Abstract
The Food and Drug Administration (FDA) had approved fingolimod usage for multiple sclerosis in 2010. Melanoma after the usage of fingolimod immunomodulation was reported rarely in clinical trials, and only two case reports exist in the published literature, both occurring in Europe. Most of the incidences reported in clinical trials were in-situ, whereas both case reports were of malignant melanoma. Fingolimod has been found to inhibit metastatic melanoma growth in a mouse model that depends on vascular endothelial growth factor (VEGF)-induced angiogenesis for metastasis. However, there are numerous pathways of angiogenesis and tumour growth found in vivo by which melanoma can expand that do not mandate VEGF. We report a case of superficial spreading malignant melanoma occurring after fingolimod therapy in the USA.
Background
Fingolimod (Gilenya) is an immunosuppressive drug that the Food and Drug Administration (FDA) approved for the treatment of multiple sclerosis in 2010.1 Fingolimod is an oral immunomodulator that once activated via phosphorylation in vivo triggers internalisation of the sphingosine-1-phosphate (S1P) type 1 receptor, a receptor that normally signals lymphocytes to migrate from the lymph nodes to carry out their immunological or in the case of multiple sclerosis, autoimmunological activities in the brain and spinal cord.1 2 Potent inhibition of angiogenesis has also been demonstrated with Fingolimod both by antagonism of the S1P receptor and the vascular endothelial growth factor (VEGF)-induced migration of vascular endothelial cells.3 In a mouse model of syngeneic B16/BL6 murine melanoma fingolimod significantly inhibited metastatic tumour growth and although not significant, tended to inhibit primary tumour growth.3
The most common adverse events of fingolimod found in various clinical trials are headache, influenza, diarrhoea, nausea, cough, back pain and elevation of liver enzymes.1 2 4–7 However, serious adverse events such as skin cancers including development of basal cell carcinoma, squamous cell carcinoma and melanoma were reported in the clinical trials using fingolimod for multiple sclerosis and are summarised in table 1. However, the scope of this case report with review of the literature was to highlight the reported development of melanoma in patients who use fingolimod and to present a case thereof.
Table 1.
Summary of fingolimod clinical trials and incidence of skin cancer
| Incidence of skin cancer |
||||
|---|---|---|---|---|
| Clinical trial | Drug and dose (sample size) | Melanoma (including in situ) | Basal cell carcinoma | SCC (including in situ) |
| Phase II RCT proof of concept core study (2006) Kappos et al4 |
Fingolimod 5 mg once daily (n=92) | 0 | 0 | 1* |
| Fingolimod 1.25 mg once daily (n=93) | 0 | 0 | 0 | |
| Placebo (n=92) | 0 | 0 | 0 | |
| TRANSFORMS (2010) Cohen et al2 |
Fingolimod 1.25 mg once daily (n=420) | 0 | 2 (0.5%) | 0 |
| Fingolimod 0.5 mg once daily (n=429) | 3 (0.7%) | 3 (0.7%) | 0 | |
| Interferon -β-1a 30 μg weekly (n=431) | 0 | 1 (0.2%) | 1 (0.2) | |
| FREEDOMS (2010) Kappos et al5 |
Fingolimod 1.25 mg once daily (n=429) | 1 (0.2%) | 1 (0.2%) | 1 (0.2%) |
| Fingolimod 0.5 mg once daily (n=425) | 0 | 4 (0.9%) | 0 | |
| Placebo (n=418) | 1 (0.2%) | 1 (0.2%) | 0 | |
| FREEDOMS II (2014) Calabresi et al7 |
Fingolimod 1.25 mg once daily (n=370) | 0 | 6 (1.6%) | 3 (0.8%) |
| Fingolimod 0.5 mg once daily (n=358) | 0 | 10 (2.8%) | 1 (0.3%) | |
| Placebo (n=355) | 0 | 2 (0.6%) | 2 (0.6%) | |
| INFORMS (2016) Lublin et al6 |
Fingolimod 0.5 mg once daily (n=336)† | 1 (0.3%) | 14 (4.2%) | 6 (1.8%) |
| Placebo (n=487) | 0 | 9 (1.8%) | 1 (0.2%) | |
Bolded numbers in figure 1 correspond to incidence of melanoma in the groups of patients taking fingolimod in the reported clinical trials.
*Location of SCC not listed.
†Patients switched from fingolimod 1.25 mg to 0.5 mg daily per protocol change at 14 months into study.
RCT, randomised controlled trial; SCC, squamous cell carcinoma.
In the TRANSFORMS (Trial Assessing Injectable Interferon versus FTY720 Oral in Relapsing–Remitting Multiple Sclerosis) trial, a 12-month phase III randomised controlled trial comparing fingolimod 1.25 mg daily (n=420) to 0.5 mg daily (n=429) to interferon-β-1a 30 µg weekly (n=431) in patients with multiple sclerosis Cohen et al found the incidence of melanoma to be 0% vs 0.7% vs 0%, respectively. Interestingly, all reported cases of melanoma were in situ. Similarly, the phase III INFORMS (Investigating FTY720 Oral in Primary Progressive MS) randomised placebo-controlled trial in patients with multiple sclerosis took place between September 2008 and August 2011. Patients took either fingolimod 0.5 mg daily (n=336) or placebo (n=487) with 0.3% vs 0% developing melanoma, respectively.6 Additionally, in FREEDOMS (FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis), a 24-month phase III clinical trial evaluating the effects of daily therapy with fingolimod 1.25 mg (n=429), 0.5 mg (n=425) or placebo (n=418) found the incidence of melanoma to be 0.2%, 0% and 0.2%, respectively.5 Although, there were two such clinical trials for which the development of melanoma after fingolimod therapy was not found, other skin cancers were indeed reported which included a 6-month phase 2 trial,4 and the 24-month FREEDOMS II phase 3 trial.7
Case presentation
A 52-year-old white woman presented for full-body skin examination after her husband noticed a mole on her back had become larger and oddly coloured, but otherwise asymptomatic. The patient's full body skin examination was 2 years prior during which time no suspicious naevi were identified. The patient had no history of skin cancer. She was started on fingolimod 0.5 mg daily for multiple sclerosis 61 months prior to presentation. Physical examination showed >50 naevi and numerous lentigines; the lesion of concern was a 10×10 mm bright pink triangular plaque with brown-to-black irregularly shaped macule and focal crust (figure 1).
Figure 1.
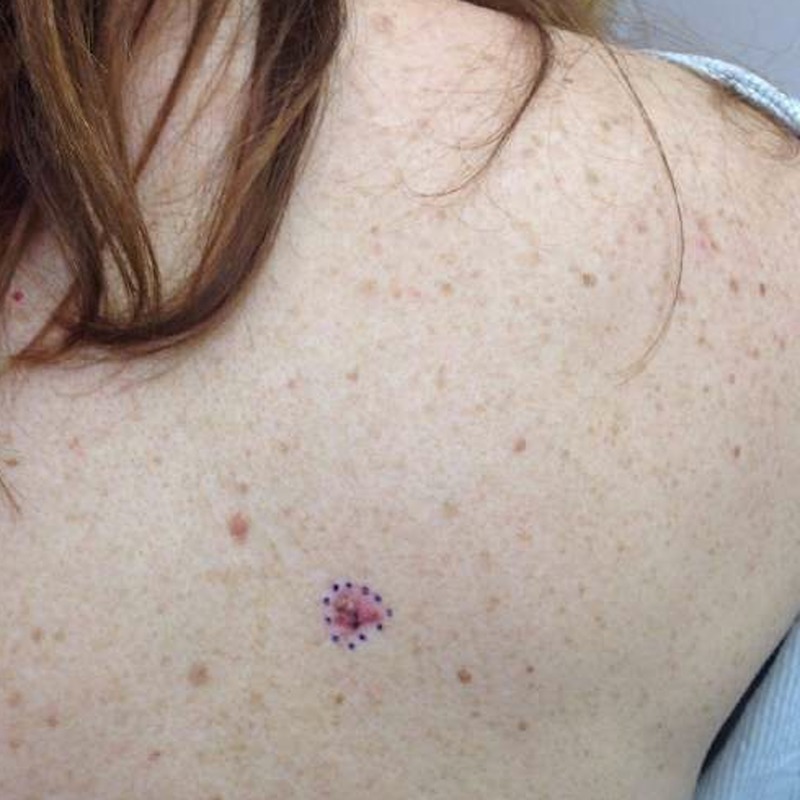
Lesion of concern. Bright pink triangular plaque measuring 10×10 mm with brown-to-black irregularly shaped macule and focal crust.
Investigations
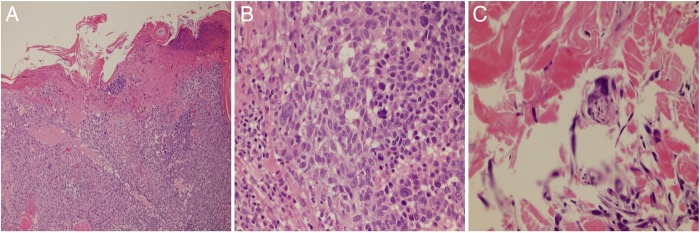
Dermatopathology showed superficial spreading malignant melanoma with ulceration (figure 2A–C). Although there was no vascular or lymphatic invasion seen, perineural invasion was noted. The melanocytes distributed in an irregular pattern singly and in nests at all levels of the epidermis which was highlighted with MART-1/Melan A immunostain. In the dermis there were irregular nests of single scattered melanocytes some of which had a spindled appearance. Mitotic figures were identified. The Breslow depth was 2.125 mm, Clark level IV. She had normal complete blood count (CBC), complete metabolic panel (CMP) and chest X-ray was negative.
Figure 2.
Dermatopathology. (A) Proliferation melanocytes distributed in an irregular pattern at all levels of the epidermis, ulcer present. (B) Proliferation melanocytes with mitotic figures. (C) Perineural invasion.
Treatment
Patient was referred to surgical oncology for management. Intraoperative lymphatic mapping with Lymphazurin blue dye showed sentinel lymph nodes in the left axilla and right neck that were biopsied and found to be free of melanoma. The melanoma was then treated by wide excision with 2 cm margins around the biopsy site down to the level of the muscle fascia with histology showing clear margins. The defect was then closed using complex plastic advancement flap for a final wide excision dimension measuring 5×13 cm. The tumour stage is stage IIB with T3bN0M0.
Outcome and follow-up
The patient was recently seen in surgical clinic 1 month postoperative and showed no evidence of recurrence with proper wound healing. She no longer takes fingolimod and will be followed in dermatology clinic every 3 months for full body skin examinations.
Discussion
A literature search yielded two case reports of patients developing melanoma after initiation of fingolimod for multiple sclerosis, both occurring in Europe. Conzett et al report a melanoma arising from another naevus which was identified 57 months after starting fingolimod 1.25 mg daily in a 41-year-old white woman in Switzerland with <50 naevi, without freckles or sun damage. The melanoma appeared as an atypical network of irregular brown globules and a central hypopigmented whitish-red zone under dermoscopy. Dermatopathology revealed melanoma ex naevo, Breslow depth 0.9 mm with atypical pleomorphic melanocytes consisting of single cells and nests of various sizes and architecture. Melan-A immunostain demonstrated focal transepidermal migration of single cells and small nests.8 Haebich et al, reported a case of melanoma in a 41-year-old person in the UK also arising from a longstanding mole diagnosed 2 months after initiation on fingolimod 0.5 mg daily. The melanoma appeared as a 10×20 mm with central darker black area, redness surrounding the edges without ulceration and a blue–white veil on dermoscopy. Dermatopathology showed superficial spreading malignant melanoma Breslow depth 1.5 mm with of atypical melanocytes, marked junctional activity, focal pagetoid spread in the epidermis, mitoses in upper dermal nests and focal lymphovascular invasion.9
The two cases of melanoma after fingolimod therapy presented above are the only such cases found on review of the published literature per PubMed; however, this is the first case reported in the USA, as all others occurred in Europe. One case of melanoma was diagnosed 57 months after starting fingolimod 1.25 mg daily and with Breslow depth of 0.9 mm8 while the other case occurred just 2 months into fingolimod 0.5 mg daily with a Breslow depth of 1.5.9 Considering all three cases reports, the combined melanoma events were calculated to occur at 0.3 events per patient-year discussed which can be sparingly compared with the melanoma incidence in patients with multiple sclerosis of 0.22% per year found in a 2015 meta-analysis.10 Although, melanoma after treatment with fingolimod for multiple sclerosis was reported in some of the safety and efficacy trials for the drug, all but one of the five cases of melanoma found in patients taking fingolimod during these clinical trials were in situ,2 5 6 possibly due to the increased monitoring for adverse events during study periods. While, two such trials indeed showed an increased incidence of basal cell carcinoma in patients taking fingolimod compared with placebo,6 7 there was no such finding in regards to melanoma. Perhaps, suggesting a longer treatment period required for the development of melanoma as the longest clinical trial was 35 months and a dose change from 1.25 mg daily to 0.5 mg daily took place 14 months into the trial.6 However, there is no clear time trend to the development of melanoma after starting fingolimod, as the development is likely related to unclear patient-specific factors while undergoing immunomodulation.
Although, LaMotagne et al3 describe a syngeneic B16/BL6 murine melanoma in a mouse model for which fingolimod significantly inhibited metastatic tumour growth and although not significant, tended to inhibit primary tumour growth, in vitro studies did not show direct induction of apoptosis. This syngeneic B16/BL6 murine melanoma model is a highly metastatic cell line11 that has only been shown to be responsive to blocking VEGF-induced angiogenesis,3 which is not representative of the various pathways of angiogenesis and tumour growth found in vivo. Perhaps, this inhibition of angiogenesis-dependent metastasis in a mouse model explains why most of the cases of melanoma reported in these rather short clinical trials were in-situ, suggesting that VEGF-independent mechanism for angiogenesis must be utilised in order for malignant melanoma to occur. The link between the development of melanoma and fingolimod is not clearly understood but has been postulated to be due to the lack of immunosurveillance necessary for the body to recognise and clear tumour formation prior to immunoescape.8 Therefore, the longer immunosurveillance is poor and especially in combination with mutanogenic ultraviolet (UV) radiation exposure, the more likely UV-associated malignancies such as melanoma are to form and persist via immunoescape. As a precautionary measure we support that patients should have regular full body skin examinations with their dermatologist while taking fingolimod for multiple sclerosis such to decrease morbidity and mortality associated with skin malignancy.
Learning points.
Fingolimod immunomodulation may impart increased risk for melanoma.
The patient-specific risk factors that combine with fingolimod usage to impart the increased risk are not clearly understood.
Patients taking fingolimod should have regular full body skin examinations with a dermatologist to monitor for skin cancers, especially if expected therapy duration will be more than 6 months.
Physicians should discuss the possible risk of melanoma with patients when starting fingolimod.
Footnotes
Contributors: Data interpretation, drafting of the manuscript and review of the literature first performed by CLR. Revising the manuscript critically for important intellectual content, patient consenting, and correspondence were carried out by MG.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. FDA approves first oral drug to reduce MS relapses [Internet]. Fda.gov. 2016 (cited 23 June 2016). http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm226755.htm.
- 2.Cohen J, Barkhof F, Comi G et al. , TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–15. 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- 3.LaMontagne K, Littlewood-Evans A, Schnell C et al. . Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res 2006;66:221–31. 10.1158/0008-5472.CAN-05-2001 [DOI] [PubMed] [Google Scholar]
- 4.Kappos L, Antel J, Comi G et al. , FTY720 D2201 Study Group. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355:1124–40. 10.1056/NEJMoa052643 [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Radue EW, O'Connor P et al. , FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 6.Lublin F, Miller D, Freedman M et al. . Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1075–84. 10.1016/S0140-6736(15)01314-8 [DOI] [PubMed] [Google Scholar]
- 7.Calabresi PA, Radue EW, Goodin D et al. . Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:545–56. 10.1016/S1474-4422(14)70049-3 [DOI] [PubMed] [Google Scholar]
- 8.Conzett KB, Kolm I, Jelcic I et al. . Melanoma occurring during treatment with fingolimod for multiple sclerosis: a case report. Arch Dermatol 2011;147:991 10.1001/archdermatol.2011.212 [DOI] [PubMed] [Google Scholar]
- 9.Haebich G, Mughal A, Tofazzal N. Superficial spreading malignant melanoma in a patient on fingolimod therapy for multiple sclerosis. Clin Exp Dermatol 2016;41:433–4. 10.1111/ced.12770 [DOI] [PubMed] [Google Scholar]
- 10.Marrie RA, Reider N, Cohen J et al. . A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler 2015;21:294–304. 10.1177/1352458514564489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmons JJ, Cohessy S, Wong ET. Injection of syngeneic murine melanoma cells to determine their metastatic potential in the lungs. J Vis Exp 2016; doi:10.3791/54039 10.3791/54039 [DOI] [PMC free article] [PubMed] [Google Scholar]