Abstract
Malnutrition is a major public health problem especially in the developing countries. The objective of the study was to compare WHO/UNICEF recommended therapeutic food with home based therapeutic food in the management of severe acute malnutrition. It was a randomized controlled trial at tertiary care level hospital with nutritional rehabilitation centre. Children (6 month to 5 years) having severe acute malnutrition were included in the study. Group A (n=74 children) was given WHO recommended therapeutic food and group B (n=75 children) was given home based therapeutic food. The mean rate of weight gain, gain in height and increase in mid-upper arm circumference were significantly higher (p<0.05) in the group received home based therapeutic food. Mean duration to achieve target weight was 21.44±3.33 days in group A and 16.28±2.11 days in group B (p<o.ooo1). Group B children had higher rate of increase in urinary creatinine (p<0.0001). Affordability (p<0.0001), Feasibility (p=0.027) and Average frequency of feeding per day (p<0.0001) was found significantly higher in group B. Difficulty in making therapeutic food was significantly low in group B (p<0.05). Both kinds of therapeutic food were effective for the management of severe acute malnutrition, but the home based therapeutic food was found to be more effective. This could be explained by better acceptability in terms of better palatability, more affordability, increased frequency of feeding, and having less difficulty in making.
Keywords: Severe acute malnutrition, WHO recommended therapeutic food, Home based therapeutic food, Urinary creatinine
Introduction
Malnutrition is a major public health problem especially in the developing countries and is an underlying factor in over 50% of death due to preventable causes that occur in children under 5 years of age [1,2].
Irrespective of significant advances in economic prosperity and in the field of medical therapeutics, malnutrition remains a significant public health problem in India. Approximately 8.1 million children under the age of 5 years (6.4%) suffer from severe acute malnutrition (SAM) and it is one of the important co-morbidities leading to hospital admissions in developing countries [3].
Severe acute malnutrition is also associated with high mortality rate, ranging from 73 to 187 per 1000 [4]. Considering the seriousness of situation, it is obvious that prevention and treatment of SAM should be one of the important priorities of the health planners.
World health organization (WHO) recommends initial management of SAM patients at a referral center followed by management at home once the child gets stabilized [5]. Developing countries like India have large number of SAM children with large proportion of them living in rural areas. Their parents have lack of education and proper resources. In such situation, inpatient treatment is associated with high cost, which is unacceptable to the family. Delayed institutional management on the other hand leads to increased morbidity and mortality. Considering the grimness of current scenario, improvement in standard guidelines for the management of SAM is the need of hour.
The aim of our study was to compare the difference in outcome of WHO recommended therapeutic food (TF) with a locally available, safe, palatable, affordable and culturally acceptable energy dense food which has qualities similar to WHO recommended TF in terms of nutrition in SAM children.
Methods
All malnourished children (6 month to 5 years) presented to pediatric outpatient clinic in a tertiary institute in India were assessed and only SAM children were included in the study. SAM was defined as per WHO criteria. Children with weight for height <3SD, mid arm circumference <115 mm, bilateral pedal edema or visible severe wasting were included and WHO multicentric growth standards were used as reference criteria [6]. Children with cerebral palsy, chronic illness, malignancy, metabolic disorder, chromosomal disorder and HIV positive children were excluded.
Randomization of study subjects was done by random number table method. All SAM children who passed appetite test or had no complications were included in the study. The patients who failed appetite test or had complications were initially admitted in pediatric ward till they get stabilized with return of appetite, afterwards they were shifted to nutritional rehabilitation centre (NRC). The study was done from July 2011 to September 2012. Sample size calculated was 160 calculated as follows:
Zα = Z value of alpha error; Zβ = z value of beta error; P1 = proportion of success with new treatment; P2 = proportion of success with standard treatment; Q1 = 1-P1; Q2 = 1-P2; L = P1-P2
Institutional Ethical Committee approved the study. Written Consent was taken from patient’s parent by explaining them nature and purpose of study in their language. Initially, it was assumed that there is no difference between the outcomes of the two groups. Group A (n=80) (WHO recommended therapeutic food group) received only therapeutic food recommended by WHO/UNICEF. No other food was allowed except for breast milk. Patients admitted in our hospital received therapeutic food from NRC kitchen. Patients who were managed on out-patient basis or after discharged from hospital received therapeutic food at home. Mothers were educated for making therapeutic food at home by a standard method.
Group B (n=80) (home based therapeutic food group) received energy dense home based therapeutic food, which was equivalent to WHO/UNICEF recommended therapeutic food in terms of energy and protein. This food was not simply the home diet but was fortified and energy dense food, containing adequate amount of protein, carbohydrate, fat and multivitamins. Various combinations of food items were made to provide adequate amount of energy, protein, carbohydrate, fat, and multivitamins for the management of SAM, which can be easily cooked at home, depending upon local availability and cultural acceptability. These recipes were taught at NRC kitchen for 2–3 days, so that mothers were confident enough to cook at home.
Duration of therapy was taken as 8 weeks. Target weight gain was taken as 15% of weight on the day of admission or weight on edema free day as recommended by the WHO [7]. Each patient was followed weekly to record the weight gain, height gain, change in mid upper arm circumference (MUAC), loss of edema, non-responders and to ensure that patient’s mother/care-taker followed our instructions strictly. Patients were telephonically reminded about their visits and ensured for good compliance.
Non-responder was defined as child having weight gain < 5 gm/kg/day [7]. Defaulter was defined as child who left out the study before completion of 4 weeks [7]. Each patient was followed till 8 weeks (Figure 1). All data were collected on preformed Performa.
Figure 1.
Flow chart depicting methodology
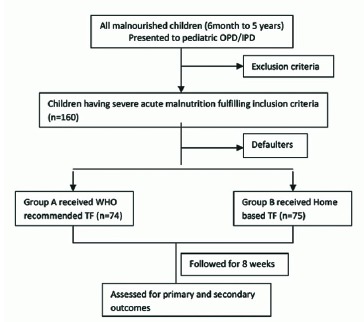
IPD- Inpatient department, OPD- Outpatient department, TF- Therapeutic food, WHO- World Health Organization
Twenty-four-hour urinary creatinine was selected as marker of malnutrition. Because it represents the total body muscle mass [8]. It helps in interpreting and monitoring acute malnutrition. Its value decreases in malnutrition and increases with increase in muscle mass, considering normal renal functions. Twenty-four-hour urine creatinine was measured on day 1 of enrollment and at the end of 8 weeks. Different parameters were used for assessment of outcome and acceptability (Table 1).
Table 1.
Primary and Secondary assessment parameters
| Primary assessment (for outcome) | Secondary assessment (for acceptability) |
|---|---|
| 1. Rate of weight gain | 1. Affordability |
| 2. Duration required to achieve target weight | 2. Feasibility |
| 3. Rate of height/length gain | 3. Palatability |
| 4. Rate of gain in MUAC | 4. Frequency of feeding |
| 5. Non-responders | 5. Difficulty in making |
| 6. Death |
Statistical analysis: All parameters were assessed by collecting data in a preformed working Performa and a set of questionnaire. Fisher’s exact test was applied to compare the proportions of two groups. Unpaired t-test was used to compare the means of two groups. P value <0.05 was taken as significant, p<0.01 as highly significant, and p<0.0001 as most significant. Confidence interval was taken as 95%.
Results
Both groups were found statistically comparable to each other (Table 2). Mean rate of weight gains in group A and B were 7.20 gram/kg/day and 9.51gram/kg/day respectively (p<0.0001) whereas average height gains were 0.17 cms/week and 0.18 cms/week respectively (p=0.0002). Rate of increase in mid upper arm circumference in group A and B were 0.122±0.014cm/week and 0.129±0.015cm/week respectively (P =0.0038). Mean duration to achieve target weight was 21.44±3.33 days in group A and 16.28±2.11 days in group B (p<0.0001). Group B children had higher rate of increase in urinary creatinine (p<0.0001). 96 % family found home based TF palatable as compared to 68.9% in case of WHO TF (p<0.0001). For 93.3% of families home based TF was affordable while only 45.9% families found WHO TF affordable (p<0.0001). Only 10.6% family found difficulty in making home based TF while 29.7% found WHO TF difficult to make. Frequency of feeding was 8.42±1.68 per day in group B as compared to 7.20±1.65 feeds per day in group A (p<0.0001) (Table 3 & 4).
Table 2.
Comparison of parameters in group A and B
| Parameters | Group A (WHO recommended therapeutic food) | Group B (Home based therapeutic food) | ‘P’ value |
|---|---|---|---|
| Mean weight (Kg) | 7.77 ± 1.28 | 7.30 ± 1.62 | 0.47 |
| Mean age (months) | 23.78 ± 9.06 | 24.24 ±12.31 | 0.46 |
| Male/female | 47/27 | 45/30 | 0.78 |
| Z-score (−3 to > −4) | 53/74 | 48/75 | 0.38 |
| Breast feed babies | 35/74 | 39/75 | 0.62 |
| Edema | 21/74 | 18/75 | 0.58 |
| Hospitalized patients | 29/74 | 28/75 | 0.87 |
| Associated Acute diarrhea | 38/74 | 36/75 | 0.74 |
| Mean income per capita Rs | 4613 ± 2475 | 4008 ± 2223 | 0.12 |
| Availability of clean water | 74/74 | 75/75 | - |
| Educated mother | 24 | 19 | 0.37 |
Table 3.
Primary assessment (Outcome measurement)
| Parameters | Group A (WHO recommended therapeutic food) | Group B (Home based therapeutic food) |
|---|---|---|
| Mean rate of weight gain (gm/kg/day) | 7.20 ± 1.35 | 9.51 ± 1.45*** |
| Mean duration of treatment (days) | 21.44 ± 3.33 | 16.28± 2.11*** |
| Rate of height/length gain (cms/week) | 0.17±0.01 | 0.18±0.02** |
| Rate of increase in MUAC (cms/week) | 0.122±0.014 | 0.129±0.015* |
| Mean rate of increase in 24 hour urinary creatinine (mg/kg/day) | 7.05±1.23 | 9.02±1.33*** |
| Non-responders | 2 | 1 |
| Mortality | 0 | 0 |
P<0.01,
P<0.001,
P<0.0001
Table 4.
Secondary assessment (Acceptability measurement)
| Parameters | Group-A, n=74 (%) | Group-B, n=75 (%) | ‘p’ value |
|---|---|---|---|
| Palatability | 51 (68.9) | 72 (96)*** | P<0.0001 |
| Affordability | 34 (45.9) | 70 (93.3)*** | P<0.0001 |
| Feasibility | 53 (71.6) | 65 (86.6) | P=0.027 |
| Frequency of feeding (number of feeds per day) | 7.20±1.65 | 8.42±1.68*** | P<0.0001 |
| Making difficulty | 22 (29.7) | 8 (10.6)* | P=0.004 |
P<0.01,
P<0.001,
P<0.0001
Discussion
In the early 1960s and 1970s, a debate started over hospital inpatient treatment of SAM versus community based treatment, considering the poor success rate of hospital inpatient treatment in non-emergency settings. It was questioned whether hospital is the best place to treat SAM. This concern prompted to decentralize the management of SAM from hospitals to the either homes of affected children or simpler NRC/primary health care centers [9].
Bhutta et al Analyzed data from 21 studies on management of SAM and concluded that use of prepared balanced foods such as spreads and ready-to-use foods is feasible in community settings [6].
There have been many studies comparing hospital and home strategies for rehabilitating SAM patients involving interventions in different combinations and almost all have found home based therapy superior to inpatient care. Some of these studies involved use of ready to use therapeutic food at home whereas others have used simply therapeutic food prepared at home [10–15]. National workshop on development of guidelines for home based care and standard treatment of children suffering from severe acute malnutrition concluded that home based management could be feasible, acceptable, and cost effective option for children with uncomplicated SAM [16]. After comparing both groups, we found that both kinds of therapeutic foods were effective for the management of SAM, but the home based therapeutic food was found to be more effective. This can be explained by better acceptability in terms of better palatability, more affordability, increased frequency of feeding, and having less difficulty in making.
It is important to understand that the caregivers of the malnourished patients mostly come from poor families and cannot afford to leave home for long periods of time to stay with their malnourished child during treatment as it leads to loss of their daily wages. However, if these children are caught early in uncomplicated stages of malnutrition, the technical aspects of treatment can be made simpler. All that is required is a balanced diet of sufficient quantity and quality. The home based therapeutic food is cheaper to produce and easier to administer, making success rates high and treatment costs low. It could also benefit children by reducing exposure to hospital-acquired infections.
Conclusion
We found that both kinds of therapeutic foods were effective for the management of SAM, but the home based therapeutic food was found to be more effective. Our study further adds to the literature that home based therapeutic food is superior to WHO recommended therapeutic food in many terms. The limitation of our study is that it was of a short duration of follow up.
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003; 361(9376):2226–2234. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004; 80(1):193–198. [DOI] [PubMed] [Google Scholar]
- 3.Lahariya C, Khandekar J. How the findings of national family health survey-3 can act as a trigger for improving the status of anemic mothers and ndernourished children in India: a review. Indian J Med Sci. 2007; 61(9):535–44. [PubMed] [Google Scholar]
- 4.Pelletier DL. The relationship between child anthropometry and mortality in developing countries: implications for policy, programs and future research. J Nutr 1994; 124 (suppl): 2047S–2081S. [DOI] [PubMed] [Google Scholar]
- 5.Collins S, Dent N, Binns P, Bahwere P, Sadler K, and Hallam A. Management of severe acute malnutrition in children. Lancet 2006; 368:1992–2000. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008; 371: 417–440. [DOI] [PubMed] [Google Scholar]
- 7.WHO child growth standards and the identification of severe acute malnutrition in infants and children A Joint Statement by the World Health Organization and the United Nations Children’s Fund. 2009. http://www.who.int/maternal_child_adolescent/documents/9789241598163/en/. [PubMed]
- 8.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983; 37(3):478–494. [DOI] [PubMed] [Google Scholar]
- 9.Ashworth A. Efficacy and effectiveness of community-based treatment of severe malnutrition. Food Nutr Bull 2006; 27(3 Suppl): S24–S48. [DOI] [PubMed] [Google Scholar]
- 10.Gaboulaud V, Dan-Bouzoua N, Brasher C, Fedida G, Gergonne B, Brown V. Could nutritional rehabilitation at home complement or replace centre-based therapeutic feeding programmes for severe malnutrition? J Trop Pediatr 2007; 53: 49–51. [DOI] [PubMed] [Google Scholar]
- 11.Simpore J, Kabore F, Zongo F, Dansou D, Bere A, Pignatelli S, et al. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr J. 2006. January 23; 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amthor RE, Cole SM, Manary MJ. The use of home based therapy with ready to use therapeutic food to treat malnutrition in a rural area during a food crisis. J Am Diet Assoc 2009; 109:464–467. [DOI] [PubMed] [Google Scholar]
- 13.Jilcott SB, Ickes SB, Ammerman AS, Myhre JA. Iterative design, implementation and evaluation of a supplemental feeding program for underweight children ages 6–59 months in Western Uganda. Matern Child Health J. 2010; 14: 299–306. [DOI] [PubMed] [Google Scholar]
- 14.Linemann Z, Matilsky D, Ndekha M, Manary M, Maleta K, Manary MJ. A large scale operational of home based therapy with ready to use therapeutic food in childhood malnutrition in Malawi. Mat Child Nutr 2007; 3:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciliberto MA, Manary MJ, Ndekha MJ, Briend A, Ashorn P. Home-based therapy for oedematous malnutrition with ready-to-use therapeutic food. Acta Paediatr. 2006; 95:1012–1015. [DOI] [PubMed] [Google Scholar]
- 16.Gupta P, Shah D, Sachdev HP, Kapil U. National workshop on “Development of guidelines for effective home based care and treatment of children suffering from severe acute malnutrition”. Indian Pediatr 2006; 43:131–139. [PubMed] [Google Scholar]


