Abstract
Objective
Clinical trials have not consistently demonstrated differences between tumour necrosis factor inhibitor (TNFi) plus methotrexate and triple therapy (methotrexate plus hydroxychloroquine plus sulfasalazine) in rheumatoid arthritis (RA). The study objective was to estimate the efficacy, radiographic benefits, safety and patient-reported outcomes of TNFi–methotrexate versus triple therapy in patients with RA.
Methods
A systematic review and network meta-analysis (NMA) of randomised controlled trials of TNFi–methotrexate or triple therapy as one of the treatment arms in patients with an inadequate response to or who were naive to methotrexate was conducted. American College of Rheumatology 70% response criteria (ACR70) at 6 months was the prespecified primary endpoint to evaluate depth of response. Data from direct and indirect comparisons between TNFi–methotrexate and triple therapy were pooled and quantitatively analysed using fixed-effects and random-effects Bayesian models.
Results
We analysed 33 studies in patients with inadequate response to methotrexate and 19 in patients naive to methotrexate. In inadequate responders, triple therapy was associated with lower odds of achieving ACR70 at 6 months compared with TNFi–methotrexate (OR 0.35, 95% credible interval (CrI) 0.19 to 0.64). Most secondary endpoints tended to favour TNFi–methotrexate in terms of OR direction; however, no clear increased likelihood of achieving these endpoints was observed for either therapy. The odds of infection were lower with triple therapy than with TNFi−methotrexate (OR 0.08, 95% CrI 0.00 to 0.57). There were no differences observed between the two regimens in patients naive to methotrexate.
Conclusions
In this NMA, triple therapy was associated with 65% lower odds of achieving ACR70 at 6 months compared with TNFi–methotrexate in patients with inadequate response to methotrexate. Although secondary endpoints numerically favoured TNFi–methotrexate, no clear differences were observed. The odds of infection were greater with TNFi–methotrexate. No differences were observed for patients naive to methotrexate. These results may help inform care of patients who fail methotrexate first-line therapy.
Keywords: Rheumatoid Arthritis, DMARDs (synthetic), DMARDs (biologic), Methotrexate, TNF-alpha
Key messages.
What is already known about this subject?
Previous studies have reported that tumour necrosis factor inhibitor (TNFi)–methotrexate and triple therapy (methotrexate/hydroxychloroquine/sulfasalazine) are equivalent in patients with rheumatoid arthritis (RA); however, many of these studies were not adequately powered while also suggesting deeper responses associated with TNFi–methotrexate.
What does this study add?
In this network meta-analysis, American College of Rheumatology 70% response criteria (ACR70) at 6 months (primary endpoint) was more likely with TNFi–methotrexate versus triple therapy in patients with an adequate response to methotrexate. Other endpoints did not favour either therapy, although the calculated ORs favoured TNFi–methotrexate in their directionality.
How might this impact on clinical practice?
The greater depth of response suggested by TNFi–methotrexate compared with triple therapy may inform therapy in patients with RA who failed previous methotrexate therapy.
Introduction
The current treatment paradigm for patients with rheumatoid arthritis (RA) consists of disease-modifying antirheumatic drugs (DMARDs), including conventional synthetic DMARDs (csDMARDs) and biologic DMARDs (bDMARDs). The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) propose methotrexate as the first step in the treatment of patients with active RA.1 2 Although ∼30% of patients achieve full clinical remission with methotrexate monotherapy, 70% require a step-up treatment that includes the addition of either csDMARDs or bDMARDs.3 Several studies have suggested that initial combination therapy is superior to initial methotrexate monotherapy, whether this is a combination of csDMARDs or the combination of methotrexate with a tumour necrosis factor inhibitor (TNFi).4–12 In patients with inadequate response to methotrexate, the addition of another agent(s) can lead to improved outcomes. Adding a TNFi was superior to adding placebo to methotrexate monotherapy13; similarly, it has been shown that csDMARD triple therapy (adding hydroxychloroquine and sulfasalazine to methotrexate) is superior to methotrexate monotherapy.14 However, it is uncertain whether methotrexate plus TNFi is superior to a combination of csDMARDs or vice versa. Using a completers analysis, the Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial concluded that there was no difference between triple therapy and TNFi (specifically, etanercept) plus methotrexate for the endpoints ACR 20% response criteria (ACR20), ACR50, or Disease Activity Score including 28-joint count (DAS28; the primary endpoint) at weeks 48 and 102 in a population of patients who were mostly methotrexate-naive.5 However, there were differences favouring etanercept–methotrexate in several endpoints—radiographic progression and the percentage of patients achieving ACR70—suggesting that etanercept plus methotrexate was more effective than triple therapy in achieving clinically relevant outcomes over time.5 In the TEAR trial, more patients dropped out than the 10% expected (33% by week 48 and 37% by week 102); it is therefore possible that true differences between the two treatments were missed at weeks 48 and 102 because of inadequate power.5 Two randomised controlled trials (RCTs) compared triple therapy to TNFi–methotrexate as second-line therapy. In the Swefot trial in patients with early RA, TNFi–methotrexate was found initially to be superior to triple therapy in patients for whom methotrexate monotherapy had failed, but by year 2 this difference was no longer significant. This study had lower enrolment than originally planned, and a type 2 error at year 2 could not be excluded.3 15 Similar results were reported from the Rheumatoid Arthritis Comparison of Active Therapies (RACAT) trial, which also did not reach its target sample size for the primary endpoint of a 0.3-unit difference in DAS28 at week 48.16
Network meta-analysis (NMA) is a model that allows indirect comparisons of interventions through the use of a network of interventions and comparisons.17 NMA can provide estimates of relative efficacy between all interventions connected through the network of trials, even those that have never been compared directly.17–19 Thus, through combining direct and indirect evidence, NMAs have the potential to allow greater precision of point estimates and more power to determine between-group differences relative to single clinical trials or classical pair-wise meta-analyses.20 The objective of this NMA was to evaluate the clinical efficacy, radiographic outcomes, patient-reported outcomes (PROs) and safety of TNFi–methotrexate combination therapy compared with triple therapy in patients with active RA who were methotrexate-naive or had an inadequate response to methotrexate. As ACR and EULAR guidelines recommend methotrexate as first-line therapy in RA, the results in patients with inadequate response to methotrexate are likely more clinically relevant.
Methods
Design
We conducted a systematic literature review of EMBASE, MEDLINE and the Cochrane library, followed by an NMA. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in the development and reporting of this analysis,21 which can be found in the online supplementary appendix.
rmdopen-2016-000371supp_appendix.pdf (1.9MB, pdf)
Search strategy
The search strategy combined indexed and free-text terms for disease and interventions of interest and used the Scottish Intercollegiate Guidelines Network (SIGN) filter for RCTs in EMBASE and MEDLINE. No study filter was used in the Cochrane (Central Register of Controlled Trials (CENTRAL)) Library search. The three databases were searched through Ovid. The list of search terms can be found in the online supplementary material methods. Separate reproducible searches were conducted and were supplemented by a manual bibliography check of identified relevant review articles (including previously published systematic reviews and Cochrane reviews), and conference abstracts search in two professional conferences for 2012, 2013 and 2014. Conference abstracts were searched to complement data unavailable in the corresponding full-text publications. Studies available only as a conference meeting abstract were not included.
Eligibility criteria
Included studies were full-text manuscripts of RCTs conducted in adult patients with RA, stratified by methotrexate experience (inadequate responders vs naive). Patients were treated with either TNFi–methotrexate combination therapy or triple therapy (methotrexate + hydroxychloroquine + sulfasalazine). TNFis included in the analysis were adalimumab, certolizumab pegol, etanercept, golimumab, infliximab and biosimilar infliximab. Studies reported at least one endpoint of clinical efficacy, radiographic progression, PRO or safety at 13 weeks, 26 weeks, 1 year or 2 years of follow-up (full list in online supplementary table S1).
Study identification and data extraction
Abstracts and full-text articles were screened by two reviewers, and data were extracted by one reviewer and validated by a second independent reviewer. Any uncertainties were resolved by a third reviewer.
Endpoints
As both treatment combinations have been shown to be effective in the treatment of RA, the prespecified primary endpoint of this analysis was ACR70 at 6 months in order to compare the depth of response of each of these regimens; other endpoints and time points analysed are listed in table 1. The ACR responses were analysed at 3 months, 6 months, 1 year and 2 years.
Table 1.
Endpoints analysed in the network meta-analysis
| Endpoints analysed | Time points analysed (all endpoints) |
|---|---|
Primary endpoint
|
|
Safety
| |
Patient-reported outcomes
|
ACR70/50/20, American College of Rheumatology 70%/50%/20% response criteria; AE, adverse event; CRP, C-reactive protein; DAS28, Disease Activity Score including 28-joint count; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; mTSS, modified total Sharp score; RA, rheumatoid arthritis.
Assessment of bias
The quality of each study was assessed using the Cochrane Collaboration's tool for assessing risk of bias for RCTs (see online supplementary table S2).22 We did not assess publication bias; however, all studies that met the eligibility criteria were included in the analysis.
Network meta-analysis
For all studies, the feasibility of an NMA was assessed by the academic authors who examined box plots of clinically important baseline characteristic distributions to explore the level of heterogeneity among treatment groups. Accordingly, the following assumptions were made: the sets of trials do not differ with respect to the distribution of effect modifiers, all doses of methotrexate are equivalent and the various TNFis identified were considered as one type of therapy.
The analysis was conducted using WinBUGS V.1.4.3 (MRC Biostatistics, Cambridge Institute of Public Health, Cambridge, UK). Vague (‘flat’) distributions were used such that any parameter value was equally likely. This approach assigns more weight in assuming the therapies are equivalent compared with applying informed prior or traditional frequentist approaches. Fixed-effects and random-effects models were run for each network of evidence and fitted with preferred model fit determined by the deviance information criterion (DIC). A model with a lower DIC score was considered a better fit. Both models were run for 3 months (13 weeks), 6 months (26 weeks), 1 year and 2 years. To evaluate simulation convergence, history, trace plots and Brooks-Gelman-Rubin statistics diagnostics were examined. Three chains were run each with 40 000 iterations and the traces were visually inspected.
For dichotomous endpoints, a logit model was used to estimate the odds of attaining an endpoint. ORs and 95% credible intervals (CrIs) of triple therapy versus TNFi–methotrexate were calculated, with ORs <1 indicating a better performance for TNFi–methotrexate for clinical efficacy and radiographic outcomes, and ORs >1 indicating a better performance for TNFi–methotrexate for safety outcomes. For continuous endpoints, a weighted mean difference between mean changes from baseline for treatments at follow-up was estimated using end-of-trial mean scores or change in mean score from baseline. Values >0 for difference in mean change from baseline of triple therapy versus TNFi–methotrexate indicated better performance for TNFi–methotrexate.
Handling of missing data was conducted according to the Cochrane Handbook for Systematic Reviews.23 When an endpoint of interest was not reported adequately or not in a format we could incorporate, attempts to inform missing data were undertaken, including contacting the authors of the study and cross-checking against previously published NMAs reporting the data point of interest. We used the best available data from the specific time points indicated (ie, the Ns were not inflated to the number of randomised patients). We used the intent-to-treat method (with various imputation methods) as much as possible; for studies using completer-only or modified intent-to-treat methods, those data were used for the analysis. For studies missing SDs, an average of the SDs of all other studies in the same network was used for a particular study. Randomised arms with more than 20% crossover at any time point were not analysed beyond the time point where crossover occurred because treatment effects could not be attributed to the initial therapy once these crossovers took place.
Sensitivity analyses using non-responder imputation and completers data were compared with the main results of the study to determine whether the method used to account for missing data affected the results. Comparison between the results from the NMA and a classical pair-wise direct comparison were used to determine consistency.
Results
Studies identified
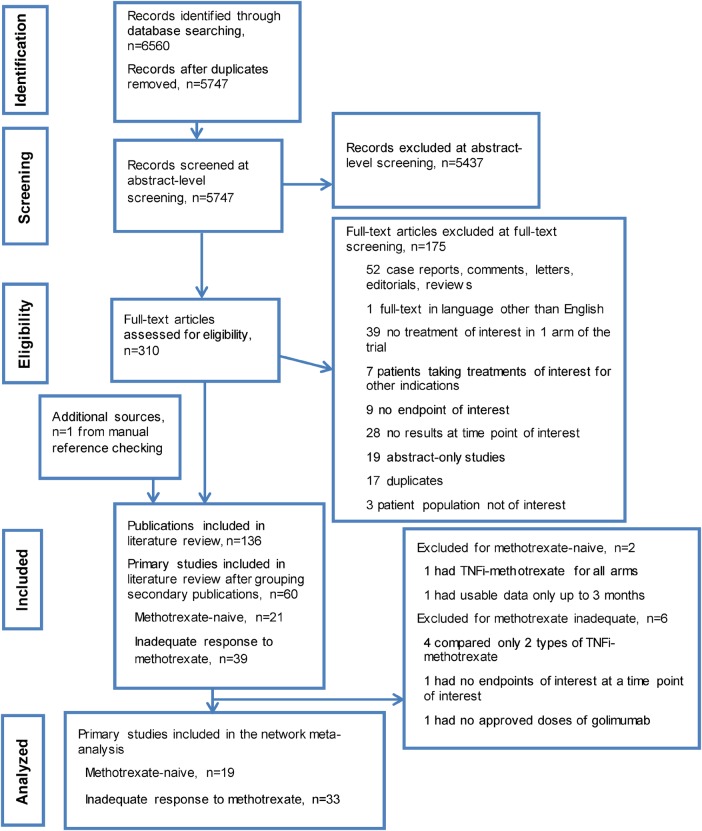
The search yielded 6560 abstracts and citations from EMBASE, MEDLINE, and the Cochrane (CENTRAL) library. After screening, 60 full-text primary studies were identified for possible inclusion, which corresponded to 136 records. Of the 60 studies, 393 24–61 were in patients with inadequate response to methotrexate, and 214 5 7 11 14 62–77 were in methotrexate-naive patients. A PRISMA diagram documenting the search, screening and trial selection process, and reasons for exclusion is shown in figure 1.21
Figure 1.
PRISMA diagram documenting the search and screening process. Articles were identified by searching MEDLINE, EMBASE and Cochrane CENTRAL databases from 1 January 1990 to 9 September 2014; manual bibliography checks of previously published systematic reviews and Cochrane reviews supplemented these searches. Conference abstracts from the American College of Rheumatology Annual Meetings and the Annual European Congresses of Rheumatology in 2012, 2013 and 2014 were used to supplement data not included in the corresponding full-text publications. TNFi, tumour necrosis factor inhibitor.
Characteristics of studies
Patients with inadequate response to methotrexate
Inadequate response to methotrexate was defined as the presence of active RA despite current use of methotrexate; however, we observed differences in definitions across the studies. The descriptions of patients included the following: patients with active RA on methotrexate before the study, patients on stable doses of methotrexate for at least 4 weeks before study enrolment, patients who had active RA despite methotrexate treatment and patients with inadequate response to methotrexate treatment. Study and baseline characteristics of the studies are listed in online supplementary table S3. The baseline mean age was 37–61 years. Female enrolment ranged from 43% to 100%; the proportion of patients who were rheumatoid factor–positive (RF+) was 61–100%; and mean disease duration was 1.3–14.3 years. Baseline DAS28–erythrocyte sedimentation rate (ESR) ranged from 4.6 to 6.9 and DAS28–C-reactive protein (CRP) from 4.3 to 6.0. Our feasibility assessments of box plots of baseline characteristics across studies (see online supplementary figures S1–S14) determined that there were no clinically meaningful differences in treatment cohorts across the studies.
Methotrexate-naive patients
The definition of patients who were naive to methotrexate differed across the trials. In most cases, the definition was no prior exposure to methotrexate; however, a few studies allowed limited exposure to methotrexate. Also included was a study enrolling patients who had not received methotrexate within 6 months before randomisation78 as well as a single study that allowed ≤40 mg of total methotrexate in <20% of patients before the study period.5 Another study allowed patients with DMARD treatment, but only 10% of patients had received methotrexate before; therefore, it was included in this group of methotrexate-naive studies.14 The baseline mean age was 47–54 years; female enrolment was 53–85%; the RF+ proportion was 49–100% (see online supplementary table S3). Mean disease duration was 0.1–10 years; baseline DAS28-ESR was 5.5–6.9 and DAS28-CRP ranged from 5.0 to 6.2. Baseline radiographic score and modified total Sharp score (mTSS) showed a wide distribution, with the majority reporting mean scores of 10–20, and others up to 44.77 The feasibility assessment of box plots of distributions of baseline characteristics (see online supplementary figures S15–S26) determined that there were no clinically meaningful differences in baseline characteristics across the studies.
Network meta-analysis results
Patients with inadequate response to methotrexate
The 39 trials from the systematic review were further assessed for the availability of appropriate treatment arms (approved doses), and if none were present, the trial was excluded from the analyses; 23 (59%) had unclear risk of bias, 15 (38%) had low risk of bias and 1 (3%) had a high risk of bias.27 Online supplementary table S4 describes special considerations for studies or treatment arms from certain studies that were not included in the NMA. Four trials were not used in the analyses because they compared two types of TNFi–methotrexate combinations.28 56 58 60 One trial was excluded because none of the endpoints of interest at the time points of interest were reported.59 Another did not evaluate an approved dose for golimumab and was excluded.38 Certain treatment arms from 10 trials evaluated non-approved doses of the TNFis, and hence could not be included in the analyses.30–32 34 45 47 48 53 57 Finally, in five trials, data were used until randomisation was truly maintained, up to 6 months in four trials33 43 48 52 and up to 1 year in another trial,49 before allowance of additional RA treatments. Thus, 33 primary studies3 15 24–27 29–37 39–55 57 61 79–111 and their follow-up analyses (67 records total) in patients with inadequate response to methotrexate were included in the NMA. The full list of studies included in the NMA and the endpoints available for analysis in each study is shown in online supplementary table S5.
Efficacy
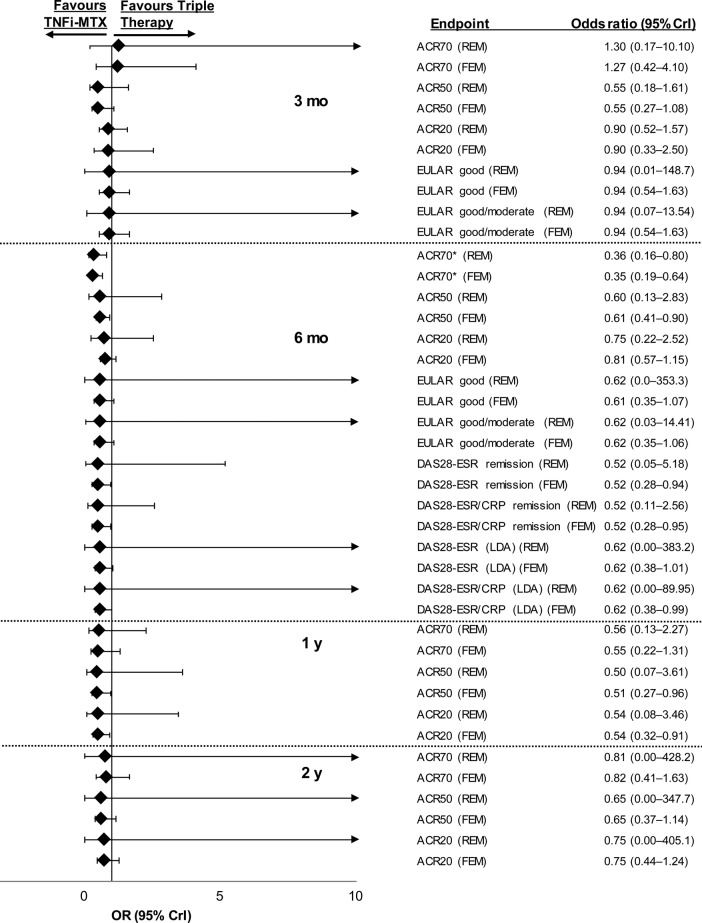
The number of studies used for each analysis along with DIC values for fixed-effects and random-effects models are shown in online supplementary table S6; the network of evidence for the primary endpoint is shown in online supplementary figure S27. Patients with inadequate response to methotrexate were less likely to achieve ACR70 with triple therapy than with TNFi–methotrexate at 6 months in the fixed-effects (OR 0.35, 95% CrI 0.19 to 0.64) and random-effects (OR 0.36, 95% CrI 0.16 to 0.80) models (figure 2; full results in online supplementary table S7). Although the values of the OR for the other efficacy endpoints of ACR20/50, DAS28-ESR remission or low disease activity, DAS28-ESR/CRP remission or low disease activity favoured TNFi–methotrexate, the CrIs extended past 1 and thus a true difference between the two regimens could not be confirmed for these endpoints. Similarly, mean differences for DAS28-ESR and DAS28-ESR/CRP at 6 months did not favour either treatment (figure 3).
Figure 2.
Relative treatment effects concerning efficacy endpoints in patients with inadequate response to methotrexate for triple therapy versus TNFi–methotrexate at 3 months, 6 months, 1 year and 2 years. ACR70/50/20, American College of Rheumatology 70%/50%/20% response criteria; CrI, credible interval; CRP, C-reactive protein; DAS28, Disease Activity Score including 28-joint count; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; FEM, fixed-effects model; LDA, low disease activity; MTX, methotrexate; REM, random-effects model; TNFi, tumour necrosis factor inhibitor. *ACR70 at 6 months was a prespecified primary endpoint.
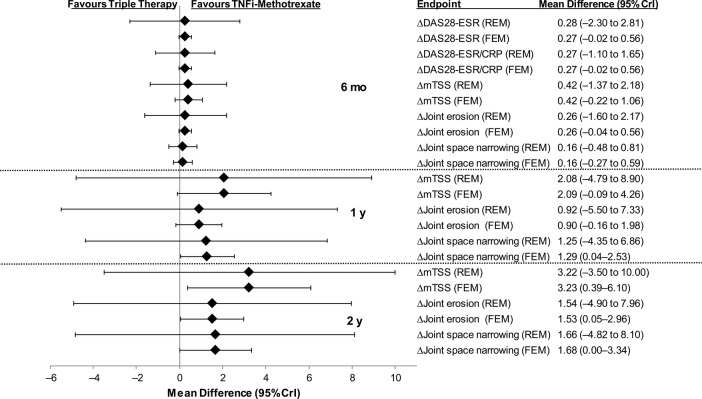
Figure 3.
Mean differences in efficacy and radiographic outcomes in triple therapy versus TNFi–methotrexate in patients with inadequate response to methotrexate. CrI, credible interval; CRP, C-reactive protein; DAS28, Disease Activity Score including 28-joint count; ESR, erythrocyte sedimentation rate; FEM, fixed-effects model; mTSS, modified total Sharp score; REM, random-effects model; TNFi, tumour necrosis factor inhibitor.
Mean differences for radiographic endpoints at 6 months, 1 year and 2 years are shown in figure 3. Overall, the mean differences between TNFi–methotrexate and triple therapy did not favour either therapy for the radiographic endpoints of mTSS, joint erosion or joint space narrowing. However, differences were observed in the fixed-effects model, but not the random-effects model for mTSS, joint erosion and joint space narrowing that favoured TNFi–methotrexate over triple therapy at 2 years.
Safety
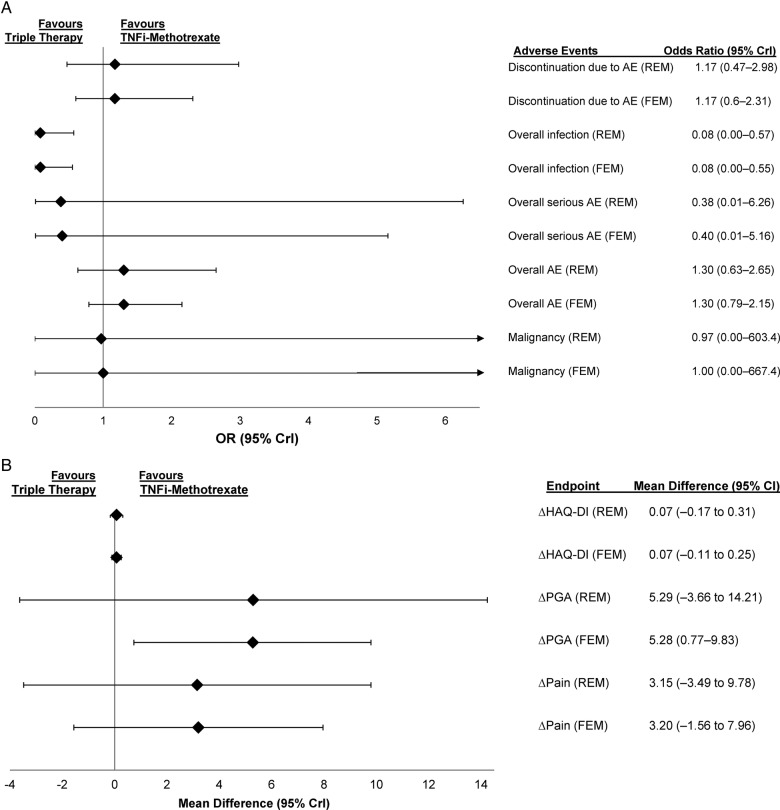
Triple therapy showed lower odds of infection in the random-effects (OR 0.08, 95% CrI 0.00 to 0.57) and fixed-effects (OR 0.08, 95% CrI 0.00 to 0.55) models. For the other safety endpoints of overall adverse events (AEs), discontinuation due to AEs, serious AEs and malignancy, the likelihood of achieving these endpoints were similar between triple therapy and TNFi–methotrexate (figure 4A; full results in online supplementary table S8).
Figure 4.
(A) ORs comparing safety outcomes and (B) mean differences in patient-reported outcome endpoints in patients treated with triple therapy versus TNFi–methotrexate in patients who had inadequate response to methotrexate at 6 months. AE, adverse event; CrI, credible interval; FEM, fixed-effects model; HAQ-DI, Health Assessment Questionnaire-Disability Index; PGA, patient general assessment; REM, random-effects model; TNFi, tumour necrosis factor inhibitor.
Patient-reported outcomes
TNFi–methotrexate was favoured for mean difference in patient's general assessment in the fixed-effects, but not random-effects model at 6 months (figure 4B); full results in online supplementary table S7). The two treatments were comparable for Health Assessment Questionnaire-Disease Index and pain scores at 6 months. Comparisons were not available for the other time points.
Methotrexate-naive patients
Further exploration of study design and treatment characteristics of the 21 studies (see online supplementary table S3) was conducted to assess if all available treatment arms or time points could be included in the analyses. Fourteen studies (67%) had low risk of bias; seven studies (33%) had an unclear risk of bias. Among the studies available, further exploration of the study design and treatment characteristics was conducted to assess if all available treatment arms or time points could be included in the analyses (see online supplementary table S8). A few considerations were given to certain studies to ensure that only approved doses were included or that study populations at all time points considered for the analysis represented the true randomised population; these are shown in the online supplementary table S9. In three trials,37 92 104 participants were allowed to switch treatments or add additional DMARDs. These studies were not analysed beyond the time point when >20% of participants no longer maintained randomisation. One trial (CONCERTO) evaluated TNFi–methotrexate in all treatment arms and was not included in the analyses.64 112–114 Another study (BeST) was excluded because patients were allowed to receive additional DMARDs in the methotrexate and TNFi–methotrexate arms after 3 months.11 115 116 One trial evaluated TNFi–methotrexate in all treatment arms resulting in it not being feasible to be included in the analyses.112 Thus, 19 primary studies and their associated follow-up analyses were included in the NMA.4 5 7 14 16 62 63 65–77 117–141
Participants in the TEAR5 trial were assessed for DAS28-ESR at 6 months. Those randomised to the two step-up arms were allowed to add on assigned therapies (etanercept or hydroxychloroquine plus sulfasalazine) if DAS28-ESR was ≥3.2 with methotrexate monotherapy. Data from the first 6 months from the two step-up arms were collapsed to form a methotrexate monotherapy arm in the NMA because no additional treatments were introduced during that time. After 6 months, TEAR was analysed as the original four-arm trial. The COmbination of Methotrexate and ETanercept (COMET) trial randomised patients into two arms from baseline to 1 year and to four arms after 1 year until study conclusion at 2 years. Both randomisations occurred at baseline; thus COMET was analysed as a two-arm trial until 1 year.4 After the 1-year time point, COMET was analysed as a four-arm trial, with arms randomised to switch treatment regimen labelled as ‘switch 1’ (combination etanercept plus methotrexate therapy for the first 52 weeks; after 52 weeks patients continued methotrexate, and etanercept was switched to placebo) and ‘switch 2’ (methotrexate monotherapy plus placebo for the first 52 weeks; after 52 weeks patients continued to receive methotrexate, and placebo was switched to etanercept). The full list of studies of methotrexate-naive patients included in the NMA and the endpoints available for analysis are shown in online supplementary table S10.
Efficacy
The number of studies used in each analysis along with DIC values for fixed-effects and random-effects models are shown in online supplementary table S11; the network of evidence for the analysis of the primary endpoint is shown in online supplementary figure S28. Full results of the NMA are shown in online supplementary table S12 and figure S29; OR values <1 indicate a better performance for TNFi–methotrexate over triple therapy. For the primary endpoint, ACR70 at 6 months in methotrexate-naive patients, the OR in the fixed-effects model was 0.76 (95% CrI 0.36 to 1.50); for the random-effects model, the OR was 0.77 (95% CrI 0.31 to 1.76). Achievement of ACR70 at 2 years was more likely in patients treated with TNFi–methotrexate compared with triple therapy in the fixed-effects model (OR, 0.44, 95% CrI 0.22 to 0.82) but not the random-effects model (OR 0.43, 95% CrI 0.14 to 1.28). Similarly, the likelihood of no radiographic progression was more likely in patients treated with TNFi–methotrexate compared with triple therapy in the fixed-effects model (OR 0.48, 95% CrI 0.25 to 0.9) but not the random-effects model (OR 0.47, 95% CrI 0.15 to 1.41). No differences were indicated based on ORs for ACR50 or ACR20 at 6 months or 2 years, or in DAS28-ESR remission at 2 years. Mean differences between TNFi–methotrexate and triple therapy did not favour either therapy in either model for the efficacy endpoints for DAS28-ESR/CRP or DAS28-ESR at 3 months, 6 months, 1 year, or 2 years.
Mean differences between TNFi–methotrexate and triple therapy for the radiographic endpoints of change in joint space narrowing, change in joint erosion or change in mTSS at 2 years did not favour either therapy in either model. (see online supplementary figure S30). No comparisons were available for these endpoints at other time points.
Safety
The ORs showed no difference in the odds of discontinuations due to AEs, overall AEs, serious infections and aspartate aminotransferase elevation between TNFi–methotrexate and triple therapy in patients naive to methotrexate (see online supplementary table S13 and figure S31A).
Patient-reported outcomes
Comparisons were available for the Health Assessment Questionnaire-Disability Index and patient general assessment (PGA) at 2 years; no difference in likelihood of achieving these outcomes was observed between TNFi–methotrexate and triple therapy (see online supplementary table S12 and figure S31B).
Sensitivity analysis
The sensitivity analysis of DAS28-ESR remission at 2 years using non-responders data from the COMET trial gave similar results for the comparison of triple therapy versus TNFi–methotrexate. The TEAR trial reported non-responder imputation and completer data for DAS28-ESR change from baseline at 3 months, 6 months, 1 year and 2 years. The results of the sensitivity analysis were consistent with the direction and magnitude of our main results, with a few exceptions, including mean change in DAS28-ESR at 3 months and 2 years when non-responders imputation data were used. At 3 months, the fixed-effects model result using TEAR last observation carried forward data was 0.21 (95% CrI −0.03 to 0.46), whereas in the sensitivity analysis using non-responders imputation data, the resulting mean difference from the fixed-effects model between TNFi–methotrexate and triple therapy was 0.30 (95% CrI 0.06 to 0.54). Although at 2 years, the observed result was similar to the results obtained from the last observation carried forward analysis, there was a change in the directionality of the relative treatment effect, with a trend of TNFi–methotrexate performing better in the sensitivity analysis using non-responders imputation data.
Discussion
This is the first study to provide a systematic, qualitative and quantitative review of the published literature across critical efficacy, radiographic, safety and PRO endpoints in patients with RA receiving TNFi–methotrexate or triple therapy. This NMA showed that in methotrexate-naive patients there was no difference in the likelihood of achieving clinical efficacy endpoints between triple therapy and TNFi–methotrexate. Although most endpoints favoured TNFi–methotrexate in terms of OR directionality, such a result is not likely to be clinically relevant. In patients with inadequate response to methotrexate, the NMA shows better outcomes for patients treated with TNFi–methotrexate versus triple therapy with respect to the primary endpoint of ACR70 at 6 months; other endpoints were favourable in terms of directionality of the OR, but the limits of the CrIs indicated no clear difference for either therapy. In patients with inadequate response to methotrexate, triple therapy had 65% lower odds than TNFi–methotrexate in achieving ACR70 at 6 months. Trends showing benefits of TNFi–methotrexate were observed across the majority of endpoints. Taken together, these results indicate that the two treatment strategies are comparable, although TNFi–methotrexate may be associated with deeper responses.
Our results suggest that the addition of a TNFi to methotrexate after inadequate response to methotrexate could result in better outcomes compared with the addition of sulfasalazine and hydroxychloroquine, chiefly with ACR70 at 6 months. Our finding is therefore applicable to most patients who do not achieve a good response with methotrexate monotherapy.3 It should be noted that current EULAR guidelines recommend that patients without unfavourable prognosis fail at least two csDMARDs before proceeding to biologic therapy2; unfortunately, this could not be examined in this analysis. Nevertheless, our results support the current ACR guidelines and the current recommendation of adding TNFi to methotrexate in patients with an unfavourable prognosis per EULAR.1 2 Notably, although the OR directionality favoured TNFi–methotrexate over triple therapy in this population at 1 and 2 years, CrIs extended past 1, indicating no increased likelihood of response for either therapy; however, comparison of these time points were limited by the number of studies and data points available for analysis. Therefore, the durability of the difference between TNFi–methotrexate and triple therapy in patients with inadequate response to methotrexate is uncertain.
Our results suggest that patients given combination therapy as first-line treatment benefit comparably from triple therapy and TNFi–methotrexate. Results of recent studies suggest that in patients naive to methotrexate, outcomes at 2 years are similar among patients treated with methotrexate monotherapy and ‘step-up’ to combination therapy and those treated early with TNFi–methotrexate combination therapy or triple therapy.5 16 Starting treatment with methotrexate monotherapy is recommended by EULAR and the ACR1 2 and is the approach most often used in clinical practice. Importantly, initial treatment with a biologic agent would not be reimbursed in most countries and is not necessary in all patients; therefore, although of interest, these findings would not be applicable to most patients with early RA naive to methotrexate. It is noteworthy that the present analysis was designed and conducted before the most recent recommendations, thus it was deemed important to the investigators to include these data. It should also be noted that the use of corticosteroids in some patients is a potential confounder to these results but due to randomisation, it is likely that steroids were used equally in all treatment groups within any trial.
In patients with inadequate response to methotrexate, the majority of safety outcomes were similar between the two treatment regimens; however, an increased risk of infection was noted for TNFi–methotrexate. Safety outcomes were also similar between the treatment regimens in patients naive to methotrexate. Improvements in PGA and pain scores were greater for patients treated with TNFi–methotrexate compared with triple therapy in those with inadequate response to methotrexate (random-effects model only); negligible differences between the two regimens were observed in PGA scores in the methotrexate-naive population.
In this NMA, we present the results from fixed-effects and random-effects models. Typically, random-effects models are preferred because it is generally accepted that baseline characteristics and potential effect modifiers do not have equal distribution across trials, unlike fixed-effects models, which assume a common treatment effect size across trials comparing the same interventions.142 In our limited sample of studies, we found the baseline clinical values across the trials considered for the NMA to be clinically uniform. Beyond examining box plots to assess the distribution of baseline characteristics, an informal graphical check of the treatment effect modifiers of clinically important baseline values was performed. As available data were sparse, we could not come to a conclusive finding of any treatment effect modifier among the available baseline values. The fixed-effects model results are most applicable to our data set because we could not ascertain any systematic differences between the patient populations included, and we compared only two or fewer studies per intervention in each network.
Advantages of NMAs over traditional meta-analyses include the ability to compare directly and indirectly.17 This NMA was designed using best practice guidelines to identify all RCTs available; all available literature was considered. Stratifying the base analysis population by prior exposure to methotrexate alleviates concerns over the prior treatment and response differences across the studies. The combinations of TNFi–methotrexate were prespecified and included only approved doses of TNFi for the treatment of RA; triple therapy was specifically a combination of methotrexate, hydroxychloroquine and sulfasalazine. All available efficacy, radiographic, PRO and safety endpoints were assessed to provide an overall understanding of the relative treatment estimates for the comparison of triple therapy to TNFi–methotrexate.
Limitations of this analysis were that few studies with a common comparator (methotrexate monotherapy) were available; in particular, there was a lack of methotrexate monotherapy arms in studies that evaluated triple therapy. Therefore, results were driven by the few studies that had head-to-head comparison between triple therapy and TNFi–methotrexate and should be interpreted cautiously. We also considered all doses of methotrexate as equal between the treatment arms in the network, which is a potential confounder to our results. Also, certain studies were not included because their endpoints differed from other studies or included patients who received other therapies or used definitions of methotrexate-naive that included patients who had received methotrexate. Furthermore, any negative results not available to the searched databases because of publication bias are excluded from our analysis. Finally, not all endpoints used for inclusion in the study could be analysed in the NMA; for safety, the only specific AEs that could be analysed in the NMA were infection, malignancy and aspartate aminotransferase elevation.
In conclusion, in patients who were naive to methotrexate treatment, neither triple therapy nor TNFi–methotrexate showed an increased likelihood of achieving ACR70 at 6 months; at the group level, these two treatment strategies appear comparable. The majority of secondary endpoints favoured TNFi–methotrexate in terms of OR directionality, although the limits of the CrIs indicated no clear likelihood of better response with either therapy. TNFi–methotrexate showed increased odds of achieving ACR70 at 6 months over triple therapy, suggesting a possible deeper response with TNFi–methotrexate over triple therapy. These conclusions are in line with the recent ACR guidelines and the EULAR recommendations for the treatment of RA in patients with poor prognostic markers. In patients without poor prognostic markers, it would be reasonable to use either therapy combination.
Acknowledgments
Medical writing support was provided by Dikran Toroser, PhD, CMPP (Amgen) and Miranda Tradewell, PhD, and Rick Davis, MS (Complete Healthcare Communications, LLC, funded by Amgen). Manpreet Sidhu, PhD, and Neil Hawkins, PhD, reviewed and provided input on the study protocol, input on analyses approaches and assumptions, and review of analyses results and interpretation.
Footnotes
Contributors: All authors meet the criteria for authorship, participated in the writing and/or critical revision of the manuscript, and have seen and approved the submitted version. JC, DC, RF, KN, JP, DHT, VT, RvV and GW contributed to the conception, design or planning of the study. KN, VT, SU and RvV were involved in data acquisition. JC, DC, RF, KN, JP, VT, SU and RvV analysed the data. JC, DC, RF and JP drafted the manuscript. All authors interpreted the results and critically revised the manuscript.
Funding: Amgen Inc.
Competing interests: RF has received grants and non-financial support from Amgen. VT, SU and KN are employees of ICON plc, which received grants and consulting fees from Amgen. RvV has received grants from Abbvie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, Roche and Union Chimique Belge, and personal fees from Abbvie, Amgen, Biotest, Bristol-Myers Squibb, Celgene, Crescendo, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Roche, Union Chimique Belge and Vertex. DHT, JC and DC are employees and stockholders of Amgen. JP received grants from Amgen and received consulting fees from Abbvie, Amgen, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche and Union Chimique Belge. GW had nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Singh JA, Saag KG, Bridges SL Jr et al. . 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheum 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Vollenhoven RF, Ernestam S, Geborek P et al. . Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374:459–66. 10.1016/S0140-6736(09)60944-2 [DOI] [PubMed] [Google Scholar]
- 4.Emery P, Breedveld FC, Hall S et al. . Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 [DOI] [PubMed] [Google Scholar]
- 5.Moreland LW, O'Dell JR, Paulus HE et al. . A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: The Treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824–35. 10.1002/art.34498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Heijde D, Klareskog L, Wajdula J et al. . Sustained halting of joint damage with combination etanercept and methotrexate: 3-year results from the TEMPO trial. Ann Rheum Dis 2006;65:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breedveld FC, Weisman MH, Kavanaugh AF et al. . The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 8.de Jong PH, Hazes JM, Han HK et al. . Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis 2014;73:1331–9. 10.1136/annrheumdis-2013-204788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keystone EC, Breedveld FC, van der Heijde D et al. . Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J Rheumatol 2014;41:5–14. 10.3899/jrheum.130543 [DOI] [PubMed] [Google Scholar]
- 10.Rantalaiho V, Korpela M, Laasonen L et al. . Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish Rheumatoid Arthritis Combination Therapy trial. Arthritis Res Ther 2010;12:R122 10.1186/ar3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF et al. . Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum 2008;58:S126–35. 10.1002/art.23364 [DOI] [PubMed] [Google Scholar]
- 12.Smolen JS, Van Der Heijde DM, St Clair EW et al. . Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum 2006;54:702–10. 10.1002/art.21678 [DOI] [PubMed] [Google Scholar]
- 13.Nam JL, Ramiro S, Gaujoux-Viala C et al. . Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2014;73:516–28. 10.1136/annrheumdis-2013-204577 [DOI] [PubMed] [Google Scholar]
- 14.O'Dell JR, Haire CE, Erikson N et al. . Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med 1996;334:1287–91. 10.1056/NEJM199605163342002 [DOI] [PubMed] [Google Scholar]
- 15.Van Vollenhoven RF, Geborek P, Forslind K et al. . Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 2012;379:1712–20. 10.1016/S0140-6736(12)60027-0 [DOI] [PubMed] [Google Scholar]
- 16.O'Dell JR, Curtis JR, Mikuls TR et al. . Validation of the methotrexate-first strategy in patients with early, poor-prognosis rheumatoid arthritis: results from a two-year randomized, double-blind trial. Arthritis Rheum 2013;65:1985–94. 10.1002/art.38012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013;346:f2914 10.1136/bmj.f2914 [DOI] [PubMed] [Google Scholar]
- 18.Buckley F, Finckh A, Huizinga TW et al. . Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J Manag Care Spec Pharm 2015;21:409–23. 10.18553/jmcp.2015.21.5.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YH, Bae SC, Song GG. Comparative efficacy and safety of tofacitinib, with or without methotrexate, in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Rheumatol Int 2015;35:1965–74. 10.1007/s00296-015-3291-4 [DOI] [PubMed] [Google Scholar]
- 20.Thorlund K, Mills EJ. Sample size and power considerations in network meta-analysis. Syst Rev 2012;1:41 10.1186/2046-4053-1-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC et al. , Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Cochrane Collaborations. Cochrane Handbook for Systematic Reviews of Interventions. http://handbook.cochrane.org/front_page.htm (accessed 23 Sep 2015).
- 24.Chen DY, Chou SJ, Hsieh TY et al. . Randomized, double-blind, placebo-controlled, comparative study of human anti-TNF antibody adalimumab in combination with methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis. J Formos Med Assoc 2009;108:310–19. 10.1016/S0929-6646(09)60071-1 [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Takeuchi T, Miyasaka N et al. . A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol 2006;33:37–44. [PubMed] [Google Scholar]
- 26.Choy E, McKenna F, Vencovsky J et al. . Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford) 2012;51:1226–34. 10.1093/rheumatology/ker519 [DOI] [PubMed] [Google Scholar]
- 27.Durez P, Nzeusseu Toukap A, Lauwerys BR et al. . A randomised comparative study of the short term clinical and biological effects of intravenous pulse methylprednisolone and infliximab in patients with active rheumatoid arthritis despite methotrexate treatment. Ann Rheum Dis 2004;63:1069–74. 10.1136/ard.2003.012914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furst DE, Gaylis N, Bray V et al. . Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis 2007;66:893–9. 10.1136/ard.2006.068304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kameda H, Ueki Y, Saito K et al. . Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomized trial. Mod Rheumatol 2010;20:531–8. 10.1007/s10165-010-0324-4 [DOI] [PubMed] [Google Scholar]
- 30.Kay J, Matteson EL, Dasgupta B et al. . Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 2008;58:964–75. 10.1002/art.23383 [DOI] [PubMed] [Google Scholar]
- 31.Kavanaugh A, St Clair EW, McCune WJ et al. . Chimeric anti-tumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol 2000;27:841–50. [PubMed] [Google Scholar]
- 32.Keystone E, Heijde DV, Mason D Jr et al. . Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319–29. 10.1002/art.23964 [DOI] [PubMed] [Google Scholar]
- 33.Keystone EC, Genovese MC, Klareskog L et al. , GO-FORWARD Study. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis 2009;68:789–96. 10.1136/ard.2008.099010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keystone EC, Kavanaugh AF, Sharp JT et al. . Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti–tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11. 10.1002/art.20217 [DOI] [PubMed] [Google Scholar]
- 35.Kim HY, Hsu PN, Barba M et al. . Randomized comparison of etanercept with usual therapy in an Asian population with active rheumatoid arthritis: the APPEAL trial. Int J Rheum Dis 2012;15:188–96. 10.1111/j.1756-185X.2011.01680.x [DOI] [PubMed] [Google Scholar]
- 36.Kim HY, Lee SK, Song YW et al. . A randomized, double-blind, placebo-controlled, phase III study of the human anti-tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR J Rheumatol 2007;10:9–16. 10.1111/j.1479-8077.2007.00248.x [DOI] [Google Scholar]
- 37.Kim J, Ryu H, Yoo DH et al. . A clinical trial and extension study of infliximab in Korean patients with active rheumatoid arthritis despite methotrexate treatment. J Korean Med Sci 2013;28:1716–22. 10.3346/jkms.2013.28.12.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kremer J, Ritchlin C, Mendelsohn A et al. . Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum 2010;62:917–28. 10.1002/art.27348 [DOI] [PubMed] [Google Scholar]
- 39.Lan JL, Chou SJ, Chen DY et al. . A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12-week, double-blind, randomized, placebo-controlled study. J Formos Med Assoc 2004;103:618–23. [PubMed] [Google Scholar]
- 40.Machado DA, Guzman RM, Xavier RM et al. . Open-label observation of addition of etanercept versus a conventional disease-modifying antirheumatic drug in subjects with active rheumatoid arthritis despite methotrexate therapy in the Latin American region. J Clin Rheumatol 2014;20:25–33. 10.1097/RHU.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 41.Maini R, St Clair EW, Breedveld F et al. . Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999;354:1932–9. 10.1016/S0140-6736(99)05246-0 [DOI] [PubMed] [Google Scholar]
- 42.Maini RN, Breedveld FC, Kalden JR et al. . Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63. [DOI] [PubMed] [Google Scholar]
- 43.O'Dell JR, Mikuls TR, Taylor TH et al. . Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013;369:307–18. 10.1056/NEJMoa1303006 [DOI] [PubMed] [Google Scholar]
- 44.Schiff M, Keiserman M, Codding C et al. . Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2008;67:1096–103. 10.1136/ard.2007.080002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smolen J, Landewe RB, Mease P et al. . Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797–804. 10.1136/ard.2008.101659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam LS, Shang Q, Li EK et al. . Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis—a randomized trial. J Rheumatol 2012;39:2267–75. 10.3899/jrheum.120541 [DOI] [PubMed] [Google Scholar]
- 47.Tanaka C, Shiozawa K, Hashiramoto A et al. . A study on the selection of DMARDs for the combination therapy with adalimumab. Kobe J Med Sci 2012;58:E41–50. [PubMed] [Google Scholar]
- 48.Tanaka Y, Harigai M, Takeuchi T et al. . Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO-FORTH study. Ann Rheum Dis 2012;71:817–24. 10.1136/ard.2011.200317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor PC, Steuer A, Gruber J et al. . Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo-controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum 2004;50:1107–16. 10.1002/art.20123 [DOI] [PubMed] [Google Scholar]
- 50.van Riel PL, Taggart AJ, Sany J et al. , Add Enbrel or Replace Methotrexate Study Investigators. Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study. Ann Rheum Dis 2006;65:1478–83. 10.1136/ard.2005.043299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Vollenhoven RF, Fleischmann R, Cohen S et al. . Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. 10.1056/NEJMoa1112072 [DOI] [PubMed] [Google Scholar]
- 52.Weinblatt ME, Bingham CO III, Mendelsohn AM et al. . Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis 2013;72:381–9. 10.1136/annrheumdis-2012-201411 [DOI] [PubMed] [Google Scholar]
- 53.Weinblatt ME, Keystone EC, Furst DE et al. . Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45. 10.1002/art.10697 [DOI] [PubMed] [Google Scholar]
- 54.Weinblatt ME, Schiff M, Valente R et al. . Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum 2013;65:28–38. 10.1002/art.37711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinblatt ME, Kremer JM, Bankhurst AD et al. . A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. 10.1056/NEJM199901283400401 [DOI] [PubMed] [Google Scholar]
- 56.Weinblatt ME, Schiff MH, Ruderman EM et al. . Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum 2008;58:1921–30. 10.1002/art.23493 [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto K, Takeuchi T, Yamanaka H et al. . Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis: the HIKARI randomized, placebo-controlled trial. Mod Rheumatol 2014;24:552–60. 10.3109/14397595.2013.843764 [DOI] [PubMed] [Google Scholar]
- 58.Yoo DH, Hrycaj P, Miranda P et al. . A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613–20. 10.1136/annrheumdis-2012-203090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang FC, Hou Y, Huang F et al. . Infliximab versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a preliminary study from China. APLAR J Rheumatol 2006;9:127–30. 10.1111/j.1479-8077.2006.00186.x [DOI] [Google Scholar]
- 60.Takeuchi T, Miyasaka N, Inoue K et al. . Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol 2009;19:478–87. 10.1007/s10165-009-0195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westhovens R, Yocum D, Han J et al. . The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 2006;54:1075–86. 10.1002/art.21734 [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi T, Yamanaka H, Ishiguro N et al. . Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis 2014;73:536–43. 10.1136/annrheumdis-2012-202433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hørslev-Petersen K, Hetland ML, Junker P et al. , OPERA Study-Group. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis 2014;73:654–61. 10.1136/annrheumdis-2012-202735 [DOI] [PubMed] [Google Scholar]
- 64.Burmester G, Kivitz A, Kupper H et al. . Efficacy, pharmacokinetics, and safety of different doses of methotrexate in combination with adalimumab: results from the CONCERTO trial [abstract]. Ann Rheum Dis 2013;72(Suppl 1):A72–A3. [Google Scholar]
- 65.Detert J, Bastian H, Listing J et al. . Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844–50. 10.1136/annrheumdis-2012-201612 [DOI] [PubMed] [Google Scholar]
- 66.Kavanaugh A, Fleischmann R, Emery P et al. . Efficacy of addition, or continuation, of adalimumab in patients who did not achieve stable low disease activity with methotrexate or adalimumab plus methotrexate in the optima study. Ann Rheum Dis 2013;71:511–12. [Google Scholar]
- 67.Soubrier M, Puéchal X, Sibilia J et al. . Evaluation of two strategies (initial methotrexate monotherapy vs its combination with adalimumab) in management of early active rheumatoid arthritis: data from the GUEPARD trial. Rheumatology (Oxford) 2009;48:1429–34. 10.1093/rheumatology/kep261 [DOI] [PubMed] [Google Scholar]
- 68.Bejarano V, Quinn M, Conaghan PG et al. . Effect of the early use of the anti-tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Rheum 2008;59:1467–74. 10.1002/art.24106 [DOI] [PubMed] [Google Scholar]
- 69.Iannone F, La Montagna G, Bagnato G et al. . Safety of etanercept and methotrexate in patients with rheumatoid arthritis and hepatitis C virus infection: a multicenter randomized clinical trial. J Rheumatol 2014;41:286–92. 10.3899/jrheum.130658 [DOI] [PubMed] [Google Scholar]
- 70.Klareskog L, van der Heijde D, de Jager JP et al. . Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. 10.1016/S0140-6736(04)15640-7 [DOI] [PubMed] [Google Scholar]
- 71.Emery P, Fleischmann RM, Moreland LW et al. . Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272–83. 10.1002/art.24638 [DOI] [PubMed] [Google Scholar]
- 72.Nam JL, Villeneuve E, Hensor EMA et al. . Remission induction comparing infliximab and high-dose intravenous steroid, followed by treat-to-target: a double-blind, randomised, controlled trial in new-onset, treatment-naive, rheumatoid arthritis (the IDEA study). Ann Rheum Dis 2014;73:75–85. 10.1136/annrheumdis-2013-203440 [DOI] [PubMed] [Google Scholar]
- 73.Durez P, Malghem J, Nzeusseu Toukap A et al. . Treatment of early rheumatoid arthritis: a randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum 2007;56:3919–27. 10.1002/art.23055 [DOI] [PubMed] [Google Scholar]
- 74.Quinn MA, Conaghan PG, O'Connor PJ et al. . Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:27–35. 10.1002/art.20712 [DOI] [PubMed] [Google Scholar]
- 75.St Clair EW, van der Heijde DM, Smolen JS et al. . Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43. 10.1002/art.20568 [DOI] [PubMed] [Google Scholar]
- 76.Leirisalo-Repo M, Kautiainen H, Laasonen L et al. . Infliximab for 6 months added on combination therapy in early rheumatoid arthritis: 2-year results from an investigator-initiated, randomised, double-blind, placebo-controlled study (the NEO-RACo Study). Ann Rheum Dis 2013;72:851–7. 10.1136/annrheumdis-2012-201365 [DOI] [PubMed] [Google Scholar]
- 77.Saunders SA, Capell HA, Stirling A et al. . Triple therapy in early active rheumatoid arthritis: a randomized, single-blind, controlled trial comparing step-up and parallel treatment strategies. Arthritis Rheum 2008;58:1310–17. 10.1002/art.23449 [DOI] [PubMed] [Google Scholar]
- 78.Klareskog L, van der Heijde D, De Jager J et al. . Clinical outcomes of a double-blind study of etanercept and methotrexate, alone and combined, in patients with active rheumatoid arthritis (TEMPO trial), year 2 results. Ann Rheum Dis 2004;63:OP0003 10.1136/ard.2004.028225 [DOI] [Google Scholar]
- 79.Yamanaka H, Yamamoto K, Takeuchi T et al. . Improved physical function, pain, and health related quality of life with certolizumab pegol in Japanese rheumatoid arthritis patients without methotrexate co-administration: results from the Hikari study. Ann Rheum Dis 2013;71:664. [Google Scholar]
- 80.Van Vollenhoven RF, Wallenstein G, Lee EB et al. . Effects of tofacitinib (CP-690,550), an oral janus kinase inhibitor, or adalimumab on patient reported outcomes in a phase 3 study of active rheumatoid arthritis. Ann Rheum Dis 2013;71:206. [Google Scholar]
- 81.Tanaka Y, Yamamoto K, Takeuchi T et al. . Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52-week results from an open-label extension of the J-RAPID study. Mod Rheumatol 2014;24:734–43. 10.3109/14397595.2014.881709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breedveld FC, Emery P, Keystone E et al. . Infliximab in active early rheumatoid arthritis. Ann Rheum Dis 2004;63:149–55. 10.1136/ard.2003.013961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kameda H, Kanbe K, Sato E et al. . Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol 2011;38:1585–92. 10.3899/jrheum.110014 [DOI] [PubMed] [Google Scholar]
- 84.Keystone EC, Combe B, Smolen J et al. . Sustained efficacy of certolizumab pegol added to methotrexate in the treatment of rheumatoid arthritis: 2-year results from the RAPID 1 trial. Rheumatology (Oxford) 2012;51:1628–38. 10.1093/rheumatology/kes082 [DOI] [PubMed] [Google Scholar]
- 85.Strand V, Mease P, Burmester GR et al. . Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther 2009;11:R170 10.1186/ar2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conaghan PG, Emery P, Østergaard M et al. . Assessment by MRI of inflammation and damage in rheumatoid arthritis patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Ann Rheum Dis 2011;70:1968–74. 10.1136/ard.2010.146068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conaghan PG, Emery P, Ostergaard M et al. . Assessment of inflammation and damage by MRI in established RA patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Arthritis Rheum 2010;62:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Genovese MC, Han C, Keystone EC et al. . Effect of golimumab on patient-reported outcomes in rheumatoid arthritis: results from the GO-FORWARD study. J Rheumatol 2012;39:1185–91. 10.3899/jrheum.111195 [DOI] [PubMed] [Google Scholar]
- 89.Genovese MC, Keystone EC, Hsia EC et al. . Impact of golimumab on physical function, health-related quality of life, productivity and employment in rheumatoid arthritis patients: week 52 results from the GO-FORWARD study. Int J Rheum Dis 2010;13:88. [Google Scholar]
- 90.Keystone EC, Genovese MC, Hall S et al. . Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: results through 2 years of the Go-Forward study extension. J Rheumatol 2013;40:1097–103. 10.3899/jrheum.120584 [DOI] [PubMed] [Google Scholar]
- 91.Rubbert A, Keystone E, Genovese M et al. . Golimumab administered subcutaneously every 4 weeks in active rheumatoid arthritis despite methotrexate: week 24 results of Go-Forward study. Rheumatology 2009;48:i92. [Google Scholar]
- 92.Bae SC, Gun SC, Mok CC et al. . Improved health outcomes with etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskel Disord 2013;14:13 10.1186/1471-2474-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atsumi T, Yamamoto K, Takeuchi T et al. . The first early rheumatoid arthritis, certolizumab pegol, multicenter, double-blind, randomized, parallel-group study: C-Opera, in patients fulfilling the 2010 ACR/EULAR classification criteria, demonstrates inhibition of joint damage progression. Ann Rheum Dis 2014;73:484 10.1136/annrheumdis-2015-207511 [DOI] [Google Scholar]
- 94.Bingham CO III, Weinblatt ME, Mendelsohn A et al. . Predictors of significant disease activity score-28 (using C-reactive protein) remission achieved with intravenous golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: results of the phase iii, multicentre, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2013;52:i82–i3. [Google Scholar]
- 95.Bingham CO III, Weinblatt M, Han C et al. . The effect of intravenous golimumab on health-related quality of life in rheumatoid arthritis: 24-week results of the phase III GO-FURTHER trial. J Rheumatol 2014;41:1067–76. 10.3899/jrheum.130864 [DOI] [PubMed] [Google Scholar]
- 96.Bingham CO III, Weinblatt M, Mendelsohn A et al. . Predictors of significant disease activity score-28 (using C-reactive protein) remission achieved with intravenous golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: results of the phase 3, multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2012;64:S558. [Google Scholar]
- 97.Bingham CO III, Westhovens R, Mendelsohn AM et al. . Sustained and consistent clinical benefit with intravenous golimumab therapy in patients with active rheumatoid arthritis despite methotrexate therapy: results through 1-year of a phase 3, randomized, multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2013;65:S374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karlsson JA, Neovius M, Nilsson JÅ et al. . Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in early rheumatoid arthritis: 2-year quality-of-life results of the randomised, controlled, SWEFOT trial. Ann Rheum Dis 2013;72:1927–33. 10.1136/annrheumdis-2012-202062 [DOI] [PubMed] [Google Scholar]
- 99.Lipsky PE, van der Heijde DM, St Clair EW et al. , Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594–602. [DOI] [PubMed] [Google Scholar]
- 100.Maini RN, Breedveld FC, Kalden JR et al. . Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004;50:1051–65. 10.1002/art.20159 [DOI] [PubMed] [Google Scholar]
- 101.Rezaei H, Saevarsdottir S, Geborek P et al. . Evaluation of hand bone loss by digital X-ray radiogrammetry as a complement to clinical and radiographic assessment in early rheumatoid arthritis: results from the SWEFOT trial. BMC Musculoskel Disord 2013;14:79 10.1186/1471-2474-14-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schiff M, Weinblatt ME, Valente R et al. . Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 2014;73:86–94. 10.1136/annrheumdis-2013-203843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smolen J, Keystone E, Schiff M et al. . Sustained long-term efficacy of certolizumab pegol plus methotrexate in the treatment of rheumatoid arthritis: a 2-year analysis of the Rapid 1 study. Rheumatology 2009;48:i85–6. [Google Scholar]
- 104.Strand V, Smolen JS, van Vollenhoven RF et al. . Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis 2011;70:996–1002. 10.1136/ard.2010.143586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanaka Y, Harigai M, Takeuchi T et al. . 52-week results of clinical, radiographic and pharmacokinetic assessments: golimumab, a human anti-TNF monoclonal antibody, administered subcutaneously every four weeks in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2013;71:372 10.1136/annrheumdis-2012-eular.1626 [DOI] [Google Scholar]
- 106.Tanaka Y, Harigai M, Takeuchi T et al. . Golimumab administered subcutaneously every four weeks in patients with active rheumatoid arthritis despite methotrexate therapy: long-term clinical, radiographic and safety results, including evaluation of remission using the new ACR/EULAR criteria. Ann Rheum Dis 2013;72:372. [Google Scholar]
- 107.Taylor PC, Steuer A, Gruber J et al. . Ultrasonographic and radiographic results from a two-year controlled trial of immediate or one-year-delayed addition of infliximab to ongoing methotrexate therapy in patients with erosive early rheumatoid arthritis. Arthritis Rheum 2006;54:47–53. 10.1002/art.21544 [DOI] [PubMed] [Google Scholar]
- 108.van Riel PL, Freundlich B, MacPeek D et al. . Patient-reported health outcomes in a trial of etanercept monotherapy versus combination therapy with etanercept and methotrexate for rheumatoid arthritis: the ADORE trial. Ann Rheum Dis 2008;67:1104–10. 10.1136/ard.2006.068585 [DOI] [PubMed] [Google Scholar]
- 109.Weinblatt M, Bingham CO III, Mendelsohn A et al. . Intravenous golimumab inhibits radiographic progression and maintains clinical efficacy and safety in patients with active rheumatoid arthritis despite methotrexate therapy: 1-year results of a phase 3 trial. Arthritis Rheum 2012;64:S353–S4. [Google Scholar]
- 110.Westhovens R, Weinblatt ME, Han C et al. . Intravenously administered golimumab significantly improves health related quality of life and work productivity in patients with rheumatoid arthritis: results of a phase III, placebo-controlled trial. Value Health 2012;15:A42. [Google Scholar]
- 111.Westhovens R, Weinblatt ME, Han C et al. . Health-related quality of life of patients with rheumatoid arthritis achieving DAS28 remission, improvement in physical function and no radiographic progression after treatment with intravenous golimumab [abstract]. Ann Rheum Dis 2014;73:479–80. 10.1136/annrheumdis-2014-eular.3819 [DOI] [Google Scholar]
- 112.Burmester G, Kivitz AJ, Van Vollenhoven RF et al. . Methotrexate dose has minimal effects on methotrexate-related toxicity in patients with early rheumatoid arthritis treated in combination with adalimumab-results of CONCERTO trial [abstract]. Ann Rheum Dis 2013;72(Suppl 10):A246. [Google Scholar]
- 113.Burmester GR, Weinblatt ME, McInnes IB et al. . Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis 2013;72:1445–52. 10.1136/annrheumdis-2012-202450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fleischmann RM, Kivitz A, Van Vollenhoven RF et al. . No differences in patient-reported outcomes by methotrexate dose among early rheumatoid arthritis patients treated concomitantly with adalimumab: results from the CONCERTO trial [abstract]. Arthritis Rheum 2013;65:S574–S5. [Google Scholar]
- 115.Goekoop-Ruiterman YP, De Vries-Bouwstra JK, Allaart CF et al. . Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med 2007;146:406–15. 10.7326/0003-4819-146-6-200703200-00005 [DOI] [PubMed] [Google Scholar]
- 116.van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP et al. . Patient-reported outcomes in a randomized trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Care Res (Hoboken) 2009;61:4–12. 10.1002/art.24367 [DOI] [PubMed] [Google Scholar]
- 117.Horslev-Petersen K, Hetland ML, Junker P et al. . Remission rates increase substantially by adding adalimumab to methotrexate and intra-articular glucocorticoid in patients with early rheumatoid arthritis: 1-year results of the investigator-initiated, double-blinded randomized clinical trial (OPERA). Scand J Rheumatol 2012;41:17–18. [Google Scholar]
- 118.Horslev-Petersen K, Hetland ML, Junker P et al. . Adalimumab added to methotrexate and intra-articular glucocorticoid increases remission rates at one year in early, DMARD-naive patients with rheumatoid arthritis: an investigator-initiated randomized, controlled, double-blinded study [abstract]. Arthritis Rheum 2011;65:394. [Google Scholar]
- 119.Horslev-Petersen K, Hetland ML, Junker P et al. . Very high remission rates are achieved by methotrexate and intraarticular glucocorticoids independent of induction therapy with adalimumab; year 2 clinical results of an investigator-initiated randomised, controlled clinical trial of early, rheumatoid arthritis (OPERA). Arthritis Rheum 2013;65:S1148. [Google Scholar]
- 120.Horslev-Petersen K, Hetland ML, Junker P et al. . Improved remission rates acquired by adding adalimumab to methotrexate and intraarticular glucocorticoid cannot be maintained after withdrawal of adalimumab. A 2-year investigator initiated randomised, controlled study on early rheumatoid arthritis [abstract]. Ann Rheum Dis 2013;72:A236. [Google Scholar]
- 121.Detert J, Bastian H, Listing J et al. . Efficacy of an induction therapy with adalimumab plus methotrexate versus methotrexate monotherapy in recent onset rheumatoid arthritis—an investigator initiated randomized controlled trial [abstract]. Arthritis Rheum 2011;63:1697. [Google Scholar]
- 122.Detert J, Bastian H, Listing J et al. . Induction therapy with adalimumab plus methotrexate versus methotrexate monotherapy in recent onset rheumatoid arthritis (RA)-an investigator initiated randomized controlled trial. Ann Rheum Dis 2013;71:102–3. 10.1136/annrheumdis-2012-eular.1512 [DOI] [PubMed] [Google Scholar]
- 123.Kavanaugh A, Emery P, Fleischmann R et al. . Withdrawal of adalimumab in early rheumatoid arthritis patients who attained stable low disease activity with adalimumab plus methotrexate: results of a phase 4, double-blind, placebo-controlled trial. Arthritis Rheum 2011;63:1699. [Google Scholar]
- 124.Kavanaugh A, Fleischmann R, Emery P et al. . Clinical and functional improvements in early RA following treatment with adalimumab plus methotrexate compared with methotrexate monotherapy: 26-week results of the Optima trial. Arthritis Rheum 2010;62:1791 10.1002/art.27673 [DOI] [Google Scholar]
- 125.Kavanaugh A, Fleischmann RM, Emery P et al. . Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64–71. 10.1136/annrheumdis-2011-201247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smolen JS, Fleischmann RM, Emery P et al. . Treating rheumatoid arthritis to target: outcomes and predictors in early rheumatoid arthritis patients treated with adalimumab plus methotrexate, methotrexate alone or methotrexate plus subsequent adalimumab. Rheumatology (Oxford) 2012;51:iii114–iii5. [Google Scholar]
- 127.Emery P, Kavanaugh AF, Smolen J et al. . Combination therapy with adalimumab-methotrexate significantly improved work ability, physical function, fatigue, and other patient-reported outcomes in early rheumatoid arthritis: results from a 26-week analysis. Arthritis Rheum 2011;63:2189. [Google Scholar]
- 128.Kimel M, Cifaldi M, Chen N et al. . Adalimumab plus methotrexate improved SF-36 scores and reduced the effect of rheumatoid arthritis (RA) on work activity for patients with early RA. J Rheumatol 2008;35:206–15. [PubMed] [Google Scholar]
- 129.Strand V, Rentz AM, Cifaldi MA et al. . Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol 2012;39:63–72. 10.3899/jrheum.101161 [DOI] [PubMed] [Google Scholar]
- 130.Emery P, Breedveld F, van der Heijde D et al. , Combination of Methotrexate and Etanercept in Early Rheumatoid Arthritis Trial Group. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum 2010;62:674–82. 10.1002/art.27268 [DOI] [PubMed] [Google Scholar]
- 131.Kekow J, Moots RJ, Emery P et al. . Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis 2010;69:222–5. 10.1136/ard.2008.102509 [DOI] [PubMed] [Google Scholar]
- 132.Anis A, Zhang W, Emery P et al. . The effect of etanercept on work productivity in patients with early active rheumatoid arthritis: results from the COMET study. Rheumatology (Oxford) 2009;48:1283–9. 10.1093/rheumatology/kep239 [DOI] [PubMed] [Google Scholar]
- 133.van der Heijde D, Klareskog L, Boers M et al. , TEMPO Investigators. Comparison of different definitions to classify remission and sustained remission: 1 year TEMPO results. Ann Rheum Dis 2005;64:1582–7. 10.1136/ard.2004.034371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van der Heijde D, Klareskog L, Rodriguez-Valverde V et al. , TEMPO Study Investigators. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 2006;54:1063–74. 10.1002/art.21655 [DOI] [PubMed] [Google Scholar]
- 135.van der Heijde D, Klareskog L, Singh A et al. . Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis 2006;65:328–34. 10.1136/ard.2005.035709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Emery P, Fleischmann RM, Doyle MK et al. . Golimumab, a human anti-tumor necrosis factor monoclonal antibody, injected subcutaneously every 4 weeks in patients with active rheumatoid arthritis who had never taken methotrexate: 1-year and 2-year clinical, radiologic, and physical function findings of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res 2013;65:1732–42. 10.1002/acr.22072 [DOI] [PubMed] [Google Scholar]
- 137.Emery P, Fleischmann RM, Moreland LW et al. . Golimumab, a new human anti-TNF-alpha monoclonal antibody, in methotrexate-naive active rheumatoid arthritis (Go-Before study). Rheumatology 2009;48:i93 10.1093/rheumatology/kep727 [DOI] [Google Scholar]
- 138.Østergaard M, Emery P, Conaghan PG et al. . Significant improvement in synovitis, osteitis, and bone erosion following golimumab and methotrexate combination therapy as compared with methotrexate alone: a magnetic resonance imaging study of 318 methotrexate-naive rheumatoid arthritis patients. Arthritis Rheum 2011;63:3712–22. 10.1002/art.30592 [DOI] [PubMed] [Google Scholar]
- 139.Haugeberg G, Conaghan PG, Quinn M et al. . Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2009;68:1898–901. 10.1136/ard.2008.106484 [DOI] [PubMed] [Google Scholar]
- 140.Valleala H, Kautiainen H, Mottonen T et al. . Bone mineral density in patients with recent onset, active rheumatoid arthritis: two-year data of the NEO-RACo study. Arthritis Rheum 2009;60:1008. [Google Scholar]
- 141.Moreland LW, O'Dell JR, Paulus HE et al. . Two-year radiographic results from the TEAR trial. Arthritis Rheum 2010;62:1368. [Google Scholar]
- 142.Jansen JP, Trikalinos T, Cappelleri JC et al. . Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform healthcare decision-making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014;17:157–73. 10.1016/j.jval.2014.01.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2016-000371supp_appendix.pdf (1.9MB, pdf)