Abstract
Nutrients (protein, carbohydrates and fats) have traditionally been thought of as fuels simply providing the energy for cellular metabolic activity. According to the classic view, if nutrients are available, then anabolic pathways are activated, and if nutrients are not available, catabolic pathways are activated. However, it is becoming increasingly clear that nutrient effects on bone cells (stem cells, osteoblasts and osteoclasts) are complex, some nutrients promote bone formation, whereas others interfere with bone formation or actually promote bone break down. At an organ level, nutrient intake can suppress bone breakdown and modulate the activity of the calcium/vitamin D/parathyroid hormone axis. At a cellular level, nutrient intake can impact cellular energetics either through a direct mechanism (binding or uptake of the nutrient into the cell) or indirect (by elevating nutrient-related hormones such as insulin, insulin-like growth factor 1 or incretin hormones). It is also becoming clear that within a nutrient class (for example, protein), individual components (that is, amino acids) can have markedly different effects on cell function and impact bone formation. The focus of this review will be on one nutrient class in particular, dietary protein. As the prevalence of inadequate dietary protein intake increases with age, these findings may have translational implications as to the optimal dietary protein content in the setting of age-associated bone loss.
Introduction
Although calcium and vitamin D are widely accepted as important nutrients for bone formation/maintenance, the impact of other nutrients on skeletal health is increasingly recognized. This recognition relates to our changing perception of the skeleton as mainly a calcium/phosphate reservoir serving a biomechanical function to an important endocrine/paracrine organ playing a role in the body's overall energy balance.1,2 It should be kept in mind though that widespread nutritional excess is a relatively recent phenomenon. Although many current studies focus on excess-associated diseases such as obesity and diabetes mellitus, the skeleton evolved in the setting of caloric restriction or intermittent nutrient intake.
Therefore, from the nutrient/bone perspective, there are three different scenarios that could potentially impact overall skeletal health: (1) total caloric intake, irrespective of dietary composition will impact the balance between bone formation vs breakdown; (2) physiological dietary intake, carbohydrates, fats or proteins impact bone turnover in different ways; (3) pathophysiological dietary intake; excessive dietary intake of carbohydrates, fats or protein and associated conditions such as diabetes mellitus and obesity can negatively impact bone mass.
Caloric intake effects on bone are complex, fasting promotes bone breakdown,3 moderate caloric intake inhibits bone breakdown4 and increased intake of high-quality nutrients promotes bone formation.5 Caloric restriction effects on bone appear to be time dependent with short-to-medium-term restriction leading to a decrease in cortical bone mass;3 however, continued caloric restriction in rodents past 1 year leads to a preservation of bone mass compared with controls.6
Acutely, nutrient ingestion (for example, glucose and amino acids) predominantly suppresses bone breakdown by inhibiting osteoclastic activity.4,7 Chronically, however, continued nutrient ingestion could increase bone formation by modulating osteoblastic activity/mineralization.8
In addition to macronutrients, a number of additional, trace elements, vitamins such as vitamin K and ions such as zinc have also been evaluated for potential roles in bone development and formation,9,10 but will not be discussed in detail here.
Although all macronutrients (dietary protein/fat/carbohydrate) impact bone turnover, this article will focus mainly on the effects of dietary protein on bone with only a brief discussion of the other nutrients.
Dietary carbohydrates
Carbohydrate effects on bone mass can be either through direct action on bone cells (osteoblasts and osteoclasts) or indirect by increasing hormones that lower blood sugar (for example, insulin and incretins), which themselves have effects on bone turnover.
Bone resorption by osteoclasts is dependent on acid secretion, which is an energy-dependent process as evidenced by the large number of mitochondria present in osteoclasts.11,12 Glucose increases bone resorption dose-dependently between 0.1 and 7 mM, whereas fatty acids and essential amino acids do not increase bone resorption,12 in fact some amino acids can inhibit osteoclastic resorption.13
Osteoblast function is also modulated by glucose. An endocrine regulatory loop has been proposed where the osteoblast-related protein, osteocalcin, is released into the circulation and stimulates insulin secretion, which in turn promotes glucose utilization by osteoblasts and bone formation.8 Elevations of glucose lead to an increase in osteoblastic differentiation and mineralization. Immature osteoblasts have been reported to have insulin receptors and glucose transporters (GLUTs 1, 2 and 4) with GLUT 4 expression increasing with osteoblast maturity.2 Glucose appears to be the main substrate utilized by these cells and anabolic agents such as parathyroid hormone (PTH) increase aerobic glycolysis.14 Glucose in addition to serving as important energy source for the osteoblast also aids this process by generating reactive oxygen species (ROS). Osteoblast mineralization is facilitated by the generation of ROS as evidenced by the fact that an excess of antioxidants such as N-acetyl-cysteine can inhibit mineralization.15 Interestingly, although dietary ingestion of proteins, fatty acids and carbohydrates all generate ROS, it is carbohydrates that generate the most ROS.16,17 Thus, from an integrative physiological perspective, an elevation of blood glucose would lead to the increased insulin secretion, which together with glucose promotes osteoblastic differentiation/mineralization. Increased glucose also increases osteoclastic activity, which increases undercarboxylated osteocalcin further promoting insulin release and synergistically promoting osteoblast differentiation (Figure 1). The control point to avoid this being a continuous positive feedback loop that resulted in hypoglycemia is serum glucose,18 as serum glucose drops insulin secretion is inhibited. Thus, osteoblastic mineralization would only occur when glucose was elevated and inhibited as blood glucose and insulin levels dropped. A corollary to this statement is that in situations when blood glucose and insulin remained high (DM type II) then increased bone mineralization could be observed.19
Figure 1.
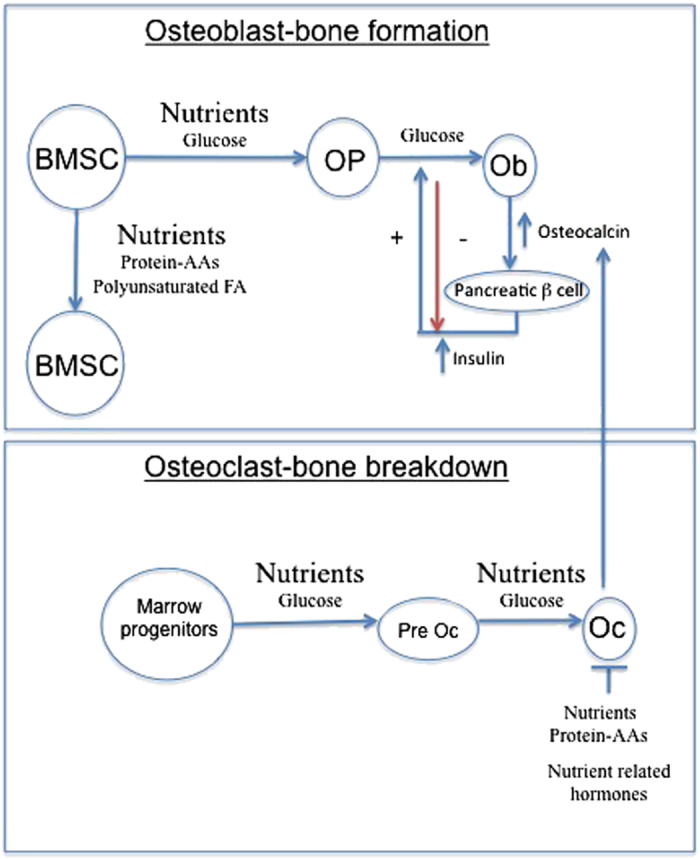
Nutrients impact multiple cell types in bones. Nutrients can modulate bone marrow stem cell function, osteoblast differentiation and osteoclast activity. Glucose has direct effects on osteoclast and osteoblasts, and indirect effects by increasing nutrient-related hormones such as insulin and incretin hormones. Amino acids and polyunsaturated fatty acids (n-3 PUFA in particular) can modulate MSC function.
In contrast to osteoblasts, mesenchymal stem cells (MSCs) are not greatly impacted by glucose unless it is in excess.20 With respect to MSCs, the data would suggest that protein/amino acids and fatty acids have a more important role that glucose in promoting stem cell proliferation (discussed below). Thus, the impact/role of nutrients on bone cell function would vary as these cells underwent proliferation vs differentiation.
Glucose also has a number of indirect effects on bone turnover. Multiple nutrient-related hormones have been implicated in meal-related inhibition of bone resorption including glucose-dependent insulinotropic peptide (GIP), GLP2 and insulin.7,21,22 In human studies, both glucose and protein were found to significantly decrease bone breakdown, whereas nutrient ingestion was not found to affect bone formation (as assessed by measuring osteocalcin levels).23 In another human study, in which oral glucose also significantly reduced bone breakdown (as assessed by decreased collagen fragments, C-telopeptide), this glucose-dependent suppression was abolished by a somatostatin analog. These findings suggest that somatostatin blocked the secretion of a nutrient-induced hormone, which was responsible for suppressing bone breakdown (that is, nutrient effects were indirect).24 Insulin infusion has also been found to modestly decrease bone breakdown (∼30% of the decline in resorption markers that occurs post-prandially).23 Data from our laboratory and from others suggest that the acute suppression of bone turnover may be mediated in part by incretin hormones such as GIP and glucagon-like peptide 2 (GLP2).7,21 Our group has coined the term ‘entero-osseous' axis to describe this close association between nutrient ingestion and bone turnover. In essence, during fasting, bone resorption occurs and the skeleton is a source of ions and minerals, and during times of nutrient ingestion, catabolic activity is decreased and anabolic activity increases related to the type and quantity of nutrients ingested.
Dietary fat
Similar to the negative effects of high glucose on bone, high-fat diets lead to lower bone mass, possibly through decreased differentiation of osteoblasts from MSCs, markers of bone formation are reduced as is the anabolic hormone insulin-like growth factor 1 (IGF-1).25 In addition, high-fat diets may increase osteoclastic bone resorption contributing to the loss of bone mineral density observed with these diets.26 High-fat diets are associated with an increase in inflammatory cytokines, which might have a role in subsequent bone loss. Overall, the net effect of obesity on bone health appears to be negative despite it being associated with higher bone mass.27 Evolutionarily obesity-related bone problems were not widespread until relatively recently. Much of the available research on the physiological role fats and fatty acids have in normal bone turnover has focused on polyunsaturated fatty acids (PUFAs), in particular comparing n-6 vs n-3 PUFA. In animal models, increasing dietary n-3 and decreasing n-6 PUFA probably has a beneficial effect on bone density and strength.28 In humans, there is limited clinical trial data to support a beneficial effect of n-3 PUFA.29 However, dietary n-3 PUFA (for example, eicosapentaenoic and docosahexaenoic acids) have anti-inflammatory properties, which could be beneficial for increasing bone mass.30,31
In addition to the direct effects that lipids may have on bone cells, adipocytes also secrete many adipokines and cytokines that can also modulate bone turnover including leptin, adiponectin, visfatin, omentin and fibroblast growth factor 21.32 Effects of some of these adipokines on bone metabolism may be complex and for some like leptin may relate to whether the effects are central33 or peripheral.34,35 The rest of this discussion however will focus on protein effects on bone.
Protein
High-protein diets have long been thought to have a negative influence on bone mass. The rationale for this belief was that the high-protein intake would necessitate bone dissolution to buffer the acid load. Evidence provided for this idea was that high-protein diets induced an increase in urinary calcium, presumably a result of increased bone breakdown. However, recent studies have led us to change our perspective on the impact of dietary protein on bone mass.36,37 Low-protein diets (<0.8 g kg−1) are associated with low intestinal calcium absorption and thus induce secondary hyperparathyroidism with elevations in PTH and 1,25 dihydroxyvitamin D.38 High-protein diets (>2 g kg−1) actually increase intestinal calcium absorption (the increased urinary calcium reflects absorption rather than resorption38) and these subjects have low PTH and 1,25 dihydoxyvitamin D values. The impact of dietary protein on urinary calcium excretion increases in a linear manner between low- and high-protein intake such that on average every 50 g increase in protein intake results in a 1.6 mmol increase in 24-h urinary calcium excretion.39 Thus, average dietary protein intakes (representative of up to 50% of the US population) would not appear to have any impact on calcium or bone turnover.
Interestingly, in addition to the direct effects of protein intake on skeletal mass, dietary protein may also modulate other anabolic signals for bone. In a study by Chevalley et al.40 where dietary protein and calcium intakes, physical activity and bone mineral content (BMC) were assessed in 232 pre pubertal boys, the anabolic effects of physical activity on BMC were enhanced in those subjects with higher median dietary protein intake. These beneficial effects of higher-protein intake and physical activity on BMC seem to persist and lead to beneficial microstructural changes in bone as assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT).41
In contrast, in studies where other dietary factors are controlled, low dietary protein intake has been consistently shown to be associated with both lower bone mineral density42 and higher rates of bone loss.43 Further, in a case–control study examining over 2500 male and female subjects divided according to their dietary protein intake (low to high by quartiles), the adjusted odds ratio of having a hip fracture for subjects between 50 and 69 years of age consuming the highest percentage of dietary protein was only 0.35 (95% confidence interval: 0.21–0.59; P<0.001). Thus, a high-protein diet was linked to a very significant drop in hip fractures in this age group.44 Dietary protein requirements increase with age and the prevalence of dietary protein insufficiency increases particularly in those over 70 years of age and in the institutionalized individuals.45 Further, an interesting study by Amman et al.46 showed that low dietary protein intakes could also impair the normal anabolic response on bone mass of PTH injections in a rodent model. This report would suggest that decreased dietary protein intake could potentially negatively affect the response of anabolic agents used in the treatment of osteoporosis.
There are also data that support a negative association between low total caloric intake and low bone mass. Thus, a caloric supplement (300 additional calories, predominantly fat and carbohydrate) was found to prevent bone loss but not to increase bone mass or stimulate bone formation in elderly (⩾70 years old) undernourished (body mass index⩽21) female patients.47
A study by Elefteriou et al.48 provides molecular evidence supporting the importance of protein (amino acids) in bone formation. In this study, ATF4, a transcription factor that stimulates amino-acid uptake and collagen synthesis in osteoblasts, was not activated in patients with Coffin–Lowry syndrome (CLS, patients with RSK2 mutations). CLS patients have a skeletal phenotype (kyphoscoliosis, pectus carinatum, short stature and so on) and, at least in the CLS mouse models (ATF4−/− or RSK2−/−), this skeletal phenotype is reversed by feeding the mice a high-protein diet. The increase in ambient amino acids from high dietary proteins apparently compensates for the decreased osteoblast amino-acid uptake (from failure to activate ATF4) and corrects the phenotype. Increased amino-acid uptake directly increases collagen type I synthesis and osteocalcin expression, the latter suggesting that amino acids themselves can promote osteoblastic differentiation.
Protein can also have indirect effects on bone mass by raising anabolic hormones such as insulin and IGF-1. Gluconeogenic amino acids (particularly alanine and glutamine) increase insulin secretion without any significant change in plasma glucose.49,50 With respect to IGF-1, aromatic amino acids appear to be the most potent in stimulating IGF-1 secretion, in contrast to branched-chain amino acids.51,52 In addition, protein ingestion also increases release of incretin hormones, which themselves have anabolic effects on bone.53,54
Direct Cellular Effects of Amino Acids
Studies going back to as early as 1920 identified a relationship between protein intake and calcium homeostasis,55 but the mechanism was not known. Dietary protein is important in the regulation of the parathyroid gland as evidenced by the elevation of parathyroid hormone in absence of adequate dietary protein.56
The CaSR is a G-protein-coupled receptor expressed in the parathyroid cells that modulates PTH secretion in response to extracellular calcium concentration. However, the CaSR is known to be expressed in other tissues including the intestines and the kidneys,57 and has also been shown to bind L-amino acids. Aromatic amino acids serve as the most potent activators of these receptors (Table 1), though most amino acids besides basic and branch-chain amino acids elicit a response.58 Not only does amino-acid activation of the CaSR influence the parathyroid gland and PTH secretion but it also influences the rate of calcium urinary excretion. Earlier studies have shown that high-protein intakes lead to increased calcium excretion.59 High plasma levels of amino acids result in the increased activation of basolateral CaSRs in the cortical thick ascending limb resulting in suppression of calcium reabsorption and increased calcium excretion.60 The influence of amino acids on renal calcium homeostasis further indicates the importance of amino-acid signaling.
Table 1. Common amino acids.
| Family | Aliphatic | Aromatic | Basic | Acidic | Sulfur containing | Hydroxyl containing |
|---|---|---|---|---|---|---|
| Alanine | Tyrosine | Arginine | Aspargine | Cysteine | Serine | |
| Leucine (BCAA) | Tryptophan | Histidine (aromatic) | Aspartic acid | Methionine | Threonine | |
| Isoleucine (BCAA) | Phenylalanine | Lysine | Glutamic acid | |||
| Valine (BCAA) | ||||||
| Proline | ||||||
| Glycine |
The CasR has also been shown to be present in bone marrow cells61 in addition to cartilage and bone (osteoblasts) cells.62 However, other investigators63 have failed to find CasR messenger RNA (mRNA) expression in osteoblastic cell lines (ROS 17/2.8 or MC3T3-E1). Interestingly, even though these investigators failed to detect CasR expression in osteoblasts, they found that they functionally responded as if the CasR were present. Thus, in the preosteoblastic cell line MC3T3-E1, the addition of calcium, gadolinium, aluminum and neomycin (activators of CasR) increased DNA synthesis even though no CasR mRNA was detected in these cells.63 Thus, these authors proposed that a molecularly distinct but functionally similar extracellular calcium sensor was present in osteoblasts. In a follow-up paper,64 these authors found that osteoblasts derived from CasR knockout mice were still able to respond to divalent cations, suggesting that additional mechanisms existed in these cells for sensing changes in extracellular calcium. These authors have also suggested that the GPRC6A receptor is the receptor that they have identified as being important as a divalent cation sensor because mice in which GPRC6A has been knocked out have impaired mineralization.65 Interestingly, the GPRC6A receptor may also be the osteocalcin receptor.66,67 Thus, in certain cells, individual amino acids could be binding to specific extracellular receptors and acting as a ‘hormone' rather than as a nutrient. Amino acids can thus exert direct effects on several tissues where the CasR is expressed including the pancreatic islet and liver, and have a role in energy balance.68 In summary, the cellular mechanisms mediating these direct AA effects fall into three general categories (Figure 2): (1) calcium receptors: L-type amino acids have been shown to modulate several members of the class 3 G-protein receptor superfamily. Members of this family with amino acid binding include the CasR, the taste receptors T1R1 and T1R3, and member 6A of the G-protein-coupled receptor family C, GPRC6A.68 It has been suggested that the T1Rs (T1R1 and T1R3 or umami taste receptor) developed evolutionarily, as a means of detecting amino acids in the diet. Thus, sweet and umami taste receptors promote ingestion of beneficial nutrients, whereas many of the toxic compounds have a bitter taste. T1R knockout mice lose all appetite for amino acids (dietary protein)69; (2) CAT proteins (cationic amino-acid transporter): these transport proteins are widely expressed and belong to the solute carrier family 7 (SLC7) with four members CAT-1, 2A, 2B and 3. All these members have similar affinity for cationic L-amino acids, which are transported in a sodium-independent manner. Once taken up by the cells, amino acids are utilized in a variety of cellular processes and help to regulate cellular energy balance. The metabolism of these AA alters the AMP/ATP ratio. Adenosine 5′-monophosphate-activated protein kinase (AMPK) consists of three subunits (a-, b- and g-subunits) and serves as a cellular ‘energy sensor'. When ATP levels drop (and AMP levels rise), AMPK is activated and serves to activate catabolic pathways and inhibit anabolic pathways.70 Thus, AA uptake by the cell results in an increase in ATP levels and an inhibition of AMPK; and (3) amino acids can also have indirect effects on amino acids by modulating the hormonal production of (for example, arginine stimulation of nitric oxide production) or because of the acid load changing the local pH. Although these effects do occur, they are not felt to be a major mechanism of AA action.
Figure 2.
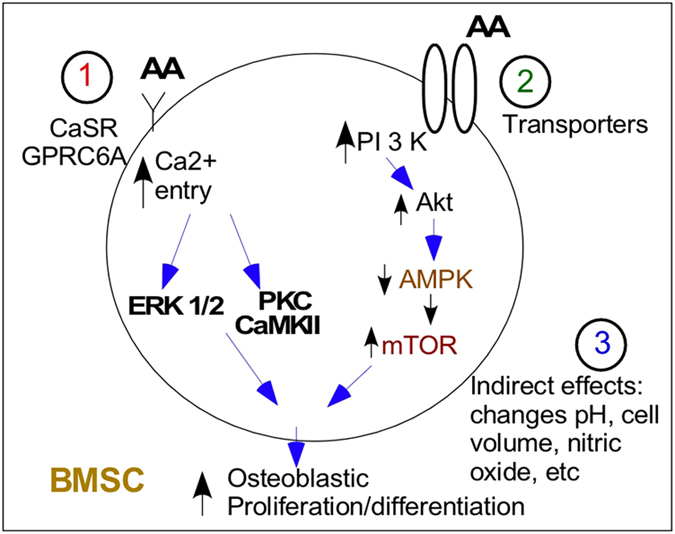
Direct and indirect amino-acid effects on BMSC function. Amino acids can (1) bind to the calcium receptor decrease the threshold for activation of this receptor by calcium or induce calcium oscillations; (2) be taken up directly by a series of cation transporters where they affect energy balance or mitochondrial function; or (3) have indirect effects by increasing production of, for example, nitric oxide (with arginine uptake), induce changes in cell pH or by being exchanged with sodium (sodium/AA transporter) alter cell swelling, cytoskeleton and so on.
Effects of arginine and taurine
Although arginine is not in the earlier discussed category of aromatic amino acids, arginine has an impact on both osteoblastic differentiation and osteogenesis. First, arginine has been shown to increase IGF-1 in mouse osteoblast-like cells and may directly or indirectly increase bone formation by increased IGF-1 production. Treatment with arginine also increased alkaline phosphatase activity and collagen synthesis in these same cells.71 More recently, the role of arginine in shifting BMSC differentiation away from adipocytic differentiation and towards osteoblastic differentiation has been explored.
In the bone marrow, runt-related transcription factor 2 (Runx2), Dlx5 and osterix promote osteogenesis through osteoblastic differentiation, whereas fatty acid-binding protein 4 (Fabp4), CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor-gamma-2 promote (PPARγ2) adipogenesis.72 The Wnt signaling pathway has an important role in the balance of adipogenesis and osteoblastogenesis in BMSCs. Wnt signaling can occur through a canonical pathway in which Runx2 is upregulated and PPARγ downregulated.73 In the non-canonical pathway, Wnt signaling results in the binding of the nuclear factor of activated T cells (NFATc). NFATc have been shown to enhance osteoblast proliferation as well as osteoclastogenesis.74 Arginine stimulates osteogenesis from MSCs by increasing mRNA levels of Runx2, Dlx5 and osterix as well as the induction of the osteogenic markers type Iα, osteocalcin and ALP.75 Not only does arginine promotes osteogenesis but also decreases adipocytic differentiation of MSCs by decreasing the expression of C/EBPα, PPARγ and Fabp4. Arginine accomplishes this shift in MSCs towards osteoblastogenesis by stimulation of the nan-cononical Wnt signaling pathway and by NFATc1 activation.75
Although taurine is not technically classified as an amino acid due to a lack of a carboxyl group, but is a derivative of cysteine and thus remains relevant in this discussion. Taurine is widely involved in many cellular processes and has an established effect on osteoblast-like cells that includes the stimulation of ALP activity and collagen synthesis as well as the inhibition of osteoclastogenesis.76,77 Recently, taurine has been found to promote MSC by upregulation of the ERK pathway. Taurine significantly increased osteogenesis through the activation of ERK1/2 and resulting the upregulation of Runx2, Osx and ALP.78 Taurine serves as another example of the role of protein nutrition on differentiation of MSCs.
Amino-Acid Effect on Osteoclastic Differentiation
Our laboratory recently showed the direct effect of amino acids on osteoclast differentiation and activity, which determines the rate of bone breakdown. Real-time PCR was used to analyze the response of various osteoclastic differentiation markers in response to amino acids with a focus on aromatic amino acids.
Early markers of osteoclast differentiation including vitronectin, calcitonin and carbonic anhydrase II were all found to be downregulated by aromatic amino acids, indicating that aromatic amino acids could inhibit bone resorption through this mechanism. Gene expression of the late markers of osteoclastic differentiation, matrix metalloproteinase 9 and cathepsin K was similarly downregulated. This indicates that the aromatic amino acids influence and likely suppress the attachment of osteoclasts and the formation of the resorption site resulting in the increase in bone mass seen with aromatic amino-acid supplementation.13
Amino-Acid Impact on MSCs in an Aging Model
Our laboratory also evaluated the effect of oxygen tension on MSC signaling pathways. Oxygen tension in the bone marrow niches in which the MSCs normally reside is low (1–3%). Under these hypoxic conditions that in fact more closely resemble normal ‘young' physiologic conditions, stimulation with aromatic amino acids had little effect on MSC pro-survival markers (Akt, STAT3, CAV1 or NOTCH1). In contrast, if oxygen tension is raised (21%), aromatic amino acids such as tyrosine and tryptophan induce increased expression pro-survival proteins in MSCs. With aging, there is niche breakdown that leads to an increase in oxidative stress, suggesting that ‘normoxic' conditions might be a more appropriate model for aged MSCs.79 Aromatic amino acids have been shown to function as antioxidants in physiologically relevant concentrations. These finding would support a protective role for aromatic amino acids against the increased oxidative stress in MSCs caused by aging.
Presumably under conditions of oxidative stress, these aromatic amino acids would be oxidized and result in an accumulation of their oxidation products in the marrow. A recent study by our laboratory examined the effect of these AA oxidation products on MSC function. In particular, tryptophan and its oxidations products were examined because it is the only amino acid to decline significantly (14%) with increasing age.80 This drop in tryptophan levels with aging has been associated with an increase in kynurenine levels, an oxidation product of tryptophan.81 We found that while tryptophan enhanced MSC proliferation, kynurenine had the opposite effect, significantly inhibiting MSC proliferation,82 suggesting a possible mechanism for age-related loss of bone mass.
Summary and Research Questions
In summary, data from our lab and others support a role for distinct nutrient effects at different stages of cell differentiation. Glucose is an important nutrient for osteoclast activity and osteoblast differentiation. In contrast, protein and PUFA appear to also have a role in MSC proliferation and function. Because of the higher caloric and or nutritional quality of these nutrients, they serve as anabolic signals for bone growth (Figure 1). Further, as a nutrient, protein's role goes beyond simple energy provision as the amino acids that compose dietary proteins serve a distinct role in several signaling pathways in the maintenance and protection of bone. As the roles of dietary protein and amino acids in nutrition and osteogenesis are further defined, new therapeutic agents for the treatment of osteoporosis and specific dietary recommendations for prevention of bone loss may be discovered and established. The different subclasses of amino acids have varying effects in the body, particularly the aromatic amino acids and the branched-chain amino acids. The differing effects of the subtypes of amino acids may lead to observations about the differing effects of protein intake that varies according to the source of this protein.83 Thus, in addition to increasing the protein intake in the elderly, paying attention to the amino-acid composition of the dietary supplements may be relevant to the prevention of age-associated osteoporosis. Finally, the best way to administer dietary protein also needs to be clarified. In aging studies, intermittent fasting provides many of the benefits in as much as longevity as continuous caloric restriction. Historically, protein was generally ingested by humans in an intermittent rather than in a daily manner. In fact, if some amino acids are truly acting as ‘hormones' and binding to extracellular receptors then providing amino acids in a continuous manner is more likely to lead to receptor downregulation and loss of a beneficial effect. Thus, further studies are needed to clarify the timing of dietary protein intake.
Acknowledgments
Funding for our research was provided by the National Institute on Aging (P01 AG036675).
Footnotes
The authors declare no conflict of interest.
References
- Riddle RC, Frey JL, Tomlinson RE, Ferron M, Li Y, DiGirolamo DJ et al. Tsc2 is a molecular checkpoint controlling osteoblast development and glucose homeostasis. Mol Cell Biol 2014; 34: 1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Riddle RC, Clemens TL. Bone and the regulation of global energy balance. J Intern Med 2015; 277: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res 2008; 23: 870–878. [DOI] [PubMed] [Google Scholar]
- Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 2002; 30: 886–890. [DOI] [PubMed] [Google Scholar]
- Bonjour JP. Dietary protein: an essential nutrient for bone health. J Am Coll Nutr 2005; 24: (6 Suppl): 526S–536S. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology 2008; 149: 634–641. [DOI] [PubMed] [Google Scholar]
- Clowes JA, Khosla S, Eastell R. Potential role of pancreatic and enteric hormones in regulating bone turnover. J Bone Miner Res 2005; 20: 1497–1506. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res 2011; 26: 677–680. [DOI] [PubMed] [Google Scholar]
- Berger PK, Pollock NK, Laing EM, Chertin V, Bernard PJ, Grider A et al. Zinc supplementation increases procollagen type 1 amino-terminal propeptide in premenarcheal girls: a randomized controlled trial. J Nutr 2015; 145: 2699–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman KD, O'Connor E. Does high vitamin K1 intake protect against bone loss in later life? Nutr Rev 2008; 66: 532–538. [DOI] [PubMed] [Google Scholar]
- Larsen KI, Falany M, Wang W, Williams JP. Glucose is a key metabolic regulator of osteoclasts; glucose stimulated increases in ATP/ADP ratio and calmodulin kinase II activity. Biochem Cell Biol 2005; 83: 667–673. [DOI] [PubMed] [Google Scholar]
- Williams JP, Blair HC, McDonald JM, McKenna MA, Jordan SE, Williford J et al. Regulation of osteoclastic bone resorption by glucose. Biochem Biophys Res Commun 1997; 235: 646–651. [DOI] [PubMed] [Google Scholar]
- Refaey ME, Zhong Q, Ding KH, Shi XM, Xu J, Bollag WB et al. Impact of dietary aromatic amino acids on osteoclastic activity. Calcif Tissue Int 2014; 95: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen E, Long F. Aerobic glycolysis in osteoblasts. Curr Osteoporos Rep 2014; 12: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki N, Yamashita A, Niimi S, Yamazaki T. Involvement of reactive oxygen species in osteoblastic differentiation of MC3T3-E1 cells accompanied by mitochondrial morphological dynamics. Biomed Res 2013; 34: 161–166. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr 2002; 75: 767–772. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000; 85: 2970–2973. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Zawalich KC, Ganesan S, Calle R, Zawalich WS. Physiology and pathophysiology of insulin secretion. Diabetes Care 1990; 13: 655–666. [DOI] [PubMed] [Google Scholar]
- Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 2016; 82: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, Schneider D et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun 2007; 363: 209–215. [DOI] [PubMed] [Google Scholar]
- Reid IR, Cornish J, Baldock PA. Nutrition-related peptides and bone homeostasis. J Bone Miner Res 2006; 21: 495–500. [DOI] [PubMed] [Google Scholar]
- Yavropoulou MP, Yovos JG. Incretins and bone: evolving concepts in nutrient-dependent regulation of bone turnover. Hormones (Athens) 2013; 12: 214–223. [DOI] [PubMed] [Google Scholar]
- Clowes JA, Robinson RT, Heller SR, Eastell R, Blumsohn A. Acute changes of bone turnover and PTH induced by insulin and glucose: euglycemic and hypoglycemic hyperinsulinemic clamp studies. J Clin Endocrinol Metab 2002; 87: 3324–3329. [DOI] [PubMed] [Google Scholar]
- Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab 2003; 88: 4867–4873. [DOI] [PubMed] [Google Scholar]
- Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A et al. Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res 2010; 25: 275–284. [DOI] [PubMed] [Google Scholar]
- Shu L, Beier E, Sheu T, Zhang H, Zuscik MJ, Puzas EJ et al. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int 2015; 96: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res 2011; 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BY, Cohen DJ, Ward WE, Ma DW. Investigating the role of polyunsaturated fatty acids in bone development using animal models. Molecules 2013; 18: 14203–14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard TS, Pan X, Cheek F, Ing SW, Jackson RD. A systematic review of omega-3 fatty acids and osteoporosis. Br J Nutr 2012; 107(Suppl 2): S253–S260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly OJ, Gilman JC, Kim Y, Ilich JZ. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res 2013; 33: 521–533. [DOI] [PubMed] [Google Scholar]
- Kruger MC, Coetzee M, Haag M, Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res 2010; 49: 438–449. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci 2015; 36: 461–470. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000; 100: 197–207. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int 2008; 19: 905–912. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004; 34: 376–383. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Layman DK. Amount and type of protein influences bone health. Am J Clin Nutr 2008; 87: 1567S–1570S. [DOI] [PubMed] [Google Scholar]
- Rizzoli R. Nutritional aspects of bone health. Best Pract Res Clin Endocrinol Metab 2014; 28: 795–808. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 2005; 90: 26–31. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr 2003; 78: (3 Suppl): 584S–592S. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J Bone Miner Res 2008; 23: 131–142. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Bonjour JP, van Rietbergen B, Ferrari S, Rizzoli R. Tracking of environmental determinants of bone structure and strength development in healthy boys: an eight-year follow up study on the positive interaction between physical activity and protein intake from prepuberty to mid-late adolescence. J Bone Miner Res 2014; 29: 2182–2192. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, Looker AC, Insogna KL. Low dietary protein and low bone density. Calcif Tissue Int 2000; 66: 313. [DOI] [PubMed] [Google Scholar]
- Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000; 15: 2504–2512. [DOI] [PubMed] [Google Scholar]
- Wengreen HJ, Munger RG, West NA, Cutler DR, Corcoran CD, Zhang J et al. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res 2004; 19: 537–545. [DOI] [PubMed] [Google Scholar]
- Surdykowski AK, Kenny AM, Insogna KL, Kerstetter JE. Optimizing bone health in older adults: the importance of dietary protein. Aging Health 2010; 6: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann P, Zacchetti G, Gasser JA, Lavet C, Rizzoli R. Protein malnutrition attenuates bone anabolic response to PTH in female rats. Endocrinology 2015; 156: 419–428. [DOI] [PubMed] [Google Scholar]
- Hampson G, Martin FC, Moffat K, Vaja S, Sankaralingam S, Cheung J et al. Effects of dietary improvement on bone metabolism in elderly underweight women with osteoporosis: a randomised controlled trial. Osteoporos Int 2003; 14: 750–756. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab 2006; 4: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M, Brehm A, Krssak M, Anderwald C, Bernroider E, Nowotny P et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 2003; 46: 917–925. [DOI] [PubMed] [Google Scholar]
- Rietman A, Schwarz J, Tome D, Kok FJ, Mensink M. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr 2014; 68: 973–979. [DOI] [PubMed] [Google Scholar]
- Bihuniak JD, Insogna KL. The effects of dietary protein and amino acids on skeletal metabolism. Mol Cell Endocrinol 2015; 410: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Rasmussen HM, Dallal GE. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int 2007; 18: 955–961. [DOI] [PubMed] [Google Scholar]
- Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF et al. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab 2008; 295: E779–E784. [DOI] [PubMed] [Google Scholar]
- Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD et al. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 2006; 147: 3173–3180. [DOI] [PubMed] [Google Scholar]
- Sherman HC. Calcium requirement of maintenance in man. J Biol Chem 1920; 44: 21–27. [Google Scholar]
- Kerstetter JE, Caseria DM, Mitnick ME, Ellison AF, Gay LF, Liskov TA et al. Increased circulating concentrations of parathyroid hormone in healthy, young women consuming a protein-restricted diet. Am J Clin Nutr 1997; 66: 1188–1196. [DOI] [PubMed] [Google Scholar]
- Quarles LD. Extracellular calcium-sensing receptors in the parathyroid gland, kidney, and other tissues. Curr Opin Nephrol Hypertens 2003; 12: 349–355. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Mun HC, Lok HC. Aromatic L-amino acids activate the calcium-sensing receptor. J Nutr 2007; 137: (6 Suppl 1): 1524S–1527S; discussion 48S. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, Allen LH. Protein intake and calcium homeostasis. Adv Nutr Res 1994; 9: 167–181. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr 2002; 56: 1072–1080. [DOI] [PubMed] [Google Scholar]
- House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res 1997; 12: 1959–1970. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt SA et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 1999; 140: 5883–5893. [DOI] [PubMed] [Google Scholar]
- Quarles LD, Hartle JE 2nd, Siddhanti SR, Guo R, Hinson TK. A distinct cation-sensing mechanism in MC3T3-E1 osteoblasts functionally related to the calcium receptor. J Bone Miner Res 1997; 12: 393–402. [DOI] [PubMed] [Google Scholar]
- Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD. Sensing of extracellular cations in CasR-deficient osteoblasts. Evidence for a novel cation-sensing mechanism. J Biol Chem 2000; 275: 3256–3263. [DOI] [PubMed] [Google Scholar]
- Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 2008; 3: e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res 2011; 26: 1680–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone 2016; 82: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave AD, Hampson DR. Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol Metab 2006; 17: 398–407. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 2003; 112: 293–301. [DOI] [PubMed] [Google Scholar]
- Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab 2006; 17: 205–215. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Rizzoli R, Manen D, Caverzasio J, Bonjour JP. Arginine increases insulin-like growth factor-I production and collagen synthesis in osteoblast-like cells. Bone 1998; 23: 103–109. [DOI] [PubMed] [Google Scholar]
- Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 2009; 5: 442–447. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional regulation of osteoblasts. Ann NY Acad Sci 2007; 1116: 196–207. [DOI] [PubMed] [Google Scholar]
- Winslow MM, Pan M, Starbuck M, Gallo EM, Deng L, Karsenty G et al. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell 2006; 10: 771–782. [DOI] [PubMed] [Google Scholar]
- Huh JE, Choi JY, Shin YO, Park DS, Kang JW, Nam D et al. Arginine enhances osteoblastogenesis and inhibits adipogenesis through the regulation of Wnt and NFATc signaling in human mesenchymal stem cells. Int J Mol Sci 2014; 15: 13010–13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LQ, Liu W, Cui RR, Wang D, Meng JC, Xie H et al. Taurine inhibits osteoclastogenesis through the taurine transporter. Amino Acids 2010; 39: 89–99. [DOI] [PubMed] [Google Scholar]
- Yuan LQ, Xie H, Luo XH, Wu XP, Zhou HD, Lu Y et al. Taurine transporter is expressed in osteoblasts. Amino Acids 2006; 31: 157–163. [DOI] [PubMed] [Google Scholar]
- Zhou C, Zhang X, Xu L, Wu T, Cui L, Xu D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids 2014; 46: 1673–1680. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free Radic Res 2006; 40: 1250–1258. [DOI] [PubMed] [Google Scholar]
- Caballero B, Gleason RE, Wurtman RJ. Plasma amino acid concentrations in healthy elderly men and women. Am J Clin Nutr 1991; 53: 1249–1252. [DOI] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J 2011; 278: 4425–4434. [DOI] [PubMed] [Google Scholar]
- El Refaey M, Watkins CP, Kennedy EJ, Chang A, Zhong Q, Ding KH et al. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol Cell Endocrinol 2015; 410: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsetmo L, Barr SI, Berger C, Kreiger N, Rahme E, Adachi JD et al. Associations of protein intake and protein source with bone mineral density and fracture risk: a population-based cohort study. J Nutr Health Aging 2015; 19: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]


