Abstract
Introduction
Decreasing the diagnostic delay in axial spondyloarthritis (axSpA) remains a major challenge. Here, we assessed the value of serum inflammatory biomarkers to distinguish early axSpA from other pathologies in a large cohort of patients referred with early back pain.
Methods
Serum c reactive protein (CRP), erythrocyte sedimentation rate (ESR) and calprotectin were determined in the SPondyloArthritis Caught Early (SPACE) cohort (n=310), an early back pain inception cohort. Additionally, explorative serum biomarkers derived from the literature (interleukin-27 (IL-27), human β-defensin-2 (hBD-2) and lipcolin-2 (LCN-2)) were determined by ELISA in full-blown patients with ankylosing spondylitis (AS) (n=21) and healthy controls (n=20).
Results
Serum CRP and ESR levels were not elevated in early axSpA versus ‘control’ back pain patients. Serum calprotectin was elevated in early axSpA versus controls (p=0.01) but failed to identify early axSpA at the individual level (positive predictive value of 38.7%). As to explorative biomarkers, serum levels of IL-27 were not detectable, and hBD-2 and LCN-2 serum levels were not elevated in full-blown AS versus healthy controls (p=0.572, p=0.562, respectively). Therefore, these markers were not further determined in the SPACE cohort.
Conclusions
None of the candidate serum inflammatory markers were useful as diagnostic markers in the early phase of axSpA.
Keywords: Spondyloarthritis, Inflammation, Low Back Pain
Key messages.
What is already known about this subject?
Some serum markers of inflammation are elevated in a fraction of the patients with full blown axial spondyloarthritis in comparison with healthy individuals. However, there are no established diagnostic serum biomarkers allowing the identification of axial spondyloarthritis in patient with early back pain.
What does this study add?
This study demonstrated that serum biomarkers of inflammation, including CRP and ESR, are not elevated in patients with early axial spondyloarthritis. Calprotectin levels were slightly elevated, but lacked specificity and sensitivity to be applied as a diagnostic biomarker.
How might this impact clinical practice?
Taken together with a series of other studies, our data suggest that the disease processes driving axial spondyloarthritis are not reflected by alterations in the peripheral blood compartment. Direct visualization of these processes, for example by molecular imagingof affected tissues, might be a more successful approach to identify biomarkers for axial spondyloarthritis.
Introduction
Reliable diagnosis of axial spondyloarthritis (axSpA) in the earliest phases of the disease remains an important unmet medical need. Ample evidence indicates that (1) signs and symptoms of active disease are as severe in early disease as in full-blown disease, with similar impact on function and quality of life1–3 and (2) active early axSpA can be effectively treated with non-steroidal anti-inflammatory drugs (NSAIDs) and/or tumour necrosis factor inhibitors (TNFi).4–6 Moreover, current treatments fail to significantly inhibit pathological new bone formation when started in full-blown disease;7–9 although awaiting formal proof, starting the same treatments in very early disease may also impact on structural progression.10 11 Although the time between first symptoms and diagnosis of axSpA has already been significantly reduced over the past decade by a combination of early referral strategies and the use of MRI to image axial inflammation,12–16 it remains a challenge to further reduce the diagnostic delay and to reliably distinguish early back pain due to axSpA from other causes of back pain.17–19
One potential way to address this challenge is the use of serum inflammatory biomarkers. A couple of inflammatory biomarkers have been reported to be elevated in active, full-blown axSpA. C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are both acute-phase reactants which are elevated in active SpA and decrease on effective treatment.20–23 Moreover, calprotectin (also called S100A8/A9), a calcium binding protein, is expressed and secreted during macrophage infiltration in SpA synovitis.24–26 Calprotectin was recently shown to be a good serum biomarker for treatment responses in proof-of-concept trials in SpA and to independently predict radiographic progression in axSpA.27 28 Despite their value at the group level, the value of these serum biomarkers for diagnosis of axSpA in patients with early back pain remains unknown as they are neither very sensitive (eg, only one-third of patients with active ankylosing spondylitis (AS) have an elevated CRP)20 22 nor specific as they reflect inflammation (whatever the origin) rather than axSpA as such.
Besides these inflammatory biomarkers that were already extensively studied in AS, our recent literature review identified a few other potentially interesting serum biomarkers (M. Turina et al, submitted for publication). Lin et al29 recently described that levels of interleukin-27 (IL-27), a heterodimeric cytokine composed of p28 and Epstein-Barr Virus-induced gene 3 (EBI3) which belongs to the IL-12 family, were elevated in full-blown AS when compared with healthy controls but this finding has not yet been confirmed in an independent study. Human β defensin-2 (hBD-2) and lipocalin-2 (LCN-2) are two antimicrobial peptides which are up-regulated by IL-17 and are consistently found at elevated levels in serum of patients with active psoriasis and inflammatory bowel disease (IBD).30–35 Considering the central role of IL-17 in the pathophysiology of axSpA36–38 and the pathophysiological and clinical overlap of axSpA with psoriasis and IBD,39 these two biomarkers could also be of potential interest.
Accordingly, the aim of the present study was to assess the biomarker value of established inflammatory serum markers (CRP, ESR and calprotectin) as well as more exploratory biomarkers (IL-27, hBD-2 and LCN-2) for the diagnosis of axSpA in patients with early back pain.
Methods
Patients and samples
Serum was collected from 350 individuals after obtaining written informed consent to participate in the studies. Cohort 1 (SPondyloArthritis Caught Early (SPACE)) consisted of 310 individuals with ‘early’ back pain (defined as at least 3 and maximally 24 months) and an age of onset below 45 years, referred for early evaluation of potential AxSpA.40 This study protocol is approved by the local Ethics Committees of the participating centers. Cohort 2 consisted of 21 established patients with AS according to the modified New York (mNY) criteria; all patients were TNFi naïve.41 Full descriptions of these cohorts were reported previously.40 41 Finally, we also obtained serum from 19 healthy controls. Serum samples of cohorts 2 and 3 were retrieved from our biobank for this analysis according to the study protocol as approved by the Medical Ethics Committee of the Academic Medical Center/University of Amsterdam (2013_057).
Serum inflammatory biomarkers
From cohort 1, serum CRP and ESR levels were determined by local laboratories. Serum calprotectin levels were determined by ELISA (Hycult Biotech, Uden, the Netherlands) using a 1:60 dilution and according to the manufacturer's protocol.27 28
From cohort 2 and the healthy controls, serum IL-27, hBD-2 and LCN-2 levels were determined by ELISA according to the manufacturer's protocol (eBiosciences, San Diego, California; Phoenix pharmaceuticals, . Belmont California; and Research & Diagnostic Systems, Minneapolis, USA, respectively). The dilutions were 1:2, 1:50 and 1:50, respectively.
Statistical analysis
Data between groups were compared using Mann-Whitney U tests. P values <0.05 were considered statistically significant. Data were presented as box plots (Tukey) indicating the median and IQRs. Whiskers represent 1.5 IQR and black dots represent outliers. Statistical analyses were performed with SPSS V.21.0 (SPSS , Chicago, USA).
First, we compared CRP, ESR and calprotectin levels in patients of the SPACE cohort fulfilling the ASAS criteria versus those who did not fulfil the criteria. Second, we performed a subanalysis between patients fulfilling the imaging and a clinical arm of the ASAS criteria. The imaging arm can be fulfilled only if abnormal MRI (according to the ASAS/OMERACT definition) or X-sacroiliac joints (X-SIJs) (according to mNY) abnormalities are visible, and since calprotectin is an independent marker for axial spinal progression, it might better associate with the imaging arm of the ASAS criteria. Therefore, we conducted similar analyses in patients fulfilling the imaging arm (n=36) of the ASAS axSpA criteria versus those not fulfilling the criteria (n=191). Finally, we have tested the potential value of serum IL-27, hBD-2 and LCN-2 by comparing these levels in established active AS (cohort 2) versus healthy controls. Active disease in AS was defined as a Bath ankylosing spondylitis disease activity score (BASDAI) score >4.
Results
The baseline characteristics of cohort 1 are shown in table 1. Of note, 119 patients fulfilled the ASAS axSpA criteria (of which 36 fulfilled the imaging arm) and 191 did not fulfil the ASAS axSpA criteria.
Table 1.
Demographics and disease activity measures of the SPACE cohort (cohort 1)
| Cohort 1 (SPACE) |
||
|---|---|---|
| Fulfilling ASAS axSpA criteria |
||
| Yes (n=119) | No (n=191) | |
| Demographics | ||
| Male gender, n (%) | 51 (43) | 53 (28) |
| Age, mean (SD), years | 32.15 (8.44) | 31.10 (8.30) |
| HLA-B27 positive, n (%) | 102 (86) | 18 (9) |
| IBP, n (%) | 94 (79) | 113 (59) |
| IBD, n (%) | 3 (3) | 19 (10) |
| Psoriasis, n (%) | 15 (13) | 13 (7) |
| Uveitis (n%) | 18 (15) | 6 (3) |
| NSAIDs (past or present), n (%) | 89 (75) | 128 (67) |
| DMARDs (past or present), n (%) | 7 (6) | 11 (6) |
| TNFi (past or present), n (%) | 0 (0) | 1 (<1) |
| Disease activity measures | ||
| Back pain duration, months, mean (SD) | 13 (7) | 13 (7) |
| Swollen joint count, 0–66 joints, mean (SD) | 0.14 (0.58) | 0.29 (1.65) |
| Tender joint counts, 0–68 joints, mean (SD) | 2.71 (6.12) | 2.27 (4.62) |
| PGA, 0–100mm VAS, mean (SD) | 4.06 (2.62) | 5.46 (2.45) |
| BASDAI, 0–10 cm, mean (SD) | 3.94 (2.41) | 5.52 (5.11) |
| ASDAS, 0–10 cm VAS, mean (SD) | 1.42 (0.57) | 1.39 (0.53) |
ASAS, Assessment of SpondyloArthritis international Society; AxSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DMARDs, disease-modifying antirheumatic drugs; HLA-B27, human leucocyte antigen-B27; IBD, inflammatory bowel disease; IBP, inflammatory back pain; NSAIDs, non-steroidal anti-inflammatory drugs; PGA patient global assessment of disease activity; SpA, spondyloarthritis; SPACE, SPondyloArthritis Caught Early cohort; TNFi, tumour necrosis factor inhibitors; VAS, visual analogue score.
CRP, ESR and calprotectin levels in early AxSPA
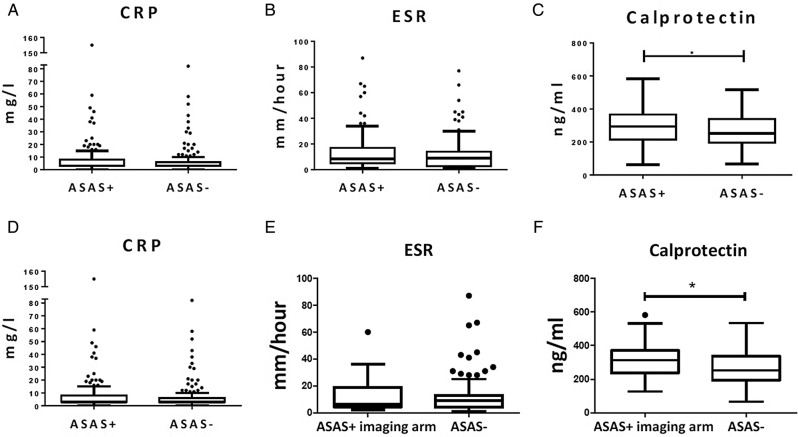
We first assessed whether CRP, ESR and calprotectin levels, known to be elevated in full-blown ankylosing spondylitis, were also elevated in patients with early back pain fulfilling the ASAS axSpA criteria.42 The median (IQR) levels of CRP (3.00 (3.00–8.00) mg/L vs 3.00 (3.00–6.00) mg/L; p=0.317) (figure 1A) and ESR (8.50 (5.00–17.00) mm/hour vs 9.00 (2.75–14.00) mm/hour; p=0.208) (figure 1B) were not significantly different between patients fulfilling and not fulfilling the ASAS axSpA criteria in the SPACE cohort. In contrast, calprotectin levels (294 214–367 ng/mL vs 251 196–339 ng/mL; p=0.01) were significantly higher in patients with early back pain fulfilling versus those not fulfilling the ASAS axSpA criteria (figure 1C). However, the discriminating value of calprotectin at the individual level was low: using a cut-off value for calprotectin at a specificity of 90% (412.40 ng/mL), the sensitivity was 10.0% and the positive predictive value (PPV) 38.7%. The post-test probability of having axSpA is thus not increased in comparison with the pretest probability (119 of 310 or 38.4%).
Figure 1.
Serum levels of (A), C reactive protein (CRP), (B), erythrocyte sedimentation rate (ESR) and (C), calprotectin of cohort 1 (SPACE) with patients with early back pain fulfilling the ASAS axial spondyloarthritis (axSpA) criteria (n=119) versus patients with early back pain not fulfilling the ASAS axial SpA criteria (n=191); and serum levels of (D), CRP, (E), ESR and (F), calprotectin of cohort 1 (SPACE) with patients fulfilling the ASAS axSpA criteria according to the imaging arm (n=36) versus patients not fulfilling the ASAS axial SpA criteria (n=191). Boxplot (Tukey): Data are presented as median (IQR). Whiskers represent 1.5 IQR and black dots represent outliers. *p<0.05 by Mann-Whitney U test. ASAS, Assessment of SpondyloArthritis international Society.
CRP, ESR and calprotectin levels in the imaging arm of early AxSPA
We conducted similar analyses in patients fulfilling the imaging arm (n=36) of the ASAS axSpA criteria versus those not fulfilling the criteria (n=191). Again, levels of CRP (4.00 (3.00–9.00) mg/L vs 3.00 (3.00–6.00) mg/L; p=0.175) (figure 1D) and ESR (6.50 (4.25–19.00) mm/hour vs 9.00 (4.00–13.00) mm/hour; p=0.512) (figure 1E) were not different between the early patients with axSpA fulfilling the ASAS imaging arm and controls not fulfilling the ASAS criteria (figures 1D and E). Levels of calprotectin (313 (237–371) ng/mL vs 253 (195–338) ng/mL; p=0.01) were significantly higher in patients with early back pain fulfilling the imaging arm of the ASAS axSpA criteria versus those not fulfilling the criteria (figure 1F). As for the global axSpA group, however, the discriminatory value at the individual level was low. Using again a cut-off for calprotectin at the 90% specificity level (249.95 ng/mL) as an example, the PPV was 80% but the sensitivity was only 7.7%.
IL-27, hBD-2 and LCN-2 in full-blown as versus healthy controls
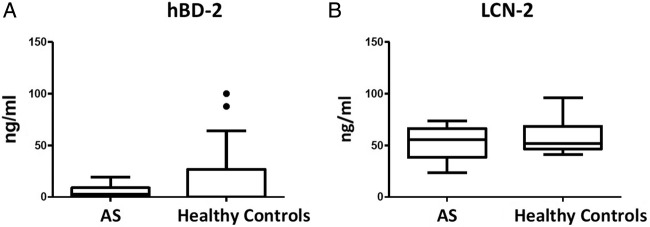
Since CRP, ESR and calprotectin were not useful as diagnostic biomarkers for axSpA in the SPACE cohort, we explored the potential value of three additional potential biomarkers. To this purpose, we first assessed their serum levels in established active AS (cohort 2) versus controls. IL-27 was undetectable in all but one serum sample of AS and healthy controls (data not shown). Serum levels of hBD-2 (median and IQR: 2.61 (0.00–8.93) ng/mL vs 0.00 (0.00–26.65) ng/mL; p=0.572) (figure 2A) and LCN-2 (55.47 (38.14–66.30) ng/mL vs 51.82 (46.59–68.57) ng/mL; p=0.562) (figure 2B) were clearly detectable but were not elevated in AS versus healthy controls. Since none of the three markers were elevated in AS, we did not proceed with testing the serum levels in the early back pain (SPACE) cohort.
Figure 2.
Serum levels of (A), human β defensin-2 (hBD-2) and (B), lipocalin-2 (LCN-2) in active full-blown ankylosing spondylitis (AS, n=21) versus healthy controls (n=20). Data are presented as median (IQR). Boxplot (Tukey): data are presented as median (IQR). Whiskers represent 1.5 IQR and black dots represent outliers. *p<0.05 by Mann-Whitney U test. *p<0.05 by Mann-Whitney U test.
Discussion
We set up this study to assess whether inflammatory serum biomarkers can contribute to the diagnosis of axSpA in individuals presenting with early back pain. A first important finding is that serum CRP and ESR levels are not elevated in patients with early axSpA versus patients with back pain from different origins, despite the fact that elevated CRP is one of the features included in the ASAS axSpA criteria. Currently, there are no known true reliable and robust biomarkers available in established axial SpA (or ankylosing spondylitis). Therefore, testing several other biomarkers in early axial SpA was not useful. Here, we solely selected those biomarkers with some evidence as potential value in established axial SpA, including CRP, ESR and calprotectin in patients with early axial SpA. Accordingly, specificity, sensitivity and PPV analyses did not allow discrimination between patients and controls (data not shown). Importantly, however, the values of CRP and ESR were assessed here in a univariate analysis as the aim was to identify biomarkers that can easily and reproducibly be used to screen patients with early back pain to diagnose axSpA early. Although CRP and ESR do not appear to be useful tools for this purpose, this does not imply that these markers cannot be useful in individual patients when combined with other SpA features in a multivariate approach.
A second interesting finding is that, in contrast to CRP and ESR, calprotectin levels were significantly increased in early axSpA versus controls with back pain. This finding is consistent with a series of previous studies suggesting that calprotectin may slightly outperform CRP as a marker of tissue inflammation in SpA,26–28 43 potentially because this protein is released during infiltration of myeloid cells in tissues and may thus more directly reflect some of the pathological processes in SpA. However, the difference in serum calprotectin levels detected at the group level between early axSpA and controls was not robust enough in terms of sensitivity and specificity to translate in a useful discriminative tool to identify patients with axSpA. These findings are consistent with studies on other serum inflammatory markers including IL-6,44 45 α-2-macroglobulin,46 MMP-347–50 and PTX-3,51 which have been explored already in established axSpA but were not robust enough to apply in a diagnostic setting in early axSpA.
The lack of increase in serum levels of inflammatory biomarkers in axSpA versus controls is most likely related to two issues. First, these biomarkers do not reflect an SpA-specific process but rather inflammation in general and therefore lack specificity. Intercurrent infections or the presence of another chronic inflammatory disease may lead to elevated CRP, ESR and/or calprotectin in patients with mechanical back pain. One way to circumvent this issue would be to measure factors that are more specifically related to the immunopathology of axSpA, such as biomarkers reflecting the activation of the IL-23/IL-17 axis. Despite their value in psoriasis and IBD, however, the IL-17-driven antimicrobial peptides hBD-2 and LCN-2 were not elevated in axSpA. This might be due to the fact that these peptides are mainly produced by epithelial cells, including keratinocytes and gut epithelial cells, and that IL-17 may act on different cell types in axSpA. Further investigations in the mechanistic aspects of SpA immunopathology remain warranted to identify novel potential biomarkers.
A second reason for the absence of elevated serum levels of inflammatory markers such as CRP and ESR may be the fact that inflammation is restricted to specific tissue compartments and does not extend to the systemic circulation and/or lymphoid organs in SpA. This concept is supported by the fact that (1) in this study a majority of early patients with axSpA have CRP and ESR levels within the normal range despite active disease (n=171, 55.2%), (2) even in the subset of patients with positive MRI demonstrating active inflammation in the SI joints, CRP, ESR and calprotectin levels were not further increased and (3) also in established SpA with active disease CRP levels are elevated in only one-third of the patients.20–23 Accordingly, we previously focused our research on the immunopathology of affected tissues, such as the synovial membrane and found striking and reproducible alterations which, however, were not reflected in the peripheral blood compartment.52 53 If this concept is correct, the search for diagnostic markers should probably be focused on non-invasive measurements of tissue pathology (including MRI, positron emission tomography-CT and other types of molecular imaging) rather than on serum biomarkers.
Acknowledgments
The authors would like to thank D Pots and IC Blijdorp for their help in this study. The authors would also like to thank KL Germar for providing language help and for critically proofreading the article.
Footnotes
Contributors: MT, NY and DB were involved in study concept and design. MT, NY, FvG, MvO, IB and ML acquired the data. MT, NY, FvG, MvO, IB, RR, ML, RL and DB were involved in analysis and interpretation of data. All authors were involved in drafting the article or revising it critically for important intellectual content and all authors approved the final version to be published.
Competing interests: MCT was supported by an unrestricted fellowship from Janssen and DB was supported by a VICI grant from The Netherlands Organization for Scientific Research (NWO), by a Consolidator Grant from the European Research Council (ERC), and by a grant from the Dutch Arthritis Foundation (Reumafonds). NY, FvG, MvO, IJB, RR, CL and RL have no conflict of interests.
Patient consent: Obtained.
Ethics approval: LUMC, AMC, Regional Committee for Medical and Health Research Ethics (Oslo, Norway).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Rudwaleit M, Haibel H, Baraliakos X et al. . The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. 10.1002/art.24483 [DOI] [PubMed] [Google Scholar]
- 2.Wallis D, Haroon N, Ayearst R et al. . Ankylosing spondylitis and nonradiographic axial spondyloarthritis: part of a common spectrum or distinct diseases? J Rheumatol 2013;40:2038–41. 10.3899/jrheum.130588 [DOI] [PubMed] [Google Scholar]
- 3.Boonen A, Sieper J, van der Heijde D et al. . The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015;44:556–62. 10.1016/j.semarthrit.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 4.Van Den Bosch F, Kruithof E, Baeten D et al. . Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum 2002;46:755–65. 10.1002/art.511 [DOI] [PubMed] [Google Scholar]
- 5.Braun J, Brandt J, Listing J et al. . Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. 10.1016/S0140-6736(02)08215-6 [DOI] [PubMed] [Google Scholar]
- 6.Sieper J, van der Heijde D, Dougados M et al. . Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 2013;72:815–22. 10.1136/annrheumdis-2012-201766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Heijde D, Landewé R, Einstein S et al. . Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31. 10.1002/art.23471 [DOI] [PubMed] [Google Scholar]
- 8.Van der Heijde D, Salonen D, Weissman BN et al. . Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127 10.1186/ar2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Heijde D, Landewé R, Baraliakos X et al. . Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. 10.1002/art.23901 [DOI] [PubMed] [Google Scholar]
- 10.Maas F, Spoorenberg A, Brouwer E et al. . Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-α blocking therapy: a prospective longitudinal observational cohort study. PLoS ONE 2015;10:e0122693 10.1371/journal.pone.0122693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksymowych WP, Zheng Y, Wichuk S et al. . OP0144 The effect of TNF inhibition on radiographic progression in ankylosing spondylitis: an observational cohort study of 384 patients. Ann Rheum Dis 2015;74(Suppl 2):123 10.1136/annrheumdis-2015-eular.6285 [DOI] [Google Scholar]
- 12.Brandt HC, Spiller I, Song IH et al. . Performance of referral recommendations in patients with chronic back pain and suspected axial spondyloarthritis. Ann Rheum Dis 2007;66:1479–84. 10.1136/ard.2006.068734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poddubnyy D, Vahldiek J, Spiller I et al. . Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol 2011;38:2452–60. 10.3899/jrheum.110070 [DOI] [PubMed] [Google Scholar]
- 14.Van Hoeven L, Vergouwe Y, de Buck PDM et al. . External validation of a referral rule for axial spondyloarthritis in primary care patients with chronic low back pain. PLoS ONE 2015;10:e0131963 10.1371/journal.pone.0131963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudwaleit M, Jurik AG, Hermann KG et al. . Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. 10.1136/ard.2009.110767 [DOI] [PubMed] [Google Scholar]
- 16.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005;52:1000–8. 10.1002/art.20990 [DOI] [PubMed] [Google Scholar]
- 17.Feldtkeller E, Khan MA, van der Heijde D et al. . Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003;23:61–6. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen J, Hetland ML. Decreases in diagnostic delay are supported by sensitivity analyses. Ann Rheum Dis 2014;73:e45–e45. 10.1136/annrheumdis-2014-205590 [DOI] [PubMed] [Google Scholar]
- 19.Salvadorini G. Bandinelli F, Delle Sedie A et al. . Ankylosing spondylitis: how diagnostic and therapeutic delay have changed over the last six decades. Clin Exp Rheumatol 2012;30:561–5. [PubMed] [Google Scholar]
- 20.Benhamou M, Gossec L, Dougados M. Clinical relevance of C reactive protein in ankylosing spondylitis and evaluation of the NSAIDs/cyclo-oxygenase-2 inhibitors’ treatment effect on C reactive protein. Rheumatology (Oxford) 2010;49:536–41. 10.1093/rheumatology/kep393 [DOI] [PubMed] [Google Scholar]
- 21.Visvanathan S, Wagner C, Marini JC et al. . Inflammatory biomarkers, disease activity and spinal disease measures in patients with ankylosing spondylitis after treatment with infliximab. Ann Rheum Dis 2008;67:511–17. 10.1136/ard.2007.071605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vries MK, van Eijk IC, van der Horst-Bruinsma IE et al. . Erythrocyte sedimentation rate, C reactive protein level, and serum amyloid a protein for patient selection and monitoring of anti-tumor necrosis factor treatment in ankylosing spondylitis. Arthritis Rheum 2009;61:1484–90. 10.1002/art.24838 [DOI] [PubMed] [Google Scholar]
- 23.Poddubnyy DA, Rudwaleit M, Listing J et al. . Comparison of a high sensitivity and standard C reactive protein measurement in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis 2010;69:1338–41. 10.1136/ard.2009.120139 [DOI] [PubMed] [Google Scholar]
- 24.Kane D, Roth J, Frosch M et al. . Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum 2003;48:1676–85. 10.1002/art.10988 [DOI] [PubMed] [Google Scholar]
- 25.Kruithof E, De Rycke L, Vandooren B et al. . Identification of synovial biomarkers of response to experimental treatment in early-phase clinical trials in spondylarthritis. Arthritis Rheum 2006;54:1795–804. 10.1002/art.21914 [DOI] [PubMed] [Google Scholar]
- 26.De Rycke L, Baeten D, Foell D et al. . Differential expression and response to anti-TNF alpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 2005;206:17–27. 10.1002/path.1758 [DOI] [PubMed] [Google Scholar]
- 27.Turina MC, Yeremenko N, Paramarta JE et al. . Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther 2014;16:413 10.1186/s13075-014-0413-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turina MC, Sieper J, Yeremenko N et al. . Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis 2014;73:1746–8. 10.1136/annrheumdis-2014-205506 [DOI] [PubMed] [Google Scholar]
- 29.Lin TT, Lu J, Qi CY et al. . Elevated serum level of IL-27 and VEGF in patients with ankylosing spondylitis and associate with disease activity. Clin Exp Med 2014;15:227–31. 10.1007/s10238-014-0281-x [DOI] [PubMed] [Google Scholar]
- 30.Gambichler T, Bechara FG, Scola N et al. . Serum levels of antimicrobial peptides and proteins do not correlate with psoriasis severity and are increased after treatment with fumaric acid esters. Arch Dermatol Res 2012;304:471–4. 10.1007/s00403-012-1227-3 [DOI] [PubMed] [Google Scholar]
- 31.Jansen PA, Rodijk-Olthuis D, Hollox EJ et al. . Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One 2009;4:e4725 10.1371/journal.pone.0004725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamata M, Tada Y, Tatsuta A et al. . Serum lipocalin-2 levels are increased in patients with psoriasis. Clin Exp Dermatol 2012;37:296–9. 10.1111/j.1365-2230.2011.04265.x [DOI] [PubMed] [Google Scholar]
- 33.Romaní J, Caixàs A, Ceperuelo-Mallafré V et al. . Circulating levels of lipocalin-2 and retinol-binding protein-4 are increased in psoriatic patients and correlated with baseline PASI. Arch Dermatol Res 2013;305:105–12. 10.1007/s00403-012-1306-5 [DOI] [PubMed] [Google Scholar]
- 34.Oikonomou KA, Kapsoritakis AN, Theodoridou C et al. . Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol Japan 2012;47:519–30. 10.1007/s00535-011-0516-5 [DOI] [PubMed] [Google Scholar]
- 35.Yeşil A, Gönen C, Senateş E et al. . Relationship between neutrophil gelatinase-associated lipocalin (NGAL) levels and inflammatory bowel disease type and activity. Dig Dis Sci 2013;58:2587–93. 10.1007/s10620-013-2676-z [DOI] [PubMed] [Google Scholar]
- 36.Hreggvidsdottir HS, Noordenbos T, Baeten DL. Inflammatory pathways in spondyloarthritis. Mol Immunol 2014;57:28–37. 10.1016/j.molimm.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 37.Baeten D, Baraliakos X, Braun J et al. . Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:1705–13. 10.1016/S0140-6736(13)61134-4 [DOI] [PubMed] [Google Scholar]
- 38.Baraliakos X, Borah B, Braun J et al. . Long-term effects of secukinumab on MRI findings in relation to clinical efficacy in subjects with active ankylosing spondylitis: an observational study. Ann Rheum Dis 2016;75:408–12. 10.1136/annrheumdis-2015-207544 [DOI] [PubMed] [Google Scholar]
- 39.Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–37. 10.1016/S0140-6736(11)60071-8 [DOI] [PubMed] [Google Scholar]
- 40.Van den Berg R, de Hooge M, van Gaalen F et al. . Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2013;52:1492–9. 10.1093/rheumatology/ket164 [DOI] [PubMed] [Google Scholar]
- 41.Salinas GF, De Rycke L, Barendregt B et al. . Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann Rheum Dis 2013;72:1037–43. 10.1136/annrheumdis-2011-201270 [DOI] [PubMed] [Google Scholar]
- 42.Sieper J, Rudwaleit M, Baraliakos X et al. . The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68(Suppl 2):ii1–44. [DOI] [PubMed] [Google Scholar]
- 43.Kruithof E, De Rycke L, Roth J et al. . Immunomodulatory effects of etanercept on peripheral joint synovitis in the spondylarthropathies. Arthritis Rheum 2005;52:3898–909. 10.1002/art.21426 [DOI] [PubMed] [Google Scholar]
- 44.Gratacós J, Collado A, Filella F et al. . Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol 1994;33:927–31. 10.1093/rheumatology/33.10.927 [DOI] [PubMed] [Google Scholar]
- 45.Pedersen SJ, Hetland ML, Sørensen IJ et al. . Circulating levels of interleukin-6, vascular endothelial growth factor, YKL-40, matrix metalloproteinase-3, and total aggrecan in spondyloarthritis patients during 3 years of treatment with TNFα inhibitors. Clin Rheumatol 2010;29:1301–9. 10.1007/s10067-010-1528-x [DOI] [PubMed] [Google Scholar]
- 46.Surrall K, Bird H, Dixon J. Caeruloplasmin, prealbumin and u2-macroglobulin as potential indices of disease activity in different arthritides. Clin Rheumatol 1987;6:64–9. 10.1007/BF02201003 [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Gu J, Rihl M et al. . Serum levels of matrix metalloproteinase 3 and macrophage colony-stimulating factor 1 correlate with disease activity in ankylosing spondylitis. Arthritis Rheum 2004;51:691–9. 10.1002/art.20696 [DOI] [PubMed] [Google Scholar]
- 48.Maksymowych WP, Landewé R, Conner-Spady B et al. . Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum 2007;56:1846–53. 10.1002/art.22589 [DOI] [PubMed] [Google Scholar]
- 49.Vandooren B, Kruithof E, Yu DTY et al. . Involvement of matrix metalloproteinases and their inhibitors in peripheral synovitis and downregulation by tumor necrosis factor alpha blockade in spondylarthropathy. Arthritis Rheum 2004;50:2942–53. 10.1002/art.20477 [DOI] [PubMed] [Google Scholar]
- 50.Chen C-H, Lin K-C, Yu DTY et al. . Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in ankylosing spondylitis: MMP-3 is a reproducibly sensitive and specific biomarker of disease activity. Rheumatology (Oxford) 2006;45:414–20. 10.1093/rheumatology/kei208 [DOI] [PubMed] [Google Scholar]
- 51.Deban L, Jaillon S, Garlanda C et al. . Pentraxins in innate immunity: lessons from PTX3. Cell Tissue Res 2011;343:237–49. 10.1007/s00441-010-1018-0 [DOI] [PubMed] [Google Scholar]
- 52.Noordenbos T, Yeremenko N, Gofita I et al. . Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum 2012;64:99–109. 10.1002/art.33396 [DOI] [PubMed] [Google Scholar]
- 53.Yeremenko N, Noordenbos T, Cantaert T et al. . Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum 2013;65:174–85. 10.1002/art.37704 [DOI] [PubMed] [Google Scholar]