Abstract
A 35-year-old man with a 12-year history of idiopathic myelofibrosis (IMF) presented in 2014 with fatigue and abdominal distension. CT scan revealed massive hepatosplenomegaly with focal splenic lesions, soft tissue around renal pelvis, mesenteric masses compressing bowel loops and perilymphatic nodules in lungs. There was portal hypertension, ascites, pleural effusion, bilateral psoas abscesses and necrotic retroperitoneal lymphadenopathy. MRI additionally revealed hypointense periportal infiltrative lesions in liver, not seen on CT scan. None of these lesions showed diffusion restriction. Biopsy from mesenteric masses revealed extramedullary haematopoeisis. Aspiration from psoas abscess confirmed tuberculosis. Follow-up after 6 weeks of ruxolitinib (JAK2 tyrosine kinase inhibitor) and 9 months of antitubercular therapy revealed resolution of psoas abscesses and lymph nodes. Mild reduction was noted in mesenteric masses and ascites while perirenal soft tissue had increased. Follow-up imaging after another 1 year of ruloxitinib showed new-onset bilateral paravertebral and presacral foci of extramedullary haematopoeisis.
Background
Idiopathic myelofibrosis (IMF) can be complicated by infections, portal hypertension, extramedullary haematopoeisis (EMH) and malignant transformation to lymphoma/leukaemia. While there are reports of IMF with EMH and tuberculosis (TB) and IMF with EMH and portal hypertension, there are currently no case reports of all three complications of IMF that is, EMH, TB and portal hypertension (PHT) occurring simultaneously in one patient.
Also, the utility of diffusion restriction in differentiating lymphoma versus EMH has not been reported previously. The initial pattern of involvement in this patient suggested lymphoma as mesenteric masses; periportal infiltrative lesions and perilymphatic nodules are more commonly reported in lymphoma as compared to EMH. Moreover, the typical paravertebral and presacral foci of EMH were absent in this patient at initial presentation. However, absence of diffusion restriction on MRI favoured a benign pathology such as EMH, as proved by biopsy.
We also present the long-term imaging follow-up in this patient post antitubercular treatment (ATT) and JAK2 inhibitor therapy, which has also not been reported previously.
Case presentation
A 35-year-old man with a 12-year history of IMF and a homozygous gain-of-function JAK-2 tyrosine kinase activation mutation presented 2 years ago with anaemia, fatigue, low-grade fever and progressive abdominal distension. The patient had been managed with hydroxyurea at the time of his initial presentation. CT scan revealed massive hepatosplenomegaly, soft tissue around bilateral renal pelvis, multiple mesenteric masses and few well-defined perilymphatic nodules in right lung. The liver measured 30 cm and spleen measured 25 cm. No focal lesions were seen in liver on CT scan while there were multiple hypodense focal lesions in spleen. The mesenteric masses were isoattenuating to muscle without any intralesional foci of fat, calcification or haemorrhage. These masses were displacing the bowel loops widely without any bowel obstruction. There was also mild, low-attenuation ascites. The splenoportal axis was dilated with the portal vein measuring 17 mm. There was focal narrowing of the portal vein at the porta without any obvious portal vein thrombosis or varices. The pericalyceal soft tissue was more marked in the left kidney with mild left hydroureteronephrosis. Chest CT sections showed well-defined perilymphatic nodules in the right lung abutting major fissure and in right upper lobe with right pleural effusion. In addition, there was bilateral psoas abscess with necrotic para-aortic lymphadenopathy (figure 1). Diagnostic considerations on CT-included lymphoma/leukaemia or EMH with secondary TB infection and PHT.
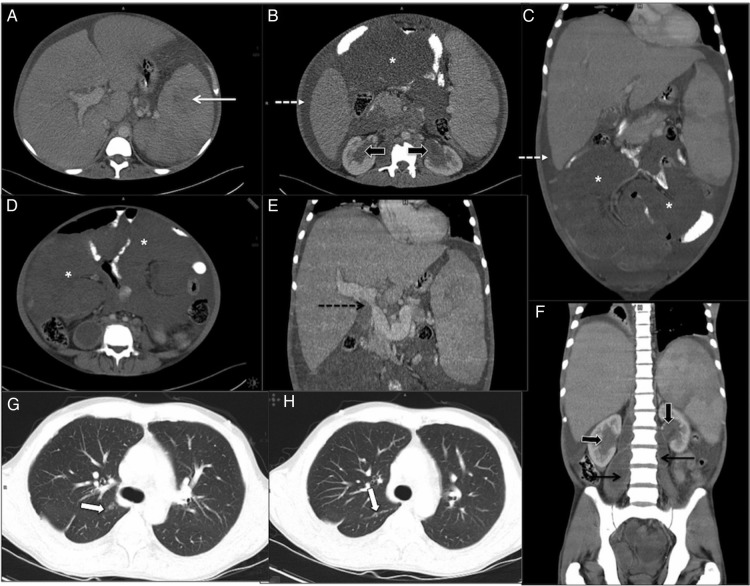
Figure 1.
(A–H): Contrast-enhanced CT in 35-year-old man with idiopathic myelofibrosis (IMF). There was hepatosplenomegaly with focal hypodense lesions in spleen (white arrow, A), multiple mesenteric masses (*, (D)), soft tissue thickening around both renal pelvis (block arrows, (B and F)) and ill-defined lung nodules in right upper lobe and abutting major fissure (white block arrows (G and H)), which were all considered either foci of extramedullary hematopoeisis (EMH) or lymphoma at initial presentation. There was also bilateral psoas abscess (black arrow, (F)) with necrotic paraortic nodes (not shown) suggestive of tuberculosis. Splenoportal axis was dilated with focal narrowing of portal vein at porta (black dotted arrow, (E)) suggestive of portal hypertension and ascites (white dotted arrow, (B and C)).
On MRI, the liver showed a heterogeneous appearance with confluent, periportal T2-weighted (T2-W) hypointense lesions that were not seen on CT. The splenic focal lesions and pericalycceal soft tissues were hypointense on T2-W images while mesenteric masses were isointense on T1-W images and mildly hyperintense on T2-W images. None of these lesions showed any diffusion restriction on diffusion-weighted imaging (DWI), favouring a benign pathology over lymphoma or leukaemic granulocytic sarcomas. Diffusion restriction was noted in bilateral psoas abscesses (figure 2). Biopsy from mesenteric masses showed features suggestive of EMH (figure 3).
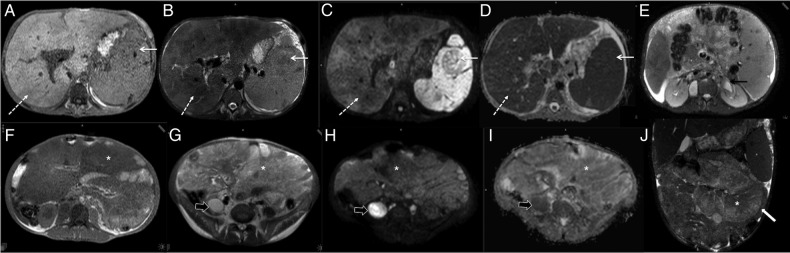
Figure 2.
(A–J): MRIs at initial presentation. (A–D) showing periportal lesions in liver (dashed white arrow) and in focal lesions in spleen (solid white arrow). These are isointense on T1-W image (arrows, (A)), hypointense on T2-W (arrows, (B)), dark on both diffusion b-800 images (arrow, (C)) and on ADC map (arrows, (D)). T2-W axial image (E) at more caudal level shows hypointense pericalyceal tissue (black arrow). (F–J) showing mesenteric masses (*). These masses are hypointense on T1-W (F), mildly hyperintense on T2-W (G), dark on both diffusion b-800 images (H) and on ADC map (I). T2-W coronal image (H) better shows mesenteric masses (*) displacing small bowel loops (white block arrow). Also note diffusion restriction in psoas abscess (black block arrows (G–I). ADC, apparent diffusion coefficient; T1-W and T2-W, T1-weighted and T2-weighted.
Figure 3.
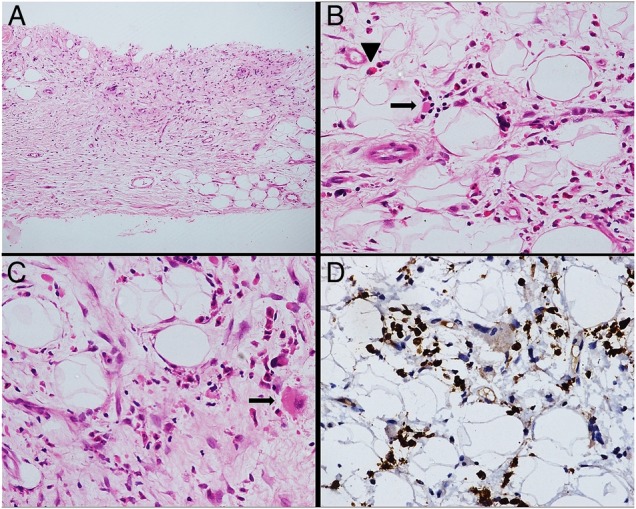
(A–D): Histopathology. Trucut biopsy from mesenteric mass shows fibroadipose tissue infiltrated by a polymorphous cell population ((A), H&E,×100) comprising of myeloid series of cells including eosinophil precursors (arrowhead), erythroid colonies (arrow) ((B), H&E,×400) and occasional megakaryocytes (arrow) ((C), H&E,×400). Antimyeloperoxidase immunohistochemical stain highlights the myeloid series of cells ((D), IHC,×400).
Aspiration from right psoas abscess confirmed TB. While biopsy from other lesions was not performed, they were also considered to be part of EMH rather than TB or lymphoma.
The patient was initially treated with 6 weeks of ruloxitinib (JAK2 tyrosine kinase inhibitor) and antibiotics. Following worsening of tubercular symptoms, ruloxitinib was stopped and the patient was given anti-ATT for 9 months along with hydroxyurea. Follow-up after 1 year of ATT revealed complete resolution of psoas abscesses and necrotic lymph nodes. There was reduction in ascites with residual peritoneal nodularity while the perirenal and pericalyceal soft tissue increased on follow-up. The hepatosplenomegaly and focal splenic lesions were unchanged (figure 4). The patient was then restarted on ruloxitinib 10 mg twice a day for treatment of IMF.
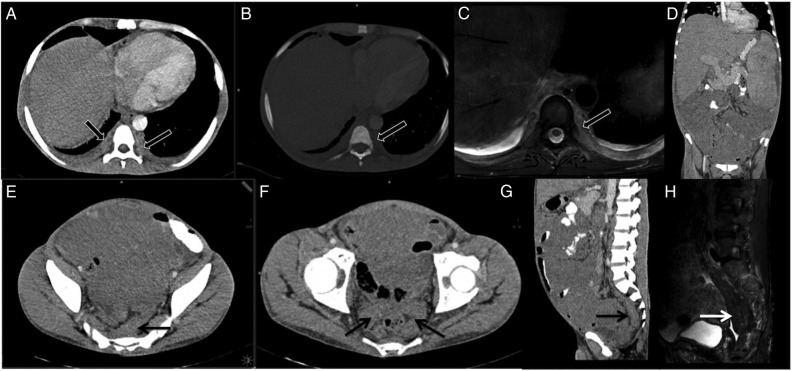
Figure 4.
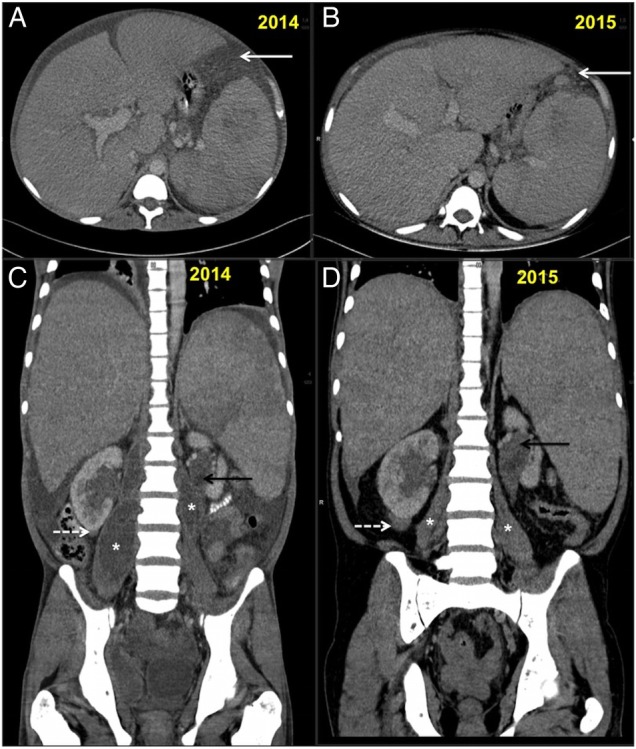
(A–D): Follow-up CT scan abdomen in 2015 and comparison with 2014 scans. (A and B) showing serial axial sections for comparison. There is partial resolution of ascites with residual peritoneal nodularity (white arrows (A and B)). (C and D) showing serial coronal sections for comparison. There is resolution of ascites and bilateral psoas abscesses (*). The right infrarenal (white dashed arrow) and left parapelvic soft tissue (back arrow) increased on follow-up while spleen size was unchanged.
The patient subsequently presented a year later with paraparesis, bilateral S1 radiculopathy and haematochezia. Follow-up imaging revealed new onset of bilateral paravertebral and presacral foci of extramedullary haematopoeisis and left pleural effusion (figure 5). The previously seen mesenteric masses, perirenal tissue and hepatopslenomegaly were unchanged while haematochezia was secondary to PHT-induced haemorrhoids.
Figure 5.
(A–H): Follow-up imaging in 2016. (A–C) showing bilateral paravertebral foci of extramedullary haematopoeisis along dorsal spine and ribs (block arrows). There was no bone destruction (B) and these masses were mildly hyperintense on T2-W MRI (C). (E–H) showing presacral (arrow, (E and G)) and perirectal (arrow, (F)) soft tissue which was hypointense on T2-W MRI (white arrow, (H)) s/o fibrotic or burnt-out hematopoetic tissue. The mesenteric masses and hepatosplenomegaly and perirenal tissue were unchanged on follow-up (D). T2-W, T2-weighted image.
The patient was managed with banding of haemorrhoids and discharged on ruloxitinib and hydroxyurea and referred for palliative radiotherapy for presacral masses.
Differential diagnosis
Based on distribution and extent of lesions, primary differential diagnosis of lymphoma or EMH was considered. We also considered a possibility of leukaemic transformation with chloromas/granulocytic sarcomas. The primary entity was complicated by TB in form of bilateral psoas abscesses and necrotic retroperitoneal nodes and portal hypertension. The diagnostic considerations in favour of lymphoma and leukaemia in this patient included typical distribution and extent of lesions (periportal, splenic, pericalyceal, mesenteric masses and perilymphatic nodules). EMH is more typically found in paravertebral and presacral location, both of which were absent in this patient at initial presentation. Hepatic involvement in EMH commonly manifests as focal masses while periportal infiltration is more common in lymphoma. Also while mesenteric masses have been described in EMH in a setting of IMF, they are also common in lymphoma and complicated TB. Finally, pulmonary involvement in EMH typically occurs as air-space opacities and interstitial septal thickening while our patient had perilymphatic nodules, which are more common in lymphoma. Pulmonary TB also manifests as centrilobular nodules rather than perilymphatic nodules as seen in this patient.
Discussion
IMF is a chronic clonal myeloproliferative disorder characterised by progressive marrow fibrosis and ineffective haematopoeisis.1 JAK2-V617F mutation is positive in about 50–60% of patients.2 Owing to progressive marrow fibrosis, there is formation of ectopic haematopoeitic tissue termed as EMH. The most common sites of EMH are spleen and liver followed by lymph nodes, paravertebral and presacral locations. However, EMH has also been described in unusual sites such as kidneys, adrenals, mesentery, gastrointestinal system, central nervous system, middle ear, skin, ovary and breast.1 3–5 Unusual sites or disseminated foci of EMH are more common in IMF due to high volume of circulating haematopoietic growth factors and cytokines. The hypothesis behind EMH include (1) displacement of pleuripotent haematopoietic stem cells from marrow and subsequent infiltration into mesenchymal organs and (2) reactivation of embryonic rests of totipotent haematopoietic stem cells in various organs secondary to ineffective marrow haematopoeisis.6 7
Hepatosplenomegaly is the commonest imaging manifestation of EMH.3 While focal lesions have been described in liver and spleen, periportal and peribiliary forms of EMH are uncommonly seen on imaging despite sinusoidal infiltration by haematopoietic cells at microscopic level.8 9 Wong et al10 published 10 cases of hepatic EMH, of which only 1 case had periportal involvement while others had focal hepatic masses.
Holden et al11 described a similar case of diffuse mesenteric EMH but their patient died in 2 weeks due to complications of portal HTN. Kim et al2 also described a case of EMH complicated by TB, in whom the ascites was secondary to TB. However, death occurred within 2 months, unlike our patient who responded to ATT and was also available for follow-up 2 years after initial presentation.
Symptomatic PHT is reported in 11% of cases with IMF and has been attributed to either sinusoidal infiltration and fibrosis causing increased intrahepatic resistance or due to increased blood flow from the enlarged spleen or due to portal vein thrombosis in EMH.12 13 Complications of PHT are common in EMH as seen in this patient too who developed bleeding from haemorrhoids on follow-up. Ascites in IMF is seen in 11% of patients with EMH and can be secondary to PHT or due to peritoneal form of EMH, rupture of hepatosplenic nodules or due to secondary infection.2 11 14–16 In our case, the ascites was likely to be due to peritoneal EMH rather than TB, as it was refractory to ATT and showed residual peritoneal nodularity on follow-up.
The diagnostic role of DWI in differentiating malignant mesenteric masses from EMH has not been described previously. While EMH can have variable T1 signal depending on the proportion of fat and fibrosis,11 they are usually isointense on T1-W and mildly hyperintense on T2-W image as seen in our case. Moreover, they do not show diffusion restriction, which can be useful in differentiating them from lymphoma or chloromas.
IMF is often treated with ruxolitinib, which is a JAK2 inhibitor. While ruxolitinb has been known to correlate with decreased spleen size, improved quality of life and overall survival benefit,17 18 the imaging features of EMH on ruxolitinib has also not been reported previously in any of these case reports. On 2 years follow-up, there was no significant decrease in the size of spleen. The patient also developed more typical paravertebral and presacral foci of EMH on follow-up suggestive of progressive disease.
Learning points.
Idiopathic myelofibrosis (IMF) can be invariably complicated by extramedullary haematopoeisis (EMH), portal hypertension, opportunistic infections and secondary lymphomatous/leukaemic transformation.
EMH in IMF can occur in unusual sites such as mesentery, kidneys and lungs and mimic lymphoma/leukaemia.
Diffusion-weighted imaging can help differentiate EMH from malignancy as EMH does not show diffusion restriction.
Long-term follow-up in this patient revealed progressive increase in extent of EMH despite adequate JAK2 inhibitor therapy.
Footnotes
Contributors: AP carried out the literature search and primarily wrote the manuscript. SHC supervised and edited the manuscript. AN gave input on pathology of the condition. PM also edited the manuscript, gave inputs from haematology and on patient management.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Choi H, David CL, Katz RL et al. Case 69: extramedullary hematopoiesis1. Radiology 2004;231:52–6. 10.1148/radiol.2311020673 [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ, Lee Y, Kim SH et al. Extramedullary peritoneal hematopoiesis combined with tuberculosis in a patient with primary myelofibrosis. Med Oncol 2009;26:238–41. 10.1007/s12032-008-9099-2 [DOI] [PubMed] [Google Scholar]
- 3.Georgiades CS, Neyman EG, Francis IR et al. Typical and atypical presentations of extramedullary hemopoiesis. Am J Roentgenol 2002;179:1239–43. 10.2214/ajr.179.5.1791239 [DOI] [PubMed] [Google Scholar]
- 4.Ginzel AW, Kransdorf MJ, Peterson JJ et al. Mass-like extramedullary hematopoiesis: imaging features. Skeletal Radiol 2012;41:911–6. 10.1007/s00256-011-1323-z [DOI] [PubMed] [Google Scholar]
- 5.Berkmen YM, Zalta BA. Case 126: extramedullary hematopoiesis. Radiology 2007;245:905–8. 10.1148/radiol.2453040715 [DOI] [PubMed] [Google Scholar]
- 6.Kwak HS, Lee JM. CT findings of extramedullary hematopoiesis in the thorax, liver and kidneys, in a patient with idiopathic myelofibrosis. J Korean Med Sci 2000;15:460–2. 10.3346/jkms.2000.15.4.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzankov A, Krugmann J, Steurer M et al. Idiopathic myelofibrosis with nodal, serosal and parenchymatous infiltration. Case report and review of the literature. Acta Haematol 2002;107:173–6. 10.1159/000057636 [DOI] [PubMed] [Google Scholar]
- 8.Ha DH. Periportal extramedullary hematopoiesis. J Korean Soc Radiol 2009;61:401 10.3348/jksr.2009.61.6.401 [DOI] [Google Scholar]
- 9.La Fianza A, van der Byl G, Maccabelli G et al. CT and MR findings in extramedullary haematopoiesis with biliary system encasement: a case report. J Radiol Case Rep 2010;4:1–8. 10.3941/jrcr.v4i11.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong Y, Chen F, Tai KS et al. Imaging features of focal intrahepatic extramedullary haematopoiesis. Br J Radiol 1999;72:906–10. 10.1259/bjr.72.861.10645201 [DOI] [PubMed] [Google Scholar]
- 11.Holden C, Hennessy O, Lee WK. Diffuse mesenteric extramedullary hematopoiesis with ascites: sonography, CT, and MRI findings. AJR Am J Roentgenol 2006;186:507–9. 10.2214/AJR.04.1788 [DOI] [PubMed] [Google Scholar]
- 12.Roux D, Merlio JP, Quinton A et al. Agnogenic myeloid metaplasia, portal hypertension, and sinusoidal abnormalities. Gastroenterology 1987;92:1067–72. 10.1016/0016-5085(87)90984-X [DOI] [PubMed] [Google Scholar]
- 13.Abu-Hilal M, Tawaker J. Portal hypertension secondary to myelofibrosis with myeloid metaplasia: a study of 13 cases. World J Gastroenterol 2009;15:3128–33. 10.3748/wjg.15.3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guermazi A, de Kerviler E, Cazals-Hatem D et al. Imaging findings in patients with myelofibrosis. Eur Radiol 1999;9:1366–75. 10.1007/s003300050850 [DOI] [PubMed] [Google Scholar]
- 15.Lioté F, Yeni P, Teillet-Thiebaud F et al. Ascites revealing peritoneal and hepatic extramedullary hematopoiesis with peliosis in agnogenic myeloid metaplasia: case report and review of the literature. Am J Med 1991;90:111–7. 10.1016/0002-9343(91)90513-W [DOI] [PubMed] [Google Scholar]
- 16.Henriquez-Camacho C, Martinez-Barranco P, Velasco M et al. Nontuberculous mycobacterial infection in a patient with myelofibrosis: case report and concise review. Clin Case Rep 2015;3:438–41. 10.1002/ccr3.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verstovsek S, Mesa RA, Gotlib J et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica 2015;100:479–88. 10.3324/haematol.2014.115840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arana Yi C, Tam CS, Verstovsek S. Efficacy and safety of ruxolitinib in the treatment of patients with myelofibrosis. Future Oncol 2015;11:719–33. 10.2217/fon.14.272 [DOI] [PMC free article] [PubMed] [Google Scholar]