Abstract
Idiopathic intracranial hypertension (IIH) is a rare neurological disorder in children. It is characterized by raised intracranial pressure (ICP) in the absence of brain parenchymal lesion, vascular malformations, hydrocephalus, or central nervous system (CNS) infection. The diagnosis is usually confirmed by high opening pressure of cerebrospinal fluid (CSF) with exclusion of secondary causes of intracranial hypertension. If not treated properly, it may lead to severe visual dysfunction. Here we review the etiology, clinical presentation, diagnostic criteria and management of IIH in children through illustration of the clinical and radiological presentation of a 13-year-old overweight girl who presented with severe headache, diplopia and bilateral papilledema. Otherwise, she had unremarkable neurological and systemic examinations. Lumbar puncture showed a high CSF opening pressure (360–540 mmH2O). Her investigations showed normal complete blood count (CBC), normal renal, liver, and thyroid function tests. Cerebrospinal fluid (CSF) and blood chemistry were unremarkable. Magnetic resonant image (MRI) of the brain demonstrated empty sella turcica, tortuous optic nerves, and flattening of the posterior sclera. Magnetic resonant venography (MRV) showed focal narrowing of the distal transverse sinuses and absence of venous sinus thrombosis. She required treatment with acetazolamide and prednisolone. With medical treatment, weight reduction, and exercise, our patient had a remarkable improvement in her symptoms with resolution of papilledema in two months. This review highlights the importance of early recognition and management of IIH to prevent permanent visual loss.
Keywords: Idiopathic intracranial hypertension, Pseudo tumor cerebri, Child, Primary intracranial hypertension, Secondary intracranial hypertension
Index Case
A 13-year-old girl, who was previously healthy, presented to the Pediatric Emergency Department at King Khalid University Hospital, Riyadh with a three-day history of severe headache followed by double vision, not associated with fever, vomiting, alteration in the level of consciousness, or abnormal movements.
Physical examination revealed a conscious child with weight of 62.6 kg (90th – 95th percentile), height of 150 cm (10th – 25th percentile) and a body mass index (BMI) of 27.8 kg/m2. Eye examination showed binocular diplopia for far vision more than near with mild limitation in abduction in extreme gazes bilaterally. Visual acuity was 20/20 in both eyes with normal color vision and pupillary light responses. Fundus examination showed elevated disc in the left eye more than the right with hyperemia and blurry margin nasally, and a healthy retina bilaterally (Figure 1). Humphrey visual fields 30-2 were also normal. Rest of neurological and systemic examinations were unremarkable. Her investigations showed normal CBC, renal and liver function tests. Thyroid function was normal with negative thyroid antibodies. Vitamin A level was 1.94 mmol/l (normal 1–2 mmol/l) and vitamin D level was 18.6 nmol/(75–250 nmol/l). Antinuclear antibodies (ANA), anti-double stranded DNA (dsDNA), complements 3 and 4 (C3, C4) were all normal. The initial lumbar puncture demonstrated an opening CSF pressure of 360 mmH2O. Thirty ml of CSF was drained and acetazolamide (125 mg twice daily) was started. Computed tomographic (CT) scan of the brain was unremarkable. Brain MRI showed partial empty sella, flattening of posterior sclera and prominence of the optic nerve head as well as tortuosity of optic nerves and prominent perioptic nerve sheath. Magnetic resonant venography (MRV) showed bilateral focal narrowing of distal transverse sinuses with no evidence of cerebral sinovenous thrombosis (Figure 2). Her symptoms worsened over few days with increased severity of headache and papilledema. Lumbar puncture was repeated and the opening CSF pressure was found to be 540 mmH2O; then 25 ml of CSF was drained. She received 60 mg of oral prednisolone for seven days. The dose of acetazolamide was gradually increased to 1000 mg twice daily. She improved gradually with resolution of papilledema in a period of 2 months from the start of her symptoms. She was able to reduce her weight by 10 kg in a period of 2 months with dietary advice and exercise. Her recent follow-up in the clinic showed no visual symptoms with significant resolution of papilledema (Figure 1).
Figure 1.
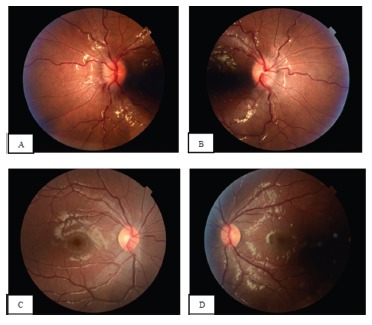
Fundus photography of the right (A) and left (B) eyes showing bilateral papilledema with optic nerve head elevation, peripapillary hemorrhages and vessel tortuosity.
Fundus photography of the right eye (C) and left eye (D) 4 months after treatment showing significant improvement of disc edema. Some peripapillary nerve fiber layer opacification remains.
Figure 2.
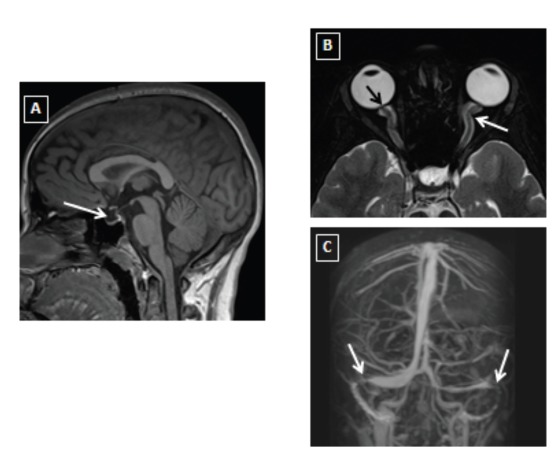
Brain MRI (A, sagittal T1WI) showing partial empty sella (white arrow). Axial T2 fat saturation for orbits (B) showing flattening of posterior globe and prominence of the optic nerve head (black arrow) as well as tortuosity of optic nerve and prominent perioptic nerve sheath (white arrow). MRV (C) showing focal narrowing of bilateral distal transverse sinuses (white arrow).
Pathogenesis
The exact pathogenesis of IIH is unknown. Normally, intracranial pressure remains constant and maintained at a normal range because of cerebral auto regulation [12]. Intracranial pressure (ICP) is determined by the production and absorption of CSF. According to the Monro-Kellie rule, an increase in ICP is related to increase in CSF, brain tissue, or blood volume [13]. Different hypotheses and theories have been proposed, such as excess of CSF production, CSF outflow reduction, increase in cerebral blood volume, and increase in brain water content, obstruction to venous system, endocrinological causes, metabolic causes, and chronic inflammation [14–20].
Obesity is an important risk factor for the development of IIH in post-pubertal females. In one study, it was found that 91% of IIH patients aged 15–17 years were obese [3]. Aylward et al [9] compared the BMI in 203 pediatric patients with IIH in pre- and post-pubertal female patients and they found that the BMI was significantly higher in the post-pubertal group. Other studies also supported that obesity is not a risk factor in pre-pubertal patients [3, 21].
IIH can be attributed to certain medications or medical illnesses. In that case, it is referred to as secondary intracranial hypertension (SIH). In a large cohort study of 203 pediatric patients with IIH, (30%) of the cases were classified as SIH [9]. Various systemic diseases have been associated with pediatric IIH, including endocrine conditions such as hypoparathyroidism, thyroid replacement therapy, and treatment with recombinant human growth hormone [22]. Other medical conditions include chiari malformation, prior meningitis, hydrocephalus, craniosynostosis, traumatic brain injury, superior sagittal sinus thrombosis, leukemia, Lyme disease, congestive heart failure, renal failure, and kidney transplantation [9]. Several medications are associated with SIH. The medical conditions and medications associated with IIH are summarized in Table 1 [8, 9, 22–24].
Table 1.
Reported risk factors for secondary intracranial hypertension
| Medication | Infection | Medical and surgical conditions |
|---|---|---|
| Amiodarone Cyclosporine Nalidixic acid Nitrofurantoin Oral contraceptive pills Steroids therapy Tetracyclines Vitamin A analogues Lithium Penicillin Phenytoin Sulphonamides |
Coxsackie B encephalitis HIV Infectious mononucleosis Lyme disease Malaria Poliomyelitis Prior meningitis Syphilis |
Adrenal insufficiency Chiari malformation Chronic anemia Congestive heart failure Craniosynostosis Cushing’s disease Guillain-Barre syndrome Hydrocephalus Leukemia Polycystic ovary syndrome Renal failure Superior sagittal sinus thrombosis Systemic lupus erythematosus Traumatic brain injury Trisomy 21 Vitamin D deficiency |
Clinical Presentation
The most common presenting symptom in children is headache which has been documented in up to 91% of the cases [7–9]. It is usually throbbing, intermittent, diffuse in nature, and worse upon awakening. Retro-orbital, neck, and back pains may also occur [25]. Nausea and vomiting are very common symptoms. Other complaints are blurred or double vision, transient visual obscurations, tinnitus, and neck stiffness [26, 27]. Atypical presentations of IIH without headache have been reported, and patients might present with some degree of visual loss [28]. In young patients, IIH can present only with irritability [22]. Unlike patients with intracranial mass lesions, the level of consciousness is usually intact in children with IIH [6]. Symptoms such as seizures and focal neurologic deficits are likely to point towards intracranial mass lesions [8].
Children with suspected IIH should have careful ophthalmological and full neurological examination. The examination of children with IIH is usually normal except for reduced visual acuity, visual fields defects, unilateral or bilateral sixth nerve palsy, and papilledema [8]. Also, children with suspected IIH should undergo detailed general examination, including blood pressure measurement and BMI assessment. The examination should be directed to identify the secondary causes, such as otitis media, mastoiditis, sinusitis, or other causes listed in Table 1.
Papilledema is the most important sign in children with IIH. However, the absence of papilledema has been documented. Faz et al [29] reported that papilledema was absent in 48% of their cases. It is typically bilateral but can also be unilateral and can be absent in infants with unfused sutures [30]. However, the absence of papilledema does not rule out the disease [26]. Sixth cranial nerve palsy has been reported in 46% to 60% of cases [7, 31]. Visual acuity loss was reported in 6 to 20% of cases, whereas visual field defect was reported in up to 91% of cases [8].
Investigations
Once IIH is clinically suspected, then urgent investigations should be done. Initial neuroimaging should start with CT scan; if unremarkable, then lumbar puncture with opening pressure should be done [23]. Further tests are indicated to rule out the secondary causes like CBC, urea and electrolyte, bone profile, plasma glucose, and thyroid function tests [32].
Neuroimaging
Brain MRI is indicated to rule out any evidence of hydrocephalus, intraparenchymal lesions, or abnormal meningeal enhancement [33]. Although CT scan is fast and inexpensive neuroimaging modality, it should be avoided when possible to minimize exposure to radiation [34]. Magnetic resonant venography (MRV) is also recommended to exclude cerebral venous sinus thrombosis [35]. Findings that suggest IIH in brain MRI include posterior globe flattening, intraocular protrusion of the optic nerve, horizontal tortuosity of the optic nerve, enlargement of optic nerve sheath, decreased in the size of the pituitary gland and transverse venous sinus stenosis [35–36].
Lumbar Puncture
Lumbar puncture in children is challenging. It should be done under sedation to avoid falsely elevated CSF pressure due to crying. It is used to measure the CSF opening pressure and to exclude meningitis. It is preferred to be done in lateral decubitus position with the legs in flexion position [8].
Interpreting the results of CSF opening pressure in children is difficult. Most of the studies suggest that 280 mmH2O is considered as the upper limit of CSF opening pressure in children between 1 and 18 years [37, 38]. In normal neonates, value above 76 mmH2O is considered abnormal [39]. CSF Samples should always be sent for routine biochemistry and microbiology analysis. The composition of CSF should be unremarkable with respect to cell count, protein, and glucose.
Diagnostic Criteria
New diagnostic criteria for pediatric IIH have been recently proposed by Friedman and his colleagues [33]. It includes specific recommendations for CSF opening pressure in pediatric population and addresses some issues when the diagnosis of IIH is not clear and atypical. The new diagnostic criteria are shown in (Table 2).
Table 2.
Diagnostic criteria for idiopathic intracranial hypertension (IIH)
| 1. Required for the diagnosis of IIH |
| A. Papilledema |
| B. Normal neurologic examination except for cranial nerve abnormalities |
| C. Neuroimaging: Normal brain parenchyma without evidence of hydrocephalus, mass, or structural lesion and no abnormal meningeal enhancement on MRI, with and without gadolinium, for typical patients (female and obese), and MRI, with and without gadolinium, and magnetic resonance venography (MRV) for others. If MRI is unavailable or contraindicated, contrast-enhanced CT may be used |
| D. Normal CSF composition |
| E. Elevated lumbar puncture opening pressure (≥250 mm CSF in adults and ≥280 mm CSF in children [250 mm CSF if the child is not sedated and not obese]) in a properly performed lumbar puncture |
| 2. Diagnosis of IIH without papilledema |
| In the absence of papilledema, a diagnosis of IIH syndrome can be made if B–E from above are satisfied, and in addition the patient has a unilateral or bilateral abducens nerve palsy |
| In the absence of papilledema or sixth nerve palsy, a diagnosis of IIH syndrome can be suggested but not made if B–E from above are satisfied, and in addition at least 3 of the following neuroimaging criteria are satisfied: |
| i. Empty sella |
| ii. Flattening of the posterior aspect of the globe |
| iii. Distention of the perioptic subarachnoid space with or without a tortuous optic nerve |
| iv. Transverse venous sinus stenosis |
| A diagnosis of IIH is definite if the patient fulfills criteria A–E. |
| The diagnosis is considered probable if criteria A–D are met but the measured CSF pressure is lower than specified for a definite diagnosis. |
Adapted from Friedman et al [33]
Management
The best approach to manage IIH in children is through a multidisciplinary team that includes a pediatrician, pediatric neurologist, ophthalmologist, orthoptist, nutritionist, and neurosurgeon. In asymptomatic patients with normal vision and mild papilledema, no treatment is needed and only serial ophthalmological evaluation is required [40]. Treatment is indicated when there is an evidence of visual loss, moderate to severe papilledema, or persistent headaches [40]. Different treatment modalities can be used. Generally, the selection of medical, surgical, or combined treatments depends on the severity of the visual symptoms and signs. In most cases, medical treatment is used first; surgical intervention is indicated if medical treatment fails or if the visual function is deteriorating. Life style modification such as weight reduction, especially in overweight patients was found to be beneficial. [41]. One case series showed that reversal of papilledema was achieved after reduction of 6% in body weight [42]. Another study showed that weight reduction can improve the symptoms and reduce ICP in overweight women with IIH [43].
Medical treatment
Treatment should aim at lowering ICP, relieve symptoms, and preserve visual function. The length of treatment varies between cases and may last up to 14 months [32]. Carbonic anhydrase inhibitors have been used to reduce ICP and to treat papilledema in IIH [44]. Acetazolamide is the most commonly used drug as a first-line treatment [8]. Commonly used medications are summarized in (Table 3).
Table 3.
Commonly used medications in the management of IIH.
| Medication | Dose | Side effects | Monitoring | Comments |
|---|---|---|---|---|
| Acetazolamide | 25 mg/kg/d, which can be increased until a clinical response is seen, maximum dose is 100 mg/kg/d [8] | Gastro intestinal upset Paresthesia of the lips, fingers, and toes Anorexia Electrolyte imbalance [8] |
Blood electrolytes If drug will be used for more than 6 months, then kidney ultrasound is recommended to rule out kidney stones [45] |
Success rate reported to range between 47% and 67%. [46] |
| Furosemide | 1–2 mg/kg/d with or without acetazolamide. [45] | Metabolic alkalosis
Hypokalaemia Hyponatraemia Hyperglycaemia Hypotension [46] |
Blood electrolytes | Few reports indicated that combining acetazolamide with furosemide reduce pressure more effectively than with acetazolamide alone. [47] |
| Topiramate | 1.5–3.0 mg/kg/d in two divided doses, the dose should increase 25 mg/w. No more than 200 mg/d [8] | Paresthesias Drowsiness Lethargy Kidney stones [48] |
Contraindicated in liver failure and in pregnancy [48]
Associated with weight loss [8] |
|
| Corticosteroids | Prednisolone: 1–2 mg/kg/d Dexamethasone: 2 mg qds or 0.1–0.75 mg/kg/d in 4 divided doses [46] | Weight gain Immunosuppression Endocrine disturbances |
Blood pressure Electrolytes Urine for glucose |
Indicated for acute, severe visual loss, and when surgery is not immediately possible [8]
- Not for chronic use |
Abbreviations: w; week, d; day.
Surgical treatment
Surgical procedures, such as optic nerve sheath fenestration (ONSF) and CSF shunting can be considered when medical treatment fails. Optic nerve sheath fenestration (ONSF) is indicated in cases of acute, severe or progressive vision loss despite medical treatment [8]. It is performed by making an incision in the optic nerve sheath, which improves CSF drainage and decreases the pressure on the optic nerves. About 50% of patients who underwent surgery on one side reported bilateral improvement in visual acuity [8]. The complication rate of this procedure ranges from 4.8% to 45%, with a mean of 12.9% [22]. The most commonly reported complications are diplopia, anisocoria, and corneal drusen [49]. Rarely central retinal artery occlusion, acute angle closure glaucoma, and optic neuropathy may occur [50].
Most commonly used CSF shunting procedures are lumboperitoneal (LP) and ventriculoperitoneal (VP) shunts. LP shunt has been reported to be the most successful in alleviating patient symptoms [8]. Complications of LP shunt include shunt obstruction, lumbar radiculopathy, infection, and tonsillar herniation [8]. Ventriculoperitoneal (VP) shunt was less studied than LP shunt in the management of IIH, and is effective in improving headache and visual function in patients with IIH [49]. Improvement of headache after VP shunt has been reported in 60% to 90% of the patients [49]. Transverse sinus stenting may be an alternative treatment option in patients with refractory IIH who fail medical treatment. [51]. Interestingly, in morbidly obese IIH children with unsuccessful trials of weight loss, bariatric surgery can be considered with positive effects. This is also indicated if obesity is associated with other complications other than IIH, such as diabetes or sleep apneas [52, 53].
Emergency management
The management is directed towards lowering ICP and preserving visual function. Neurosurgical consultation for CSF shunting may be required if there is deterioration of visual function. High dose of oral or intravenous steroids as well as intravenous acetazolamide can be used in such cases.
Prognosis
With early diagnosis and treatment, most children with mild to moderate visual field defects will have complete resolution of their symptoms [8]. Permanent visual loss or blindness is the most serious morbidity, and is mainly related to how severe the papilledema was at presentation. High risk groups for irreversible visual loss include: blacks, male gender, morbidly obese, anemic patient with fulminant IIH [53]. Permanent loss of visual acuity has been reported in up to 10% of the patients and visual field loss persists in up to 17 % of the patients [27,31,50].
Conclusion
Idiopathic intracranial hypertension is a rare neurological disorder in children with many unanswered questions. Recognizing high risk groups for irreversible visual loss is critical to guide management. Early intervention helps prevent deterioration in visual function.
References
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59(10):1492–5. [DOI] [PubMed] [Google Scholar]
- 2.McKiernan SP, DiFazio MP. Index of suspicion. Case 3. Diagnosis: Infantile pseudotumor cerebri. Pediatr Rev. 2001;22(6):211–5. [PubMed] [Google Scholar]
- 3.Balcer LJ, Liu GT, Forman S, Pun K, Volpe NJ, Galetta SL, et al. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52(4):870–2. [DOI] [PubMed] [Google Scholar]
- 4.Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014;71(6):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52(6):597–617. [DOI] [PubMed] [Google Scholar]
- 7.Babikian P, Corbett J, Bell W. Idiopathic intracranial hypertension in children: the Iowa experience. J Child Neurol. 1994;9(2):144–9. [DOI] [PubMed] [Google Scholar]
- 8.Ko MW, Liu GT. Pediatric idiopathic intracranial hypertension (pseudotumor cerebri). Horm Res Paediatr. 2010;74(6):381–9. [DOI] [PubMed] [Google Scholar]
- 9.Aylward SC, Waslo CS, Au JN, Tanne E. Manifestations of Pediatric Intracranial Hypertension From the Intracranial Hypertension Registry. Pediatr Neurol. 2016;61:76–82. [DOI] [PubMed] [Google Scholar]
- 10.Schexnayder LK, Chapman K. Presentation, investigation and management of idiopathic intracranial hypertension in children. Current Paediatrics. 2006;16(5):336–41. [Google Scholar]
- 11.Tibussek D, Distelmaier F, von Kries R, Mayatepek E. Pseudotumor cerebri in childhood and adolescence -- results of a Germany-wide ESPED-survey. Klin Padiatr. 2013;225(2):81–5. [DOI] [PubMed] [Google Scholar]
- 12.Walker RWH. Idiopathic intracranial hypertension: any light on the mechanism of the raised pressure? J Neurol Neurosur Ps. 2001;71(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746–8. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson JO. Pathogenesis of pseudotumor cerebri syndromes. Neurology. 1981;31(7):877–80. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese VP, Selhorst JB, Harbison JW. CSF infusion test in pseudotumor cerebri. Trans Am Neurol Assoc. 1978;103:146–50. [PubMed] [Google Scholar]
- 16.Dandy WE. Intracranial pressure without brain tumor - Diagnosis and treatment. Ann Surg. 1937;106:492–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joynt RJ, Sahs AL. Brain swelling of unknown cause. Neurology. 1956;6(11):801–3. [PubMed] [Google Scholar]
- 18.Bateman GA, Smith RL, Siddique SH. Idiopathic hydrocephalus in children and idiopathic intracranial hypertension in adults: two manifestations of the same pathophysiological process? J Neurosurg. 2007;107(6 Suppl):439–44. [DOI] [PubMed] [Google Scholar]
- 19.Oldstone MB. Disturbance of pituitary-adrenal interrelationships in benign intracranial hypertension (pseudotumor cerebri). J Clin Endocrinol Metab. 1966;26(12):1366–9. [DOI] [PubMed] [Google Scholar]
- 20.Dhungana S, Sharrack B, Woodroofe N. Cytokines and chemokines in idiopathic intracranial hypertension. Headache. 2009;49(2):282–5. [DOI] [PubMed] [Google Scholar]
- 21.Bursztyn LLCD, Sharan S, Walsh L, LaRoche GR, Robitaille J, De Becker I. Has rising pediatric obesity increased the incidence of idiopathic intracranial hypertension in children? Can J Ophthalmol. 2014;49(1):87–91. [DOI] [PubMed] [Google Scholar]
- 22.Spennato P, Ruggiero C, Parlato RS, Buonocore MC, Varone A, Cianciulli E, et al. Pseudotumor cerebri. Childs Nerv Syst. 2011;27(2):215–35. [DOI] [PubMed] [Google Scholar]
- 23.Rogers DL. A review of pediatric idiopathic intracranial hypertension. Pediatr Clin North Am. 2014;61(3):579–90. [DOI] [PubMed] [Google Scholar]
- 24.Rook BS, Phillips PH. Pediatric pseudotumor cerebri. Curr Opin Ophthalmol. 2016;27(5):416–9. [DOI] [PubMed] [Google Scholar]
- 25.Binder DK, Horton JC, Lawton MT, McDermott MW. Idiopathic intracranial hypertension. Neurosurgery. 2004;54(3):538–51; discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 26.Lessell S. Pediatric pseudotumor cerebri (idiopathic intracranial hypertension). Surv Ophthalmol. 1992;37(3):155–66. [DOI] [PubMed] [Google Scholar]
- 27.Phillips PH, Repka MX, Lambert SR. Pseudotumor cerebri in children. J AAPOS. 1998;2(1):33–8. [DOI] [PubMed] [Google Scholar]
- 28.Lim M, Kurian M, Penn A, Calver D, Lin JP. Visual failure without headache in idiopathic intracranial hypertension. Arch Dis Child. 2005;90(2):206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faz G, Butler IJ, Koenig MK. Incidence of papilledema and obesity in children diagnosed with idiopathic “benign” intracranial hypertension: case series and review. J Child Neurol. 2010;25(11):1389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Digre KB, Nakamoto BK, Warner JEA, Langeberg WJ, Baggaley SK, Katz BJ. A Comparison of Idiopathic Intracranial Hypertension With and Without Papilledema. Headache. 2009;49(2):185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999;127(2):178–82. [DOI] [PubMed] [Google Scholar]
- 32.Babiker MO, Prasad M, MacLeod S, Chow G, Whitehouse WP. Fifteen-minute consultation: the child with idiopathic intracranial hypertension. Arch Dis Child Educ Pract Ed. 2014;99(5):166–72. [DOI] [PubMed] [Google Scholar]
- 33.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–65. [DOI] [PubMed] [Google Scholar]
- 34.Wolf A, Hutcheson KA. Advances in evaluation and management of pediatric idiopathic intracranial hypertension. Curr Opin Ophthalmol. 2008;19(5):391–7. [DOI] [PubMed] [Google Scholar]
- 35.Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain Imaging in Idiopathic Intracranial Hypertension. J Neuroophthalmol. 2015;35(4):400–11. [DOI] [PubMed] [Google Scholar]
- 36.Gorkem SB, Doganay S, Canpolat M, Koc G, Dogan MS, Per H, et al. MR imaging findings in children with pseudotumor cerebri and comparison with healthy controls. Childs Nerv Syst. 2015;31(3):373–80. [DOI] [PubMed] [Google Scholar]
- 37.Avery RA, Shah SS, Licht DJ, Seiden JA, Huh JW, Boswinkel J, et al. Reference Range for Cerebrospinal Fluid Opening Pressure in Children. New Engl J Med. 2010;363(9):891–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MW, Vedanarayanan VV. Cerebrospinal fluid opening pressure in children: experience in a controlled setting. Pediatr Neurol. 2011;45(4):238–40. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser AM, Whitelaw AG. Normal cerebrospinal fluid pressure in the newborn. Neuropediatrics. 1986;17(2):100–2. [DOI] [PubMed] [Google Scholar]
- 40.Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuroophthalmol. 2004;24(2):138–45. [DOI] [PubMed] [Google Scholar]
- 41.Pollak L, Zohar E, Glovinsky Y, Huna-Baron R. Reevaluation of presentation and course of idiopathic intracranial hypertension--a large cohort comprehensive study. Acta Neurol Scand. 2013;127(6):406–12. [DOI] [PubMed] [Google Scholar]
- 42.Johnson LN, Krohel GB, Madsen RW, March GA, Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri). Ophthalmology. 1998;105(12):2313–7. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair AJ, Burdon MA, Nightingale PG, Ball AK, Good P, Matthews TD, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966;25(4):430–6. [DOI] [PubMed] [Google Scholar]
- 45.Ozge A, Bolay H. Intracranial hypotension and hypertension in children and adolescents. Curr Pain Headache Rep. 2014;18(7):430. [DOI] [PubMed] [Google Scholar]
- 46.Matthews YY. Drugs used in childhood idiopathic or benign intracranial hypertension. Arch Dis Childhood-E. 2008;93(1):19–25. [DOI] [PubMed] [Google Scholar]
- 47.Schoeman JF. Childhood pseudotumor cerebri: clinical and intracranial pressure response to acetazolamide and furosemide treatment in a case series. J Child Neurol. 1994;9(2):130–4. [DOI] [PubMed] [Google Scholar]
- 48.Thurtell MJ, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri): recognition, treatment, and ongoing management. Curr Treat Options Neurol. 2013;15(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Julayanont P, Karukote A, Ruthirago D, Panikkath D, Panikkath R. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesler A, Fattal-Valevski A. Idiopathic intracranial hypertension in the pediatric population. J Child Neurol. 2002;17(10):745–8. [DOI] [PubMed] [Google Scholar]
- 51.Bussière M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2010;31:645–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egan RJ, Meredith HE, Coulston JE, Bennetto L, Morgan JD, Norton SA. The effects of laparoscopic adjustable gastric banding on idiopathic intracranial hypertension. Obes Surg 2011;21(2):161–6. [DOI] [PubMed] [Google Scholar]
- 53.Fridley J, Foroozan R, Sherman V, Brandt ML, Yoshor D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg 2011;114(1):34–9. [DOI] [PubMed] [Google Scholar]


