Abstract
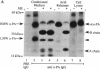
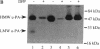
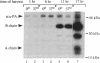
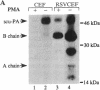
Transformed cells produce elevated levels of the urokinase-type plasminogen activator (u-PA), which has been linked with the invasive or migratory phenotype of these cells. The u-PA is secreted and normally maintained in the inactive, single-chain form (scu-PA) and it has been assumed that natural activation occurs via a plasmin-mediated cleavage converting scu-PA to the active, two-chain form (tcu-PA). We now demonstrate that secreted scu-PA in Rous sarcoma virus-transformed chicken embryo fibroblast (RSVCEF) cultures is activated by an endogenous, plasmin-independent mechanism. Normal CEFs and CEFs infected with a temperature-sensitive RSV mutant and incubated at the nonpermissive temperature do not activate scu-PA. Conditioned medium harvested from plasmin-free cultures of RSVCEFs contains active tcu-PA as determined by two independent methods. The scu-PA is progressively converted with time in culture and requires the presence of intact cells or a plasma membrane-enriched fraction. When added to RSVCEF cultures, a synthetic peptide corresponding to residues 20-41 of the growth factor domain of chicken u-PA blocks the conversion to tcu-PA, and scu-PA accumulates in the cultures. These results suggest that scu-PA is secreted by cells, becomes bound to a u-PA receptor, and is proteolytically converted to active tcu-PA by a catalytic mechanism on the surface of RSV-transformed fibroblasts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Ellis V., Scully M. F., Kakkar V. V. Plasminogen activation initiated by single-chain urokinase-type plasminogen activator. Potentiation by U937 monocytes. J Biol Chem. 1989 Feb 5;264(4):2185–2188. [PubMed] [Google Scholar]
- Fairbairn S., Gilbert R., Ojakian G., Schwimmer R., Quigley J. P. The extracellular matrix of normal chick embryo fibroblasts: its effect on transformed chick fibroblasts and its proteolytic degradation by the transformants. J Cell Biol. 1985 Nov;101(5 Pt 1):1790–1798. doi: 10.1083/jcb.101.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb R. H., Quigley J. P. Purification of plasminogen activator from Rous sarcoma virus transformed chick embryo fibroblasts treated with the tumor promoter phorbol 12-myristate 13-acetate. Biochemistry. 1980 Nov 25;19(24):5463–5471. doi: 10.1021/bi00565a001. [DOI] [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Leslie N. D., Kessler C. A., Bell S. M., Degen J. L. The chicken urokinase-type plasminogen activator gene. J Biol Chem. 1990 Jan 25;265(3):1339–1344. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Keski-Oja J., Moses H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988 May;106(5):1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Quigley J. P. Association of a protease (plasminogen activator) with a specific membrane fraction isolated from transformed cells. J Cell Biol. 1976 Nov;71(2):472–486. doi: 10.1083/jcb.71.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Berkenpas M. B., Aimes R. T., Chen J. M. Serine protease and metallo protease cascade systems involved in pericellular proteolysis. Cell Differ Dev. 1990 Dec 2;32(3):263–275. doi: 10.1016/0922-3371(90)90039-y. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Ossowski L., Reich E. Plasminogen, the serum proenzyme activated by factors from cells transformed by oncogenic viruses. J Biol Chem. 1974 Jul 10;249(13):4306–4311. [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane B. F., Rozhin J., Robinson D., Honn K. V. Role for cathepsin B and cystatins in tumor growth and progression. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):193–198. [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R. K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Tacchetti C., Cubellis M. V., Corti A., Hearing V. J., Cassani G., Appella E., Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986 Jun 6;45(5):675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Testa J. E., Quigley J. P. The role of urokinase-type plasminogen activator in aggressive tumor cell behavior. Cancer Metastasis Rev. 1990 Dec;9(4):353–367. doi: 10.1007/BF00049524. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]