Abstract
Reduced cortical dopamine levels have been observed in individuals with attention deficit hyperactivity disorder (ADHD). Global dopamine depletions by 6-hydroxydopamine (6-OHDA; with noradrenergic protection) in neonatal rats produces locomotor hyperactivity, with less known about how cortical depletion modulates risky behaviors. Here, we determined the effect of a medial prefrontal cortex (PFC) 6-OHDA depletions (30–60%) or sham microinjection at postnatal day 11 on behavior in male and female juvenile rats. Separate groups were studied for delay discounting (impulsive choice), novelty-preference, and preferences for cues and environments associated with cocaine (10, 20, and 40 mg/kg), their extinction, and reinstatement with place conditioning. Because PFC D1 receptors play a role in these behaviors, confocal microscopy was used to measure D1-immunoreactive projections to the nucleus accumbens core. Both 6-OHDA males and females increased delay discounting relative to sham controls, although only 6-OHDA females increased novelty preferences. Preferences for cocaine-associated environments, their extinction, and reinstatement with a priming dose of cocaine were reduced in 6-OHDA subjects overall. However, impulsive choice at 5 s positively correlated with preferences for cocaine-associated environments in 6-OHDA subjects, but not sham controls. As possible compensation for low dopamine levels, D1-immunoreactivity on traced neurons increased in 6-OHDA females; dopamine levels did not remain low in adolescent 6-OHDA males and D1 did not change. We believe that these modest depletions restricted to the PFC demonstrate the role of dopamine, and not norepinephrine, in understanding these behaviors in other animal models where cortical dopamine is reduced during development.
Keywords: ADHD, D1, Prefrontal cortex, Sex differences
1. Introduction
The dual hypothesis of attention deficit hyperactivity disorder (ADHD) posits that difficulties in reward processing and its associated circuitry can be differentiated from executive difficulties in ADHD children [1]. The reward-related structures include orbitofrontal regions that include the ventromedial PFC, the accumbens, amygdala and other structures. Reward-related deficits that are found in ADHD are associated with these regions and include increased delay discounting [1,2], elevated novelty-seeking [3], and insensitivity to changes in reward contingencies [4]. As these children grow older, the risk for substance use disorder increases with ADHD females more at-risk than ADHD males [5]. Notably, hyperactivity is delegated as an executive function deficit within this framework.
Dysfunctional processing within the dopamine system is present in ADHD (reviewed by [60]). Relative to adult control subjects, ADHD adults have reduced [18F]fluorodopa in the prefrontal cortex, suggestive of lower dopamine levels [6]. These dopamine deficits are also likely in children with ADHD, where reduced cerebral blood flow within the orbitofrontal region that includes the prefrontal cortex (PFC) can be normalized with drugs like methylphenidate that pharmacologically increases catecholamines [7,8].
Preclinical efforts to understand the role of dopamine in ADHD are based on relatively non-specific changes within the dopamine system [9]. For example, the dopamine transporter knockout is associated with excessive locomotion [10]. The spontaneously hypertensive rat (SHR) exhibits hyperactivity, inattention, impulsivity, poor stability of performance, and reduced PFC dopamine release and noradrenergic changes relative to Wistar Kyoto rat controls [11–13]. Dopamine levels in the PFC and striatum are initially increased in the juvenile SHR rat and decrease with age [14]. In the following study, we determined whether a more focal dopamine deficit localized to the PFC during the juvenile stage would produce reward-related behavioral changes.
Few preclinical studies have examined the effects of specific developmental reductions of dopamine on reward-related behavior during the juvenile period. Dopamine deficits produced during early development differ from those produced during adulthood [15,16]. Adult animals whose dopamine is depleted with 6-hydroxydopamine (6-OHDA) typically exhibit adipsia, aphagia, and motor impairment [15]. In contrast, whole brain neonatal dopamine depletions with 6-OHDA produce hyperactivity during the juvenile and early adolescent period [17–19], with no signs of drinking/eating disturbances [16,20]. These early lesions are associated with sex differences. For example, developmental PFC 6-OHDA lesions (i.e., 50% reduction at postnatal day [P]12–14) mildly enhance locomotor activity in P60 males [21], but not females [22]. 6-OHDA at P12 also reverses sex differences in nicotine self-administration [22]. The role of low dopamine in a number of ADHD-like behaviors in juveniles – including females – is not known. Therefore, we investigated the effects of an early 6-OHDA lesion on delay discounting, novelty preferences, activity, and place conditioning to cocaine in male and female juvenile rats.
One explanation for these differences is that the timing of the depletion differentially alters the maturation of local circuitry in the brain, especially at the receptor level. Elevated D1 on PFC neurons increases distraction [23,24], impulsive choice [25,26], working memory [27], and place preferences for cocaine-associated environments [26]. In addition, normal adolescent increases in PFC D1 receptors are related to a peak in cocaine sensitivity [28]. Here, we postulated that increases in the D1 dopamine receptor in the PFC may be related to the hypothesized changes. Such D1 increases have been reported following developmental 6-OHDA lesions in some [29], but not all studies [30]. D1 receptors typically increase during adolescence on plPFC projections to the nucleus accumbens [28,31], raising the possibility that PFC dopamine depletions might facilitate an increase in D1 in compensation.
2. Materials and methods
2.1. Subjects
Sprague-Dawley male and female rats were pretreated with desipramine (20 mg/ml in a 25 μl injection) to protect noradrenergic terminals. Subjects were bilaterally injected with 0.5 μg in 0.5 μl of either 6-OHDA or ascorbic acid saline (0.1%) in nitrogen at P11. Briefly, rats were anesthetized by hypothermia and a gas-tight Hamilton syringe was lowered into the plPFC at age-appropriate, stereotaxic coordinates: P11: AP: +2.8, ML: ±0.5, DV: 2.6 [32]. Solutions were slowly infused over the course of 5 min (i.e., 0.1 μl/min) and the needle left at the site for an additional 5 min, followed by slow retraction. Subjects were sutured and returned to their home cage with the dam upon awakening. The plPFC was targeted for depletion because it is associated with expression of learned associations, including drug cues (e.g., [33]). Subjects were tested beginning at P21, representing the juvenile stage of development [34] when the majority of children are diagnosed with ADHD [35]. Most of the female rats do not begin cycling until P35–37 in our lab. The majority of the tests were completed prior to this age, although estrogen differences may have increased variability in delay discounting.
2.2. Verification of DA depletion
The degree of depletion was verified with HPLC–ED of micropunches (0.98 mm) from the plPFC regions for each subject tested behaviorally using standard methods [36]. These data were then used as a covariate for the behavioral analyses to account for any differences in dopamine levels. Briefly, homogenized samples were injected into HPLC–EC system (BAS). Analytes were separated on a reverse-phase 150 mm × 1 mm (i.d.) column (BAS; C18, 3 μm particle size), with a mobile phase consisting of 0.1 M monochloroacetic acid buffer, 0.5 mM EDTA, 0.08% w/v octyl-sodium sulfate, and 7% v/v methanol, with a pH of 3, run at a flow rate of 90 μl/min. DA was oxidized with a glassy carbon electrode maintained at a potential of +0.7 V vs. an Ag/AgCl reference electrode. Amounts were based on a 4-point standard curve performed prior to every HPLC run.
2.3. Specific experimental details
2.3.1. Experiment 1. Delayed discounting
Subjects (n = 5–9/group; males and females) began training at P25 for increases in impulsive choice with a delay discounting task [37]. Subjects were trained to run down an arm of a T-maze to receive either a small reward (i.e., Reese’s pieces) in one arm or a large reward in the other arm. The T-maze was selected over an operant paradigm as the T-maze requires fewer training days, which is vitally important for the developmental assessments. The number of days it took to reach criterion of choosing the large reward of 10 of 12 trials across two days was recorded (e.g., 0 delay). Once subjects reached this criterion, one of three delay periods of 5, 10, or 15 s was initiated for the large reward while the small reward was available immediately. These periods are based on [37] and are sufficient to detect differences between groups [26]. Different groups of animals were used for each delay condition as our pilot studies demonstrated carry-over effects between delay conditions.
2.3.2. Experiment 2. Novelty-preferences
Subjects (n = 5–7/group) were tested for novelty preferences based on previous methods [38]. Briefly, a two chamber apparatus that differed by wall patterns was used (7 × 8.5 in. each side; Med Associates, St. Albans, VT). Subjects were habituated to the first distinct environment for 20 min on three consecutive days. The side of the habituation chamber was counterbalanced across subjects. Subjects then remained in the home cage for 24 h. On day five, the door connecting to the second chamber was opened and the total time spent on the novel side was used as an index of novelty preference. Subjects were tested at P25.
2.3.3. Experiment 3. Place conditioning
Subjects (n = 8–12/dose males and females) were initially placed into the conditioning chambers at P25. To investigate shifts in sensitivity to drug-associated cues, subjects underwent unbiased place conditioning to 10, 20, and 40 mg/kg cocaine [28]. The conditioning chamber consisted of two large (24 × 18 × 33) side compartments separated by a small (12 × 18 × 33 cm) middle compartment. The two compartments differed in floor texture, lighting, and wall coloring (black vs. white) and these drug-associated environments were counterbalanced within a condition. Through Pavlovian conditioning [39], repeated pairing of the environment with a rewarding drug allows the environment to develop conditioned incentive properties as a drug-related cue [40]. On Day 1, rats freely explored the apparatus to initially screen for baseline preferences for either side, which was defined a priori by spending greater than 18 min of the 30 min session on one side. If preferences were detected, these subjects were eliminated from further testing. Two days of conditioning to saline in the morning in one side and drug-paired chamber four hours later on the other side for 60 min each, and testing on the fourth day in a drug-free state (for 30 min) where the subjects had free access to all three chambers. Time spent in each compartment was analyzed to reflect conditioning to the environmental cues associated with each compartment. Relative to time spent on the saline-associated side of the chamber, time spent in the drug-conditioned side was considered a drug preference, whereas time spent on the saline-conditioned side was considered an aversion. A total of n = 106 subjects were included, in part, to yield a sufficient number of subjects to test extinction and reinstatement in Experiment 4.
2.3.4. Experiment 4. Extinction and reinstatement to drug-associated cues
Subjects demonstrating a significant place preference in Experiment 3 as indicated by a ratio (see formula below) greater than 0.54 were then used in extinction and reinstatement experiments, following our previous methods [28]:
Extinction testing started 24 h after testing for place conditioning. Subjects were allowed to explore the entire apparatus in a drug-free state for 30 min and time spent in each chamber recorded (i.e., an explicitly paired procedure). This test was repeated daily until each animal achieved extinction, defined as a preference ratio below 0.54 for two consecutive days, based on our methods [31] and those of Sanchez et al. [41]. Once subjects extinguished their preference to drug-associated cues, responses were reinstated with a challenge with 5 mg/kg cocaine (24 h after last extinction trial). Five mg/kg is a low priming dose commonly used for reinstatement studies (e.g., [41]).
2.3.5. Experiment 5. Relationship between delayed discounting and place preference for cocaine-associated environment
A subset of the animals from Experiment 1 (the latter half of those tested) was tested with place conditioning to a probe dose of 10 mg/kg cocaine after they had completed the delay discounting (~P45). Correlational analyses were then performed to determine if there is a correlation between delay discounting and place preference to an environment associated with cocaine.
2.3.6. Experiment 6. Retrograde tracers and confocal microscopy
Animals at P39 (n = 6–7/sex and condition) were given a 1 μl/side stereotaxic injection of a retrograde tracer (fluospheres, Molecular Probes) into the NAc (AP: +1.8; ML: 0.7; DV: −6.0, at a 3° angle) following ketamine/xylazine anesthesia according to our earlier methods [28]. Animals were intracardially perfused with ice-cold PBS followed by 4% paraformaldehyde, and brains sliced in 40 μm sections on a freezing microtome. Sections were incubated overnight in rat anti-D1 IgG (1:250; Sigma), washed, and incubated for 60 min with anti-rat Alexa 563-coupled IgG (1:400; Molecular Probes, Eugene, OR). Sections were then washed and mounted on slides using FluoroGel mounting medium (Fisher Scientific, Pittsburgh, PA). High-magnification digitized images at 40× oil-immersion were acquired in 2 μm step intervals for 20 μm within the plPFC. Within each region of interest, defined in part by a preponderance of traced neurons within the target region, a second z-stack was obtained in FITC for D1-immunoreactivity (IR). Three ROIs were generated within each subject and the number of traced, D1-IR, and co-localized cells counted. In each section, the entire plPFC was outlined at 4× magnification and the total number of IR cells was measured at 20× exclusively within the outlined area. CamKIIa was visualized using a red filter channel, while D1R was visualized using a green filter channel. Double-labeled cells were confirmed using an overlay of images from two filters for each field of view. Investigators were strictly blinded to the conditions for all analyses. Tracings of the plPFC boundaries were used for calculation of the area (a) in each section. The density of IR cells for each cell type (cells/mm2) was based on the total number of IR cells divided by Σa for each subject (the sum of areas obtained from all outlined regions). Volume of the plPFC was calculated according to the Cavalieri principle (50) as v = z × i × Σa, where z is the thickness of the section (40 μm) and i is the section interval (24; i.e., number of serial sections between each section and the following one within a compartment).
2.4. Statistical analysis
Place conditioning data was analyzed by a mixed ANCOVA (SPSS v 20) with depletion (Sham/6-OHDA), sex (male/female), and cocaine dose (10, 20, 40 mg) as between-subjects measures, pre- and post-conditioning as a repeated measures variable, and dopamine levels as a covariate to account for degree of depletion. Post-hoc analyses were corrected with Bonferroni’s, based on our previous methods [28,42]. Between-subject ANOVAs were used for delay discounting and novelty preference. Significance was set at P < 0.05.
3. Results
3.1. DA depletion
During juvenile assessment, the DA depletion was 40.1 ± 8.1% of sham-lesioned controls (P < 0.005; 4.67 ± 0.94 [6-OHDA] vs. 11.66 ± 2.2 [sham-lesioned] ng/mg wet tissue). DA and norepinephrine levels were also analyzed as the animals completed the studies at different ages in the delayed discounting paradigm (average age of sacrifice ~P55); for these analyses, age was used as a covariate. Age did influence catecholamine levels independently of condition (Age: F1,55 = 9.11 and 5.87, P = 0.004 and 0.019 for DA and norepinephrine). A depletion × sex interaction was not significant (F1,55 = 2.72, P = 0.1), although the trend is evident. DA content in males reverted to control levels in 6-OHDA males (6-OHDA-lesioned: 34.2 ± 4.9 vs. sham-lesioned: 31.3 ± 4.4), but remained lower in 6-OHDA females (6-OHDA-lesioned: 21.5 ± 5.4 vs. sham-lesioned: 36.4 ± 5.0 ng/mg wet tissue weight). Norepinephrine content levels did not significantly differ by depletion or sex (Ps = 0.7 and 0.9) and were: 6-OHDA-lesioned males: 88.2 ± 12.1 vs. sham-lesioned males: 84.8 ± 10.5; 6-OHDA-lesioned females: 87.5 ± 12.9 vs. sham-lesioned females: 83.0 ± 12.1 ng/mg wet tissue weight).
3.2. Specific experimental results
3.2.1. Experiment 1. Delayed discounting
We observed a significant effect of depletion on the number of small reinforcements selected (F1,63 = 6.402, P = 0.01), where 6-OHDA rats demonstrated more delay discounting relative to the sham-lesioned controls (Fig. 1). In addition, a significant effect of delay itself was observed where subjects selected the smaller reward more frequently as the delay interval increased (F2, 63 = 8.64, P < 0.001). These variables did not interact, nor was sex a significant influence. No significant differences were found for the number of days required to reach criterion as a result of depletion, sex, or delay (P > 0.5). On average, 6-OHDA male rats required 9.6 ± 0.9 days to reach criterion, whereas vehicles required 9.4 ± 1.7 days. Female 6-OHDA rats took 9.6 ± 0.9 days and female sham-lesioned rats required 8.6 ± 0.8 days. These data support the conclusion that 6-OHDA did not produce learning or motivational deficits.
Fig. 1.
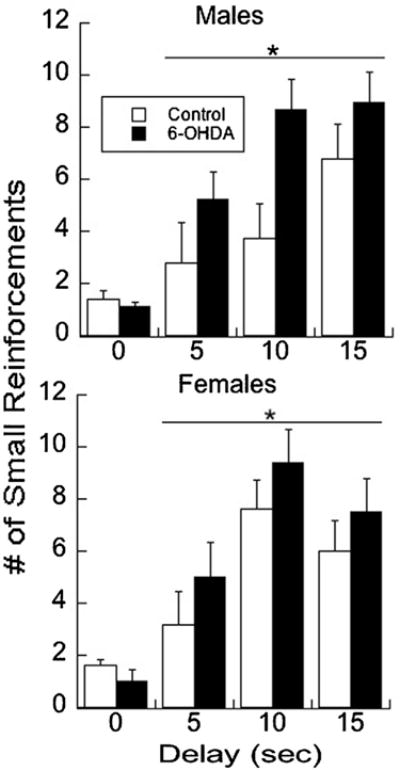
6-OHDA treatment increased delayed discounting in male and female juvenile rats relative to sham-lesioned controls. Each subject met criterion of two or less small reinforcements (e.g., 0 delay) before exposure to the delay condition of 5, 10, or 15 s. Means ± SE are presented. * P < 0.05.
3.2.2. Experiment 2. Novelty preferences and locomotor activity
Female 6-OHDA subjects also demonstrated preferences for novel environments and spent significantly more time in the novel side of the chamber relative to sham-lesioned controls and males in both groups (depletion × sex interaction: F1,19 = 4.26, P = 0.05; Fig. 2). Locomotor activity habituated across the 20 min in all groups (F3, 60 = 38.6, P < 0.001), but none of the groups differed from each other on Day 1 of habituation (F1,19 = 0.9, P = 1.0).
Fig. 2.
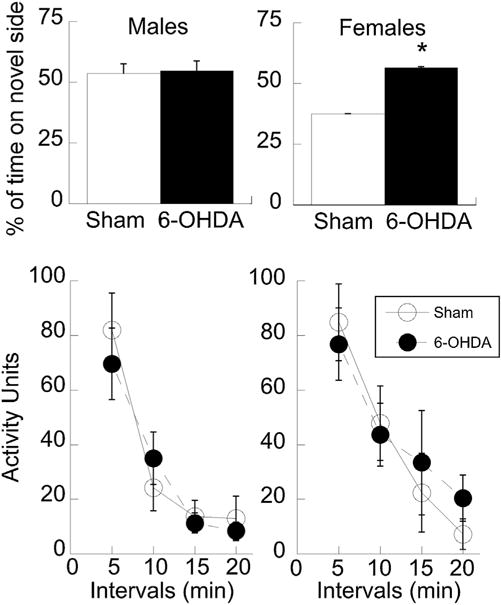
Females that received 6-OHDA showed increased novelty preference relative to sham-lesioned controls, while there were no differences in males (top). Data are expressed as a percentage of time spent in the novel side for the 20 min session. General activity was not influenced by 6-OHDA treatment for either sex (bottom). Means ± SE are presented. * P < 0.05.
3.2.3. Experiment 3. Place conditioning
An ANCOVA was used to analyze the place conditioning data, where levels of dopamine and norepinephrine served as covariates, lesion (6-OHDA/sham), dose (10, 20, 40 mg/kg cocaine), sex (male/female), and the within-subject variables of conditioning (pre-/post-) were assessed. Dopamine had a significant influence on behavior (F1,58 = 4.80, P < 0.05). Data in Fig. 3 are corrected for individual dopamine values, whereas norepinephrine did not have a significant influence (P = 0.43). Overall, 6-OHDA reduced cocaine conditioning in both sexes (effect of depletion: F1,58 = 5.32, P < 0.05), as neither sex nor dose of cocaine had an effect (Ps > 0.4).
Fig. 3.
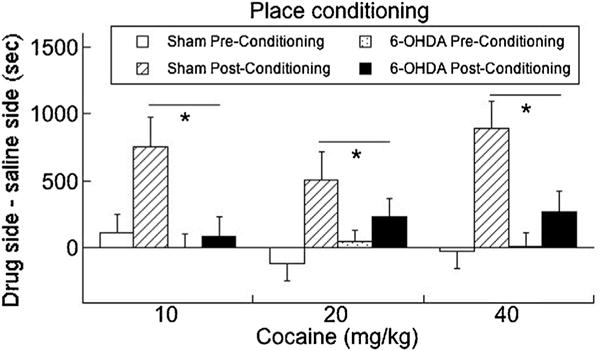
6-OHDA treatment reduced cocaine place conditioning relative to sham-lesioned controls. As no sex differences were observed, data are collapsed across males and females. Means ± SE are presented. An overall main effect of depletion on behavior was observed, with post-hoc tests revealing differences in the post-conditioning effects only. * P < 0.05.
3.2.4. Experiment 4. Extinction and reinstatement to drug-associated context
Extinction was defined as a preference ratio of 0.54 or less for two consecutive days. Extinction data were analyzed with a Kaplan–Meier survival analysis to determine how many days it took for subjects to extinguish to environments previously associated with 10, 20, or 40 mg/kg cocaine in males and females [31]. Table 1 shows that 6-OHDA subjects extinguished faster than sham-lesioned control subjects, especially at the lower dose of cocaine in males.
Table 1.
Kaplan–Meier survival analysis for days to extinguish preferences for cocaine environments.
| Cocaine dose (mg/kg) | Condition | 10 | 20 | 40 |
|---|---|---|---|---|
| Males | Sham control (n) | 9.0 ± 1.0 (5) | 5.3 ± 0.7 (7) | 4.0 ± 0.9 (5) |
| 6-OHDA (n) | 3.7 ± 0.6 (6) | 3.5 ± 0.7 (6) | 5.2 ± 1.0 (6) | |
| Females | Sham control (n) | 3.2 ± 0.5 (5) | 6.5 ± 1.0 (6) | 6.2 ± 1.7 (6) |
| 6-OHDA (n) | 4.9 ± 0.6 (8) | 7.0 ± 1.8 (5) | 4.3 ± 0.4 (6) |
Reinstatement to the priming dose of 5 mg/kg cocaine was less robust in 6-OHDA subjects (F1,49 = 4.22, P = 0.04; Fig. 4), with a trend for an interaction by sex (P = 0.07).
Fig. 4.
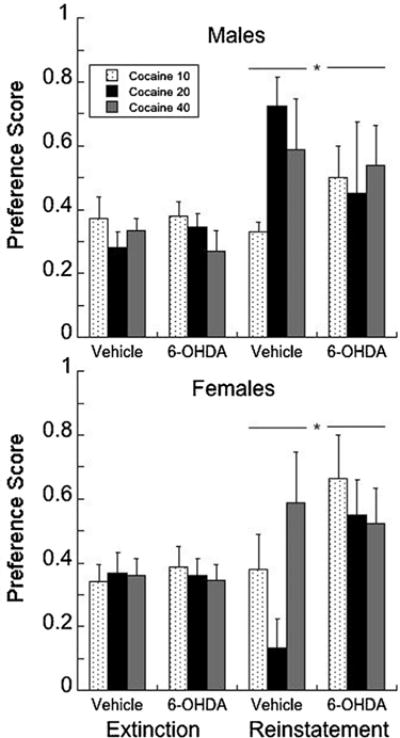
Subjects that had a significant place preference in Fig. 3 were extinguished and then the response probed with a priming dose of 5 mg/kg cocaine to determine the level of reinstatement. A preference score (calculated as the time on [drug side − time on saline side])/[drug + saline)]) of less than 0.54 was used as the criterion for extinction; anything above a score of 0.54 is considered a preference.
3.2.5. Experiment 5. Relationship between delayed discounting and place preference for cocaine-associated environment
To determine whether such a relationship existed in 6-OHDA or sham-lesioned controls, correlational analyses were preformed on a subset of subjects that were initially assessed for delayed discounting or novelty preferences prior to place conditioning to a probe dose of 10 mg/kg cocaine. Impulsive choice at the 5 s delay is highly predictive of place preferences for cocaine-associated environments in 6-OHDA subjects (Pearson’s r = 0.77; P = 0.002) but not in sham-lesioned controls (r = −0.38; P > 0.3). Sex did not influence this relationship and no correlations exist between place conditioning and impulsivity at the 10 and 15 s delay when impulsive choice is elevated for both 6-OHDA and sham-lesioned animals to the same degree (r = −0.23–0.37).
3.2.6. Experiment 6. Retrograde tracers and confocal microscopy
A depletion × sex interaction was observed for D1-immunopositive traced neurons (F1,17 = 6.76, P < 0.05; Fig. 5), such that 6-OHDA males had fewer D1-immunopositive traced cells in the plPFC than controls, while the reverse was true for the females. No other significant changes were observed for the number of D1-immunopositive cells or the number of traced neurons as a function of depletion.
Fig. 5.
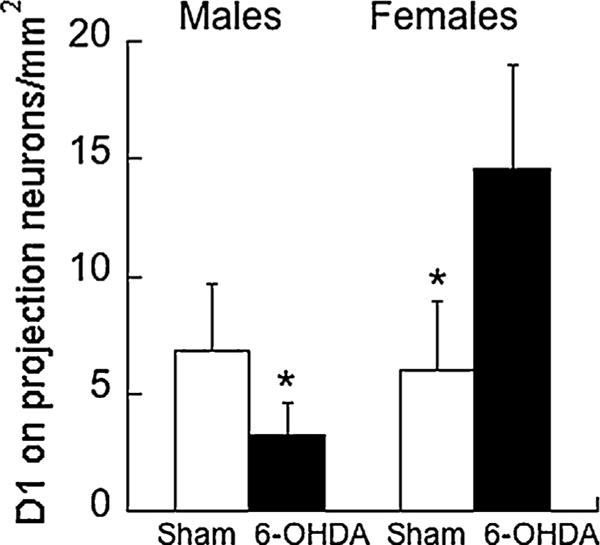
Fewer D1-immunopositive cells that project from the plPFC to the nucleus accumbens core were found in 6-OHDA males compared to sham-lesioned controls. The reverse was true for the females with more D1-immunopositive projection cells than controls. Means ± SE are presented. * P < 0.05.
4. Discussion
In the current study we demonstrated that attenuated PFC dopamine, induced by 6-OHDA lesions at P11, increased some, but not all, ADHD-like behaviors in juvenile rats. This moderate PFC dopamine depletion (~60% of sham controls) impaired delay discounting in both male and female rats. Delay discounting was more robust in 6-OHDA males than females, although this apparent sex difference was not significant. 6-OHDA females demonstrated greater preferences for novelty than 6-OHDA males. Locomotion in our juveniles was not significantly affected, similar to another developmental PFC 6-OHDA lesion study where 50% reduction in dopamine at postnatal day [P](12–14) mildly enhanced locomotor activity in P60 male [21] but not females [22]. Consistent with the dual-pathway framework, locomotor activity was not elevated in 6-OHDA animals relative to sham controls. Together, these observations are consistent with a motivational dysfunction model of ADHD for behavioral symptoms, which includes impaired discounting and a diminished sensitivity to reward that are related to hypofunction in PFC regions (as determined by blood flow changes) [1,43–45].
The observations in 6-OHDA females are also similar to dopamine changes in PFC activity in the SHR rat. Relative to Wistar controls, electrically-stimulated levels of dopamine are reduced [46] and both D1 mRNA [14,47] and the D1-associated protein calcyon are increased in the SHR PFC [48]. However, changes in PFC dopamine levels in the SHR transition from elevated dopamine during the juvenile period before declining with maturation when the rats are typically tested [14]. Our findings in 6-OHDA juvenile females can possibly inform mechanism development in the PFC of the SHR rat. Specifically, low levels of dopamine lead to an up-regulation of D1 receptors on plPFC output into the accumbens. This early change in D1 (measured here in adolescence) is associated with increased discounting and novelty preferences in 6-OHDA females and following viral-mediated transfer of the D1 into glutamatergic neurons into the plPFC [26]. In adult rats, we have shown that D1 increases the amount of cocaine self-administered and its breakpoint, suggesting greater motivation to seek cocaine [26]. However, others have found that 6-OHDA at P12 reduces nicotine self-administration in adult females [22]. These authors suggest that females are more protected from 6-OHDA due to the presence of estrogen (evident in adulthood), which itself increases nicotine self-administration [49]. We would predict that 6-OHDA females would increase intake of other drugs of abuse, especially cocaine, given the greater change in risky behaviors observed in 6-OHDA female, but not male, rats. This hypothesis is consistent with the greater risk for substance use disorders in females with ADHD [5], but requires additional investigation in older animals.
While the observations in 6-OHDA in the females are consistent with some behaviors found in ADHD, the results from male 6-OHDA rats are not. Male 6-OHDA rats demonstrated delay discounting as found in ADHD [50], but failed to show novelty preferences or differences in place conditioning. Mechanistically, male 6-OHDA rats did not show the hypothesized increase in plPFC D1 receptors. Delay discounting in adult rats have been associated with increased dopamine (extracellular levels of DOPAC were measured) and decreased serotonin in the orbital frontal cortex, but increased serotonin extracellular levels and reduced dopamine in the plPFC [51]. The orbital frontal cortex is not involved in non-cued tasks of delay discounting [52], such as the T-maze task used here. Moreover, 6-OHDA depletions in the current study were specific to the medial PFC (unpublished observations based on tyrosine hydroxylase staining). While both sexes had reduced dopamine levels following 6-OHDA initially, it is possible that secondary changes in serotonin may further mediate impulsivity change in 6-OHDA males (although not determined). Developmental 6-OHDA depletions decreased serotonergic innervation of the plPFC [53], although increased serotonin innervation in the striatum has been reported [20]. Systemic increases in serotonin levels decrease delay discounting in a T-maze task [54], with similar findings following more localized injections of a serotonin 5-HT2a agonist in the 5-choice serial reaction time task [55]. We do not have serotonin levels from these animals, leaving this question unanswered. If serotonin levels are increased following 6-OHDA in males, as found in the striatum [20], this may explain increased discounting. In contrast, novelty preferences found in the rat-bred line of high-novelty seeking rats show that c-fos responses are diminished within certain serotoninergic cell body regions [56]. These data imply that increased serotonin could reduce novelty preferences, or at a minimum, modify any changes that may be caused by 6-OHDA dopamine depletions.
Reduced sensitivity to cocaine-associated cues and contexts in juvenile 6-OHDA females and males relative to sham-lesioned subjects undermines the hypothesis that this model may increase risk for substance abuse. Clinical observations show that generally impulsive disorders emerge early in life (~5 years of age), while substance use has an adolescent onset [57]. Our animal model shows a significant relationship between delay discounting scores and preferences for cocaine-associated environments when assessed at ~P40 in 6-OHDA, but not sham-lesioned, animals. Delay discounting at 5 s, sensitivity to doses of cocaine during self-administration, and motivation to take cocaine are partially mediated by increased D1 receptors in the adult plPFC [26]. Since early PFC 6-OHDA lesions increase D1 receptors in the female plPFC, these studies raise the possibility that delay discounting may predict children at-risk for substance use.
While the correlation between impulsive choice and preferences for cocaine-associated cues and contexts is promising on an individual level, we did not observe an increase in preferences for cocaine-associated environments on a group level. One possibility is that sensitivity to the rewarding effects of cocaine and its ability to form preferences for cues and contexts may not manifest until subjects approach adolescence [57] when the risk is the highest in rats [28]. We tested this possibility by examining place conditioned responses to 10 mg/kg cocaine in adolescent 6-OHDA females. These 6-OHDA females failed to show preferences for cocaine-associated contexts (data not shown). Second, reduced preferences for cocaine-associated cues and contexts may also be the indirect result of subjects spending more time searching both compartments more due to lower dopamine levels that prevents the recollection of a reward-association [58]. Third, learning deficits could impair initial conditioning processes, but no evidence of such a learning deficit was observed in the T-maze task of delay discounting as subjects required the same number of days to reach criterion. Fourth, low dopamine impairs working memory in adult rats [22,59], but what happens during juvenility following early life 6-OHDA is not known. Working memory impairment prevents the recollection of a reward-association [58]. Extinction was rapid in both 6-OHDA groups. However, reinstatement of the previously conditioned preference for a cocaine-associated environment (a prerequisite for inclusion in this part of the study) was lower in 6-OHDA males relative to male controls, with a trend supporting the opposite effect in females (P = 0.07). Together, the most likely conclusion is that working memory may be impaired in 6-OHDA males, but not females. Future studies can test for this specific deficit.
5. Conclusions
The data presented show that reduced levels of dopamine in the plPFC during early development influences specific behaviors (delayed discounting, novelty preferences) that are relevant to ADHD. Sex differences were also observed in depleted subjects, where novelty-preferences were elevated in females, but not males. Together, early postnatal 6-OHDA lesions selectively into the plPFC may provide a novel model of examining dopamine-related dysfunction as it is relevant to ADHD.
HIGHLIGHTS.
Moderate depletion of dopamine in development increases delay discounting.
6-Hydroxydopamine increases cortical D1 receptors in females, but not males.
6-OHDA increases novelty preference in females, not males.
Acknowledgments
We gratefully acknowledge funding from NIH NIDA R01 DA15403 and DA-026485, and the Simches family, all of whom had no input to the design or outcome of the study.
Footnotes
Conflict of interest statement
The authors declare no competing financial interests in relation to the work described.
References
- 1.Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neurodevelopmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cognit Sci. 2006;10:117–23. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray LK, Kollins SH. Effects of methylphenidate on sensitivity to reinforcement in children diagnosed with attention deficit hyperactivity disorder: an application of the matching law. J Appl Behav Anal. 2000;33:573–91. doi: 10.1901/jaba.2000.33-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Klein KL, et al. Psychopathology in females with attention-deficit/hyperactivity disorder: a controlled, five-year prospective study. Biol Psychiatry. 2006;60:1098–105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–7. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BN, Lee JS, Cho SC, Lee DS. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42:19–29. doi: 10.3349/ymj.2001.42.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Kim BN, Lee JS, Shin MS, Cho SC, Lee DS. Regional cerebral perfusion abnormalities in attention deficit/hyperactivity disorder. Statistical parametric mapping analysis. Eur Arch Psychiatry Clin Neurosci. 2002;252:219–25. doi: 10.1007/s00406-002-0384-3. [DOI] [PubMed] [Google Scholar]
- 9.Paule MG, Rowland AS, Ferguson SA, Chelonis JJ, Tannock R, Swanson JM, et al. Attention deficit/hyperactivity disorder: characteristics, interventions and models. Neurotoxicol Teratol. 2000;22:631–51. doi: 10.1016/s0892-0362(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 11.Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Behav Brain Res. 2002;130:191–6. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- 12.Russell VA. Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods. 2007;161:185–98. doi: 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Miller EM, Pomerleau F, Huettl P, Russell VA, Gerhardt GA, Glaser PE. The spontaneously hypertensive and Wistar Kyoto rat models of ADHD exhibit sub-regional differences in dopamine release and uptake in the striatum and nucleus accumbens. Neuropharmacology. 2012;63:1327–34. doi: 10.1016/j.neuropharm.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 2004;11:97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL. The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles. Brain Res Brain Res Rev. 2005;48:57–73. doi: 10.1016/j.brainresrev.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Neal-Beliveau BS, Joyce JN. Timing: a critical determinant of the functional consequences of neonatal 6-OHDA lesions. Neurotoxicol Teratol. 1999;21:129–40. doi: 10.1016/s0892-0362(98)00044-0. In process citation. [DOI] [PubMed] [Google Scholar]
- 17.Shaywitz B, Yager R, Klopper J. Selective brain dopamine depletion in developing rats: an experimental model of minimal brain dysfunction. Science. 1976;191:305–7. doi: 10.1126/science.942800. [DOI] [PubMed] [Google Scholar]
- 18.Davids E, Zhang K, Kula NS, Tarazi FI, Baldessarini RJ. Effects of norepinephrine and serotonin transporter inhibitors on hyperactivity induced by neonatal 6-hydroxydopamine lesioning in rats. J Pharmacol Exp Ther. 2002;301:1097–102. doi: 10.1124/jpet.301.3.1097. [DOI] [PubMed] [Google Scholar]
- 19.Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl) 2002;160:92–8. doi: 10.1007/s00213-001-0962-5. [DOI] [PubMed] [Google Scholar]
- 20.Stachowiak MK, Bruno JP, Snyder AM, Stricker EM, Zigmond MJ. Apparent sprouting of striatal serotonergic terminals after dopamine-depleting brain lesions in neonatal rats. Brain Res. 1984;291:164–7. doi: 10.1016/0006-8993(84)90665-6. [DOI] [PubMed] [Google Scholar]
- 21.Boyce PJ, Finlay JM. Extracellular dopamine and norepinephrine in the developing rat prefrontal cortex: transient effects of early partial loss of dopamine. Brain Res Bull. 2009;79:104–10. doi: 10.1016/j.brainresbull.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, et al. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154:885–97. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–72. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 24.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- 25.Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, et al. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex. 2009;20:1064–70. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- 26.Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology (Berl) 2014;231:1615–26. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–9. [PubMed] [Google Scholar]
- 28.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. II. Effects of neonatal 6-hydroxydopamine treatments on D1 and D2 receptors, adenylate cyclase activity and presynaptic dopamine function. Brain Res Dev Brain Res. 1990;52:273–7. doi: 10.1016/0165-3806(90)90245-t. [DOI] [PubMed] [Google Scholar]
- 30.Duncan GE, Breese GR, Criswell HE, Johnson KB, Schambra UB, Mueller RA, et al. D1 dopamine receptor binding and mRNA levels are not altered after neonatal 6-hydroxydopamine treatment: evidence against dopamine-mediated induction of D1 dopamine receptors during postnatal development. J Neurochem. 1993;61:1255–62. doi: 10.1111/j.1471-4159.1993.tb13616.x. [DOI] [PubMed] [Google Scholar]
- 31.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–5. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherwood N, Timeras P. A stereotaxic atlas of the developing rat brain. Los Angeles, CA: University of California Press; 1970. [Google Scholar]
- 33.Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–97. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 35.Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2004;7:77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- 36.Andersen SL, Gazzara RA. The ontogeny of apomorphine-induced alterations in dopamine release: I. Effects on spontaneous release. J Neurochem. 1993;61:2247–55. doi: 10.1111/j.1471-4159.1993.tb07466.x. [DOI] [PubMed] [Google Scholar]
- 37.Olmstead MC, Hellemans KG, Paine TA. Alcohol-induced impulsivity in rats: an effect of cue salience? Psychopharmacology (Berl) 2006;184:221–8. doi: 10.1007/s00213-005-0215-0. [DOI] [PubMed] [Google Scholar]
- 38.Macri S, Laviola G, Leussis MP, Andersen SL. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology. 2010;35:392–402. doi: 10.1016/j.psyneuen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 40.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez CJ, Bailie TM, Wu WR, Li N, Sorg BA. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 42.Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–4. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- 43.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–6. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 44.Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry Allied Disciplines. 2009;50:669–78. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 45.Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J. Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Brain Res. 1995;676:343–51. doi: 10.1016/0006-8993(95)00135-d. [DOI] [PubMed] [Google Scholar]
- 47.Levy F. Pharmacological and therapeutic directions in ADHD: specificity in the PFC. Behav Brain Funct: BBF. 2008;4:12. doi: 10.1186/1744-9081-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heijtz RD, Alexeyenko A, Castellanos FX. Calcyon mRNA expression in the frontal-striatal circuitry and its relationship to vesicular processes and ADHD. Behav Brain Funct: BBF. 2007;3:33. doi: 10.1186/1744-9081-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230:140–2. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- 50.Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, et al. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2005;16:106–14. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- 52.Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham MG, Connor CM, Zhang K, Benes FM. Diminished serotonergic innervation of adult medial prefrontal cortex after 6-OHDA lesions in the newborn rat. Brain Res Dev Brain Res. 2005;157:124–31. doi: 10.1016/j.devbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Bizot JC, Thiebot MH, Le Bihan C, Soubrie P, Simon P. Effects of imipramine-like drugs and serotonin uptake blockers on delay of reward in rats. Possible implication in the behavioral mechanism of action of antidepressants. J Pharmacol Exp Ther. 1988;246:1144–51. [PubMed] [Google Scholar]
- 55.Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol. 2011;22:805–13. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- 56.Kerman IA, Clinton SM, Bedrosian TA, Abraham AD, Rosenthal DT, Akil H, et al. High novelty-seeking predicts aggression and gene expression differences within defined serotonergic cell groups. Brain Res. 2011;1419:34–45. doi: 10.1016/j.brainres.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence. Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huston JP, Silva MA, Topic B, Muller CP. What’s conditioned in conditioned place preference? Trends Pharmacol Sci. 2013;34:162–6. doi: 10.1016/j.tips.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Kadowaki Horita T, Kobayashi M, Mori A, Jenner P, Kanda T. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology (Berl) 2013;230:345–52. doi: 10.1007/s00213-013-3158-x. [DOI] [PubMed] [Google Scholar]
- 60.Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobeh Rev. 2010;34:744–54. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]


