Abstract
The intracellular calcium ions (Ca2+) act as second messenger to regulate gene transcription, cell proliferation, migration and death. Accumulating evidences have demonstrated that intracellular Ca2+ homeostasis is altered in cancer cells and the alteration is involved in tumor initiation, angiogenesis, progression and metastasis. Targeting derailed Ca2+ signaling for cancer therapy has become an emerging research area. This review summarizes some important Ca2+ channels, transporters and Ca2+-ATPases, which have been reported to be altered in human cancer patients. It discusses the current research effort toward evaluation of the blockers, inhibitors or regulators for Ca2+ channels/transporters or Ca2+-ATPase pumps as anti-cancer drugs. This review is also aimed to stimulate interest in, and support for research into the understanding of cellular mechanisms underlying the regulation of Ca2+ signaling in different cancer cells, and to search for novel therapies to cure these malignancies by targeting Ca2+ channels or transporters.
Abbreviations: 20-GPPD, 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol; CaM, calmodulin; CaMKII, calmodulin-dependent protein kinase II; CBD, cannabidiol; CBG, cannabigerol; CPZ, capsazepine; CRAC, Ca2+ release-activated Ca2+ channel; CTL, cytotoxic T cells; CYP3A4, cytochrome P450 3A4; ER/SR, endoplasmic/sarcoplasmic reticulum; HCX, H+/Ca2+ exchangers; IP3, inositol 1,4,5-trisphosphate; IP3R (1, 2, 3), IP3 receptor (type 1, type 2, type 3); mAb, monoclonal antibody; MCU, mitochondrial Ca2+ uniporter; MCUR1, MCU uniporter regulator 1; MICU (1, 2, 3), mitochondrial calcium uptake (type 1, type 2, type 3); MLCK, myosin light-chain kinase; NCX, Na+/Ca2+ exchanger; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma cells; PKC, protein kinase C; PM, plasma membrane; PMCA, plasma membrane Ca2+-ATPase; PTP, permeability transition pore; ROS, reactive oxygen species; RyR, ryanodine receptor; SERCA, SR/ER Ca2+-ATPase; SOCE, store-operated Ca2+ entry; SPCA, secretory pathway Ca2+-ATPase; TEA, tetraethylammonium; TG, thapsigargin; TPC2, two-pore channel 2; TRIM, 1-(2-(trifluoromethyl) phenyl) imidazole; TRP (A, C, M, ML, N, P, V), transient receptor potential (ankyrin, canonical, melastatin, mucolipin, no mechanoreceptor potential C, polycystic, vanilloid); VGCC, voltage-gated Ca2+ channel
KEY WORDS: Ca2+ channels, Store-operated Ca2+ entry, Cell proliferation, Migration, Apoptosis, Channel blockers;, Cancer therapy
Graphical abstract
Ca2+ plays vital roles in normal cell physiology, such as gene transcription, cell proliferation and migration. Abnormal Ca2+ signaling by virtue of altered channel expression or activation contributes to carcinogenesis and promotes tumor development. Targeting the dysregulated Ca2+ channels/transporters/pumps may provide a promising chemotherapy for cancer treatment.
1. Introduction
Intracellular calcium ions (Ca2+), the most abundant second messenger in human body, have a substantial diversity of roles in fundamental cellular physiology, including gene expression, cell cycle control, cell motility, autophagy and apoptosis1. Since the cytosol Ca2+ is maintained very low (~10−7 mol/L), a small fraction of Ca2+ either through release from intracellular organelles (~10−5 mol/L) or through influx from extracellular reservoir (~10−3 mol/L) can generate marked signals to activate downstream signaling cascade. Increase in Ca2+ levels are highly localized, such as the microdomains in the vicinity of inositol 1,4,5-trisphosphate receptor (IP3R) or store-operated Ca2+ entry (SOCE) channel2. Alternatively, local changes in intracellular Ca2+ can diffuse across the cell as a wave and elicit an effect at a distant site3. The prolonged intracellular elevation of Ca2+ can be toxic and triggers cell death4. Therefore, the Ca2+ signals in the form of waves, spikes or oscillations must be spatially-temporally tightly regulated5.
Among the three Ca2+ signal forms, intracellular Ca2+ oscillations provide efficient means to transmit intracellular biological information. For example, our previous study showed that intracellular Ca2+ oscillations provided essential proliferation signals for esophageal cancer cells6. In particular, the frequency, amplitude, and duration of these intracellular Ca2+ oscillations compose the specific Ca2+ codes for selective activation of transcription factors for gene transcription, cell proliferation and migration7, 8. The decoding of the oscillatory form is achieved by intracellular downstream effectors, including calmodulin (CaM), nuclear factor of activated T-cells (NFAT), nuclear factor-κB (NF-κB), calmodulin-dependent protein kinase II (CaMKII) and calpain, which differ in their on- and off-rates for Ca2+ and subsequently activate different cellular processes9, 10, 11. Furthermore, different Ca2+ regulated kinases and enzymes often occupy distinct locations within the cell. Therefore, the size, kinetics and spatial profile of a cytoplasmic Ca2+ signal are all important in determining which Ca2+-dependent response will be activated, when and for how long. Intracellular Ca2+ oscillations can reduce the effective Ca2+ threshold for signaling transduction, thereby increasing signal detection at low levels of stimulation12.
Disruption of normal Ca2+ signaling contributes to the development of malignant phenotypes13. In order to proliferate at high rates, to increase cell motility and invasion, to escape death, to fool immune-attack, or to have neovascularization, tumors remodel their Ca2+ signaling network. There has been an increasing awareness that tumorigenic pathways are associated with altered expression level or abnormal activation of Ca2+ channels, transporters or Ca2+-ATPases6, 14, 15, 16, 17, 18, 19, 20. Correction of these derailed Ca2+ signals could provide potential cancer therapies. In this review, we will summarize the Ca2+ channels, transporters and Ca2+-ATPases, which are altered and play important roles in cancer biology. We will also discuss the current effort in this emerging research area toward evaluation of a certain numbers of Ca2+ channel blockers or regulators and Ca2+-ATPase pump inhibitors as anti-cancer drugs.
2. Altered Ca2+ channels/transporters in cancer
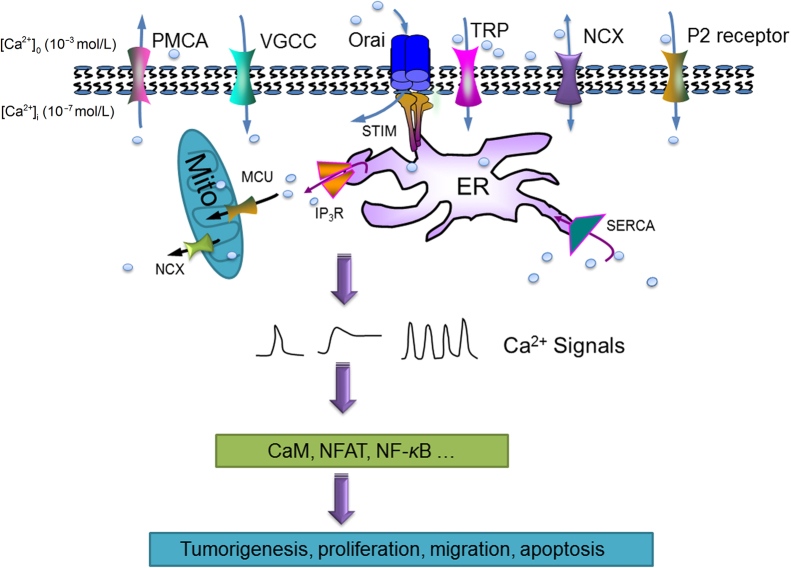
The intracellular Ca2+ homeostasis is governed by a network composed of various Ca2+ channels and transporters: (1) IP3R or ryanodine receptor (RyR) mediating Ca2+ release from endoplasmic/sarcoplasmic reticulum (ER/SR); (2) Ca2+-ATPase pumping Ca2+ from cytosol back to ER/SR or extracellular space; (3) Ca2+ channels or transporters allowing Ca2+ influx across plasma membrane (PM) from extracellular Ca2+ reservoir, such as voltage-gated Ca2+ channel (VGCC), transient receptor potential channel (TRP), Ca2+ release-activated Ca2+ channel (CRAC), Na+/Ca2+ exchanger (NCX) and purinergic receptor; (4) mitochondrial Ca2+ uniporter (MCU) regulating mitochondrial Ca2+ uptakes (Fig. 1). It is beyond the scope of this brief review to cover all the Ca2+ channels, transporters or Ca2+-ATPases for intracellular Ca2+ homeostasis; instead, we will focus on a few important ones, which have been reported to be dysregulated in cancer cells (Table 1).
Figure 1.
Important Ca2+ channels/transporters/pumps in cancer cells. The dynamic of intracellular Ca2+ is governed by a series of proteins: (1) IP3Rs mediating Ca2+ release from endoplasmic reticulum (ER); (2) Ca2+-ATPases pumping Ca2+ from cytosol to the ER or to extracellular space; (3) plasma membrane Ca2+ channels or transporters, such as VGCCs, TRPs, CRACs, NCXs and P2 receptors; (4) mitochondrial Ca2+ uniporter. The tightly regulated Ca2+ signals in the form of waves, spikes or oscillations can regulate a wide range of cellular events, including gene transcription, proliferation, migration and apoptosis. Targeting the dysregulated Ca2+ channels/transporters/pumps may provide a promising chemotherapy for cancer patients.
Table 1.
Altered Ca2+ channels/transporters/pumps in cancers.
| Channel/transporter | Cancer type | Changes | Ref. | |
|---|---|---|---|---|
| IP3R | IP3R1 | Glioma | Decreased | 24 |
| IP3R2 | Lymphocytic leukemia | Increased | 28 | |
| IP3R3 | Glioma, gastric, colon, head and neck cancer | Increased/Mutation | 24, 25, 26, 27 | |
| Ca2+-ATPase | SERCA2 | Colon cancer | Increased | 32 |
| SERCA3 | Gastric, lung, choroid plexus tumors, and in myeloid leukemia | Decreased | 31 | |
| SPCA1 | Breast cancer | Increased | 35 | |
| SPCA2 | Breast cancer | Increased | 36 | |
| PMCA1 | Oral cancer | Decreased | 39 | |
| PMCA2 | Breast cancer | mRNA elevated | 37 | |
| PMCA4 | Colon cancer | Decreased | 38 | |
| VGCC | Cav1.2 | Colon and esophageal cancer | Increased | 42 |
| Cav2.3 | Glioma | Increased | 42 | |
| Cav3.1 | Glioma | Increased | 46 | |
| Cav3.2 | Prostate, ovarian, glioma, breast, esophageal, hepatoma, melanoma, and colon cancer | Increased | 42, 43, 44, 45 | |
| TRP | TRPC1 | Breast cancer | Increased | 49 |
| TRPC3 | Ovarian and breast cancer | Increased | 50, 51 | |
| TRPC6 | Esophageal, glioma and breast cancer | Increased | 51, 57, 90 | |
| TRPM1 | Melanoma | Decreased | 91 | |
| TRPM7 | Pancreatic and breast cancer | Increased | 60, 92 | |
| TRPM8 | Pancreatic, prostate, bladder, breast, melanoma, colon and lung cancer | Increased | 58, 92, 93 | |
| TRPV1 | Bladder and prostate cancer | Decreased/Increased | 94, 95 | |
| TRPV2 | Bladder, prostate cancer and hepatocarcinoma | Decreased/Increased | 96 | |
| TRPV4 | Non-melanoma skin cancer, tumor endothelial cell derived prostate and breast cancer | Decreased | 97, 98, 99 | |
| TRPV6 | Breast, prostate, lung, thyroid, colon and ovarian cancer | Increased | 100, 101, 102, 103 | |
| Orai & STIM | Orai1 | Pancreatic adenocarcinoma, glioma, melanoma, breast, esophageal, renal, and NSCLC | Increased/Constitutive activated | 6, 36, 65, 66, 74, 104, 105, 106 |
| Orai3 | Breast, prostate and lung cancer | Increased | 68, 76, 78 | |
| STIM1 | Hepatoma, melanoma, cervical, colorectal cancer, breast and pancreatic adenocarcinoma | Increased | 66, 69, 104, 107, 108, 109 | |
| STIM2 | Breast, colorectal cancer and melanoma | Increased/Decreased | 73, 74, 109, 110 | |
| Purinergic receptor | P2X3 | Hepatoma | Increased | 84 |
| P2X5 | Melanoma, colorectal, brain, breast and renal cancer | Increased | 42 | |
| P2X7 | Neuroblastoma, melanoma, leukemia, breast, prostate, papillary thyroid, pancreatic, colon, renal, cervical and B chronic cancer | Increased | 42, 80 | |
| P2Y2 | Highly metastatic breast cancer, hepatoma and colon cancer | Increased | 81, 82, 83 | |
| P2Y4 | Colon cancer | Increased | 83 | |
| MCU | Breast, colon and prostate cancer | Decreased/Increased | 87, 88 | |
2.1. IP3Rs --- ER Ca2+ release channels
ER and SR are the major intracellular Ca2+ storage organelles in non-excitable and excitable cells, respectively. While RyRs are predominantly expressed in excitable cells, IP3Rs are the main intracellular Ca2+ release channels in non-excitable cells, including many cancer cells. So far, three isoforms of IP3R, i.e. IP3R1, IP3R2 and IP3R3 have been identified, which display different affinity to IP3 with similar but not identical functional properties21. The general domain structure of IP3Rs includes the IP3 binding site at the N-terminal region, the six transmembrane spanning domains at the C-terminal, a large number of cytoplasmic regulatory sites and protein-binding domains. Among these protein-binding domains, a significant one between amino acid 1390–1409 mediates the interaction with the BH4 domain of anti-apoptotic protein Bcl-2. IP3R-mediated ER Ca2+ release participates in the overall intracellular Ca2+ signaling network and regulates fundamental cellular functions, such as cell proliferation and differentiation22. Moreover, recent studies have demonstrated that mitochondria and ER can form structural link. Together with other interacting proteins, such as BCL-2 and BAX/BAM, the Ca2+ channels residing in the two organelles are assembled in a macromolecular complex in which the IP3R directly stimulates the mitochondrial Ca2+ uptake23. Through this ER/mitochondrial crosstalk, IP3Rs can further determine the cell fate by controlling the mitochondrial Ca2+elevation and metabolism.
Altered IP3R activity and/or the remodeling of IP3R-expression profile have been found in a number of cancers (Table 1). Compared with normal brain tissues, human glioblastoma samples present decreased IP3R1 and increased IP3R3. Kang et al.24 demonstrated that caffeine could inhibit IP3R3-mediated Ca2+ release, thus reduced migration of cultured glioblastoma cells and greatly increased mean survival in a mouse xenograft model of glioblastoma. The upregulation of IP3R3 has been observed in gastric cancer cell lines established from malignant ascites, but not in a cell line established from primary tumor or normal gastric epithelial cells25. In a clinical association study, Shibao et al.26 examined whether gain of expression of IP3R isoforms is associated with development of colorectal cancer using colorectal carcinomas tissues surgically resected from over a hundred patients. They found that IP3R1 and IP3R2 were expressed in both normal colorectal mucosa and colorectal cancer, while IP3R3 was only observed in colorectal cancer, especially in the advancing margins of tumors correlated with depth of invasion, lymph node metastasis, liver metastasis, and TNM stage. High expression of IP3R3 is associated with aggressiveness of colorectal carcinoma since it is related with decreased 5-year survival. A mutation in IP3R3 encoding gene ITPR3 was identified in genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma27. Similarly like IP3R1 and IP3R3, the dysregulation of IP3R2 was observed in chronic lymphocytic leukemia cells28. The expression of IP3R2 is significantly upregulated, which in turn may enhance mitochondrial function and energy production to accommodate for the higher metabolic activity and the induced proliferation of leukemia cells. It is notable that the role of IP3R2 may be complicated. Ouyang et al., recently reported that IP3R2 mediated Ca2+ signaling is required to reinforce the developmentally important transcription factor TCF-1 for normal development and thymocyte neoplasia prevention. Mice without IP3Rs in thymocyte developed aggressive T-cell malignancies that resemble human T-cell acute lymphoblastic leukemia29.
2.2. Ca2+-ATPases
The low cytosolic Ca2+ concentration is maintained by the Ca2+ transport system, i.e. Ca2+-ATPases. They rapidly pump cytosolic Ca2+ ions back into intracellular organelles, e.g. ER, or to extrude Ca2+ ions to extracellular space. These Ca2+-ATPases belong to the superfamily of P-type ATPase (E1E2-type) and can be further divided into three subtypes according to their subcellular localizations: plasma membrane Ca2+-ATPase (PMCA), ER/SR Ca2+-ATPase (SERCA), golgi/golgi-derived vesicles secretory pathway Ca2+-ATPase (SPCA). Each subtype contains multiple isoforms and splice variants, which present tissue-specific expression, regulation, and kinetic characteristics. As such, the Ca2+-ATPases contribute to a highly complex and fine-tuned intracellular Ca2+ signaling network30.
Among the Ca2+-ATPases family members, SERCA is the best characterized one. SERCA is responsible for replenishing ER Ca2+ stores, maintaining protein proper folding/maturation whereas dysregulated SERCA results in depleted or overloaded ER lumen Ca2+ stores, increased ER stress, dysregulated chaperones and synthesis of lipids. Mutations and altered expression levels of SERCA isoforms have been identified in various cancers, such as cancers of colon, gastric, lung, myeloid leukemia and choroid plexus tumors31 (Table 1). Overexpression of SERCA2 was found in colorectal cancer cells, which may drive proliferation and migration32. On the other hand, SERCA3 was reported to have progressively lost during multistage process of colon tumorigenesis after initial increased expression during cell differentiation33. In B lymphocytes, SERCA3 was also found to be downregulated after the infection of Epstein Barr virus, a human gammaherpesvirus involved in various malignancies including Burkitt׳s and other lymphomas34.
Altered expression of SPCA isoforms occurs in various types of cancer including breast, colon and prostate20. Clinical data showed that SPCA1 is highly expressed in basal-like breast cancers and has a low expression in the luminal subtypes35. Feng et al.36 identified up-regulation of SPCA2 in breast cancer-derived cells and human breast tumors. Knockdown of SPCA2 resulted in attenuated growth as well as decreased colony formation of MCF7 cells in soft agar and reduced tumor formation in xenografted mice. Furthermore, overexpression of SPCA2 is able to confer increased proliferation and colony formation capability in soft agar assay in MCF10A cells, a nonmalignant mammary epithelial cell line. On the other hand, knockdown of SPCA2 and low Ca2+ conditions are able to decrease activity of ERK1/2 pathway, which may result in decreased proliferation in breast cancer cells. SPCA2 appears to have the unique ability to elicit store-independent Ca2+ entry, i.e. a constitutive Ca2+ entry pathway, which in turn promotes proliferative potential of cancer cells36.
The association between altered PMCAs and cancer has been reported in a few studies as well (Table 1). PMCA2, the isoform predominantly expressed in mammary epithelia for the apical efflux of Ca2+ during lactation, is highly expressed in certain numbers of breast cancer cell lines37. In these breast cancer cell lines, PMCA2 is constitutively expressed at levels as over 100-fold high as in non-tumorigenic lines. As such, PMCA2 can keep low cytosolic Ca2+ levels and bypass apoptosis by preventing increased uptake of Ca2+ into mitochondria. On the contrary, PMCA4 and PMCA1 are down-regulated in colon or oral squamous cell carcinoma, respectively, which increase cytosolic Ca2+ to enhance cell proliferation38, 39. These observations suggest that different cancer cells may develop different means to fulfill special needs for intracellular Ca2+ signaling, and either up or down regulation of Ca2+-ATPases is used to facilitate a particular type of cancer cells to escape from normal cellular control and to promote tumorigenesis.
2.3. Plasma membrane Ca2+ channels
2.3.1. Voltage-gated Ca2+ channels
VGCCs (also known as Cav family) mediate a fast Ca2+ influx in response to membrane depolarization. There are 6 subfamilies of VGCCs, i.e. L-, N-, P-, Q-, R- and T-type. The Cav1 conducts L-type Ca2+ currents, initiating muscle contraction and endocrine secretion; the Cav2 conducts N-, P/Q- and R-type Ca2+ currents, initiating rapid synaptic transmission; the Cav3 conducts T-type Ca2+ currents, which are characterized by more rapid activation and inactivation by membrane depolarization40.
A recent meta-analysis of microarray datasets revealed VGCCs mRNA gene profile of different types of cancers41. L-type Ca2+ channels are implicated in the development and progression of several tumors, such as significantly up-regulated in colon and esophageal cancers. Novel splice variants of T-type Ca2+ channel are commonly found in human glioma, breast, ovarian, prostate colon and esophageal cancer cells. For example, Cav3.1a transcripts predominate in the normal adult brain and Cav3.1b is mostly fetal-specific; but human glioma and glioma cell lines contains Cav3.1bc as predominant splice and Cav3.1ac as a novel splice variant, which is absent in normal brain or fetal astrocytes42, 43, 44, 45, 46.
2.3.2. TRP channels
The super family of TRP channels include more than 30 members, which can be further divided into 7 subgroups, i.e. TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystic), TRPN (no mechanoreceptor potential C), and TRPV (vanilloid)47. Mammalian TRP proteins form homo- or hetero tetrameric as non-selective Ca2+-permeable cation channels, which can be activated and regulated by a wide variety of stimuli, such as Ca2+, temperature, pH, ROS, chemical and mechanical stress. As such, they are perfect candidates for cellular sensors and are believed to actively participate in the tumor--microenvironment cross-talk48.
As summarized in Table 1, a large number of TRP members present altered expression and/or channel activity in a variety of cancers. For example, the presence of the TRPC, TRPM, and TRPV subfamilies correlates with malignant growth and cancer progression49, 50, 51, 52, 53. TRPM7, 8 and TRPV2, 6 are associated with the prostate cancer progression and TRPC6 as a novel therapeutic target for esophageal carcinoma18, 54, 55, 56, 57, 58. In breast cancer, TRPC1, TRPC6, TRPM7, TRPM8, and TRPV6 are overexpressed, and their expression profiles are associated with pathologic parameters, suggesting their use as prognostic markers53, 59. The expression profile of TRPM7/8 depends on both invasive and hormonal status: in non-invasive estrogen-positive cells, TRPM7 is highly expressed and mediates Ca2+ influx, which results in proliferation and poor differentiation60; on the other hand, TRPM8 is highly expressed in non-invasive well-differentiated estrogen-positive breast cancer, and may serve as a good prognostic marker for this type cancer61; in aggressive estrogen-negative cancers, they regulate cell migration through an interaction with cytoskeleton proteins62. Besides mentioned above, there are still some of other TRP family proteins involved and contributed to cancer development, which were summarized in Table 1. Therefore the expression of TRP channels has been proposed as a tool for diagnosis or predicting prognosis in several cancers, and targeting TRP channels has been suggested as a novel therapeutic strategy48.
2.3.3. Orai and STIM
Another type of Ca2+ influx channels, including CRAC, arachidonate-regulated Ca2+ entry channel and SOCE channel has been demonstrated to heavily involve in cancer biology (Table 1). The activation of canonic SOCE pathway contains several steps, i.e. reduction or depletion of ER Ca2+ stores, translocation of ER-localized Ca2+ sensor STIM1 to ER-plasma membrane junctions, aggregation and conformational changes of cell surface Orai channels, and final Ca2+ influx. To date, three isoforms of Orai (Orai1, Orai2 and Orai3) and two isoforms of STIM (STIM1 and STIM2) have been identified in mammals63.
Among these SOCE machinery proteins, Orai1 and STIM1 are the two well characterized. Yang et al.64 first reported that STIM1 and Orai1 played a crucial role in breast cancer migration and metastasis. We also showed that Orai1 expression was elevated in tumor tissues compared with normal adjacent tissues removed from patients suffering from esophageal squamous cell carcinoma; more importantly, the elevated Orai1 expression was associated with poor prognosis. Inhibition of Orai1 channel either by pharmacological compounds or knocking-down of Orai1 expression could block cancer cell proliferation and migration in vitro and tumor growth in vivo6. Later, Orai1 and STIM1 were also found to promote cell proliferation, migration, invasion and apoptotic resistance in glioblastoma65, pancreatic adenocarcinoma66, prostate cancer67, 68, hepatocellular carcinoma69, and clear cell renal cell carcinoma70. Interestingly, STIM1 and STIM2 are involved in the anti-tumor activity of cytotoxic T cells, i.e. secretion of cytokines, such as TNFα, IL-2 and IFNγ, which induce apoptosis of cancer cells71, 72. Studies about roles of STIM2 in cancer still unconsolidated. A few reports demonstrated STIM2, as a proliferation suppressor, contributed to apoptotic resistance in colorectal cancer cells; while others revealed STIM2-mediated SOCE promote melanoma cells proliferation73, 74. This may indicate that multifaceted STIM2 exert specific functions in different types of cancer.
Compared with Orai1, much less is known about Orai2 and Orai3 in malignant diseases. Except up-regulated in non-small cell lung cancer (NSCLC) and contributed to cell proliferation and tumor grades, Orai3 is also overexpressed in breast cancer tissues, cell lines MCF-7 and T47D as respectively compared to adjacent normal tissues and non-cancerous cell line MCF-10A75, 76. Motiani et al.77,78 showed that abnormal SOCE was mediated by Orai3 in estrogen-receptor-positive breast cancer lines and this increased the proto-oncogenes NFAT transcriptional activity through MAP kinase pathway. Orai3 and Orai1 form arachidonate-regulated Ca2+ entry channel and their ratio represents an oncogenic switch, which facilitates proliferation and apoptotic resistance in prostate cancer68. More detailed discussion on recent advances in SOCE and its contribution to cancer can be found in our review article published elsewhere79.
2.3.4. Purinergic receptors
Purinergic signaling receptors for extracellular nucleotides (P1 and P2 receptors) are widely expressed by mammalian cells. The P2 receptors are divided into P2X and P2Y groups and each group contains several members with distinct ion selectivity and regulatory properties. Numerous studies have demonstrated that P2 receptors involve in cancer cells and are expressed to a very high level in some cases, such as P2X3, P2X5, P2X7 and P2Y2 and P2Y442, 80, 81, 82, 83 (Table 1). In terms of clinical evaluation, P2X3 receptor overexpression was reported to be associated with poor recurrence-free survival in hepatocellular carcinoma patients84.
2.4. Mitochondrial Ca2+ uniporter
Mitochondria are capable for rapid Ca2+ uptake and thus actively shape the overall intracellular Ca2+ signaling. Their Ca2+ uptakes are mediated largely by mitochondrial Ca2+ uniporter (MCU) and regulated by a gate keeper protein mitochondrial Ca2+ uptake 1 (MICU1)85. The accumulated mitochondrial Ca2+ ions are quickly pumped back to cytosol by mitochondrial NCX and mitochondrial H+/Ca2+ exchangers (HCX)86. A wide variety of studies reported the involvement of these mitochondrial Ca2+ handling proteins in cancer cell metabolism, apoptosis and proliferation. Overexpression of MCU is reported in the clinical estrogen receptor negative and basal-like breast cancer samples87. However, down-regulation of MCU in colon and prostate-derived cancers has been shown to promote increased proliferation and bestows resistance to cell death stimuli through diminished mitochondrial Ca2+ levels88. Down-regulation of MICU1, which results in mitochondrial Ca2+ overload, did not alter proliferation in HeLa cells; however, it triggers excessive mROS generation and significantly enhances sensitivity to apoptotic stress85, 89.
3. Drugs targeting Ca2+ channels/transporters/pumps for cancer treatment
The complexity of widespread Ca2+ channels/transporters/pumps with the diverse activation process, offers an abundance of potential targets for pharmacological regulation and cancer chemotherapy. Progress in understanding of the intracellular Ca2+ signaling network, especially the channels/transporters/pumps structure, has significantly advanced the field of drug design and development with particular focus on potentials and specific selectivity inhibitor or regulator. In this section, we attempt to summarize the known compounds or antibodies targeting these above mentioned cancer-involved Ca2+ channels/transporters/pumps, which have been studied in pre-clinical research or even in clinical trials (Table 2). A certain number of these compounds have been demonstrated with promising ability to be used in cancer therapy.
Table 2.
Summary of compounds targeting Ca2+ channels/transporters/pumps.
| Channel/Transporter | Compound | Mechanism | Cancer | Ref. | |
|---|---|---|---|---|---|
| Ca2+-ATPase | SERCA | Cyclopiazonic acid, thapsigargin, G202, KP1019 Saikosaponin-d, Alisol B | Inhibitor | Prostate, hepatoma, colon, cervical, breast cancer and nasopharyngeal | 111, 112, 113, 114, 115 |
| SERCA2 | RL71 | Inhibitor | Colon cancer | 116 | |
| PMCA | [Pt(O,O′-acac)(γ-acac)(DMS)] | Inhibitor | Breast cancer | 117 | |
| VGCC | T-type | KYS05047, mibefradil, NNC-55-0396, amlodipine | Blocker | Hepatoma, lung pancreatic cancer, epidermoid carcinoma and glioma | 118, 119, 120, 121 |
| T-type | Ghrelin | Increase protein expression | Prostate cancer | 122 | |
| TRP | TRPA1 | HC-030031 | Inhibitor | – | 123 |
| Polygodial and analog | Activator | Glioma, melanoma, uterine, lung and breast cancer | 124 | ||
| TRPC | 20-GPPD | Activator | Colon cancer | 125 | |
| TRPC | SKF96365, M804 | Blocker | Glioma | 126, 127 | |
| TRPC1 | EVP4593 | Inhibitor | Neuroblastoma | 128 | |
| TRPC4/5 | (−)-Englerin A | Activator | Renal and colon cancer | 129, 130 | |
| TRPC4/5 | M804 analog, ML204 | Inhibitor | – | 126, 131 | |
| TRPC3/6 | GSK2332255B, GSK2833503A | Inhibitor | – | 132 | |
| TRPC6 | GaQ3 | Induce protein expression | Breast, lung, osteosarcoma and hepatoma | 133 | |
| TRPV | CPZ | Inhibitor | OSCC | 134 | |
| TRPV1 | CBD, Capsaicin | Agonist | Colon cancer, renal carcinoma. | 135, 136, 137, 138 | |
| TRPV2 | 2-APB, cannabinoid, lysophospholipid and probenecid | Agonist | Glioblastoma, bladder cancer | 96, 139, 140 | |
| Ruthenium red, TEA, TRIM, 4-aminopyridine, SKF96365 and tranilast | Antagonist | Breast cancer | 96, 139, 141 | ||
| TRPV4 | GSK1016790A | Agonist | Prostate cancer | 98, 142 | |
| GSK2193874, RN-9893, BTP2 | Inhibitor | – | 143, 144, 145 | ||
| TRPM8 | CBG, M8-B | Inhibitor | Lymphoma, lung, breast, prostate and skin pancreatic, | 146, 147, 148 | |
| D-3263 | Agonist | Various advanced cancer | 93, 149 | ||
| TRPML | ML-SA1 | Agonist | – | 150 | |
| TRPML1 | MK6-83 | Agonist | – | 151 | |
| TRPV6 | TH-1177, Soricidin, SOR-C13 and SOR-C27 | Inhibitor | Ovarian, prostate and brain cancer | 152, 153, 154 | |
| Orai | CRAC | Carboxyamidotriazole, dihydropyridine, MRS-1844, MRS-1845, BTP2 | Inhibitor | Hepatoma, lung, bladder, kidney, NSCLC, glioma and leukemia | 155, 156, 157, 158, 159 |
| STIM1 | ML-9 | Translocation inhibitor | Prostate Cancer | 160, 161 | |
| Orai1-STIM1 | SKF96365 | Inhibitor | Esophageal, breast and colon cancer | 6, 64, 108 | |
| Orai1 | La3+, Gd3+, AnCoA4, SB01990, SPB06836, KM06293, RH01882, GSK-5503A, GSK-7975A, mAbs | Inhibitor | Lung cancer and glioma | 65, 162, 163, 164, 165, 166 | |
| – | 2-APB and its analogues, DPB-162AE and DPB-163AE | Inhibitor/Activator | Colon cancer and glioma | 65, 108, 167, 168 | |
| – | RO2959 | Inhibitor | – | 169 | |
| Purinergic receptor | – | Suramin | Inhibitor | Prostate cancer | 170 |
| P2X7 | AZ10606120, A-740003, A-438079, brilliant blue G, oxidized ATP | Inhibitor | Colon cancer and renal melanoma | 171, 172 | |
| P2X2/3 | A-317491, AF-353 | Inhibitor | – | 172 | |
| RyR | – | 4-Chloro-m-cresol, caffeine and its analogs | Agonist | Breast and prostate | 173, 174 |
| NCX | – | ORM-10103, KB-R7943, OSW-1, DMS, bepridil and benzothiazepine analogues, such as diltiazem, clonazepam and CGP-37157 | Blocker | Leukemia, colon and brain cancer | 175, 176, 177, 178, 179, 180 |
| SKF96365 | Enhancer | Glioma | 181 | ||
| Ghrelin | Increase protein expression | Prostate cancer | 122 | ||
| IP3R | – | Xestospongin B, xestospongin C | Inhibitor | Neuroblastoma, prostate and breast cancer | 182, 183, 184 |
| IP3R1 | 2-APB | Inhibitor | Gastric cancer | 25, 185, 186 | |
| IP3R3 | Heparin, caffeine | Inhibitor | Colon cancer and glioma | 186, 187 | |
| –Not Known | |||||
3.1. Ca2+-ATPase inhibitors
The sustained high cytoplasm Ca2+ is toxic for cells by activating cell death signaling4. Ca2+-ATPases can be easily targeted by shutting-down of these pumps to generate such toxic cytosolic Ca2+ concentrations for either apoptosis or necrosis. A PMCA selective inhibitor [Pt(O,O′-acac)(γ-acac)(DMS)] is used to rapidly induce apoptosis in MCF-7 cells, which may induce ROS in addition to cytosol Ca2+ elevation117. Our earlier work showed that the depletion of ER Ca2+ stores itself is sufficient to cause ER stress and to induce programmed cell death pathways188. A selective inhibitor of SERCA pump, thapsigargin (TG), is used to inhibit Ca2+ uptake into ER and deplete ER Ca2+ stores. The application of TG as chemotherapeutic agent has been extensively studied in prostate cancer and other cancers14,112. However, a barrier preventing the direct usage of TG as clinical effective drug is its non-selectivity since TG will destroy intracellular Ca2+ homeostasis not only in cancer cells but also in normal cells. Thus the research effort on development of TG as chemotherapy drug has been focused on tumor targeting. One successful example is G202, in which an analogue to TG is conjugated to prostate-specific membrane antigen (PSMA) targeting peptide. PSMA is a type II membrane carboxypeptidase and is overexpressed in prostate cancer cells and most tumor endothelial cells, but not in normal vasculature or normal tissue epithelium. As a prodrug, G202 itself is non-toxic since it cannot enter the cell due to its size. Once it reaches tumor, it binds with PSMA and subsequently PSMA can cleave the peptide and release active cytotoxic analog of TG. G202, later termed as mipsagargin, significantly inhibits tumor progression including prostate, breast and bladder cancers, while presenting minimally toxicity to the host animals112. G202 has showed promising results in several pre-clinical studies and is currently in phase II clinical trial for prostate cancer and progressive glioblastoma.
3.2. Voltage-gated Ca2+ channel inhibitors
The first well-studied family of compounds targeting Ca2+ signaling are the inhibitors for VGCCs, which are widely used in the cardiovascular or nervous system diseases189. As accumulating evidences reveal the important roles of VGCCs in a number of cancers, many investigators have launched the studies to repurpose the FDA approved drugs targeting VGCCs for cancer treatment42. In fact, as early as in 1990s, some structurally unrelated L-type VGCCs antagonists were tested for their potent inhibitory effects on breast tumor progression190. The dihydropyridine Ca2+ channel blocker, amlodipine, was found to inhibit the growth of human epidermoid carcinoma A431 cells both in vitro and in vivo, via arresting cell cycle at G1 phase and reducing phosphorylation of retinoblastoma protein, expression levels of cyclin D1 and cyclin dependent kinase 4120. Another interesting case is mibefradil, a T- and L-type Ca2+ channel blocker used for anti-hypertensive. It was later voluntarily withdrawn from market due to its side effect of inhibiting cytochrome P450 enzymes 2D6, 3A4 and p-glycoprotein. However, mibefradil was shown to be able efficiently to reduce tumor size, to improve the survival rate in glioma animal model as well as in a patient derived pancreas xenograft animal model42, 121. Therefore, mibefradil was repurposed for pancreatic cancer and high-grade glioma therapy. FDA swiftly approved mibefradil as an orphan drug for pancreatic cancer treatment in 2008 and its use for glioma treatment has also been moved into clinical trial191. Moreover, a mibefradil derived novel compound, NNC-55-0396, was developed to selectively target Ca2+ channel and exhibits less inhibitory effect on cytochrome P450 3A4. This new derived mibefradil compound appears to be a promising chemotherapy drug for that it is able to effectively inhibit angiogenesis in cancer cell lines but with minimal off-target effect42, 119.
3.3. TRP channel regulators
The imidazole compound SKF-96365 and related antimycotic compounds including econazole, miconazole, and clotrimazole can inhibit CRACs and some TRP channels192. While SKF-96365 was firstly described to block receptor-mediated Ca2+ entry in human platelets, neutrophils and endothelial cells193, it later was used to inhibit ovarian cancerous cell growth and tumorigenesis via reducing activities of different subtypes of TRPCs194. Treatment of SKF-96365 was reported to enhance radio-sensitization in glioblastoma, which contain high expressing levels of TRPC6 channels90. In addition, SKF-96365 can also cause cell cycle arrest at S and G2 phases in glioblastoma cells via enhancing reverse mode of the NCX1, independent of TRPCs181.
Using a structure-based design, a synthetic compound TH-1177 mimicking dihydropyridines was developed as a TRPV channel blocker. It inhibits prostate cancer cell proliferation in vitro and in vivo154. However, there is one issue preventing TH-1177 from further clinical application. It preferentially inhibits TRPV5 but less effective for TRPV6 channel; whereas TRPV6 is the most abundant Ca2+ entry channel in prostate cancer cells with its expression level as high as 45-fold more than TRPV5. Thus, TH-1177 was further modified to possess high selectivity for TRPV6 and this new agent has been demonstrated significantly improved inhibitory effects on cell proliferation in prostate and breast cancer152.
GSK1016790A is a selective TRPV4 channel agonist developed recently. It is at least 300-fold more potent than the commonly used TRPV4 activator 4α-PDD142. TRPV4 is able to regulate tumor angiogenesis and vessel maturation, thus GSK1016790A has been proposed to be used together with other anticancer drugs, such as cisplatin, to improve tumor penetration for more effective cancer therapy98. By mimicking the C-terminus of soricidin, two TRPV6 inhibitors, the SOR-C13 and SOR-C27 bind TRPV6 with high affinity in ovarian cancer cells. SOR-C13 is currently in phase I clinical trial for advanced cancers, in which TRPV6 channels are normally overexpressed153. Another compound in phase I clinical trial with similar mechanism is D-3263. It is an activator for TRPM8 and induces cancer cell apoptosis, which is proposed to be used for patients with solid tumors93.
Capsaicin, a well-known activator for TRPV1, was shown to induce apoptosis by its action on TRPV6 but independent on TRPV1, followed by activation of calpains in human small cell lung cancer cells (SCLC) in vitro and in vivo101. TRPV antagonist capsazepine (CPZ) was demonstrated to effectively inhibit oral squamous cell carcinoma cells (OSCC) growth in vivo. The anti-cancer mechanism of CPZ may also rely on ROS release, independent of TRPV1134. Subcutaneous injection of either capsaicin or CPZ significantly suppresses PC-3 tumor growth by inducing apoptosis of tumor cells in vivo, suggesting they are promising chemotherapy drugs195. Sesquiterpene (−)-englerin A is a selective and potent activator for TRPC4 and TRPC5, which results in massive Ca2+ influx followed by cell death and retarded tumor cell growth129, 130. However, its severe lethal side effect must be resolved before it can be considered as a potential therapeutic agent. The natural compound, 20-GPPD, a metabolite of ginseng saponin, induces TRPC-mediated Ca2+ influx and apoptosis in CT-26 cells and reduces tumor mass by 75% in vivo although the exact molecular target remains unknown125. Some cannabinoid compounds, in particular cannabidiol (CBD), stimulate TRPV1 while the non-psychotropic cannabigerol (CBG) is an antagonist of TRPM8146. CBD displayed anti-invasive action in human lung carcinoma A549 cells, dependent on the effect of CBD cannabinoid receptor and TRPV1196.
3.4. Orai inhibitors
Among many known compounds targeting CRAC or SOCE, the Orai channel blockers are particularly well studied. Because CRAC channels present highly selective Ca2+ conductance, they are subject to blockage by the trivalent ions La3+ and Gd3+. Both La3+ and Gd3+ can directly block CRAC pore formed by the I–II loop region of Orai1. However, this effect is not specific for CRAC, as La3+ and Gd3+ also block other Ca2+ channels as well, such as Cav, TRP channel and PMCA162, 163, 197.
The first Orai1 inhibitor used in cancer study is SKF-96365. Yang et al. 64, demonstrated that SKF-96365 can inhibit breast cancer cell migration in vitro and reduce tumor growth and metastasis in vivo. We also showed that SKF-96365 inhibited Orai1-mediated SOCE and intracellular Ca2+ oscillations in esophageal cancer cells and resulted in significant retarded tumor growth in nude mice6.
Another commonly used SOCE inhibitor is 2-APB, which was initially identified as a noncompetitive antagonist of IP3R (at rather high concentration ~100 μmol/L)185. The effects of 2-APB on SOCE are multifaceted. On one hand, 2-APB inhibits Orai1 current without interrupting STIM1 and Orai1 interaction198; on the other hand, it can directly activate Orai3 channel independent on store depletion or STIM1199, 200. There is also a dose-dependent bimodal effect of 2-APB on SOCE, with strong enhancement at low doses (<5 μmol/L) and transient enhancement followed by inhibition at high concentrations (>20 μmol/L)167, 201. Although several studies demonstrated that 2-APB effectively inhibit cancer cell proliferation and tumor progression, the non-selective and multiple-target nature renders 2-APB unsuitable for chemotherapy25, 108. Much effort has been devoted to develop 2-APB derived compounds to overcome the obstacle. For example, DPB-162AE and DPB-163AE are constructed as dimers of 2-APB. They are over 100-fold more potent than 2-APB on SOCE inhibition, without affecting IP3R function at such concentrations168. Nevertheless, there will be a long journey before such compounds can be developed as effective chemotherapeutic drug.
Another line of research is screening of small-molecule compounds that bound to Orai1 and/or STIM1 in a microarray system containing minimal functional domains of STIM1 and Orai1. Some novel STIM–Orai inhibitors have been identified using this high-throughput screen approach, such as AnCoA4. AnCoA4 inhibits CRACs and attenuates T-cell activation both in in vitro and in vivo. It reduces the recruitment of Orai1 into puncta and also inhibits the activity of the constitutively active Orai1V102C channels, independent on STIM1166.
ML-9 is an inhibitor for myosin light-chain kinase (MLCK) and appears to be able to disperse STIM1 puncta, thus to inhibit SOCE. Although its target and mechanism of action are unclear, ML-9 is the only known inhibitor so far to inhibit SOCE through interference with STIM1 translocation160. Later, ML-9 alone was proved to effectively induce prostate cancer cell death associated with autophagy in a concentration and Ca2+ dependent manner. Furthermore, combination of ML-9 and some existing anticancer drugs, such as docetaxel, significantly promotes cancer cell death, suggesting ML-9 as a promising adjuvant drug for chemotherapy161.
RO2959 is a novel, potent and selective SOCE inhibitor (IC50, ~40 nmol/L). It inhibits a wide range of Ca2+ dependent cellular functions including gene expression, cytokine production, and proliferation in T cells. To achieve the IC50 values at nmol/L level, it requires pre-incubation of cells for more than 30 min, which suggests that RO2959 may act on Orai1 channels indirectly169. The effect of RO2959 in cancer and molecular basis of drug action, including whether it affects the function and choreography of STIM1, are still unclear.
Another class of potent CRAC inhibitor is designed targeting the Ca2+ selectivity filter of Orai channel. In particular, E106 accounts for Ca2+ selectivity in Orai1 and can be blocked by extracellular protons202. This class of inhibitor includes SB01990, SPB06836, KM06293 and RH01882, which all present the capability to alter the pore geometry of Orai1 and diminishes SOCE164.
Two pyrazole derivatives GSK-5503A and GSK-7975A slowly inhibit Orai1- and Orai3-mediated SOCE currents without affecting STIM1–Orai1 coupling. It takes a few minutes for the two compounds to have effects, suggesting that the mechanism is likely other than through the CRAC channel activation process165. The IC50 is increased 10-fold in the Orai1E106D and in 2-APB activated Orai3 channels, both of which are poorly selective for Ca2+ and exhibit wider pores than the Orai1WT channel165, 200, 203. Interestingly, these compounds also inhibit TRPV6 channels, possibly due to similarities between CRAC and TRPV6 channels in the target site204. The Pyr analogs, including Pyr2, 3, 6 and 10, show different selectivity on TRPC3 and Orai1/STIM1-mediated Ca2+ entry. Pyr10 is potent and selective for TRPC3-mediated responses (18-fold), and Pyr6 prefers Orai1/STIM1, while Pyr3 equally blocked the two channels205. The best-studied member of this group is Pyr2 (also known as BTP2 or YM-58483), a potent inhibitor for both CRAC and TRPC-mediated Ca2+ entry127, 157, 206. However, it also proposed that a key mechanism of BTP2 inhibition of Ca2+ influx and cytokine release might be related to its ability to depolarize the cell membrane via TRPM4 activation, thereby reducing the driving force for Ca2+ entry145. Synta 66 selectively inhibits ICRAC in RBL-1 cells, which is structurally similar to BTP2 but contains a biphenyl group rather than the pyrazole ring in BTP2207. Compared with BTP2 and the GSK compounds, the speed of inhibition by Synta 66 is rather slow. It requires pre-incubation with cells for more than 1 h and its effect is poorly reversible. These data suggest that the pore blocking mechanism is unlikely the case for Synta66. Moreover, Synta66 has no effect on STIM1 puncta formation, suggesting that it does not inhibit the early steps of STIM1 activation and translocation to junctional ER-PM sites208. CM2489 and CM3457 are another two promising inhibitors for SOCE, demonstrated in lymphocytes and T cell-derived cytokine production209. CM2489 has completed phase I clinical trials for the treatment of moderate-to-severe plaque psoriasis. This is the first CRAC channel inhibitor to be tested on humans and represents a promising lead for the development of novel therapeutics for human diseases, including cancers.
Recombinant Orai1 monoclonal antibodies (mAbs) exhibit strong and specific binding against Orai1 at amino acid residues 210–217 in the second extracellular loop. These mAbs, especially 2C1.1, inhibit ICRAC in Orai1 high expressed Jurkat T cells as well as HEK293210. Functional assays indicate that these mAbs strongly inhibited NFAT-dependent gene transcription in Jurkat cells or TG- and ionomycin-induced cytokine secretion from human T cells. Taking a similar approach, another specific anti-Orai1 mAb targeting the second extracellular loop, inhibits the proliferation of T cells and cytokine production both in vitro and in vivo. Further mechanistic studies suggested that mAb-targeted Orai1 proteins are internalized, which resulting in loss of functional SOCE activity211. Although the anti-Orai1 mAbs-based potential therapy is limited in immune diseases, it raises high hope of similar application for cancer treatment.
3.5. Miscellaneous
Many regulators targeting Ca2+ channels/transporters/pumps other than the above mentioned, have been used in various areas, such as immune suppression, anti-hypertension and anti-cancer. Xestospongin B as a specific IP3R inhibitor has been demonstrated to reduce proliferation and colony formation ability, while induce necrotic death, specifically in tumorigenic breast cells, compared with non-tumorigenic cell lines184. Caffeine, a RyR agonist, can induce apoptosis in prostate cancer cells, while another agonist 4-chloro-m-cresol can inhibit the breast cancer cell growth173. Several compounds targeting NCX, such as OSW-1, showed apoptosis-induce function by causing mitochondrial Ca2+ overload in leukemia174. Brilliant blue G, a P2X7 antagonist, exhibits inhibitory effect on glioma growth. Emodin, a P2X non-specific inhibitor, reduces P2X7-mediated cancer cell migration. A number of P2X7 receptor regulators, such as antagonist A-438079 and A-740003 are mainly studied in pain relief. Since the P2 receptors play important roles in a certain number of cancers, it is reasonable to believe these antagonists may be potential effective anti-cancer drugs as well172. If that holds true, these compounds could exert dual functions as both chemotherapeutic agents and pain killer.
4. Conclusion and future directions
It is becoming evident that Ca2+ channels/transporters/pumps are involved in a wide range of cancers. Dysregulated Ca2+ homeostasis may play a role more like a “driver” than a “passenger” in carcinogenesis or tumorigenesis. As summarized in this review, this relatively new field has already importantly contributed to the identification of possible chemotherapeutic agents for a certain number of cancers, with a few even moved to clinical trials. Since many of the Ca2+ channels/transporters/pumps may play a role in normal physiology and normal cell functions, one challenge in drug development targeting these Ca2+ signaling proteins is to identify their cancer specific properties. Moreover, it is also important to identify a set of the channels that contribute to the tumor development in a given patient tissue. Structure-based rational design of more potent, more specific and less off-target compounds targeting Ca2+ channels/transporters/pumps will likely provide promising leads for novel cancer treatment in the coming years.
Acknowledgements
The research in Pan׳s laboratory is partially supported by NIH R01-CA185055 (to Zui Pan) and Chaochu Cui received postgraduate student training of internationalization level promotion program from Sun Yat-sen University (02300-52114000).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Csordas G., Varnai P., Golenar T., Roy S., Purkins G., Schneider T.G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkash J., Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87:587–595. doi: 10.1016/j.lfs.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McConkey D.J., Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun. 1997;239:357–366. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- 5.Baudouin-Legros M., Brouillard F., Tondelier D., Hinzpeter A., Edelman A. Effect of ouabain on CFTR gene expression in human Calu-3 cells. Am J Physiol Cell Physiol. 2003;284:C620–C626. doi: 10.1152/ajpcell.00457.2002. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H., Zhang H., Jin F., Fang M., Huang M., Yang C.S. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5:3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parekh A.B. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Berridge M.J. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 9.Smedler E., Uhlen P. Frequency decoding of calcium oscillations. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Cullen P.J. Calcium signalling: the ups and downs of protein kinase C. Curr Biol. 2003;13:R699–R701. doi: 10.1016/j.cub.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 11.Hu Q. [Ca2+]i Oscillation frequency regulates agonist-stimulated NF-κB transcriptional activity. J Biol Chem. 1999;274:33995–33998. doi: 10.1074/jbc.274.48.33995. [DOI] [PubMed] [Google Scholar]
- 12.Dolmetsch R.E., Xu K., Lewis R.S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 13.Kondratskyi A., Yassine M., Kondratska K., Skryma R., Slomianny C., Prevarskaya N. Calcium-permeable ion channels in control of autophagy and cancer. Front Physiol. 2013;4:272. doi: 10.3389/fphys.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu J., Liu H., Fu T., Xu Y. Thapsigargin increases apoptotic cell death in human hepatoma BEL-7404 cells. Cell Res. 1995;5:59–65. [Google Scholar]
- 15.Vandewalle B., Hornez L., Wattez N., Revillion F., Lefebvre J. Vitamin-D3 derivatives and breast-tumor cell growth: effect on intracellular calcium and apoptosis. Int J Cancer. 1995;61:806–811. doi: 10.1002/ijc.2910610611. [DOI] [PubMed] [Google Scholar]
- 16.Liu L.H., Boivin G.P., Prasad V., Periasamy M., Shull G.E. Squamous cell tumors in mice heterozygous for a null allele of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump. J Biol Chem. 2001;276:26737–26740. doi: 10.1074/jbc.C100275200. [DOI] [PubMed] [Google Scholar]
- 17.Roderick H.L., Cook S.J. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 18.Gkika D., Prevarskaya N. TRP channels in prostate cancer: the good, the bad and the ugly? Asian J Androl. 2011;13:673–676. doi: 10.1038/aja.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karacosta L.G., Foster B.A., Azabdaftari G., Feliciano D.M., Edelman A.M. A regulatory feedback loop between Ca2+/calmodulin--dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression. J Biol Chem. 2012;287:24832–24843. doi: 10.1074/jbc.M112.370783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteith G.R., Davis F.M., Roberts-Thomson S.J. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–31673. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikoshiba K. IP3 receptors and their role in cell function. In: Joachim K., Marek M., editors. New Comprehensive Biochemistry. Amsterdam: Elsevier; 2007. pp. 267–285. [Google Scholar]
- 22.Vervloessem T., Yule D.I., Bultynck G., Parys J.B. The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca2+-release channel. Biochim Biophys Acta. 2015;1853:1992–2005. doi: 10.1016/j.bbamcr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang S.S., Han K.S., Ku B.M., Lee Y.K., Hong J., Shin H.Y. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010;70:1173–1183. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakakura C., Hagiwara A., Fukuda K., Shimomura K., Takagi T., Kin S. Possible involvement of inositol 1,4,5-trisphosphate receptor type 3 (IP3R3) in the peritoneal dissemination of gastric cancers. Anticancer Res. 2003;23:3691–3697. [PubMed] [Google Scholar]
- 26.Shibao K., Fiedler M.J., Nagata J., Minagawa N., Hirata K., Nakayama Y. The type III inositol 1,4,5-trisphosphate receptor is associated with aggressiveness of colorectal carcinoma. Cell calcium. 2010;48:315–323. doi: 10.1016/j.ceca.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedberg M.L., Goh G., Chiosea S.I., Bauman J.E., Freilino M.L., Zeng Y. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest. 2016;126:169–180. doi: 10.1172/JCI82066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akl H., Monaco G., La Rovere R., Welkenhuyzen K., Kiviluoto S., Vervliet T. IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang K., Leandro Gomez-Amaro R., Stachura D.L., Tang H., Peng X., Fang X. Loss of IP3R-dependent Ca2+ signalling in thymocytes leads to aberrant development and acute lymphoblastic leukemia. Nat Commun. 2014;5:4814. doi: 10.1038/ncomms5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs J. The plethora of PMCA isoforms: alternative splicing and differential expression. Biochim Biophys Acta. 2015;1853:2018–2024. doi: 10.1016/j.bbamcr.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Dang D., Rao R. Calcium-ATPases: gene disorders and dysregulation in cancer. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbamcr.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan L., Li A., Li W., Cai P., Yang B., Zhang M. Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed Pharmacother. 2014;68:1141–1148. doi: 10.1016/j.biopha.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Brouland J.P., Gelebart P., Kovacs T., Enouf J., Grossmann J., Papp B. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. Am J Pathol. 2005;167:233–242. doi: 10.1016/S0002-9440(10)62968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dellis O., Arbabian A., Brouland J.P., Kovacs T., Rowe M., Chomienne C. Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein–Barr virus latent membrane protein-1. Mol Cancer. 2009;8:59. doi: 10.1186/1476-4598-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grice D.M., Vetter I., Faddy H.M., Kenny P.A., Roberts-Thomson S.J., Monteith G.R. Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J Biol Chem. 2010;285:37458–37466. doi: 10.1074/jbc.M110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng M., Grice D.M., Faddy H.M., Nguyen N., Leitch S., Wang Y. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee W.J., Roberts-Thomson S.J., Monteith G.R. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem Biophys Res Commun. 2005;337:779–783. doi: 10.1016/j.bbrc.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 38.Aung C.S., Kruger W.A., Poronnik P., Roberts-Thomson S.J., Monteith G.R. Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochem Biophys Res Commun. 2007;355:932–936. doi: 10.1016/j.bbrc.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 39.Saito K., Uzawa K., Endo Y., Kato Y., Nakashima D., Ogawara K. Plasma membrane Ca2+ ATPase isoform 1 down-regulated in human oral cancer. Oncol Rep. 2006;15:49–55. [PubMed] [Google Scholar]
- 40.Catterall W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 41.Wang C.Y., Lai M.D., Phan N.N., Sun Z., Lin Y.C. Meta-analysis of public microarray datasets reveals voltage-gated calcium gene signatures in clinical cancer patients. PLoS One. 2015;10:e0125766. doi: 10.1371/journal.pone.0125766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kale V.P., Amin S.G., Pandey M.K. Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochim Et Biophys Acta (BBA)-Biomembr. 2015;1848:2747–2755. doi: 10.1016/j.bbamem.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Dziegielewska B., Gray L.S., Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflugers Arch. 2014;466:801–810. doi: 10.1007/s00424-014-1444-z. [DOI] [PubMed] [Google Scholar]
- 44.Ohkubo T., Yamazaki J. T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int J Oncol. 2012;41:267–275. doi: 10.3892/ijo.2012.1422. [DOI] [PubMed] [Google Scholar]
- 45.Gackiere F., Bidaux G., Delcourt P., van Coppenolle F., Katsogiannou M., Dewailly E. CaV3.2 T-type calcium channels are involved in calcium-dependent secretion of neuroendocrine prostate cancer cells. J Biol Chem. 2008;283:10162–10173. doi: 10.1074/jbc.M707159200. [DOI] [PubMed] [Google Scholar]
- 46.Latour I., Louw D.F., Beedle A.M., Hamid J., Sutherland G.R., Zamponi G.W. Expression of T-type calcium channel splice variants in human glioma. Glia. 2004;48:112–119. doi: 10.1002/glia.20063. [DOI] [PubMed] [Google Scholar]
- 47.Wu L.J., Sweet T.B., Clapham D.E. International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 49.El Hiani Y., Lehen׳kyi V., Ouadid-Ahidouch H., Ahidouch A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch Biochem Biophys. 2009;486:58–63. doi: 10.1016/j.abb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Yang S.L., Cao Q., Zhou K.C., Feng Y.J., Wang Y.Z. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28:1320–1328. doi: 10.1038/onc.2008.475. [DOI] [PubMed] [Google Scholar]
- 51.Aydar E., Yeo S., Djamgoz M., Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009;9:23. doi: 10.1186/1475-2867-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prevarskaya N., Zhang L., Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Ouadid-Ahidouch H., Dhennin-Duthille I., Gautier M., Sevestre H., Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med. 2013;19:117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Ding X., He Z., Shi Y., Wang Q., Wang Y. Targeting TRPC6 channels in oesophageal carcinoma growth. Expert Opin Ther Targets. 2010;14:513–527. doi: 10.1517/14728221003733602. [DOI] [PubMed] [Google Scholar]
- 55.Monet M., Lehen׳kyi V., Gackiere F., Firlej V., Vandenberghe M., Roudbaraki M. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L., Barritt G.J. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr-Relat Cancer. 2006;13:27–38. doi: 10.1677/erc.1.01093. [DOI] [PubMed] [Google Scholar]
- 57.Zhang S.S., Wen J., Yang F., Cai X.L., Yang H., Luo K.J. High expression of transient potential receptor C6 correlated with poor prognosis in patients with esophageal squamous cell carcinoma. Med Oncol. 2013;30:607. doi: 10.1007/s12032-013-0607-7. [DOI] [PubMed] [Google Scholar]
- 58.Tsavaler L., Shapero M.H., Morkowski S., Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–3769. [PubMed] [Google Scholar]
- 59.Dhennin-Duthille I., Gautier M., Faouzi M., Guilbert A., Brevet M., Vaudry D. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 60.Guilbert A., Gautier M., Dhennin-Duthille I., Haren N., Sevestre H., Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009;297:C493–C502. doi: 10.1152/ajpcell.00624.2008. [DOI] [PubMed] [Google Scholar]
- 61.Chodon D., Guilbert A., Dhennin-Duthille I., Gautier M., Telliez M.S., Sevestre H. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer. 2010;10:212. doi: 10.1186/1471-2407-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark K., Middelbeek J., van Leeuwen F.N. Interplay between TRP channels and the cytoskeleton in health and disease. Eur J Cell Biol. 2008;87:631–640. doi: 10.1016/j.ejcb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Bergmeier W, Weidinger C, Zee I, Feske S. Emerging roles of store-operated Ca2+ entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels (Austin) 2013;7:379-91 [DOI] [PMC free article] [PubMed]
- 64.Yang S., Zhang J.J., Huang X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 65.Motiani R.K., Hyzinski-Garcia M.C., Zhang X., Henkel M.M., Abdullaev I.F., Kuo Y.H. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013;465:1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kondratska K., Kondratskyi A., Yassine M., Lemonnier L., Lepage G., Morabito A. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim Biophys Acta. 2014;1843:2263–2269. doi: 10.1016/j.bbamcr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Flourakis M., Lehen׳kyi V., Beck B., Raphael M., Vandenberghe M., Abeele F.V. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubois C., Vanden Abeele F., Lehen׳kyi V., Gkika D., Guarmit B., Lepage G. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26:19–32. doi: 10.1016/j.ccr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 69.Yang N., Tang Y., Wang F., Zhang H., Xu D., Shen Y. Blockade of store-operated Ca2+ entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013;330:163–169. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 70.Kim J.H., Lkhagvadorj S., Lee M.R., Hwang K.H., Chung H.C., Jung J.H. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014;448:76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 71.Weidinger C., Shaw P.J., Feske S. STIM1 and STIM2-mediated Ca2+ influx regulates antitumour immunity by CD8+ T cells. EMBO Mol Med. 2013;5:1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oh-Hora M., Yamashita M., Hogan P.G., Sharma S., Lamperti E., Chung W. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aytes A., Mollevi D.G., Martinez-Iniesta M., Nadal M., Vidal A., Morales A. Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol Carcinog. 2012;51:746–753. doi: 10.1002/mc.20843. [DOI] [PubMed] [Google Scholar]
- 74.Stanisz H., Saul S., Muller C.S., Kappl R., Niemeyer B.A., Vogt T. Inverse regulation of melanoma growth and migration by Orai1/STIM2-dependent calcium entry. Pigment Cell Melanoma Res. 2014;27:442–453. doi: 10.1111/pcmr.12222. [DOI] [PubMed] [Google Scholar]
- 75.Faouzi M., Hague F., Potier M., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol. 2011;226:542–551. doi: 10.1002/jcp.22363. [DOI] [PubMed] [Google Scholar]
- 76.Ay A.S., Benzerdjerb N., Sevestre H., Ahidouch A., Ouadid-Ahidouch H. Orai3 constitutes a native store-operated calcium entry that regulates non small cell lung adenocarcinoma cell proliferation. PLoS One. 2013;8:e72889. doi: 10.1371/journal.pone.0072889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Motiani R.K., Zhang X., Harmon K.E., Keller R.S., Matrougui K., Bennett J.A. Orai3 is an estrogen receptor alpha-regulated Ca2+ channel that promotes tumorigenesis. Faseb J. 2013;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Motiani R.K., Abdullaev I.F., Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan Z., Ma J. Open Sesame: treasure in store-operated calcium entry pathway for cancer therapy. Sci China Life Sci. 2015;58:48–53. doi: 10.1007/s11427-014-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giannuzzo A., Pedersen S.F., Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:1–15. doi: 10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin H., Eun S.Y., Lee J.S., Park S.W., Lee J.H., Chang K.C. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014;16:R77. doi: 10.1186/bcr3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie R., Xu J., Wen G., Jin H., Liu X., Yang Y. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem. 2014;289:19137–19149. doi: 10.1074/jbc.M113.540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nylund G., Hultman L., Nordgren S., Delbro D.S. P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton Autacoid Pharmacol. 2007;27:79–84. doi: 10.1111/j.1474-8673.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 84.Maynard J.P., Lee J.S., Sohn B.H., Yu X., Lopez-Terrada D., Finegold M.J. P2X3 purinergic receptor overexpression is associated with poor recurrence-free survival in hepatocellular carcinoma patients. Oncotarget. 2015;6:41162–41179. doi: 10.18632/oncotarget.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Csordas G., Golenar T., Seifert E.L., Kamer K.J., Sancak Y., Perocchi F. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 87.Curry M.C., Peters A.A., Kenny P.A., Roberts-Thomson S.J., Monteith G.R. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2013;434:695–700. doi: 10.1016/j.bbrc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Marchi S., Lupini L., Patergnani S., Rimessi A., Missiroli S., Bonora M. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mallilankaraman K., Doonan P., Cardenas C., Chandramoorthy H.C., Muller M., Miller R. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding X., He Z., Zhou K., Cheng J., Yao H., Lu D. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- 91.Duncan L.M., Deeds J., Hunter J., Shao J., Holmgren L.M., Woolf E.A. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- 92.Yee N.S., Chan A.S., Yee J.D., Yee R.K. TRPM7 and TRPM8 ion channels in pancreatic adenocarcinoma: potential roles as cancer biomarkers and targets. Sci (Cairo) 2012:415158. doi: 10.6064/2012/415158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y., Qin N. TRPM8 in health and disease: cold sensing and beyond. Adv Exp Med Biol. 2011;704:185–208. doi: 10.1007/978-94-007-0265-3_10. [DOI] [PubMed] [Google Scholar]
- 94.Kalogris C., Caprodossi S., Amantini C., Lambertucci F., Nabissi M., Morelli M.B. Expression of transient receptor potential vanilloid-1 (TRPV1) in urothelial cancers of human bladder: relation to clinicopathological and molecular parameters. Histopathology. 2010;57:744–752. doi: 10.1111/j.1365-2559.2010.03683.x. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez M.G., Sanchez A.M., Collado B., Malagarie-Cazenave S., Olea N., Carmena M.J. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 96.Perálvarez-Marín A., Doñate-Macian P., Gaudet R. What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J. 2013;280:5471–5487. doi: 10.1111/febs.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fusi C., Materazzi S., Minocci D., Maio V., Oranges T., Massi D. Transient receptor potential vanilloid 4 (TRPV4) is downregulated in keratinocytes in human non-melanoma skin cancer. J Invest Dermatol. 2014;134:2408–2417. doi: 10.1038/jid.2014.145. [DOI] [PubMed] [Google Scholar]
- 98.Adapala R.K., Thoppil R.J., Ghosh K., Cappelli H.C., Dudley A.C., Paruchuri S. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35:314–322. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fiorio Pla A., Ong H.L., Cheng K.T., Brossa A., Bussolati B., Lockwich T. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–212. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raphael M., Lehen׳kyi V., Vandenberghe M., Beck B., Khalimonchyk S., Vanden Abeele F. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1413409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lau J.K., Brown K.C., Dom A.M., Witte T.R., Thornhill B.A., Crabtree C.M. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis. 2014;19:1190–1201. doi: 10.1007/s10495-014-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peters A.A., Simpson P.T., Bassett J.J., Lee J.M., Da Silva L., Reid L.E. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor-negative breast cancer. Mol Cancer Ther. 2012;11:2158–2168. doi: 10.1158/1535-7163.MCT-11-0965. [DOI] [PubMed] [Google Scholar]
- 103.Vy Lehen’kyi, Raphaël M., Prevarskaya N. The role of the TRPV6 channel in cancer. J Physiol. 2012;590:1369–1376. doi: 10.1113/jphysiol.2011.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun J., Lu F., He H., Shen J., Messina J., Mathew R. STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol. 2014;207:535–548. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moccia F., Dragoni S., Poletto V., Rosti V., Tanzi F., Ganini C. Orai1 and transient receptor potential channels as novel molecular targets to impair tumor neovascularization in renal cell carcinoma and other malignancies. Anti-Cancer Agents Med Chem. 2014;14:296–312. doi: 10.2174/18715206113139990315. [DOI] [PubMed] [Google Scholar]
- 106.Zhan Z.Y., Zhong L.X., Feng M., Wang J.F., Liu D.B., Xiong J.P. Over-expression of Orai1 mediates cell proliferation and associates with poor prognosis in human non-small cell lung carcinoma. Int J Clin Exp Pathol. 2015;8:5080–5088. [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y.F., Chiu W.T., Chen Y.T., Lin P.Y., Huang H.J., Chou C.Y. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J.Y., Sun J., Huang M.Y., Wang Y.S., Hou M.F., Sun Y. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34:4358–4367. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McAndrew D., Grice D.M., Peters A.A., Davis F.M., Stewart T., Rice M. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 110.Sobradillo D., Hernandez-Morales M., Ubierna D., Moyer M.P., Nunez L., Villalobos C. A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J Biol Chem. 2014;289:28765–28782. doi: 10.1074/jbc.M114.581678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yao S., Gallenkamp D., Wolfel K., Luke B., Schindler M., Scherkenbeck J. Synthesis and SERCA activities of structurally simplified cyclopiazonic acid analogues. Bioorg Med Chem. 2011;19:4669–4678. doi: 10.1016/j.bmc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 112.Denmeade S.R., Mhaka A.M., Rosen D.M., Brennen W.N., Dalrymple S., Dach I. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci Transl Med. 2012;4:140ra86. doi: 10.1126/scitranslmed.3003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wong V.K., Li T., Law B.Y., Ma E.D., Yip N.C., Michelangeli F. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013;4:e720. doi: 10.1038/cddis.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Law B.Y., Wang M., Ma D.L., Al-Mousa F., Michelangeli F., Cheng S.H. Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol Cancer Ther. 2010;9:718–730. doi: 10.1158/1535-7163.MCT-09-0700. [DOI] [PubMed] [Google Scholar]
- 115.Bergamo A., Masi A., Jakupec M.A., Keppler B.K., Sava G. Inhibitory effects of the ruthenium complex KP1019 in models of mammary cancer cell migration and invasion. Metal-Based Drugs. 2009:681270. doi: 10.1155/2009/681270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang B., Zhang M., Gao J., Li J., Fan L., Xiang G. Small molecule RL71 targets SERCA2 at a novel site in the treatment of human colorectal cancer. Oncotarget. 2015;6:37613–37625. doi: 10.18632/oncotarget.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muscella A., Calabriso N., Vetrugno C., Fanizzi F.P., De Pascali S.A., Storelli C. The platinum (II) complex [Pt(O,O׳-acac)(γ-acac)(DMS)] alters the intracellular calcium homeostasis in MCF-7 breast cancer cells. Biochem Pharmacol. 2011;81:91–103. doi: 10.1016/j.bcp.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 118.Rim H.K., Lee H.W., Choi I.S., Park J.Y., Choi H.W., Choi J.H. T-type Ca2+ channel blocker, KYS05047 induces G1 phase cell cycle arrest by decreasing intracellular Ca2+ levels in human lung adenocarcinoma A549 cells. Bioorg Med Chem Lett. 2012;22:7123–7126. doi: 10.1016/j.bmcl.2012.09.076. [DOI] [PubMed] [Google Scholar]
- 119.Kim K.H., Kim D., Park J.Y., Jung H.J., Cho Y.H., Kim H.K. NNC 55-0396, a T-type Ca2+ channel inhibitor, inhibits angiogenesis via suppression of hypoxia-inducible factor-1α signal transduction. J Mol Med (Berl) 2015;93:499–509. doi: 10.1007/s00109-014-1235-1. [DOI] [PubMed] [Google Scholar]
- 120.Yoshida J., Ishibashi T., Nishio M. G1 cell cycle arrest by amlodipine, a dihydropyridine Ca2+ channel blocker, in human epidermoid carcinoma A431 cells. Biochem Pharmacol. 2007;73:943–953. doi: 10.1016/j.bcp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 121.Garrido-Laguna I., Tan A.C., Villarroel M.C., Rajeshkumar N., Rubio-Viqueira B., Gray L. Activity of the T-type calcium channel antagonist Mibefradil in pancreatic cancer xenografts. Clin Cancer Res. 2008;14 [B49-B49] [Google Scholar]
- 122.Diaz-Lezama N., Hernandez-Elvira M., Sandoval A., Monroy A., Felix R., Monjaraz E. Ghrelin inhibits proliferation and increases T-type Ca2+ channel expression in PC-3 human prostate carcinoma cells. Biochem Biophys Res Commun. 2010;403:24–29. doi: 10.1016/j.bbrc.2010.10.100. [DOI] [PubMed] [Google Scholar]
- 123.Shigetomi E., Tong X., Kwan K.Y., Corey D.P., Khakh B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dasari R., De Carvalho A., Medellin D.C., Middleton K.N., Hague F., Volmar M.N. Wittig derivatization of sesquiterpenoid polygodial leads to cytostatic agents with activity against drug resistant cancer cells and capable of pyrrolylation of primary amines. Eur J Med Chem. 2015;103:226–237. doi: 10.1016/j.ejmech.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hwang J.A., Hwang M.K., Jang Y., Lee E.J., Kim J.E., Oh M.H. 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng, inhibits colon cancer growth by targeting TRPC channel-mediated calcium influx. J Nutr Biochem. 2013;24:1096–1104. doi: 10.1016/j.jnutbio.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 126.Zhu Y., Lu Y., Qu C., Miller M., Tian J., Thakur D.P. Identification and optimization of 2-aminobenzimidazole derivatives as novel inhibitors of TRPC4 and TRPC5 channels. Br J Pharmacol. 2015;172:3495–3509. doi: 10.1111/bph.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He L.P., Hewavitharana T., Soboloff J., Spassova M.A., Gill D.L. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005;280:10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- 128.Vigont V.A., Zimina O.A., Glushankova L.N., Bezprozvanny I.B., Mozhayeva G.N., Kaznacheyeva E.V. Store-operated calcium entry into SK-N-SH human neuroblastoma cells modeling huntington׳s disease. Biochem (Mosc) Suppl Ser A: Membr Cell Biol. 2012;6:206–214. [Google Scholar]
- 129.Akbulut Y., Gaunt H.J., Muraki K., Ludlow M.J., Amer M.S., Bruns A. (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015;54:3787–3791. doi: 10.1002/anie.201411511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carson C., Raman P., Tullai J., Xu L., Henault M., Thomas E. Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS One. 2015;10:e0127498. doi: 10.1371/journal.pone.0127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller M., Shi J., Zhu Y., Kustov M., Tian J.B., Stevens A. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seo K., Rainer P.P., Hahn V.S., Lee D.I., Jo S.H., Andersen A. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci U S A. 2014;111:1551–1556. doi: 10.1073/pnas.1308963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Madan E., Gogna R., Keppler B., Pati U. p53 increases intra-cellular calcium release by transcriptional regulation of calcium channel TRPC6 in GaQ3-treated cancer cells. PLoS One. 2013;8:e71016. doi: 10.1371/journal.pone.0071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gonzales C.B., Kirma N.B., De La Chapa J.J., Chen R., Henry M.A., Luo S. Vanilloids induce oral cancer apoptosis independent of TRPV1. Oral Oncol. 2014;50:437–447. doi: 10.1016/j.oraloncology.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang F., Xiao X., Cheng W., Yang W., Yu P., Song Z. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat Chem Biol. 2015;11:518–524. doi: 10.1038/nchembio.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Costa B., Giagnoni G., Franke C., Trovato A.E., Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–250. doi: 10.1038/sj.bjp.0705920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu T., Wang G., Tao H., Yang Z., Wang Y., Meng Z. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer. 2016;16:790. doi: 10.1186/s12885-016-2831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Jong P.R., Takahashi N., Harris A.R., Lee J., Bertin S., Jeffries J. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J Clin Invest. 2014;124:3793–3806. doi: 10.1172/JCI72340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Juvin V., Penna A., Chemin J., Lin Y.L., Rassendren F.A. Pharmacological characterization and molecular determinants of the activation of transient receptor potential V2 channel orthologs by 2-aminoethoxydiphenyl borate. Mol Pharmacol. 2007;72:1258–1268. doi: 10.1124/mol.107.037044. [DOI] [PubMed] [Google Scholar]
- 140.Velasco G., Hernández-Tiedra S., Dávila D., Lorente M. The use of cannabinoids as anticancer agents. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;64:259–266. doi: 10.1016/j.pnpbp.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Nie L., Oishi Y., Doi I., Shibata H., Kojima I. Inhibition of proliferation of MCF-7 breast cancer cells by a blocker of Ca2+-permeable channel. Cell Calcium. 1997;22:75–82. doi: 10.1016/s0143-4160(97)90107-x. [DOI] [PubMed] [Google Scholar]
- 142.Thorneloe K.S., Sulpizio A.C., Lin Z., Figueroa D.J., Clouse A.K., McCafferty G.P. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 143.Thorneloe K.S., Bao W., Alsaid H., Jian M.-Y., Costell M., Maniscalco K. Discovery of orally active transient receptor potential vanilloid 4 (TRPV4) blockers for the treatment of pulmonary edema in heart failure. Circulation. 2011;124:A13510. [Google Scholar]
- 144.Wei Z.L., Nguyen M.T., O׳Mahony D.J., Acevedo A., Zipfel S., Zhang Q. Identification of orally-bioavailable antagonists of the TRPV4 ion-channel. Bioorg Med Chem Lett. 2015;25:4011–4015. doi: 10.1016/j.bmcl.2015.06.098. [DOI] [PubMed] [Google Scholar]
- 145.Takezawa R., Cheng H., Beck A., Ishikawa J., Launay P., Kubota H. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol. 2006;69:1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- 146.De Petrocellis L., Ligresti A., Moriello A.S., Allara M., Bisogno T., Petrosino S. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Almeida M.C., Hew-Butler T., Soriano R.N., Rao S., Wang W., Wang J. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yee NS. Roles of TRPM8 ion channels in cancer: proliferation, survival, and invasion Cancers (Basel) 2015;7:2134-2146. [DOI] [PMC free article] [PubMed]
- 149.Tolcher A., Patnaik A., Papadopoulos K., Mays T., Stephan T., Humble D.J. 376 Preliminary results from a Phase 1 study of D-3263 HCl, a TRPM8 calcium channel agonist, in patients with advanced cancer. Eur J Cancer Suppl. 2010;8:119. [Google Scholar]
- 150.Wang W., Gao Q., Yang M., Zhang X., Yu L., Lawas M. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc Natl Acad Sci U S A. 2015;112:E1373–E1381. doi: 10.1073/pnas.1419669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen C.C., Keller M., Hess M., Schiffmann R., Urban N., Wolfgardt A. A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat Commun. 2014;5:4681. doi: 10.1038/ncomms5681. [DOI] [PubMed] [Google Scholar]
- 152.Landowski C.P., Bolanz K.A., Suzuki Y., Hediger M.A. Chemical inhibitors of the calcium entry channel TRPV6. Pharm Res. 2011;28:322–330. doi: 10.1007/s11095-010-0249-9. [DOI] [PubMed] [Google Scholar]
- 153.Bowen C.V., DeBay D., Ewart H.S., Gallant P., Gormley S., Ilenchuk T.T. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS One. 2013;8:e58866. doi: 10.1371/journal.pone.0058866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haverstick D.M., Heady T.N., Macdonald T.L., Gray L.S. Inhibition of human prostate cancer proliferation in vitro and in a mouse model by a compound synthesized to block Ca2+ entry. Cancer Res. 2000;60:1002–1008. [PubMed] [Google Scholar]
- 155.Perabo F.G., Wirger A., Kamp S., Lindner H., Schmidt D.H., Muller S.C. Carboxyamido-triazole (CAI), a signal transduction inhibitor induces growth inhibition and apoptosis in bladder cancer cells by modulation of Bcl-2. Anticancer Res. 2004;24:2869–2877. [PubMed] [Google Scholar]
- 156.Harper J.L., Camerini-Otero C.S., Li A.-H., Kim S.-A., Jacobson K.A., Daly J.W. Dihydropyridines as inhibitors of capacitative calcium entry in leukemic HL-60 cells. Biochem Pharmacol. 2003;65:329–338. doi: 10.1016/s0006-2952(02)01488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yoshino T., Ishikawa J., Ohga K., Morokata T., Takezawa R., Morio H. YM-58483, a selective CRAC channel inhibitor, prevents antigen-induced airway eosinophilia and late phase asthmatic responses via Th2 cytokine inhibition in animal models. Eur J Pharmacol. 2007;560:225–233. doi: 10.1016/j.ejphar.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 158.Dutcher J.P., Leon L., Manola J., Friedland D.M., Roth B., Wilding G. Phase II study of carboxyamidotriazole in patients with advanced renal cell carcinoma refractory to immunotherapy: e4896, an Eastern Cooperative Oncology Group Study. Cancer. 2005;104:2392–2399. doi: 10.1002/cncr.21473. [DOI] [PubMed] [Google Scholar]
- 159.Moody T.W., Chiles J., Moody E., Sieczkiewicz G.J., Kohn E.C. CAI inhibits the growth of small cell lung cancer cells. Lung Cancer. 2003;39:279–288. doi: 10.1016/s0169-5002(02)00525-1. [DOI] [PubMed] [Google Scholar]
- 160.Smyth J.T., DeHaven W.I., Bird G.S., Putney J.W. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kondratskyi A., Yassine M., Slomianny C., Kondratska K., Gordienko D., Dewailly E. Identification of ML-9 as a lysosomotropic agent targeting autophagy and cell death. Cell Death Dis. 2014;5:e1193. doi: 10.1038/cddis.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McNally B.A., Somasundaram A., Yamashita M., Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yeromin A.V., Zhang S.L., Jiang W., Yu Y., Safrina O., Cahalan M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sampath B., Sankaranarayanan K. Glu106 targeted inhibitors of ORAI1 as potential Ca release-activated Ca (CRAC) channel blockers - molecular modeling and docking studies. J Recept Signal Transduct Res. 2016:1–14. doi: 10.3109/10799893.2016.1141956. [DOI] [PubMed] [Google Scholar]
- 165.Derler I., Schindl R., Fritsch R., Heftberger P., Riedl M.C., Begg M. The action of selective CRAC channel blockers is affected by the Orai pore geometry. Cell Calcium. 2013;53:139–151. doi: 10.1016/j.ceca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sadaghiani A.M., Lee S.M., Odegaard J.I., Leveson-Gower D.B., McPherson O.M., Novick P. Identification of Orai1 channel inhibitors by using minimal functional domains to screen small molecule microarrays. Chem Biol. 2014;21:1278–1292. doi: 10.1016/j.chembiol.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 167.Prakriya M., Lewis R.S. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goto J., Suzuki A.Z., Ozaki S., Matsumoto N., Nakamura T., Ebisui E. Two novel 2-aminoethyl diphenylborinate (2-APB) analogues differentially activate and inhibit store-operated Ca2+ entry via STIM proteins. Cell Calcium. 2010;47:1–10. doi: 10.1016/j.ceca.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen G., Panicker S., Lau K.Y., Apparsundaram S., Patel V.A., Chen S.L. Characterization of a novel CRAC inhibitor that potently blocks human T cell activation and effector functions. Mol Immunol. 2013;54:355–367. doi: 10.1016/j.molimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 170.Small E.J., Meyer M., Marshall M.E., Reyno L.M., Meyers F.J., Natale R.B. Suramin therapy for patients with symptomatic hormone-refractory prostate cancer: results of a randomized phase III trial comparing suramin plus hydrocortisone to placebo plus hydrocortisone. J Clin Oncol. 2000;18:1440–1450. doi: 10.1200/JCO.2000.18.7.1440. [DOI] [PubMed] [Google Scholar]
- 171.Adinolfi E., Raffaghello L., Giuliani A.L., Cavazzini L., Capece M., Chiozzi P. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 172.Adinolfi E., Capece M., Amoroso F., De Marchi E., Franceschini A. Emerging roles of P2X receptors in cancer. Curr Med Chem. 2015;22:878–890. doi: 10.2174/0929867321666141012172913. [DOI] [PubMed] [Google Scholar]
- 173.Mariot P., Prevarskaya N., Roudbaraki M.M., Le Bourhis X., Van Coppenolle F., Vanoverberghe K. Evidence of functional ryanodine receptor involved in apoptosis of prostate cancer (LNCaP) cells. Prostate. 2000;43:205–214. doi: 10.1002/(sici)1097-0045(20000515)43:3<205::aid-pros6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 174.Abdul M., Ramlal S., Hoosein N. Ryanodine receptor expression correlates with tumor grade in breast cancer. Pathol Oncol Res. 2008;14:157–160. doi: 10.1007/s12253-008-9045-9. [DOI] [PubMed] [Google Scholar]
- 175.Garcia-Prieto C., Riaz Ahmed K.B., Chen Z., Zhou Y., Hammoudi N., Kang Y. Effective killing of leukemia cells by the natural product OSW-1 through disruption of cellular calcium homeostasis. J Biol Chem. 2013;288:3240–3250. doi: 10.1074/jbc.M112.384776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim H.L., Im D.S. N,N-dimethyl-D-erythro-sphingosine increases intracellular Ca2+ concentration via Na+–Ca2+-exchanger in HCT116 human colonolocancer cells. Arch Pharm Res. 2008;31:54–59. doi: 10.1007/s12272-008-1120-y. [DOI] [PubMed] [Google Scholar]
- 177.Lee Y.S., Wurster R.D. Bepridil enhances in vitro antitumor activity of antiestrogens in human brain tumor cells. Cancer Lett. 1996;110:243–248. doi: 10.1016/s0304-3835(96)04518-1. [DOI] [PubMed] [Google Scholar]
- 178.Cox D.A., Conforti L., Sperelakis N., Matlib M.A. Selectivity of inhibition of Na+–Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol. 1993;21:595–599. doi: 10.1097/00005344-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 179.Jost N., Nagy N., Corici C., Kohajda Z., Horvath A., Acsai K. ORM-10103, a novel specific inhibitor of the Na+/Ca2+ exchanger, decreases early and delayed afterdepolarizations in the canine heart. Br J Pharmacol. 2013;170:768–778. doi: 10.1111/bph.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brustovetsky T., Brittain M.K., Sheets P.L., Cummins T.R., Pinelis V., Brustovetsky N. KB-R7943, an inhibitor of the reverse Na+/Ca2+ exchanger, blocks N-methyl-D-aspartate receptor and inhibits mitochondrial complex I. Br J Pharmacol. 2011;162:255–270. doi: 10.1111/j.1476-5381.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song M., Chen D., Yu S.P. The TRPC channel blocker SKF 96365 inhibits glioblastoma cell growth by enhancing reverse mode of the Na+/Ca2+ exchanger and increasing intracellular Ca2+ Br J Pharmacol. 2014;171:3432–3447. doi: 10.1111/bph.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jaimovich E., Mattei C., Liberona J.L., Cardenas C., Estrada M., Barbier J. Xestospongin B, a competitive inhibitor of IP3-mediated Ca2+ signalling in cultured rat myotubes, isolated myonuclei, and neuroblastoma (NG108-15) cells. FEBS Lett. 2005;579:2051–2057. doi: 10.1016/j.febslet.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 183.Miyamoto S., Izumi M., Hori M., Kobayashi M., Ozaki H., Karaki H., Xestospongin C. a selective and membrane-permeable inhibitor of IP3 receptor, attenuates the positive inotropic effect of α-adrenergic stimulation in guinea-pig papillary muscle. Br J Pharmacol. 2000;130:650–654. doi: 10.1038/sj.bjp.0703358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cárdenas C., Müller M., McNeal A., Lovy A., Janˇa F., Bustos G. Selective vulnerability of cancer cells by inhibition of Ca2+ transfer from endoplasmic reticulum to mitochondria. Cell Rep. 2016;14:2313–2324. doi: 10.1016/j.celrep.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 186.Saleem H., Tovey S.C., Molinski T.F., Taylor C.W. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol. 2014;171:3298–3312. doi: 10.1111/bph.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kang S.S., Han K.-S., Ku B.M., Lee Y.K., Hong J., Shin H.Y. Inhibition of the Ca2+ release channel, IP3R subtype 3 by caffeine slows glioblastoma invasion and migration and extends survival. Cancer Res. 2010;70:1173–1183. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pan Z., Damron D., Nieminen A.L., Bhat M.B., Ma J. Depletion of intracellular Ca2+ by caffeine and ryanodine induces apoptosis of chinese hamster ovary cells transfected with ryanodine receptor. J Biol Chem. 2000;275:19978–19984. doi: 10.1074/jbc.M908329199. [DOI] [PubMed] [Google Scholar]
- 189.Piedras-Rentería E.S., Barrett C.F., Cao Y.-Q., Tsien R.W. Voltage-gated calcium channels, calcium signaling, and channelopathies. In: Joachim K., Marek M., editors. New Comprehensive Biochemistry. Amsterdam: Elsevier; 2007. pp. 127–166. [Google Scholar]
- 190.Strobl J.S., Kirkwood K.L., Lantz T.K., Lewine M.A., Peterson V.A., Worley J.F., 3rd Inhibition of human breast cancer cell proliferation in tissue culture by the neuroleptic agents pimozide and thioridazine. Cancer Res. 1990;50:5399–5405. [PubMed] [Google Scholar]
- 191.Krouse A.J., Gray L., Macdonald T., McCray J. Repurposing and rescuing of mibefradil, an antihypertensive, for cancer: a case study. Assay Drug Dev Technol. 2015;13:650–653. doi: 10.1089/adt.2015.29014.ajkdrrr. [DOI] [PubMed] [Google Scholar]
- 192.Bruce J.I.E., Elliott A.C. Pharmacological evaluation of the role of cytochrome P450 in intracellular calcium signalling in rat pancreatic acinar cells. Br J Pharmacol. 2000;131:761–771. doi: 10.1038/sj.bjp.0703631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Merritt J.E., Armstrong W.P., Benham C.D., Hallam T.J., Jacob R., Jaxa-Chamiec A. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zeng B., Yuan C., Yang X., Atkin S.L., Xu S.Z. TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Curr Cancer Drug Targets. 2013;13:103–116. [PubMed] [Google Scholar]
- 195.Sanchez A.M., Sanchez M.G., Malagarie-Cazenave S., Olea N., Diaz-Laviada I. Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis. 2006;11:89–99. doi: 10.1007/s10495-005-3275-z. [DOI] [PubMed] [Google Scholar]
- 196.Ramer R., Bublitz K., Freimuth N., Merkord J., Rohde H., Haustein M. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. Faseb J. 2012;26:1535–1548. doi: 10.1096/fj.11-198184. [DOI] [PubMed] [Google Scholar]
- 197.Trebak M., Bird G.S., McKay R.R., Putney J.W., Jr. Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 198.Yamashita M., Somasundaram A., Prakriya M. Competitive modulation of Ca2+ release-activated Ca2+ channel gating by STIM1 and 2-aminoethyldiphenyl borate. J Biol Chem. 2011;286:9429–9442. doi: 10.1074/jbc.M110.189035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schindl R., Bergsmann J., Frischauf I., Derler I., Fahrner M., Muik M. 2-aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. J Biol Chem. 2008;283:20261–20267. doi: 10.1074/jbc.M803101200. [DOI] [PubMed] [Google Scholar]
- 200.Yamashita M., Prakriya M. Divergence of Ca2+ selectivity and equilibrium Ca2+ blockade in a Ca2+ release-activated Ca2+ channel. J General Physiol. 2014;143:325–343. doi: 10.1085/jgp.201311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ma H.T., Venkatachalam K., Parys J.B., Gill D.L. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J Biol Chem. 2002;277:6915–6922. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 202.Scrimgeour N.R., Wilson D.P., Rychkov G.Y. Glu106 in the Orai1 pore contributes to fast Ca2+-dependent inactivation and pH dependence of Ca2+ release-activated Ca2+ (CRAC) current. Biochem J. 2012;441:743–753. doi: 10.1042/BJ20110558. [DOI] [PubMed] [Google Scholar]
- 203.Yamashita M., Navarro-Borelly L., McNally B.A., Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol. 2007;130:525–540. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jairaman A., Prakriya M. Molecular pharmacology of store-operated CRAC channels. Channels (Austin) 2013;7:402–414. doi: 10.4161/chan.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schleifer H., Doleschal B., Lichtenegger M., Oppenrieder R., Derler I., Frischauf I. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca2+ entry pathways. Br J Pharmacol. 2012;167:1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zitt C., Strauss B., Schwarz E.C., Spaeth N., Rast G., Hatzelmann A. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- 207.Ng S.W., di Capite J., Singaravelu K., Parekh A.B. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 208.Li J., McKeown L., Ojelabi O., Stacey M., Foster R., O׳Regan D. Nanomolar potency and selectivity of a Ca2+ release-activated Ca2+ channel inhibitor against store-operated Ca2+ entry and migration of vascular smooth muscle cells. Br J Pharmacol. 2011;164:382–393. doi: 10.1111/j.1476-5381.2011.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ramos S., Grigoryev S., Rogers E., Roos J., Whitten J., Stauderman K. CM3457, a potent and selective oral CRAC channel inhibitor, suppresses T and mast cell function and is efficacious in rat models of arthritis and asthma. J Immunol. 2012;188:72.3. [Google Scholar]
- 210.Lin F.F., Elliott R., Colombero A., Gaida K., Kelley L., Moksa A. Generation and characterization of fully human monoclonal antibodies against human Orai1 for autoimmune disease. J Pharmacol Exp Ther. 2013;345:225–238. doi: 10.1124/jpet.112.202788. [DOI] [PubMed] [Google Scholar]
- 211.Cox J.H., Hussell S., Sondergaard H., Roepstorff K., Bui J.V., Deer J.R. Antibody-mediated targeting of the Orai1 calcium channel inhibits T cell function. PLoS One. 2013;8:e82944. doi: 10.1371/journal.pone.0082944. [DOI] [PMC free article] [PubMed] [Google Scholar]