepatic mitochondria were incubated with the MitoHCAs (10 μmol/L) at 25 °C for 15 and 30 min. The enzyme assays were performed utilizing standardized kits. The values represent mean±SEM of three independent experiments. ***P<0.001, **P<0.01, *P<0.05 vs. the control groups.
2.4. Antiproliferative activity
Next, the antiproliferative activities of MitoHCAs against human hepatoma HepG2 cells and two normal cell lines, human hepatic L02 and diploid human WI38 fibroblasts, were investigated. With the knowledge that MitoHCAs target the mitochondria and would interfere with the MTT assay, the sulforhodamine B (SRB) method, which estimates cell number indirectly by measuring total protein content, was utilized instead, and this assay has proven to be the most suitable in the preclinical screening of novel therapeutic compounds32. In comparison to CA and butylTPP, MitoHCAs exhibited enhanced antiproliferative activities toward HepG2 cells (Table 1). MitoCA, MitoFA and MitoCaA were more toxic to the cells, resulting in IC50 values ~10 μmol/L at 24 h and ~6 μmol/L at 48 h. Moreover, MitoHCAs selectively inhibited proliferation in HepG2 cells in contrast to the non-cancerous L02 and WI38 cells; the IC50 values of the L02 and WI38 cells were 10-fold higher than for the HepG2 cells. The therapeutic index (TI) of MitoHCAs is calculated as the ratio of antiproliferative activities between normal cell and cancer cell lines (Table 2). The results revealed that the most active compound, MitoCaA, had the highest TI against HepG2 cells, with values of 12.91 at 24 h and 11.21 at 48 h, in comparison to compound CaA (TI=2.01 and 1.68, respectively). This data indicates that the TPP-carrier molecule improved the antiproliferative activities of the synthesized HCAs, which is consistent with the mitochondria acting as a key organelle in controlling apoptosis.
Table 1.
Antiproliferative activity of MitoHCAsa.
| Compd. | IC50 (μmol/L) |
|||||
|---|---|---|---|---|---|---|
| HepG2 |
L02 |
WI38 |
||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| MitoCA | 11.22±0.40 | 5.40±0.32 | 105.42±2.16 | 59.82±5.77 | 126.50±6.28 | 66.31±2.16 |
| Mitop-CoA | 26.80±1.53 | 15.33±0.91 | 185.75±6.39 | 80.57±6.32 | 270.45±8.05 | 112.57±3.87 |
| MitoFA | 10.42±0.37 | 6.49±0.28 | 89.73±3.47 | 50.72±4.49 | 98.63±2.18 | 53.52±1.71 |
| MitoCaA | 9.19±0.25 | 5.96±0.21 | 118.68±0.93 | 66.83±3.49 | 123.57±6.92 | 70.57±2.15 |
| CaA | 200.89±3.88 | 92.97±1.35 | 402.95±18.87 | 156.14±8.83 | 280.89±9.47 | 198.39±7.78 |
| ButylTPP | 25.35±1.02 | 12.81±0.87 | 120.58±9.97 | 71.42±6.64 | 178.67±3.73 | 88.51±3.95 |
Values represent mean±SEM from at least three independent experiments.
Table 2.
Therapeutic index (TI) of MitoHCAs.
| Compd. | TIa |
|
|---|---|---|
| 24 h | 48 h | |
| MitoCA | 9.40 | 11.08 |
| Mitop-CoA | 6.93 | 5.26 |
| MitoFA | 8.61 | 7.82 |
| MitoCaA | 12.91 | 11.21 |
| CaA | 2.01 | 1.68 |
| ButylTPP | 4.76 | 5.58 |
TI=IC50 L02 normal cell/IC50 HepG2 cancer cell.
2.5. Induction of apoptosis and variations in mitochondrial morphology
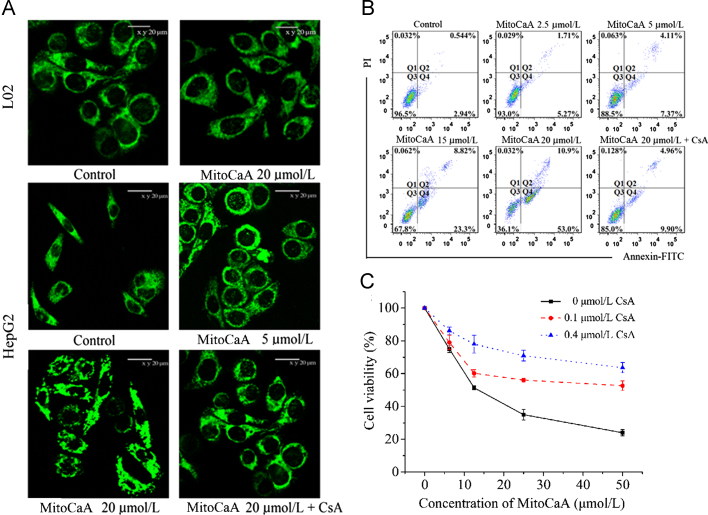
As MitoCaA displayed the optimal antiproliferative activities against HepG2 cells, the compound was further examined for its impact on cell apoptosis and mitochondrial morphology. MitoTracker Green FM was used to monitor mitochondrial morphology. MitoCaA triggered mitochondrial fragmentation after a 4-h treatment in a dose-dependent manner: treatment with 20 μmol/L MitoCaA resulted in donut-shaped morphology and a discontinuous network of mitochondria (Fig. 4A), in contrast to the filamentous or reticular mitochondria found in vehicle-treated cells. Noticeably, under the same experimental conditions, 20 μmol/L MitoCaA did not cause mitochondrial damage to normal L02 cells (Fig. 4A). Next, apoptotic cells were detected by AnnexinV–FITC/PI double staining. MitoCaA triggered apoptotic cell death in a dose-dependent manner (Fig. 4B). After treatment with 20 μmol/L MitoCaA for 24 h, FITC-positive cells accounted for 63.9% of the total cells, suggesting that apoptosis was a crucial pathway in the cytotoxicity of MitoCaA. This data suggests that MitoCaA induced apoptotic cell death through mitochondrial impairment.
Figure 4.
(A) Mitochondrial morphology of L02 cells and HepG2 cells treated with MitoCaA or MitoCaA+CsA (0.4 μmol/L) for 4 h. The mitochondrial network and morphology were visualized using MitoTracker Green FM staining and a confocal microscope. (B) Flow cytometry analyses for the apoptotic induction of HepG2 cells after 24 h treatment with MitoCaA or MitoCaA+CsA (0.4 μmol/L). (C) Cell viability of HepG2 cells after 24 h treatment with MitoCaA or MitoCaA+CsA. The values represent mean±SEM of three independent experiments.
mPTP is known to be a critical component in drug-induced cell death. The opening of mPTP occurs in response to mitochondrial damage and the release of apoptotic factors, such as cyt c28. In order to determine if mPTP induction was involved in MitoCaA-induced apoptotic cell death, the effects of CsA, a known inhibitor of mPTP opening due to its interaction with cyclophilin D28 on MitoCaA-induced cell death and mitochondrial morphology, were monitored. Interestingly, inclusion of CsA significantly attenuated cell death and mitochondrial damage in the MitoCaA model (Fig. 4). In sum, these results indicated mPTP involvement in MitoCaA-induced HepG2 cell death.
2.6. Induction of mitochondrial dysfunction
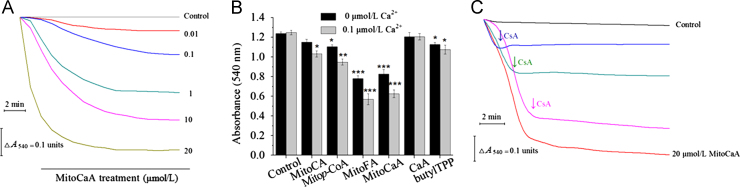
Based on the obtained results, the investigation was expanded to examine the impact of the compounds on the whole mitochondria. mPTP opening is often indicated by mitochondrial swelling. Isolated hepatic mitochondria were treated with MitoCaA (0.01–20 μmol/L) and an obvious decrease in absorbance was noted in a concentration-dependent manner, indicating large-amplitude swelling in the mitochondrial matrix (Fig. 5A). Next, the induction potency of various MitoHCAs was assessed, and neither MitoCA nor CaA induced swelling. The other compounds triggered swelling with increasing effect: butylTPP<Mitop-CoA<MitoFA≈MitoCaA (Fig. 5B). Ca2+ is a key stimulator involved in inducing the opening of the mPTP, and may trigger mPTP opening in combination with other stimuli28. During the resting state, the endogenous Ca2+ concentration should be ~0.1 μmol/L in the mitochondria33, but incubation with 0.1 μmol/L of Ca2+ had no effect on cell swelling under the tested conditions (Fig. 5B). However, treatment with MitoHCAs and Ca2+ intensified swelling, indicating that MitoHCAs had a synergistic interaction with Ca2+ to induce mPTP opening. Interestingly, the parent compound CaA failed to cause swelling, regardless of Ca2+ challenge, implying that CaA does not accumulate to high enough concentrations in the mitochondria matrix to induce swelling. In addition, swelling induced by MitoCaA was sensitive to CsA, as CsA blocked the swelling process immediately upon addition (Fig. 5C).
Figure 5.
Detection of swelling in energized hepatic mitochondria (1 mg protein/mL). (A) In the presence of the various MitoCaA concentrations. (B) In the presence of 10 μmol/L MitoHCAs alone or 10 μmol/L MitoHCAs+Ca2+. (C) Inhibition of 20 μmol/L MitoCaA-induced mitochondrial swelling by 1 μmol/L of CsA. CsA was added as indicated by the arrows. The assays were performed ≥4 times with similar results. The values represent mean±SEM of three independent experiments. ***P<0.001 vs. the control groups.
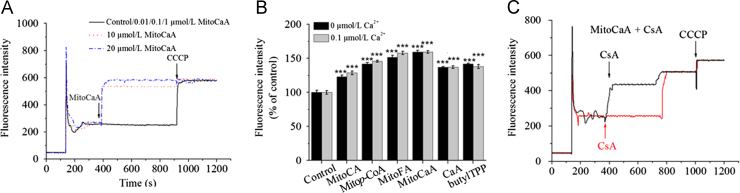
As mPTP opening may disrupt MMP, the effect of MitoHCAs on membrane potential was examined. Both 10 and 20 μmol/L MitoCaA promoted a transient decrease in MMP (Fig. 6A), with a decreasing affect among the synthesized compounds: MitoCA≈CaA≈butylTPP<Mitop-CoA<MitoFA<MitoCaA, roughly correlating with the previously noted induction of swelling (Fig. 6B). Treatment with CsA in MitoCaA-supplemented mitochondria delayed, but did not completely block, the dissipation of the MMP (Fig. 6C). In the presence of CsA, MMP was maintained for 400 s, after which the MMP level fully dissipated. 0.1 μmol/L Ca2+ was sufficient to diminish MMP in the presence of MitoHCAs, which was consistent with the synergistic induction of mPTP opening caused by Ca2+ (Fig. 6B).
Figure 6.
Collapse of mitochondrial membrane potential (ΔΨm). (A) In the presence of various MitoCaA concentrations. (B) In the presence of MitoHCAs, CaA and butylTPP (10 μmol/L) with and without Ca2+. (C) In the presence of MitoCaA (10 μmol/L) with CsA (1 μmol/L). CsA was added as indicated by the arrows. The energized mitochondria suspensions (1 mg protein /mL) were incubated with 0.15 μmol/L of Rhodamine 123. 1 μmol/L CCCP, a respiratory uncoupler, was used to fully dissipate ΔΨm. The assays were performed ≥ 4 times with similar results. The values represent mean ± SEM of three independent experiments. ***P<0.001 vs. the control groups.
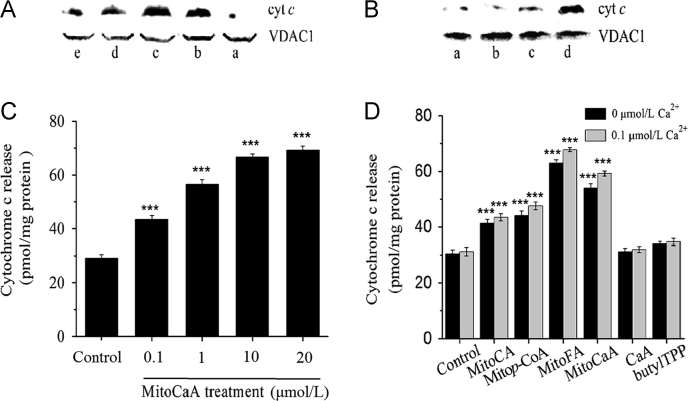
The release of cyt c is a characteristic feature of the mitochondrial apoptotic pathway, which is downstream of mPTP opening34. After incubation with MitoHCAs, mitochondria released cyt c, as visualized by immunoblotting (Fig. 7A). However, in the presence of CsA, cyt c release was blocked (Fig. 7B). The total amount of cyt c was determined by dithionite-reduction assay, and MitoCaA induced the release of cyt c in a concentration-dependent manner (Fig. 7C). Incubation with 10 μmol/L of all MitoHCAs induced cyt c release (Fig. 7D). Both MitoFA and MitoCaA triggered significant release of 60 pmol cyt c/mg protein (about 2.5-fold vs. control). However, CaA and butylTPP had no effects. Synergistic release with Ca2+ was also observed (Fig. 7D). In summary, MitoFA and MitoCaA were able to trigger mitochondrial dysfunction. Ca2+, as a key molecule in cell and mitochondria function, has synergistic actions with MitoHCAs in inducing mitochondrial damage. Further, CsA strongly prevented the mitochondrial dysfunction induced by MitoCaA.
Figure 7.
Detection of cyt c release from energized mitochondria suspensions (2 mg protein/mL). (A) In the presence of 10 μmol/L MitoHCAs. a: control; b: MitoCaA; c: MitoFA; d: Mitop-CoA; e: MitoCA. (B) a: control; b: 1 μmol/L MitoCaA; c: 20 μmol/L MitoCaA +1 μmol/L CsA; d: 20 μmol/L MitoCaA. The blots shown are representative of three independent experiments demonstrating similar results. (C) In the presence of the various concentrations of MitoCaA. (D) In the presence of MitoHCAs, CaA and butylTPP (10 μmol/L) with or without Ca2+. The quantitative assays were performed by the dithionite-reduction method. The values represent mean±SEM of three independent experiments. ***P<0.001 vs. the control groups.
3. Conclusions
Mitochondrial therapies, particularly those involving mitochondria-targeted antioxidants, have been considered to be effective in treating cancer7, 35. In this study, a TPP carrier was used to target HCAs to mitochondria. The synthesized MitoHCAs improved the inherent biological activities of the parent compounds and the order of activities was MitoCA <Mitop-CoA<MitoFA≈MitoCaA, which was the similar to the biological activity order of parent compound HCAs19. MitoFA and MitoCaA have been identified as potent mitochondrial antioxidants and antiproliferative molecules with minimal toxicity toward non-cancerous cells. The protective effects of CsA suggested that mPTP was involved in cell death and mitochondrial dysfunction. The activity and mechanism of MitoFA and MitoCaA could guide further development of HCAs as potential therapeutic drugs against hepatocellular carcinomas.
4. Experimental section
4.1. Materials and instruments
All reagents and PRMI 1640 media were obtained from Sigma–Aldrich Ltd. (Beijing, China) or J&K Scientific Ltd. (Beijing, China). Antioxidant enzyme assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The BCA protein assay kit was obtained from Beyotime Institute of Biotechnology (Jiangsu, China). Polyvinylidine fluoride membranes and Centricon YM-3 were purchased from the Millipore Corp. (USA). Rabbit anti-cyt c (AF0146) and rabbit anti-VDAC1 (DF6140) were obtained from Affinity Biosciences (OH, USA). Fetal bovine serum was purchased from Hangzhou Sijiqing Biomaterials Co., Ltd. (Hangzhou, China).
1H NMR and 13C NMR spectra were recorded using a Varian Mercury spectrometer operating at 400 MHz for 1H NMR and 100 MHz for 13C NMR. HR-MS spectra were obtained on an Orbitrap Elite (Thermo Scientific) mass spectrometer. UV/Vis spectra were recorded at 25 °C with a Perkin-Elmer Lambda 25 spectrophotometer that was equipped with temperature-controlled cell holders (USA). Fluorescence intensity and fluorescence spectra were measured at 25 °C with an Infinite M200 Pro Multimode Reader (Switzerland) and a Shimadzu RF-5301 spectrofluorimeter (Japan), respectively.
4.2. Chemistry
4.2.1. Synthesis procedures
The MitoHCAs and intermediates were synthesized according to previously reported procedures12, 29. The chemical structures were confirmed by 1H and 13C NMR, HR-MS-ESI (Supplementary Figs. S1–S4)
(E)-4-(3-Phenyl)acrylate butyltriphenylphosphonium bromate (MitoCA): 85% yield; 1H NMR (400 MHz, DMSO-d6): δ: 1.68 (m, 2H, CH2), 1.86 (m, 2H, CH2), 3.71 (m, 2H, CH2-P), 4.21 (m, 2H, OCH2), 6.54 (d, 1H, J=16Hz, H-α), 7.44–7.46 (m, 3H, H-3, H-4, H-5), 7.58 (d, 1H, J=16 Hz, H-β), 7.76–7.79 (m, 2H, H-2, H-6), 7.81–7.89 (m, 15H, PPh3); 13C NMR (100 MHz, DMSO-d6): δ: 18.93, 20.02, 29.15, 63.27, 118.35, 118.48, 119.33, 128.80, 130.65, 131.03, 134.02, 134.37, 135.35, 144.99, 166.56; HR-MS: Calcd. for C31H30O2P, 465.1978; found 465.1970, error 1.7 ppm.
(E)-4-(3-(4-Hydroxyphenyl) acrylate butyltriphenylphosphonium bromate) (Mitop-CoA): 75% yield; 1H NMR (400 MHz, DMSO-d6): δ: 1.66 (m, 2H, CH2), 1.82 (m, 2H, CH2), 3.65 (m, 2H, CH2-P), 4.17 (m, 2H, OCH2), 6.28 (d, 1H, J=16 Hz, H-α), 6.80 (d, 2H, J=8.8 Hz, H-3, H-5), 7.51 (d, 1H, J=16 Hz, H-β), 7.53 (d, 2H, J=8.8 Hz, H-2, H-6), 7.73–7.90 (m, 15H, PPh3), 10.05 (s, 1H, OH); 13C NMR (100 MHz, DMSO-d6): δ: 19.81, 19.63, 28.87, 62.59, 114.10, 115.87, 118.13, 125.13, 130.31, 133.67, 135.02, 144.87, 159.87, 166.61; HR-MS: Calcd. for C31H30O3P, 481.1927; found 481.1919, error 1.7 ppm.
(E)-4-(3-(4-Methoxyphenyl) acrylate butyltriphenylphosphonium bromate) (MitoFA): 70% yield; 1H NMR (400 MHz, CDCl3): δ: 1.72 (m, 2H, CH2), 2.16 (m, 2H, CH2), 3.93 (s, 3H, -OCH3), 4.01 (m, 2H, CH2-P), 4.23 (m, 2H, OCH2), 6.15 (d, 1H, J=15.6 Hz, H-α), 6.99–7.05 (m, 3H, H-2, H-5, H-6), 7.52 (d, 1H, J=15.6 Hz, H-β), 7.65–7.89 (m, 15H, PPh3); 13C NMR (100 MHz, CDCl3): δ: 19.17, 21.84, 29.01, 56.04, 62.82, 109.77, 114.77, 115.02, 117.69, 122.82, 126.51, 130.36, 133.54, 134.94, 145.12, 147.03, 148.42, 167.20; HR-MS: Calcd. for C32H32O4P, 511.2033; found 511.2088, error 1.0 ppm.
(E)-4-(3-(3,4-Dihydroxyphenyl) acrylate butyltriphenylphosphonium bromate) (MitoCaA): 81% yield; 1H NMR (400 MHz, DMSO-d6): δ: 1.63 (m, 2H, CH2), 1.81 (m, 2H, CH2), 3.66 (m, 2H, CH2-P), 4.14 (m, 2H, OCH2), 6.13 (d, 1H, J=16 Hz, H-α), 6.76 (d, 1H, J=8 Hz, H-5), 6.94 (d, 1H, J=8 Hz, H-6), 7.03 (s, 1H, H-2), 7.37 (d, 1H, J=15.6 Hz, H-β), 7.76–7.86 (m, 15H, PPh3), 9.19 (s, 1H, OH), 9.66 (s, 1H, OH); 13C NMR (100 MHz, DMSO-d6): δ: 18.55, 19.60, 28.84, 62.53, 113.84, 114.86, 115.87, 118.12, 121.53, 125.50, 130.29, 133.66, 135.00, 145.25, 145.70, 148.58, 166.54; HR-MS: Calcd. for C31H30O4P, 497.1876; found 497.1880, error 0.8 ppm.
4.2.2. HPLC purity analysis
HPLC analysis was performed using a Waters 600 system equipped with a Waters 2998 photodiode array detector, including a 2707 automatic injector and a computer integrating apparatus. The column was a Diamonsil C18 (150 mm×4.6 mm, 5 μm) and the column compartment was kept constant at 25 °C. The injection volume was 20.0 μL. Two mobile phases were used: (1) methanol: acetate buffer (50:50, v/v, pH=3.2), flow-rate of 1 mL/min. (2) acetonitrile:acetate buffer (50:50, v/v, pH=4.0), flow-rate of 0.5 mL/min. Detection was measured at 300 nm. The results and spectra are shown in Supplementary Fig. S2.
4.3. Biological studies
For the subsequent assays, the MitoHCAs were dissolved in DMSO, with the final concentrations of each reactant indicated in parentheses. The final DMSO concentration did not exceed 0.1% (v/v) and did not affect the results from the assays.
4.3.1. Preparation of hepatic mitochondria
Male Wister rats weighting 250±20 g, obtained from the animal center of the Medical College of Lanzhou University, were fasted overnight before cervical dislocation. All the animals were cared for and experiments were carried out in accordance with the principles and guidelines of the National Institutes of Health guide for the care and use of laboratory animals. All the protocols were approved by the Ethics Committee of Lanzhou University. Mitochondria were isolated by a standard differential centrifugation protocol36. The protein concentration was determined by biuret assay, using BSA as a standard37. For subsequent assays mitochondrial suspensions were incubated in the standard medium (in mmol/L: sucrose 250, HEPES 10, KH2PO 1, glutamate 10, malate 5; pH 7.4).
4.3.2. Mitochondrial lipid peroxidation assay
In order to quantify the amount of lipid peroxidation in mitochondria suspended in the standard medium (1 mg/mL), the TBARS assay was used38. The final concentrations of reactants are indicated in parentheses. The peroxidation reaction was initiated by addition of FeCl2 (20 μmol/L)/ascorbate (100 μmol/L) or AAPH (10 mmol/L), and inhibited by addition of the MitoHCAs (6 μmol/L). After incubation for 30 min, a TCA-TBA-HCl stock solution (15% w/v TCA; 0.375% w/v TBA; 0.25 mol/L HCl), together with 0.02% (w/v) butylated hydroxytoluene, was added. The solution was heated in a hot-water bath for 15 min. After cooling to room temperature, the precipitate was removed by centrifugation and the absorbance of the supernatant was determined at 532 nm38. The amount of malondialdehyde (MDA) was calculated using ε532=1.5×105 mol/(L•cm).
4.3.3. Measurement of mitochondrial H2O2 and production
H2O2 and production were assessed using the fluorescents probes DCFDA and DHE, respectively39. DCFDA probe (8 μmol/L) or DHE probe (5 μmol/L) was added to 1 mg/mL of energized mitochondria suspension in the standard medium. MitoHCAs were added and the suspension was incubated for either 15 or 30 min in the dark at 25 °C. DCFDA was detected with excitation and emission wavelengths of 485 nm and 521 nm, respectively. DHE was detected with excitation and emission wavelengths of 470 nm and 585 nm, respectively.
4.3.4. Measurement of mitochondrial SOD, GPx and CAT activities
Mitochondrial SOD, GSH-Px and CAT activities were determined according to the instructions from the commercial kits (Nanjing Jiancheng Bioengineering Institute). Briefly, after incubation with the tested compounds for 15 or 30 min at 30 °C, the mitochondria were sonicated at 400 A every 5 s for 5 cycles. Enzyme activities were determined as described in the instructions. Controlled experiments determined that the tested compounds did not interfere with the assays.
4.3.5. Cell culture
HepG2, L02, and WI38 cells were obtained from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science. HepG2 cells were cultured in 1640 media supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C under a 5% CO2 atmosphere. L02 and WI38 cells were cultured in DMEM media supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin at 37 °C under a 5% CO2 atmosphere.
4.3.6. SRB assay
Antiproliferative activity was determined using the SRB assay, which estimates cell number indirectly by measuring total protein concentration. 5×104 adherent cells/well were incubated in 96-well microtiter plates for 24 h. Following the addition of the test compounds the plates were incubated for an additional 24 h and 48 h, after which the plates were treated as described by the standard SRB method32. The absorbance was read at 540 nm on a microplate reader. IC50 values were determined from a log plot of percent of control versus concentration.
4.3.7. Flow cytometric analysis
HepG2 cells (5×105/well) were seeded in six-well plates and incubated for 24 h to allow for exponential growth and then treated with the test compounds for another 24 h. The cells were collected and washed with phosphate-buffered saline (PBS). Apoptotic cells were labeled with annexinV–FITC/PI according to the manufacturer׳s instructions (Beyotime Institute of Biotechnology, Jiangsu, China). Cells were analyzed on a FACSCanto flow cytometer (Becton Dickinson, LSRFortessa, USA).
4.3.8. Measurement of mitochondrial morphology
HepG2 cells were grown on coverslips at 5×105 cells/wells inside 6-well plates overnight and then treated with the test compounds for 4 h. After treatment, the media was removed from the dish and the cells were washed 3 times with pre-warmed PBS. The cells were stained and incubated at 37 °C for 30 min with 50 nmol/L MitoTracker Green FM. After staining, cells were washed twice with PBS. The coverslips were inverted onto glass slides with 10 μL of an antifade mounting medium (Beyotime). Confocal images were obtained using a confocal laser-scanning microscope (Zessi, LSM 510 META, Germany).
4.3.9. Measurement of mitochondrial membrane potential and swelling
MMP was measured using the fluorescence dye Rhodamine 12340. Briefly, 0.15 μmol/L Rhodamine 123 was added to energized isolated hepatic mitochondria (1 mg/mL) in the standard medium (excitation and emission wavelengths of 503 and 523 nm, respectively). After a short incubation for signal stabilization, the compounds were added as indicated in the legend to Fig. 6. Finally, the respiratory uncoupler CCCP (1 μmol/L) was used to fully depolarize MMP. Mitochondrial swelling was determined by measuring the apparent A540 change of the mitochondrial suspension41. Energized isolated hepatic mitochondria (1 mg/mL) were incubated in the standard medium. Further compounds were added as indicated in the Fig. 5 legend.
4.3.10. Measurement of cyt c release
Immunoblotting was performed as follows: the mitochondria (2 mg protein/mL) were incubated for 15 min at 25 °C in standard medium with the test compounds. The reaction mixtures were then centrifuged at 12,000×g for 10 min at 4 °C to obtain mitochondrial pellets. The supernatant fractions were centrifuged further at 100,000×g for 15 min at 4 °C to eliminate mitochondrial membrane fragments, and then were concentrated by ultrafiltration with a Centricon YM-3. Twenty microliter aliquots of the concentrated supernatants were subjected to electrophoresis on a 12% SDS-PAGE gel. The gel was transferred to a polyvinylidine fluoride membrane for immunoblotting analysis. VDAC1 was used as a loading control. The protein bands were visualized using the Gel Imaging System (ChemDoc-It610, UVP, USA). The dithionite-reduction assay was performed as follows: (1) energized hepatic mitochondria (2 mg/mL) were treated with MitoHCAs at the desired concentrations for 15 min; (2) mitochondria were centrifuged at 12,000×g for 10 min; (3) dithionite (1.2 mg) was added to 4.0 mL of the supernatant; (4) the amount of cyt c was determined at 550 nm42. The extinction coefficient of is 23.
4.3.11. Statistical analysis
The data are expressed as the mean±SEM. Statistical differences between two groups were assessed by the Student׳s t-test. And P<0.05 was used as the criterion for statistical significance.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (Grant No. 21302079), and the Fundamental Research Funds for the Central Universities (No. lzujbky2014151).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2016.05.002.
Appendix A. Supplementary material
Supplementary material
References
- 1.Choi J., Corder N.L.B., Koduru B., Wang Y.Y. Oxidative stress and hepatic Nox proteins in chronic hepatitis C and hepatocellular carcinoma. Free Radic Biol Med. 2014;72:267–284. doi: 10.1016/j.freeradbiomed.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardin R., Piciocchi M., Bortolami M., Kotsafti A., Barzon L., Lavezzo E. Oxidative damage in the progression of chronic liver disease to hepatocellular carcinoma: an intricate pathway. World J Gastroenterol. 2014;20:3078–3086. doi: 10.3748/wjg.v20.i12.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger C., Alhasawi A., Contavadoo M., Appanna V.D. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front Cell Dev Biol. 2015;3:40. doi: 10.3389/fcell.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang J.Y., Wu K.S., Zeng Y., Tang W.R., Du P.L., Xu Z.X. Liver cancer mortality characteristics and trends in China from 1991 to 2012. Asian Pac J Cancer Prev. 2015;16:1959–1964. doi: 10.7314/apjcp.2015.16.5.1959. [DOI] [PubMed] [Google Scholar]
- 5.Zuo T.T., Zheng R.S., Zhang S.W., Zeng H.M., Chen W.Q. Incidence and mortality of liver cancer in China in 2011. Chin J Cancer. 2015;34:508–513. doi: 10.1186/s40880-015-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu C.C., Wang J.J., Chen Y.S., Chen J.J., Tsai T.C., Lai C.C. Trends and predictors of outcomes after surgery for hepatocellular carcinoma: a nationwide population-based study in Taiwan. Eur J Surg Oncol. 2015;41:1170–1178. doi: 10.1016/j.ejso.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M., Li C., Guo W.W., Liu H.N., Liang W.Q., Gao J.Q. Mitochondrial drug delivery for cancer therapy. Acta Pharm Sin. 2016;51:257–263. [PubMed] [Google Scholar]
- 9.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 10.Modica-Napolitano J.S., Aprille J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001;49:63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu X., Altharawi A., Gut J., Rosenthal P.J., Long T.E. 1,4-Naphthoquinone cations as antiplasmodial agents: hydroxy-, acyloxy-, and alkoxy-substituted analogues. ACS Med Chem Lett. 2012;3:1029–1033. doi: 10.1021/ml300242v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biasutto L., Mattarei A., Marotta E., Bradaschia A., Sassi N., Garbisa S. Development of mitochondria-targeted derivatives of resveratrol. Bioorg Med Chem Lett. 2008;18:5594–5597. doi: 10.1016/j.bmcl.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 13.Jin H., Kanthasamy A., Ghosh A., Anantharam V., Kalyanaraman B., Kanthasamy A.G. Mitochondria-targeted antioxidants for treatment of Parkinson׳s disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842:1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar H., Kim I.S., More S.V., Kim B.W., Choi D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep. 2014;31:109–139. doi: 10.1039/c3np70065h. [DOI] [PubMed] [Google Scholar]
- 15.Butler M.S., Robertson A.A., Cooper M.A. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31:1612–1661. doi: 10.1039/c4np00064a. [DOI] [PubMed] [Google Scholar]
- 16.Jiang R.W., Lau K.M., Hon P.M., Mak T.C.W., Woo K.S., Fung K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem. 2005;12:237–246. doi: 10.2174/0929867053363397. [DOI] [PubMed] [Google Scholar]
- 17.Yi L.Z., Liang Y.Z., Wu H., Yuan D.L. The analysis of Radix Angelicae Sinensis (Danggui) J Chromatogr A. 2009;1216:1991–2001. doi: 10.1016/j.chroma.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Li F., Cao Q.E., Ding Z.T. Separation and determination of three phenylpropanoids in the traditional Chinese medicine and its preparations by capillary electrophoresis. J Chromatogr Sci. 2007;45:354–359. doi: 10.1093/chromsci/45.6.354. [DOI] [PubMed] [Google Scholar]
- 19.El-Seedi H.R., El-Said A.M., Khalifa S.A., Göransson U., Bohlin L., Borg-Karlson A.K. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J Agric Food Chem. 2012;60:10877–10895. doi: 10.1021/jf301807g. [DOI] [PubMed] [Google Scholar]
- 20.Nkondjock A. Coffee consumption and the risk of cancer: an overview. Cancer Lett. 2009;277:121–125. doi: 10.1016/j.canlet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Mu H.N., Li Q., Pan C.S., Liu Y.Y., Yan L., Hu B.H. Caffeic acid attenuates rat liver reperfusion injury through sirtuin 3-dependent regulation of mitochondrial respiratory chain. Free Radic Biol Med. 2015;85:237–249. doi: 10.1016/j.freeradbiomed.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Yang S.Y., Hong C.O., Lee G.P., Kim C.T., Lee K.W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem Toxicol. 2013;55:92–99. doi: 10.1016/j.fct.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Chang W.C., Hsieh C.H., Hsiao M.W., Lin W.C., Hung Y.C., Ye J.C. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J Obstet Gynecol. 2010;49:419–424. doi: 10.1016/S1028-4559(10)60092-7. [DOI] [PubMed] [Google Scholar]
- 24.Shailasree S., Venkataramana M., Niranjana S.R., Prakash H.S. Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol Neurobiol. 2015;51:119–130. doi: 10.1007/s12035-014-8700-2. [DOI] [PubMed] [Google Scholar]
- 25.Hemaiswarya S., Doble M. Combination of phenylpropanoids with 5-fluorouracil as anti-cancer agents against human cervical cancer (HeLa) cell line. Phytomedicine. 2013;20:151–158. doi: 10.1016/j.phymed.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso C., Santangelo R. Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol. 2014;65:185–195. doi: 10.1016/j.fct.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Saso L., Firuzi O. Pharmacological applications of antioxidants: lights and shadows. Current Drug Targets. 2014;15:1177–1199. doi: 10.2174/1389450115666141024113925. [DOI] [PubMed] [Google Scholar]
- 28.Dalla Via L., García-Argáez A.N., Martínez-Vázquez M., Grancara S., Martinis P., Toninello A. Mitochondrial permeability transition as target of anticancer drugs. Curr Pharm Des. 2014;20:223–244. doi: 10.2174/13816128113199990033. [DOI] [PubMed] [Google Scholar]
- 29.Chen L., Zhang Y.H., Kong X.W., Peng S.X., Tian J.D. Synthesis and biological evaluation of nitric oxide-releasing derivatives of oleanolic acid as inhibitors of HepG2 cell apoptosis. Bioorg Med Chem Lett. 2007;17:2979–2982. doi: 10.1016/j.bmcl.2007.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills E.D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969;113:315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagoa R., Graziani I., Lopez-Sanchez C., Garcia-Martinez V., Gutierrez-Merino C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim Biophys Acta. 2011;1807:1562–1572. doi: 10.1016/j.bbabio.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 32.van Tonder A., Joubert A.M., Cromarty A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8:47. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J.W., Zhai Y.J., Sun F. Research of mitochondrial calcium transportation. Acta Biophys Sin. 2013;29:167–180. [Google Scholar]
- 34.Yang J.C., Cortopassi G.A. Induction of the mitochondrial permeability transition causes release of the apoptogenic factor cytochrome c. Free Radic Biol Med. 1998;24:624–631. doi: 10.1016/s0891-5849(97)00367-5. [DOI] [PubMed] [Google Scholar]
- 35.Olszewska A., Szewczyk A. Mitochondria as a pharmacological target: magnum overview. IUBMB Life. 2013;65:273–281. doi: 10.1002/iub.1147. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y.B., Ye L.H., Liu H.X., Xia Q., Zhang Y., Yang X.D. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J Inorg Biochem. 2010;104:371–378. doi: 10.1016/j.jinorgbio.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 38.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 39.Degli Esposti M. Measuring mitochondrial reactive oxygen species. Methods. 2002;26:335–340. doi: 10.1016/S1046-2023(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 40.Scaduto R.C., Jr., Grotyohann L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petronilli V., Cola C., Massari S., Colonna R., Bernardi P. Physiological effectors modify voltage sensing by the cyclosporin A–sensitive permeability transition pore of mitochondria. J Biol Chem. 1993;268:21939–21945. [PubMed] [Google Scholar]
- 42.The State Pharmacopoeia Committee of China . vol. II. China Medical and Technology Press; Beijing: 2015. (Pharmacopoeia of the People׳s Republic of China). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material