Abstract
Recently there has been a strong interest in cross-frequency coupling, the interaction between neuronal oscillations in different frequency bands. In particular, measures quantifying the coupling between the phase of slow oscillations and the amplitude of fast oscillations have been applied to a wide range of data recorded from animals and humans. Some of the measures applied to detect phase-amplitude coupling have been criticized for being sensitive to nonsinusoidal properties of the oscillations and thus spuriously indicate the presence of coupling. While such instances of spurious identification of coupling have been observed, in this commentary we give concrete examples illustrating cases when the identification of cross-frequency coupling can be trusted. These examples are based on control analyses and empirical observations rather than signal-processing tools. Finally, we provide concrete advice on how to determine when measures of phase-amplitude coupling can be considered trustworthy.
Keywords: alpha, EEG, gamma, MEG, oscillations, theta
Significance Statement
Neuronal oscillations at different frequencies are thought to reflect processing within and across brain networks. To fully understand how these oscillations support neuronal computation, it is essential to understand how they interact. It is, however, not straightforward to quantify cross-frequency interactions. We here discuss the problems associated with quantifying cross-frequency coupling and put forward examples in which indices of cross-frequency interactions can be considered reliable.
Detecting phase-amplitude coupling
In the past decade, there has been an increasing interest in the role of brain oscillations in both human and animal research (Buzsáki, 2006). Since these oscillations co-occur in different frequency bands, their functional role cannot be understood in isolation, and it is imperative to uncover how they interact. There are different ways by which these oscillations can interact [e.g., phase-to-phase, amplitude-to-amplitude, and phase-to-amplitude coupling (PAC); Palva et al., 2005; Jensen and Colgin, 2007; Siegel et al., 2012]. In particular, PAC has received strong interest since the phenomenon suggests that the phase of slow oscillations correlates with neuronal activity in higher-frequency bands (Bragin et al., 1995; Canolty et al., 2006; Tort et al., 2009; Miller et al., 2010; Belluscio et al., 2012; Lisman and Jensen, 2013; von Nicolai et al., 2014; Bonnefond and Jensen, 2015; Colgin, 2015; Florin and Baillet, 2015; Hyafil et al., 2015a,b; McLelland and VanRullen, 2016; Fig. 1). Such findings have provided important insights into the temporal coordination of neuronal activity. However, the reliability of PAC has recently been questioned since the measure is sensitive to nonsinusoidal properties of the neuronal oscillations (Kramer et al., 2008; Aru et al., 2015). Indeed, recent articles have reported PAC, which can fully be explained by the lower-frequency oscillation, as having a saw-tooth like shape (Cole et al., 2016; Sheremet et al., 2016; Cole and Voytek, 2017). Although these concerns are valid, they do not exclude the existence of a true measure of PAC that is associated with neuronal activity in different frequency bands. The aim of this commentary is to provide examples for cases where measures of PAC can be considered trustworthy. We will conclude by providing concrete recommendations on how to determine whether measures of PAC should be considered trustworthy.
Figure 1.
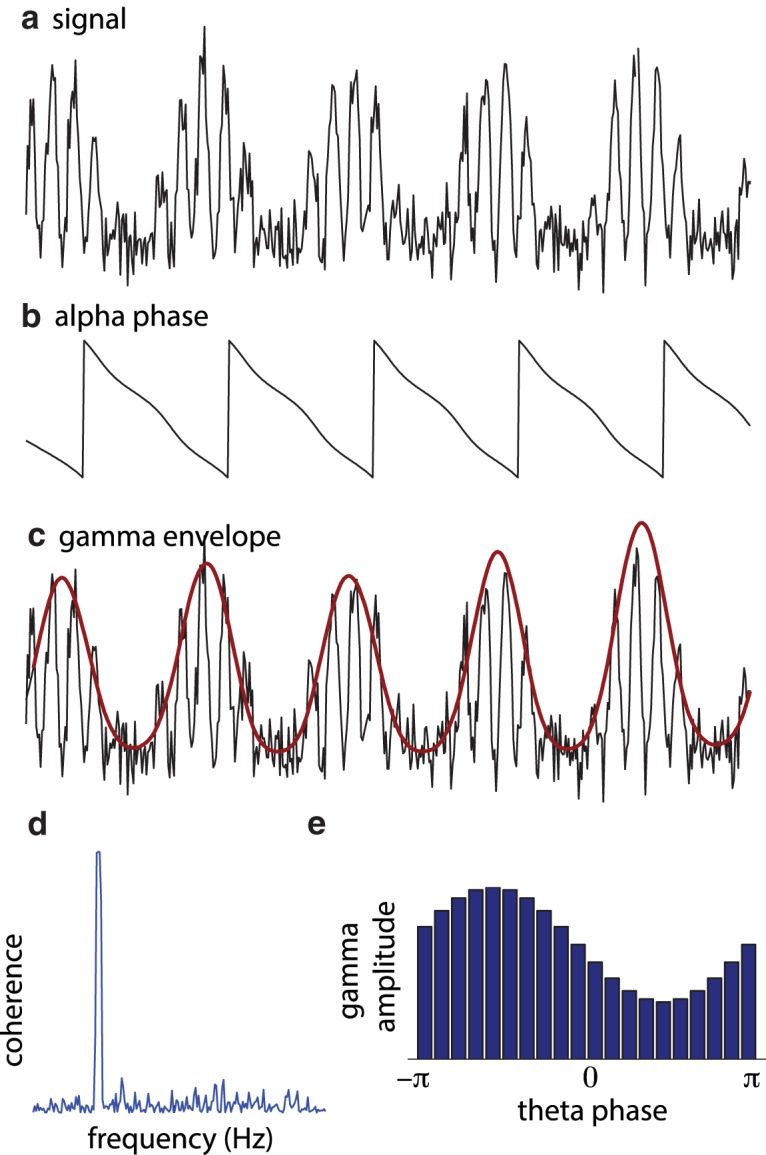
Quantifying PAC: a, The raw signal. b, c, The temporal evolution of phase (b) and power (c) are typically identified using a discrete Fourier transform, wavelet transformations, or a bandpass filter followed by a Hilbert transformation. d, e, The relationship between phase and amplitude envelope can be quantified by the phase relationship between the two signals (e.g., coherence; d; Osipova et al., 2008) or by considering the nonuniformity of the phase distribution of fast-frequency power with respect to the slow oscillations (e; Tort et al., 2010).
Even though there are several metrics by which PAC can be quantified, these different methods are qualitatively similar. The core of these methods is that they (1) estimate the phase of the slow oscillations (Fig. 1b), (2) estimate the temporal evolution of amplitude or power (the “envelope”) of the faster signal (Fig. 1c), and (3) relate the phase of the slow oscillations to the time course of power of the faster signal (Fig. 1d,e).
The time course of phase and power (points 1 and 2) can be estimated by a sliding time window subjected to a discrete Fourier transformation, a wavelet transformation (typically, a Morlet wavelet), or a bandpass filter followed by a Hilbert transformation. These three approaches, at some level, all involve a Fourier-like transformation multiplying the signal by , where f is the frequency of interest (Bruns, 2004). The slow and fast signals (point 3) are related by estimating the phase synchronization (e.g., coherence) between the slow signal and the envelope of the fast signal (Osipova et al., 2008; Fig. 1d) or by quantifying the distribution of power of the fast signal with respect to the phase of the slow signal (Canolty et al., 2006; Tort et al., 2010; Fig. 1e). Strong coherence or a nonuniform phase distribution of high-frequency power is indicative of PAC. Another method includes a general linear model approach to quantify the relationship between phase and amplitude (van Wijk et al., 2015). All these measures have in common that they are sensitive to coupling in the sense of stronger high-frequency amplitude at certain low-frequency phases than at others. When applying these PAC measures, a broad set of frequency ranges are typically explored, yielding a frequency-by-frequency measure of the coupling.
The problem: spurious identification PAC coupling
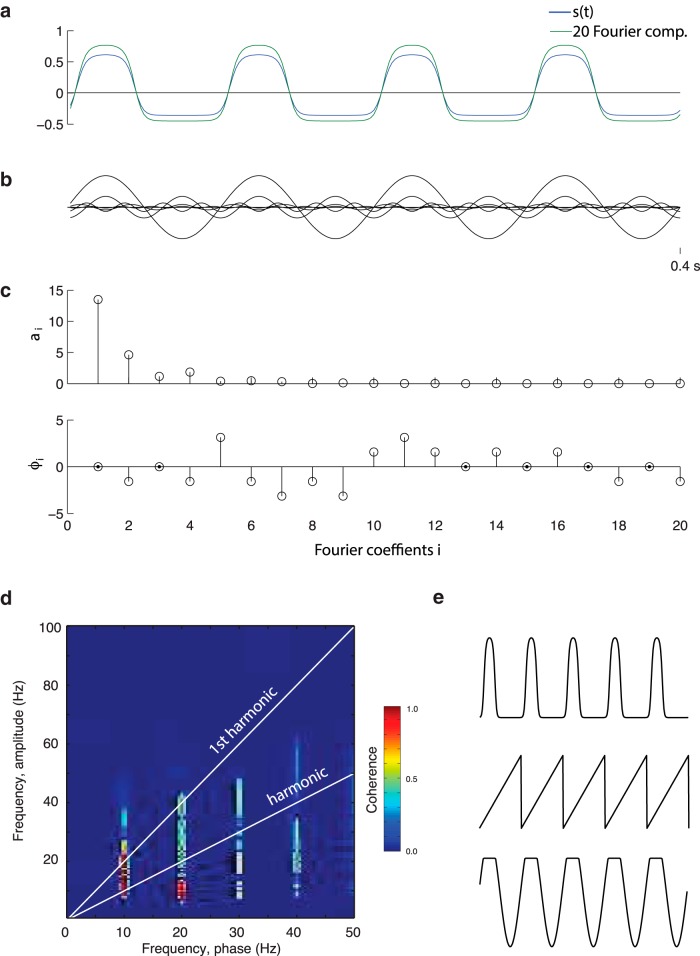
The goal of reporting PAC is to identify coupling in which amplitude in the high-frequency band is associated with actual neuronal activity in that frequency band, which is modulated by slow neural oscillations. However, the amplitude modulations at fast frequencies might not be caused by neuronal firing in that frequency range per se but could also stem from higher harmonics generated by the oscillations in the slow-frequency band. From standard Fourier analysis (Stein and Shakarchi, 2003), it follows that any periodic signal repeated at frequency f0 can be expressed as a sum of sinusoidal functions with frequencies f0, 2f0, 3f0, … (Fig. 2). Importantly, the more the periodic signal deviates from a sinusoidal function, the larger the coefficients of the higher harmonics. Any nonsinusoidal neural oscillation will therefore necessarily have power in higher harmonics. Importantly, depending on the exact waveform shape, the application of a technique like a sliding time window Fourier transform (or equivalent) will result in this higher harmonic power showing a modulation as a function of phase of the slow oscillation. The higher harmonics are not reflecting “true neuronal activity” per se, as they may not be associated with spiking or oscillations at these frequencies. Measures of PAC are sensitive to higher harmonics, as shown in Figure 2d. Note that, due to frequency smoothing as a result of estimating the envelope for the fast frequencies, the harmonic contributions are often “bleeding” together in the PAC plot. Therefore, great care must be taken when interpreting measures of PAC, in particular in the frequency bands of the higher harmonics, additionally taking into account the effective frequency smoothing (Kramer et al., 2008; Aru et al., 2015; Cole et al., 2016; Jones, 2016; Lozano-Soldevilla et al., 2016). In particular, Cole et al. (2016) and Sheremet et al. (2016) provide concrete examples from respective intracranial human and hippocampal rat recordings in which nonsinusoidal oscillations contribute to estimates of PAC.
Figure 2.
Periodic nonsinusoidal signals expressed as Fourier series. a, A periodic nonsinusoidal signal (blue line) constructed by a sigmoidal function applied to sinusoidal signal: . The signal can be expressed as a sum of sinusoids . i.e. the periodic signal can be expressed as a sum of sinusoids at the harmonic frequencies. The green line shows the sum of the first 10 harmonics. b, The first 10 harmonics. c, The coefficient for amplitude and phase (αi and ). d, The PAC for the signal is sensitive to the higher harmonics and can therefore produce a spurious phase-to-power coupling. e, Examples of nonsinusoidal functions of some neuronal relevance: periodic pulses, a saw-tooth function, and a clipped sinusoidal signal.
How are nonsinusoidal oscillations generated?
What will cause neuronal oscillations to be nonsinusoidal? One possibility is a gradual ramping up of neuronal activity within a slow cycle. This would produce a saw-tooth like shape (Fig. 2e), which indeed has been observed in the rat hippocampus (Terrazas et al., 2005; Belluscio et al., 2012; Sheremet et al., 2016) and in electrocorticography recordings in the human motor cortex (Cole et al., 2016). Another possibility is pulses of neuronal activity repeated at a fixed frequency. Yet another possibility is “clipping” in which the periodic signal is capped when exceeding a certain magnitude. This could be explained by periodic neuronal activity “maxing out” (e.g., due to a depletion of some resource). While clipping is a theoretical possibility, we are not aware of reports of such effects. As described, nonsinusoidal wave shapes will produce higher harmonics, resulting in spurious identification of PAC. As with many measures of neural activity, one must be cautious of potential confounds like these when interpreting measures of PAC. We would here like to emphasize that the possibility of spurious measures of coupling by no means precludes the existence of true PAC associated with neuronal activity in various frequency bands.
Three examples of reliable phase-to-amplitude coupling
How best to handle the concerns of spurious identification of PAC? Although advanced signal-processing techniques are being developed to alleviate problems with spurious coupling (Dvorak and Fenton, 2014; van Driel et al., 2015; Cole et al., 2016; Soto et al., 2016), it is problematic to implement signal-processing tools that unequivocally remove the effects of higher harmonics when calculating the PAC measure. However, even when signal-processing tools cannot provide a conclusive answer, there are specific empirical circumstances that might alleviate concerns about the spurious identification of coupling. We will here provide three such examples.
Example 1: neuronal spiking clocked by gamma oscillations
The core concern in PAC is that activity at higher frequencies is caused by harmonics rather than neuronal firing at higher frequencies. Intracranial animal recordings allow us to discern both spikes and local field potentials (LFPs). Such recording is routinely performed in behaving rats using electrodes implanted in the hippocampus. Such recordings have revealed that hippocampal activity in the gamma band is modulated by the phase of the theta oscillations (Bragin et al., 1995; Colgin et al., 2009; Belluscio et al., 2012; Fig. 3a). Given that hippocampal theta oscillations are clearly nonsinusoidal (Belluscio et al., 2012), higher harmonics are indeed a concern. However, Colgin et al. (2009) were able to identify reliable gamma oscillations directly visible in the LFP (Fig. 3b). Interestingly, both fast and slow gamma oscillations were identified. Furthermore, analysis of spike-triggered LFP recordings revealed that spike timing was clocked by the phase of ongoing gamma oscillations (Fig. 3c). These findings provide direct evidence for actual neuronal activity with a dominant gamma frequency component. By simultaneously considering neuronal spiking and LFPs, we can confidently conclude that the coupling between gamma activity and theta phase reported in the rat hippocampus is due to genuine oscillatory activity in the gamma band.
Figure 3.
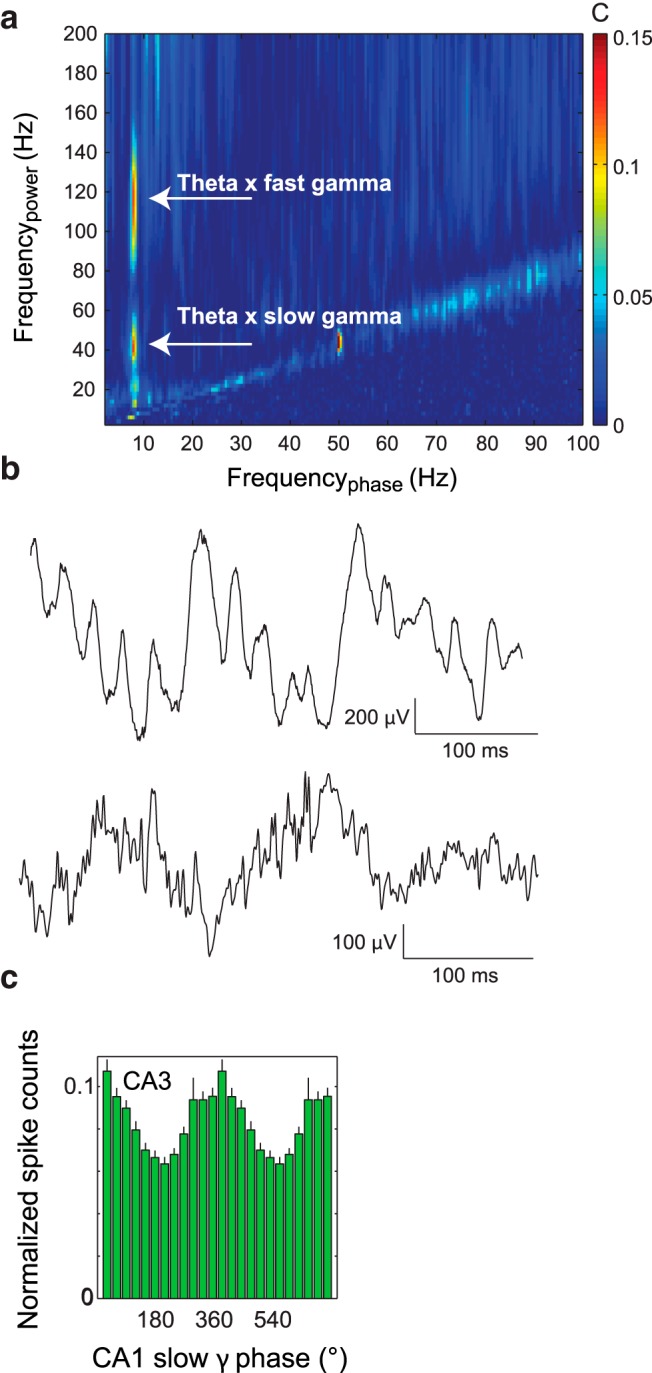
Gamma oscillations are modulated by theta phase in the rat hippocampus. a, The PAC measure applied to the LFP of rat data reveals phase-amplitude coupling. b, Gamma oscillations coupled to the phase of the theta oscillations are directly visible in the unfiltered LFPs. c, A histogram demonstrating the neuronal firing is modulated by gamma band oscillations. Reprinted by permission from Macmillan Publishers Ltd. (Colgin et al., 2009).
Example 2: slow and fast oscillations are generated by different populations
PAC is not constrained to data from single site recordings. It can also be applied to quantify the relationship between the phase of oscillations in one site and fast activity in another site (van der Meij et al. 2012). This was done in the study by Spaak et al. (2012) in which data from laminar recordings in visual cortex of the monkey were analyzed. The core finding was that the phase of alpha oscillations in the deeper layers (infragranular layers) was coupled to the gamma band activity in superficial layers (supragranular layers; Fig. 4). Importantly, the PAC between layers was stronger than the local PAC in any of the layers. This finding provides evidence for different populations generating the alpha and gamma band activity. The observation strengthens the case that gamma activity coupled to alpha phase is not spurious. Had the effect been due to higher harmonics, it would have been stronger locally than across sites. In conclusion, measures of PAC in which the slow and fast activity are generated in distinct layers, areas, or populations reduce concerns on spurious coupling.
Figure 4.
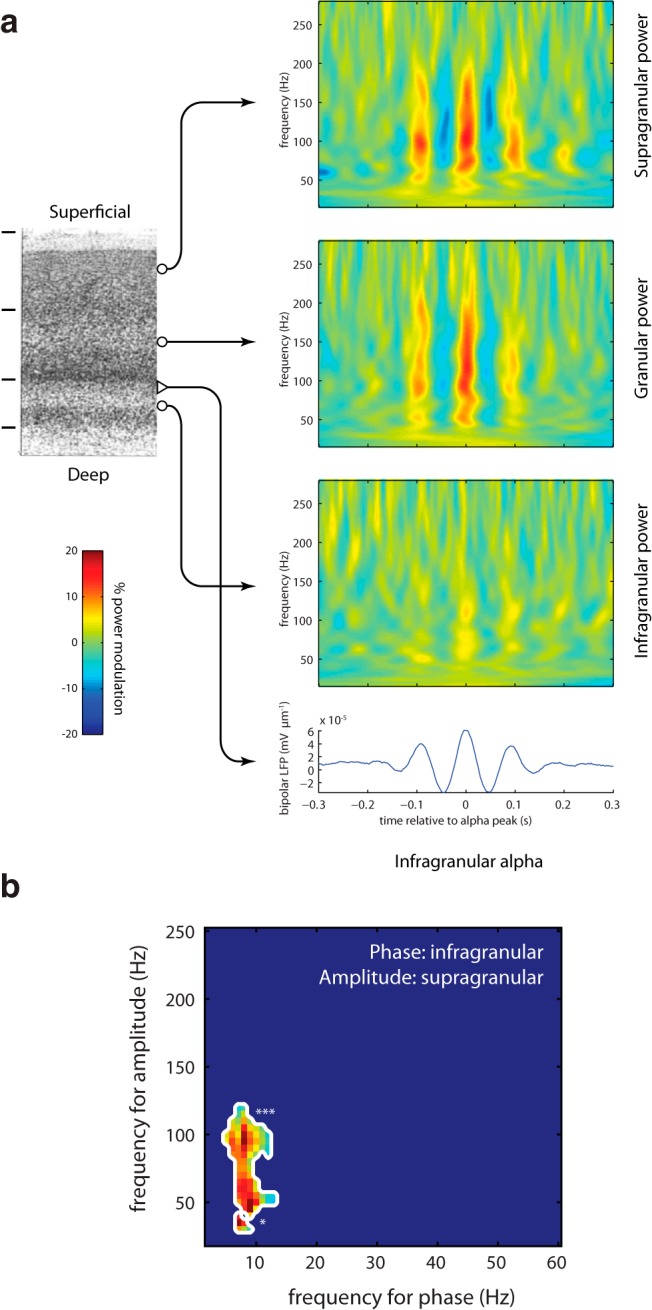
Gamma power in superficial cortical layers is coupled to alpha phase in deeper layers. a, Epochs of data in the alpha range are phase aligned according to the peaks in each epoch. For each epoch, the time–frequency representation of power is calculated and averaged. These data were obtained from laminar recordings in visual cortex of the monkey. Note that coupling was stronger across sites than within sites. This would not have been the case if the coupling was explained by nonsinusoidal alpha oscillations. b, The PAC measure applied to the data from the deep and superficial layers. Reproduced with permission from Cell Press (Spaak et al., 2012).
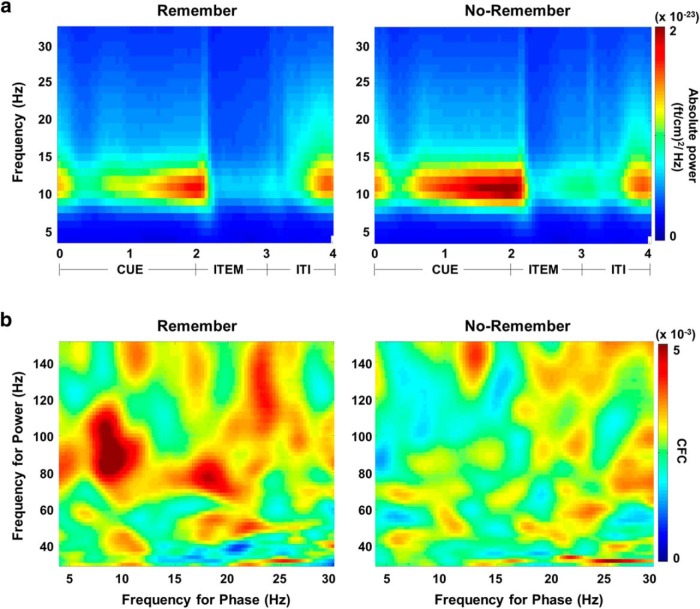
Example 3: coupling increases when low-frequency power decreases
A recent memory study relying on human MEG investigated PAC when subjects were preparing to encode upcoming visual items (Park et al., 2014, 2016). When subjects were asked to ignore the upcoming item (“No-Remember”), alpha power in visual cortex was stronger compared with when subjects were asked to remember (“Remember”) the item (Fig. 5a). However, importantly, in the Remember condition, when alpha power was low, the PAC coupling was stronger (Fig. 5b). This finding is consistent with the notion that alpha oscillations inhibit gamma band activity in a phasic manner (Mazaheri and Jensen, 2008). The stronger the alpha oscillations, the lower the gamma power and therefore the weaker the PAC in the No-Remember condition. Had the PAC been explained by the nonsinusoidal shape of the alpha oscillations, and thus by the higher harmonics of the alpha frequency, one would expect PAC to increase with alpha power since the magnitude of the higher harmonics would increase as well. Park et al. (2016) found the reverse, in support of nonspurious PAC. It does remain a theoretical possibility that low-amplitude alpha oscillations are less sinusoidal than high-amplitude alpha oscillations. However, this is not likely to explain the effect on PAC, since the lower amplitude would also impair the alpha phase estimate and thereby reduce the PAC. In sum, studies in which one can demonstrate an increase in PAC associated with a decrease in power speak to the existence of nonspurious coupling.
Figure 5.
An MEG study in which subjects were asked to encode (‘Remember’) or ignore (‘No-Remember’) upcoming visual stimulus. The ‘Remember’ condition was associated with less alpha power (a) but stronger PAC (b). Given that PAC goes up when alpha power goes down, the coupling is not likely to be explained by higher harmonics of the alpha oscillations. Reproduced with permission from Macmillan Publishers Ltd. (Park et al., 2016).
Does broadband gamma activity warrant special caution?
Transient power in higher harmonics at certain phases of a lower-frequency oscillation, together with frequency smoothing due to power estimation, might result in a typical broadband frequency response at those low-frequency phases that contain sharp transitions (e.g., the peak of a saw tooth). One should be extra careful about such broadband PAC profiles. However, even the occurrence of broadband (“gamma”) activity modulated by slow oscillations does not per se imply that the measured PAC is caused by nonsinusoidal slow oscillations. As we will explain below, the broadband response could be caused by neuronal spiking, providing a wide frequency content. Indeed, it is debated whether neuronal activity in the gamma band can be considered oscillatory or whether it is a broadband phenomenon (Hermes et al., 2015). This issue is complicated by factors such as differences between species, tasks, brain regions, and recording techniques. For instance, Colgin et al. (2009) have reported fast and slow gamma oscillations, both in a well defined, relatively narrow frequency band, in the behaving rat. These were isolated in spike-field recordings and were associated with, respectively, retrospective and prospective memory operations (Bieri et al., 2014). In contrast, several other studies report broadband activity modulated by slow oscillations (Canolty et al., 2006). The latter should, however, not be considered spurious PAC. Broadband activity modulated by a slow rhythm does speak to the temporal organization of neuronal computation since it demonstrates that the neuronal activity is synchronized more strongly at some phases than others within a cycle of a slow rhythm. This phasic synchronization is bound to modulate the communication to target regions and is likely to be important for neuronal communication (Jensen, 2005; Hyafil et al., 2015b; McLelland and VanRullen, 2016).
Conclusion
Nonsinusoidal low-frequency waveforms will have transient power at higher harmonics in certain phases of the cycle. When analyzing PAC in such signals, one might erroneously conclude that higher-frequency neural activity is clocked by a lower-frequency one. Even advanced signal-processing tools might never completely alleviate this potential artifact. Instead, one can look to other empirical factors to aid the interpretation of PAC. We have here presented three examples of reports of PAC that cannot be explained by concerns such as higher harmonics. While great care should be taken when interpreting PAC measures, there are numerous good examples of true coupling between distinct neuronal activity at slow and fast frequencies. Although there is no magic bullet, we recommend searching for complementary evidence, as follows:
Accumulate evidence from complementary modalities. For instance, human data recorded by EEG and MEG can be related to intracranial electrocorticographic recordings from patients. Use spike-field data in rodents or monkeys to elucidate whether the gamma activity in question can be associated with the coordination of neural firing.
Investigate coupling between cortical layers or regions. For instance, if alpha or theta phase drives gamma power in a neighboring region more strongly than within the phase-providing region itself, concerns on spurious PAC are reduced.
Relate modulations in low-frequency power to modulations in PAC. A lack of correlation or a negative correlation makes the interpretation as a harmonic artifact less likely.
We encourage a continued debate on when measures of PAC can be considered to reflect neuronal coupling between oscillations and fast neuronal activity. Given careful interpretation, we believe PAC measures are an essential tool for understanding the temporal organization of hierarchical neuronal computation.
Acknowledgments
Acknowledgments: We thank Laura Colgin for fruitful comments.
Synthesis
Reviewing Editor: Bradley Postle, University of Wisconsin
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Michael Cohen, Bradley Voytek
The two reviews have both found your revision to be appreciably improved over the original:
Reviewer 1: "The revision is improved. I still think the title does not really fit. I would have called it something like "Case studies of true neural phase-amplitude coupling". But I'll leave that up to the authors and editor to decide."
Reviewer 2: "The authors have addressed all of my concerns. This piece is a nice commentary on a quickly-moving area of systems neuroscience."
I am in agreement with Reviewer 1, and will request that you consider a making a few stylistic modifications to the title and to the body of your commentary, that I think will sharpen and more precisely articulate the points that you want to make. With regard to the title, R2 and I both think that the formulation "when to trust" doesn't succinctly convey the arguments, nor the contents of your paper. My suggestion would be "Discriminating valid from spurious indices of phase-amplitude coupling". Getting into the commentary itself, I would encourage you to be careful to distinguish the physical phenomenon of PAC from analytic measures that are intended to index this phenomenon. Thus, for example, in the Abstract, you presumably don't intend to mean that non-sinusoidal properties the oscillations literally "create spurious coupling", but rather that they can some measures to 'spuriously indicate the presence of coupling'. And in the following sentence, rather than "While such spurious coupling has been observed, in this commentary we give concrete examples illustrating cases when cross-frequency coupling can be trusted", I think what you intend to say is that while such instances of 'the spurious identification of coupling' have been observed, you illustrate cases in which the identification of CFC can be trusted. At the end of the Significance Statement, what you are doing is putting forward examples in which 'indices of' cross-frequency interactions can be considered reliable. I won't go on through the entirety of the manuscript, but I hope can see how being careful to distinguish the measure of the phenomenon from the phenomenon itself with help clarify your arguments.
Finally, one detail that I note in your revision is that since the time that you first submitted this manuscript to eNeuro, the manuscript that you have been citing as "Cole SR, Peterson EJ, van der Meij R, de Hemptinnee C, Starr PA, Voytek B (2016). Nonsinusoidal oscillations underlie pathological phase-amplitude coupling in the motor cortex in Parkinson's disease. BioRxiv." has now been accepted at a peer-reviewed journal, as so should be updated to: "Cole SR & Voytek B. Brain oscillations and the importance of waveform shape. Trends Cogn Sci". If you'd like to see a preprint, it's available at http://voyteklab.com/publications/
References
- Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, Singer W, Vicente R (2015) Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol 31:51–61. 10.1016/j.conb.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsaki G (2012) Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci 32:423–435. 10.1523/JNEUROSCI.4122-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri KW, Bobbitt KN, Colgin LL (2014) Slow and fast γ rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron 82:670–681. 10.1016/j.neuron.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. (2015) Gamma activity coupled to alpha phase as a mechanism for top-down controlled gating. PLoS One 10(6):e0128667 10.1371/journal.pone.0128667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G (1995) Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns A (2004) Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? J Neurosci Methods 137:321–332. 10.1016/j.jneumeth.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2006) Rhythms of the brain. Oxford/New York: Oxford UP. [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT (2006) High gamma power is phase-locked to theta oscillations in human neocortex. Science 313:1626–1628. 10.1126/science.1128115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Voytek B (2017) Brain oscillations and the importance of waveform shape. Trends Cogn Sci, in press. [DOI] [PubMed] [Google Scholar]
- Cole SR, Peterson EJ, van der Meij R, de Hemptinnee C, Starr PA, Voytek B (2016) Nonsinusoidal oscillations underlie pathological phase-amplitude coupling in the motor cortex in Parkinson’s disease. bioRxiv. Advance online publication. Retrieved December 27, 2016 10.1101/049304 [DOI] [Google Scholar]
- Colgin LL (2015) Theta-gamma coupling in the entorhinal-hippocampal system. Curr Opin Neurobiol 31:45–50. 10.1016/j.conb.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI (2009) Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462:353–357. 10.1038/nature08573 [DOI] [PubMed] [Google Scholar]
- Dvorak D, Fenton AA (2014) Toward a proper estimation of phase-amplitude coupling in neural oscillations. J Neurosci Methods 225:42–56. 10.1016/j.jneumeth.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin E, Baillet S (2015) The brain's resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations. Neuroimage 111:26–35. 10.1016/j.neuroimage.2015.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Wandell BA, Winawer J (2015) Gamma oscillations in visual cortex: the stimulus matters. Trends Cogn Sci 19:57–58. 10.1016/j.tics.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil A, Fontolan L, Kabdebon C, Gutkin B, Giraud AL (2015a) Speech encoding by coupled cortical theta and gamma oscillations. Elife 4:e06213. 10.7554/eLife.06213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil A, Giraud AL, Fontolan L, Gutkin B (2015b) Neural cross-frequency coupling: connecting architectures, mechanisms, and functions. Trends Neurosci 38:725–740. [DOI] [PubMed] [Google Scholar]
- Jensen O (2005) Reading the hippocampal code by theta phase-locking. Trends Cogn Sci 9:551–553. 10.1016/j.tics.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Jensen O, Colgin LL (2007) Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci 11:267–269. 10.1016/j.tics.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Jones SR (2016) When brain rhythms aren't “rhythmic”: implication for their mechanisms and meaning. Curr Opin Neurobiol 40:72–80. 10.1016/j.conb.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Tort AB, Kopell NJ (2008) Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods 170:352–357. 10.1016/j.jneumeth.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Jensen O (2013) The θ-γ neural code. Neuron 77:1002–1016. 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Soldevilla D, Ter Huurne N, Oostenveld R (2016) Neuronal oscillations with non-sinusoidal morphology produce spurious phase-to-amplitude coupling and directionality. Front Comput Neurosci 10:87. 10.3389/fncom.2016.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Jensen O (2008) Asymmetric amplitude modulations of brain oscillations generate slow evoked responses. J Neurosci 28:7781–7787. 10.1523/JNEUROSCI.1631-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland D, VanRullen R (2016) Theta-gamma coding meets communication-through-coherence: neuronal oscillatory multiplexing theories reconciled. PLoS Comput Biol 12:e1005162. 10.1371/journal.pcbi.1005162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Sharma M, Rao RP, den Nijs M, Fetz EE, Sejnowski TJ, Hebb AO, Ojemann JG, Makeig S, Leuthardt EC (2010) Dynamic modulation of local population activity by rhythm phase in human occipital cortex during a visual search task. Front Hum Neurosci 4:197. 10.3389/fnhum.2010.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipova D, Hermes D, Jensen O (2008) Gamma power is phase-locked to posterior alpha activity. PLoS One 3:e3990. 10.1371/journal.pone.0003990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Palva S, Kaila K (2005) Phase synchrony among neuronal oscillations in the human cortex. J Neurosci 25:3962–3972. 10.1523/JNEUROSCI.4250-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee DS, Kang E, Kang H, Hahm J, Kim JS, Chung CK, Jensen O (2014) Blocking of irrelevant memories by posterior alpha activity boosts memory encoding. Hum Brain Mapp 35:3972–3987. 10.1002/hbm.22452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee DS, Kang E, Kang H, Hahm J, Kim JS, Chung CK, Jiang H, Gross J, Jensen O (2016) Formation of visual memories controlled by gamma power phase-locked to alpha oscillations. Sci Rep 6:28092. 10.1038/srep28092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheremet A, Burke SN, Maurer AP (2016) Movement enhances the nonlinearity of hippocampal theta. J Neurosci 36:4218–4230. 10.1523/JNEUROSCI.3564-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012) Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci 13:121–134. 10.1038/nrn3137 [DOI] [PubMed] [Google Scholar]
- Soto JL, Lachaux JP, Baillet S, Jerbi K (2016) A multivariate method for estimating cross-frequency neuronal interactions and correcting linear mixing in MEG data, using canonical correlations. J Neurosci Methods 271:169–181. 10.1016/j.jneumeth.2016.07.017 [DOI] [PubMed] [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O (2012) Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr Biol 22:2313–2318. 10.1016/j.cub.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EM, Shakarchi R (2003) Fourier analysis: an introduction. Princeton, NJ: Princeton UP. [Google Scholar]
- Terrazas A, Krause M, Lipa P, Gothard KM, Barnes CA, McNaughton BL (2005) Self-motion and the hippocampal spatial metric. J Neurosci 25:8085–8096. 10.1523/JNEUROSCI.0693-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H (2009) Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A 106:20942–20947. 10.1073/pnas.0911331106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski R, Eichenbaum H, Kopell N (2010) Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104:1195–1210. 10.1152/jn.00106.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meij R, Kahana M, Maris E (2012) Phase-amplitude coupling in human electrocorticography is spatially distributed and phase diverse. J Neurosci 32:111–123. 10.1523/JNEUROSCI.4816-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel J, Cox R, Cohen MX (2015) Phase-clustering bias in phase-amplitude cross-frequency coupling and its removal. J Neurosci Methods 254:60–72. 10.1016/j.jneumeth.2015.07.014 [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Jha A, Penny W, Litvak V (2015) Parametric estimation of cross-frequency coupling. J Neurosci Methods 243:94–102. 10.1016/j.jneumeth.2015.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Nicolai C, Engler G, Sharott A, Engel AK, Moll CK, Siegel M (2014) Corticostriatal coordination through coherent phase-amplitude coupling. J Neurosci 34:5938–5948. 10.1523/JNEUROSCI.5007-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]