Abstract
Aromatic and medicinal plants produce essential oils in the form of secondary metabolites. These essential oils can be used in diverse applications in food, perfume, and cosmetic industries. The use of essential oils as antimicrobials and food preservative agents is of concern because of several reported side effects of synthetic oils. Essential oils have the potential to be used as a food preservative for cereals, grains, pulses, fruits, and vegetables. In this review, we briefly describe the results in relevant literature and summarize the uses of essential oils with special emphasis on their antibacterial, bactericidal, antifungal, fungicidal, and food preservative properties. Essential oils have pronounced antimicrobial and food preservative properties because they consist of a variety of active constituents (e.g., terpenes, terpenoids, carotenoids, coumarins, curcumins) that have great significance in the food industry. Thus, the various properties of essential oils offer the possibility of using natural, safe, eco-friendly, cost-effective, renewable, and easily biodegradable antimicrobials for food commodity preservation in the near future.
Keywords: essential oils, antibacterial, antifungal, food preservative properties, bioactivity
Introduction
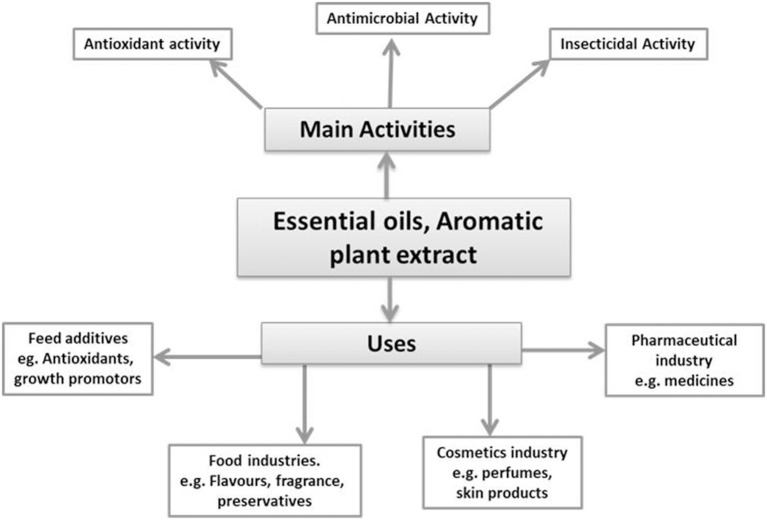
Since ancient times, commercial antimicrobial agents have been applied as a way to manage food deterioration or contamination. Nowadays, user concerns toward synthetic preservatives have resulted in increasing attention on various natural antimicrobials such as essential oils. Aromatic and medicinal plant essential oils and their components demonstrate antibacterial, antifungal, and food preservative activities against a wide range of microbial pathogens (Basim et al., 2000; Iacobellis et al., 2004; Tripathi and Kumar, 2007; Pandey et al., 2014b; Sonker et al., 2015; Gormez et al., 2016; Figure 1). These essential oils are hydrophobic liquids of aromatic compounds that are volatile and oily in nature and present in various plant parts such as twig, flower, leaf, bark, seed, and root. Many plant essential oils are useful as a flavor or aroma enhancer in cosmetics, food additives, soaps, plastics resins, and perfumes. Moreover, curiosity about essential oil applications that can act as antimicrobial agents is growing because of the broad range of activities, natural origins, and generally recognized as safe (GRAS) status of essential oils. Currently, essential oils are frequently studied for their antimicrobial (Cowan, 1999; Burt, 2004; Nedorostova et al., 2009), antifungal (Singh and Tripathi, 1999), antiulcer (Dordevic et al., 2007), antihelminthic (Inouye et al., 2001), antioxidant (Mimica-Dukic et al., 2003), anti-inflammatory (Singh et al., 1996), repellent, insecticidal, antifeedant (Isman et al., 1990; Pandey et al., 2014a), cytotoxic (Sylvestre et al., 2007), antiviral (Maurya et al., 2005), ovicidal (Pandey et al., 2011b), anesthetic (Ghelardini et al., 2001), molluscicidal (Fico et al., 2004), immunomodulatory (Mediratta et al., 2002), antinociceptive (Abdollahi et al., 2003), and larvicidal (Jantan et al., 2003) properties as well as for their use as food preservatives (Ukeh and Mordue, 2009; Pandey et al., 2014c).
Figure 1.
Different activities and uses of essential oils.
Essential oils of aromatic and medicinal plants are reported to be effective against agents affecting stored products such as insects, human pathogenic fungi, and bacteria. Essential oils of Chenopodium ambrosioides, Clausena pentaphylla, Mentha arvensis, and Ocimum sanctum are contact-sensitive and act as fumigant toxicants against Callosobruchus chinensis and C. maculatus (Pandey et al., 2011a) associated with pigeon pea seeds. Similarly, the essential oil of Tanacetum nubigenum exhibit significant repellent and fumigant toxicity against Tribolium castaneum, which affects wheat during storage (Haider et al., 2015). Eucalyptus globulus essential oil has antibacterial activity against Escherichia coli and Staphylococcus aureus, thus, it is effective against both Gram-positive and Gram-negative bacteria (Bachir and Benali, 2012). In addition, other bacterial pathogens such as Haemophilus influenzae, S. aureus, S. pneumonia, and S. pyogenes were inhibited by Eucalyptus odorata essential oil under in vitro conditions (Posadzki et al., 2012). This review highlights the use of essential oils and their antifungal, fungicidal and food preservative properties in controlling fungi associated with food commodities. Additional emphasis has been given on the efficacy of essential oils against plant pathogenic bacteria as antibacterial and bactericidal.
Essential oils and functions of their active constituents
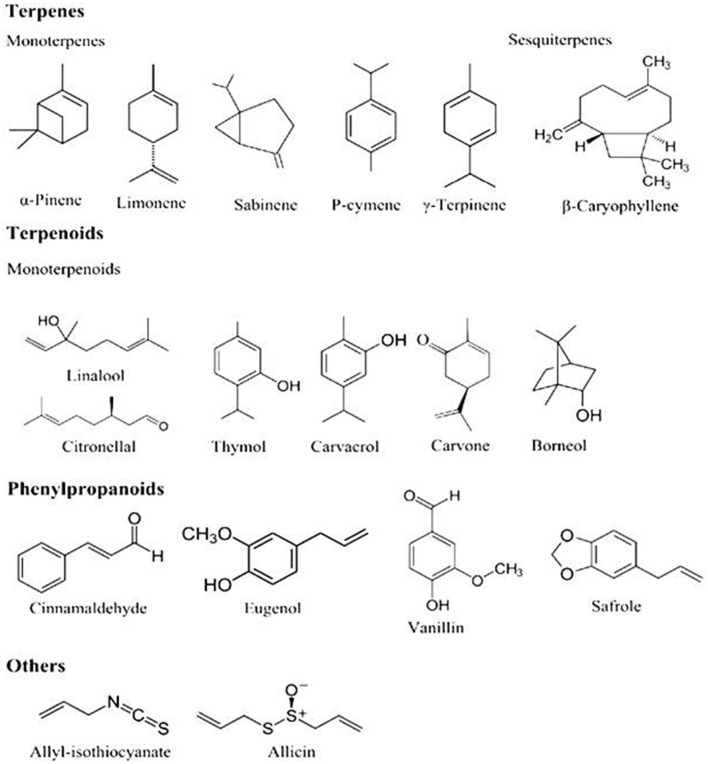
The majority of aromatic plants retain a volatile odoriferous mixture of compounds which can be extracted as an essential oil. Generally, aromatic and medicinal plants produce a wide range of secondary metabolites viz., terpenoids, alcoholic compounds (e.g., geraniol, menthol, linalool), acidic compounds (e.g., benzoic, cinnamic, myristic acids), aldehydes (e.g., citral, benzaldehyde, cinnamaldehyde, carvone camphor), ketonic bodies (e.g., thymol, eugenol), and phenols (e.g., ascaridole, anethole). Among those, terpenes (e.g., pinene, myrcene, limonene, terpinene, p-cymene), terpenoids (e.g., oxygen-containing hydrocarbons), and aromatic phenols (e.g., carvacrol, thymol, safrole, eugenol) are found to have major roles in the composition of various essential oils (Figure 2) (Koul et al., 2008). Derivatives of terpenoids and aromatic polyterpenoids are synthesized by the mevalonic acid and shikimic acid pathways, respectively (Bedi et al., 2008). Terpenoids are among an immense pool of secondary compounds produced by aromatic and medicinal plants, and they have an important role in providing resistance to pathogens. Monoterpenoids are antimicrobial in nature, result in disruptive multiplication and development of microorganisms, and interfere in physiological and biochemical processes of microorganisms (Burt, 2004). Some botanical constituents such as azadirachtin, carvone, menthol, ascaridol, methyl eugenol, toosendanin, and volkensin have reported potential to act against several bacterial and fungal pathogens as well as against insect pests (Isman, 2006; Pandey et al., 2012, 2016). Moreover, many of them have powerful bactericidal, fungicidal, and insecticidal activities and can be responsible for improved taste or toxic properties.
Figure 2.
Actives compounds of essential oils. Figure as originally published in Hyldgaard et al. (2012).
Fungi such as Aspergillus flavus, Neurospora sitophila, and Penicillium digitatum are completely inhibited by Cymbopogon citratus essential oil (Shukla, 2009; Sonker et al., 2015). Essential oils from Nigella sativa, Cymbopogon citratus, and Pulicaria undulata inhibit the growth of Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli (El-Kamali et al., 1998). Essential oils from Acorus, Artemisia, Chenopodium, Clausena, Curcuma, Cinnamon, Cymbopogon, Eupatorium, Foeniculum, Hyptis, Lippia, Ocimum, Putranjiva, Syzygium, and Vitex are known for their pronounced antimicrobial properties (Pandey et al., 2012, 2013b, 2014c; Sonker et al., 2015). The antibacterial properties of essential oils and their several active natural compounds against foodborne bacteria and their applications in food (Burt, 2004) could provide alternatives to conventional bactericides and fungicides (Perricone et al., 2015).
Potency of essential oils against phytopathogenic bacteria
In cereals, pulses, fruits, and vegetables, bacterial species can cause major loss of plant quality and quantity during cultivation, transit, and storage by 20–40% of the total harvest per year. The bacterial species responsible for many diseases and loss of crops include Clavibacter michiganensis, Pseudomonas syringae pv. tomato, P. solanacearum, P. cichorii, P. syringae pv. syringae, P. putida, Erwinia carotovora, E. amylovora, E. carotovora subsp. atroceptica, E. chrysanthemi, E. herbicola, Xanthomonas citri, X. campestris, X. axanopodis pv. malvacearum, X. axanopodis pv. vesicatoria, X. axanopodis pv. campestris, X. campestris pv. raphani, X. axanopodis pv. vitians, and X. campestris pv. zinnia. Such bacteria cause substantial losses in many crops of national and international significance (Agrios, 2005). There are many essential oils that have been evaluated for their potential for antibacterial activity against these phytopathogenic bacteria under in vitro and in vivo conditions (Dorman and Deans, 2000; Iscan et al., 2003; Kotan et al., 2013). The methods used to assess the actions of essential oils against phytopathogenic bacteria include disc diffusion, agar dilution, agar well, and broth dilution (Perricone et al., 2015). Antimicrobial studies of essential oil constituents and their mode of actions more have been extensively undertaken on bacteria; however, there is limited information available about their actions on yeasts and molds.
Generally, Gram-negative bacteria are less susceptible to essential oils than Gram-positive bacteria. The outer membrane of Gram-negative bacteria contains hydrophilic lipopolysaccharides (LPS) that acts as a barrier to macromolecules and hydrophobic compounds, thus providing increased tolerance to hydrophobic antimicrobial compounds such as those found in essential oils (Nikaido, 1994, 2003; Trombetta et al., 2005). Therefore, it is difficult to predict the susceptibility of microorganisms to essential oils due to the breadth of genetic variations among species. Antibacterial activities of essential oils against a variety of phytopathogenic bacteria are summarized in Table 1.
Table 1.
Antibacterial investigations of essential oils against phytopathogenic bacteria.
| Plant species (essential oil) tested | Plant pathogenic bacteria | Remarks | Investigators |
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| Curcuma longa | Erwinia carotovora, Pseudomonas solanacearum, Xanthomons citri, Xa. malvacearum | Oil exhibited efficacy to all bacteria at 1:10 dilution than 1:1000 dilution. | Banerjee and Nigam, 1978 |
| Carum copticum | 5 Bacteria | Oils exhibited variable degree of efficacy to test bacteria. Dethymolysed oil of Carum copticum was found to be most potent bacteriotoxicant. | Pandey et al., 1981 |
| Origanum vulgare, Satureja hortensis, Thymus vulgaris | Erwinia amylovora | The test bacterium was found to be susceptible toward all the tested oils. | Scortichini and Rossi, 1989 |
| Ocimum basilicum | Pseudomonas putida | Resistant to the bacteria. | Lachowicz et al., 1998 |
| Artemisia afra, Pteronia incana, Rosmarinus officinalis | Erwinia carotovora, Erw. chrysanthemi | All three oils showed variable range of zone of inhibition. | Mangena and Muyima, 1999 |
| Thymbra spicata | 6 Bacteria | The oil showed variable MIC and MBC values against all test bacteria in contact and volatile phase. | Basim et al., 2000 |
| Thymus vulgaris, Pelarogonium graveolens | Erwinia carotovora | Thymus vulgaris showed highest zone of inhibition, while Pelarogonium graveolens showed no inhibition against test bacteria. | Dorman and Deans, 2000 |
| Cinnamomum zeylanicum, Cymbopogon citratus | Erwinia amylovora, Erw. herbicola | Cinnamomum zeylanicum showed highest toxicity against both bacteria, while Cymbopogon citratus was found to be least toxic. | Vanneste and Boyd, 2002 |
| Rosa damascena | Xanthomonas vesicatoria XV(88.5, 56, 97.2) | Remarkably inhibited tested strains of Xa. vesicatoria. | Basim and Basim, 2003 |
| Satureja hortensis | Clavibacter michiganensis, Pseudomonas syringae pv. tomato, Xa. campestris subsp. campestris | Oil was effective against all three tested organisms. | Gulluce et al., 2003 |
| Heracleum sphondylium sub sp. ternatum | 5 Bacteria | The MIC of oil was least against 3 bacteria (Xa. compestris pv. phaseoli, Xa. compestris, Ps. syringae pv. syringae) while higher against Ps. pv. phasiolicola and Ps. syringae pv. tomato). | Iscan et al., 2003 |
| Thymol oil, Palmerosa oil, Lemongrass oil, Tea tree oil | Ralstonia solanacearum | In all the oil tested, 3 oils remarkably inhibited the growth of the test bacteria except Tea tree oil. | Pradhanang et al., 2003 |
| Rosa damascene, Thymbra spicata | Erwinia amylovora | R. damascene oil was least effective with MBC value 1386.5 μg/m than T. spicata oil (500μg/m). | Basim and Basim, 2004 |
| Coriandrum sativum, Foeniculum vulgare | 27 Bacteria | A significant antibacterial activity was observed by agar diffusion method with C. sativum oil whereas a much reduced effect was observed for F. vulgare oil. | Cantore et al., 2004 |
| Coriandrum sativum, Foeniculum vulgare, Cuminum cyminum, Carum carvi | 29 Bacteria | A significant antibacterial activity was observed against Gram+ and Gram –ve bacteria. A much reduced effect was observed for the wild fennel. | Iacobellis et al., 2004 |
| Cuminum cyminum, Carum carvi | 31 Bacteria | The activity was particularly high against the genera Clavibacter, Curtobacterium, Rhodococcus, Erwinia, Xanthomonas, Ralstonia and Agrobacterium while lower activity was observed against Pseudomonas sp. | Iacobellis et al., 2005 |
| Thymol, Palmarosa oil | Ralstonia solanacearum | Both Thymol and Palmarosa oil during soil treatment reduced bacterial wilt significantly. | Ji et al., 2005 |
| Artemisia absinthium, A. dracunculus, A. santonicum, A. spicigera | 16 Bacteria | Among all the 4 oils, A. santonicum and A. spicigera oils showed antibacterial activities over a very wide spectrum, ineffective against Ps. syringae pv. populans. | Kordali et al., 2005 |
| Ocimum gratissimum, Thymus vulgaris, Cymbopogon citratus, Zingiber officinale, Monodora myristica | 5 Bacteria | The essential oils from Ocimum gratissimum and Thymus vulgaris were highly effective against all bacteria tested, Cymbopogon citratus and Zingiber officinale were moderate effective while Monodora myristica was least effective. | Nguefack et al., 2005 |
| Artemisia turanica | Agrobacterium tumifacience | The oil did not exhibit any antibacterial effect on test strains. | Behravan et al., 2006 |
| Thymbra spicata, Thymus kotschyanus, Origanum onites, Satureja hortensis | Clavibacter michiganensis sub. sp. michiganensis, Pseudomonas syringae pv. tomato, Xanthomonas campestris pv. malvacearum | All the essential oils exhibited antibacterial activity against all pathogens except Xa. campestris pv. malvacearum. Gram+ve bacteria were more sensitive than Gram –ve bacteria. | Kizil and Uyar, 2006 |
| Ziziphora persica | 5 Bacteria | The oil showed variable range of zone of inhibition against Ps. syringae and Erw. caratovora while ineffective against other three bacteria. | Ozturk and Ercisli, 2006 |
| Origanum vulgare, Carum carvi | 6 Bacteria | Origanum vulgare exhibited highest effectiveness against all tested bacteria, Xa. vesicatoria 67 was the most sensitive to the essential oil extracted from Carum carvi. | Vasinauskiene et al., 2006 |
| Rosa brunonii | Xanthomonas campestris | 20% dilution of essential oil was found to be most effective. | Jangwan et al., 2007 |
| 24 Plants | Xanthomonas axonopodis pv. vesicatoria | Of 24 plant samples, 7 essential oils were highly active showing inhibition zone in range of 22–46.3mm and MIC of 25–200μl/ml in range. | Kotan et al., 2007 |
| Russowia sogdiana | Erwinia carotovora var. carotovora, Pseudomonas lachrymans, Xanthomonas vesicatoria, Agrobacterium tumifacience | The oil exhibited a broad spectrum of antibacterial activity against all test bacteria with MIC values ranging from 0.2 mg/ml to 0.8 mg/ml. | Tan et al., 2007 |
| Satureja thymbra | Pseudomonas putida | The treatment resulted in log reduction at the level below the detection limit formed for either 60 or 18 min. | Chorianopoulos et al., 2008 |
| Mentha arvensis, Citrus limonum, Tagetes bipinata, Lavandula latifolia | Clavibacter michiganensis sub. sp. sepedonicus (cms), C. michiganensis sub. sp. insidiosus (cmi) | Mentha arvensis and Citrus limonum were effective against cms and Tagetes bipinata and Lavandula latifolia were effective against cmi. | Pouvova et al., 2008 |
| 13 Plants | Agrobacterium tumefaciens, Erwinia carotovora var. carotovora | All the oils exhibited variable degree of toxicity toward both bacteria. | Saad et al., 2008 |
| Teucrium montanum | 5 Bacteria | Oil exhibited toxicity. The diameters of zone of inhibition ranged from (10–18 mm) with the highest zone of inhibition were observed against Azotobacter chlorococcum. Agrobacterium tumifaciens and Erwinia carotovora showed higher level of resistant. | Vukovic et al., 2008 |
| Satureja hortensis | Erwinia amylovora | The MIC and MBC of oil against Erw. amylovora were found to be 0.025 and 0.05 μl/ml respectively. | Mihajilov-Krstev et al., 2009 |
| Metasequoia glyptostroboides | Xanthomonas campestris pv. campestris KC94-17, Xa. campestris pv. vesicatoria YK93-4, Xa. oryzae pv. oryzae KX019, Xanthomonas sp. SK12 | The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of oil and the extract were ranged from 125 to 250 μl/ml and 125–500 μg/ml and 250–1000 μl/ml and 250–2000 μg/ml respectively. | Bajpai et al., 2010a |
| Cleistocalyx operculatus | Xanthomonas campestris pv. campestris KC94-17, Xa. campestris pv. vesicatoria YK93-4, Xa. oryzae pv. oryzae KX019, Xanthomonas sp. SK12 | The MIC and MBC values of the oil and extract against the tested Xanthomonas spp. ranged from 31.25 to 125 μl/ml and 62.5 to 250 μg/ml respectively. | Bajpai et al., 2010b |
| Satureja hortensis, Thymus vulgaris | Erwinia amylovora | 20 μl dose of both oil exhibited 25mm zone of inhibition. | Karami-Osboo et al., 2010 |
| Origanum sp., Thymus sp., Mellisa sp., Mentha sp. and Nepeta sp | Erwinia amylovora, Pseudomonas syringae pv. syringae | 1 μl dose of all the oils exhibited maximum efficacy and Origanum sp. oil was found to be more potent. | Kokoskova et al., 2011 |
| Chenopodium ambrosioides | Erwinia herbicola, P. putida | The MIC and MBC of the oil were 0.25 and 2.0 μl/ml for Erw. herbicola, and 0.12 and 1.0 μl/ml for Ps. putida, respectively. | Pandey et al., 2012 |
| Satureja hortensis | Clavibacter michiganensis ssp. michiganensis, Erwinia amylovora, Erw. carotovora subsp. atroceptica, Erw. chrysanthemi, Pseudomonas cichorii, Ps. syringae pv. syringae, Ps. syringae pv. tomato, Xanthomonas axanopodis pv. malvacearum, Xa. axanopodis pv. vesicatoria, Xa. axanopodis pv. campestris, Xa. campestris pv. raphani, Xa. axanopodis pv. vitians, Xa. campestris pv. zinnia, Xa. axanopodis pv. pelargonii | 12.5 μl dose of essential oil gave maximum zone of inhibition against Xanthomonas axanopodis pv. vitians (50 mm) followed by Xanthomonas axanopodis pv. campestris (45 mm), Xanthomonas axanopodis pv. pelargonii, Xanthomonas axanopodis pv. malvacearum (42 mm), Erwinia carotovora subsp. atroceptica (41mm) while zone of inhibition was <40 mm for other bacterial species. | Kotan et al., 2013 |
| Eupatorium adenophorum | Erw. herbicola Ps. putida | The MIC and MBC values were ranges of 0.25–4.0 μl/ml for the both bacterial species. | Pandey et al., 2014b |
| Nepeta hindostana | Erw. herbicola Ps. putida | The MIC and MBC values were 2 and 8 μl/ml for Erw. herbicola and 4 and >16 for Ps. putida. | Pandey et al., 2015 |
| Origanum rotundifolium | 20 plant pathogenic bacteria | The essential oil exhibits significant antibacterial effect against the test bacteria. | Gormez et al., 2016 |
Potency of essential oils against storage fungi
Fungi can act as major destroyers of food commodities, including cereals, pulses, fruits, and vegetables, through the production of mycotoxins and render food unhealthy for human consumption by adversely affecting their nutritional value (Paranagama et al., 2003; Pandey et al., 2016). During storage, spoilage of stored food commodities is a chronic problem in tropical hot and humid climates. According to the FAO, foodborne fungal pathogens and their toxic metabolites can produce qualitative and quantitative losses of up to 25% of total agricultural food commodities throughout the world (Agrios, 2005). Fungal infection in food commodities results in a reduction of food quality, color, and texture as well as a reduction in nutrients present and physiological properties of food commodities (Dhingra et al., 2001). During infection, fungi can also produce mycotoxins, which can lead to famines in developing countries (Wagacha and Muthomi, 2008). With regard to molds, food contamination by Alternaria, Aspergillus, Penicillium, Fusarium, and Rhizopus spp. is of great significance because of the related health hazards and foodborne infections (Pandey and Tripathi, 2011). Hence, during storage and transit, prevention of fungal growth by essential oils could be a cost-effective approach to combat food losses. In recent years, throughout the world, the antifungal potential of essential oils is being considered significantly important (Baruah et al., 1996; Arras and Usai, 2001; Lalitha and Raveesha, 2006; Bosquez-Molina et al., 2010). The antifungal activities of essential oils are related to the associated disintegration of fungal hyphae due to the mono- and sesquiterpene compounds present in the essential oils. Moreover, essential oils amplify membrane permeability; as such compounds can dissolve in cell membranes and cause membrane swelling, thereby reducing membrane function (Dorman and Deans, 2000). Additionally, the lipophilic property of essential oils is responsible for their antifungal activity as that property gives them the ability to penetrate cell walls and affect enzymes involved in cell-wall synthesis, thus altering the morphological characteristics of the fungi (Cox et al., 2000). The present account summarizes the investigations into essential oils tested for their antifungal activity against fungi affecting food storage (Table 2).
Table 2.
Antifungal investigations of essential oils against fungi infecting food commodities during postharvest.
| Plant species (essential oil) tested | Postharvest fungi | Remarks | Investigators |
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| Raphanus sativus | Alternaria brassicae, Fusarium avenaceum, Phoma spp. | The oil was active at 1:250 to 1:1000000 dilutions. | Nehrash, 1961 |
| Juniperus communis | Aspergillus niger | Exhibited toxicity. | Slavenas and Razinskaite, 1962 |
| Eugenia bracteata, E. heyneana | Cephalosporium sacchri, Curvularia lunata, Fusarium moniliforme | Both the oils were toxic toward test fungi. | Rao and Joseph, 1971 |
| Cymbopogon citratus, Mentha arvensis, Sweet basil | Penicillium italicum | Mentha oil was most toxic during both in vitro and in vivo testing. | Arora and Pandey, 1977 |
| Curcuma aromatica, C. caesia, Myristica fragrans | 15 Storage fungi | Myristica fragrans was most toxic. | Kher and Chaurasia, 1978 |
| Trachyspermum ammi, Oenanthera stalonifera, Anethum graveolens, Apium graveolens, Parthenium histerophorus and Psoralea corylifolia | 16 Fungal species | The essential oils exhibited significant antimycotic activity. | Sharma and Singh, 1979 |
| Cestrum diurnum | 39 Storage fungi | The oil inhibited mycelial growth of all test fungi at 0.7% concentration. | Renu et al., 1980 |
| Ageratum conyzoides | Colletotricum capsici, Penicillium italicum | The oil was found to be toxic at its MIC of 0.5% and 0.2% against C. capsici and P. italicum respectively. | Chandra and Dixit, 1981 |
| Ageratum conyzoides, Cymbopogon martini var. motia, Eupatorium capillifolium, Ocimum adscendens, | Helminthosporium oryzae | The oil of Ocimum adscendens was found most effective at 200 μl/l conc. | Asthana et al., 1982 |
| Citrus medica, Ocimum canum | Aspergillus flavus, A. versicolor | Oils completely inhibited the mycelial growth of test fungi at 2000 ppm. | Dubey et al., 1983 |
| Alpinia galanga | 24 Storage fungi | The oil showed broad antifungal spectrum at 0.4% and 0.6% conc. | Tripathi et al., 1983 |
| Lemon grass, Mentha sp., Palmarosa, Zingiber sp. | Aspergillus parasiticus | Mentha oil was most potent control the growth of A. parasiticus and aflatoxin production. | Kala et al., 1984 |
| Citrus aurantifolia | 20 Storage fungi | Fungitoxic at 2000 ppm. | Upadhyay et al., 1985 |
| Ocimum adscendens | 30 Storage fungi | Fungicidal at 400 ppm. | Asthana et al., 1986 |
| Anisomeles ovata | Aspergillus flavus | Effective at 2000 ppm. | Upadhyay et al., 1987 |
| Eucalyptus sp. | Aspergillus niger | Checked mycelial growth at 1000 ppm. | Tiwari et al., 1988 |
| Thyme, Cumin, Clove, Caraway, Rosemary, Sage | Aspergillus parasiticus | All the oils exhibited broad range of fungitoxicity. | Farag et al., 1989 |
| Amomum subulatum | A. flavus | Fungitoxic at 3000 ppm. | Mishra and Dubey, 1990 |
| Daucus carota | A. flavus | Exhibited antifungal activity at 2000 ppm. | Dwivedi et al., 1991 |
| Cinnamomum camphora | A. flavus | Fungitoxic at 400 ppm. | Mishra et al., 1991 |
| Ocimum gratissimum | A. flavus, A. niger | MIC of oil was 100 ppm against A. flavus and 900 ppm against A. niger. | Dixit and Shukla, 1992 |
| Hyptis suaveolens | 21 Storage fungi | Exhibited mycelial inhibition at 2000 ppm. | Singh et al., 1992 |
| Mixture of Apium graveolens and Cuminum cyminum | 29 Storage fungi | Mixture showed antifungal activity at the conc. of 1:1 ratio. | Mishra et al., 1993 |
| 14 Plant essential oils | 47 Storage fungi | Cymbopogon citratus oil was found to be fungistatic in nature at 1500ppm concentration against all test fungi. | Mishra and Dubey, 1994 |
| Cinnamomum zeylanicum | 35 Storage fungi | Showed strongest activity at 400 ppm. | Tiwari et al., 1994 |
| Nardostachys jatmansi | A. flavus, A. niger, F. oxysporum | Completely inhibited mycelial growth of all test fungi at 1.0 × 1000 μl/l. | Mishra et al., 1995 |
| Cymbopogon martini, Eucalyptus citriodora, Cinnamomum tamala, Mentha piperita | F. moniliforme | Mycotoxic activity of oils increased with increase concentration of oil. | Baruah et al., 1996 |
| Monarda citriodora, Melaleuca alternifolia | 15 Post-harvest fungi | Exhibited absolute toxicity. | Bishop and Thornton, 1997 |
| Thymus vulgaris | Botrytis cinerea, Rhizopus stolonifer | Absolutely inhibited the mycelial growth of test fungi. | Reddy et al., 1998 |
| Cedrus deodara, Tracchispermum ammi | A. niger, Curvularia ovoidea | The MIC of oils was found to be 1000 and 500 ppm respectively. | Singh and Tripathi, 1999 |
| Ocimum gratissimum, Zingiber cassumunar and Cymbopogon citratus | A. flavus | Oils exhibited antimycotic activity at its MIC ranging from 500 to 1300 ppm. | Dubey et al., 2000 |
| Thymus capitatus | Alternaria citri, Botrytis cinerea, Penicillium digitatum, P. italicum | Oil was effective against all test fungi at 250 ppm conc. | Arras and Usai, 2001 |
| Caesulia axillaris | A. flavus, A. niger | MIC of oil against test fungi was 1000 ppm. | Dubey et al., 2002 |
| Putranjiva roxburghii | 15 Storage fungi | The MIC and MFC of the oil were 400 and 600 ppm respectively. | Kumar and Tripathi, 2002 |
| Cinnamon and Clove oil | C. musae, F. proliferatum, Lasiodiplodia theobromae | The oils were effective at 500 ppm concentration. | Ranasinghe et al., 2002 |
| Acorus calamus, Hedychium spicatum | Helminthosporium oryzae, F. moniliforme | The oil inhibited growth of test fungi at 0.5 × 103ml/l and 1.0 × 103ml/l respectively. | Mishra et al., 2003 |
| Cymbopogon citratus | A. flavus | Oil was fungistatic and fungicidal nature at 0.60 and 1.0mg/ml concentration respectively. | Paranagama et al., 2003 |
| Cymbopogon flexuosus | 25 Fungi | Oil showed absolute toxicity against all fungi at 0.3 μl/ml conc. | Shahi et al., 2003 |
| Curcuma longa | 10 Storage fungi | The oil exhibited 10% toxicity at 3000 ppm. | Singh et al., 2003 |
| Chrysanthemum viscidehirtum | Botrytis cinerea, Phytophthora citrophthora | Chrysanthemum viscidehirtum exhibited strong activity at 150 ppm. | Chebli et al., 2004 |
| Mentha piperita | P. digitatum | M. piperita caused 100% inhibition at 1000 μg/ml conc. | Dhaliwal et al., 2004 |
| 9 Plant essential oils | P. expansum | All oils were found to be effective. | Neri et al., 2005 |
| Thymus vulgaris and T. copticum | Alternaria citri, Penicillium italicum, P. digitatum | Out of 5 oils, Thymus vulgaris and T. copticum were absolutely fungitoxic at 500 mg/l conc. | Azizi et al., 2006 |
| 15 Plant essential oils | 10 Fungi | 13 essential oils were found to be effective inhibited mycelial growth of all test fungi at 3.0% (v/v). | Lalitha and Raveesha, 2006 |
| Epicarp of Citrus sinensis | 10 Post-harvest fungi | Oil exhibited absolute toxicity toward test fungi. | Sharma and Tripathi, 2006 |
| Mentha arvensis | 9 Post-harvest fungi | Oil exhibited absolute mycelial inhibition against Aspergillus flavus, A. fumigatus, Helminthosporium oryzae and Sclerotium rolfsii at 0.10 mg/ml. | Kumar et al., 2007 |
| Satureja hortensis | A. flavus | Oil exhibited toxicity. | Dikbas et al., 2008 |
| Lippia scaberrima | Botryosphaeria parva, C. gloeosporioides | Oil was found to be effective, absolutely inhibited the mycelial growth of test fungi. | Regnier et al., 2008 |
| Mentha arvensis | A. flavus | The MIC of Mentha oil against A. flavus was recorded at 400μl/l and it exhibited broad fungitoxic activity against other 14 storage fungi. | Kumar et al., 2009 |
| Satureja hortensis, Thymus vulgaris | Botrytis cinerea | Oil was found to be toxic and significantly inhibited the growth of test fungi | Abdollahi et al., 2010 |
| Thyme and Mexican lime | C. gloeosporioides, Rhizopus stolonifer | 0.060% concentration of thyme oil stopped the mycelial growth of both test fungi | Bosquez-Molina et al., 2010 |
| Cinnamon oil | C. musae | 0.4% concentration of oil suppressed mycelial growth | Maqbool et al., 2010 |
| Mentha arvensis, Ocimum canum | A. flavus, A. ochraceus, A. niger, A. terreus | Both the oils exhibited significant growth of all the test fungi at 500 ppm concentration. | Pandey and Tripathi, 2011 |
| Eucalyptus, Clove, Cinnamon, Nutmeg, Neem, Nirgudi, Karanj, Sesame | A. flavus, A. niger, A. terreus, A. oryzae, A. fumigatus, Fusarium moniliforme, F. solani and Penicillium sp. | At 50 μl concentration of Eucalyptus, Clove, Cinnamon, Nutmeg oils were more potent against these fungi and among these cinnamon was most potent where zone of inhibition observed was in range of 22.5–67.5 mm. | Shirurkar and Wahegaonkar, 2012 |
| Clausena pentaphylla | A. flavus, A. ochraceus, A. niger, A. terreus | Oil exhibited absolute mycelial inhibition at 0.36 μl/ml and MIC and MFC values were 0.07 μl/ml against all the test fungi | Pandey et al., 2013a |
| Chenopodium ambrosioides | A. flavus, A. ochraceus, A. niger, A. terreus | Absolute mycelia inhibition for all the test fungi was found at 0.36 μl/ml | Pandey et al., 2013b |
| Cymbopogon citratus | A. flavus, A. niger, A. ochraceus | A 0.33 μl/ml dose of the oil caused 100% mycelial inhibition and MIC was reported to be 0.29 μl/ml against all the test fungi | Sonker et al., 2014 |
| Artemisia nilagirica | A. flavus, A. niger, A. ochraceus | Oil showed absolute mycelial inhibition of all the test fungi at 0.33 μl/ml, and MIC and MFC reported were 0.29 and 0.58 μl/ml, respectively for all Aspergillus species. | Sonker et al., 2015 |
| Lippia alba | A. flavus | Absolute mycelial inhibition was observed at 0.28 μl/ml. Oil was fungicidal (MIC) at 0.14 μl/ml, and fungistatic at 0.28 μl/ml. | Pandey et al., 2016 |
Potency of essential oils in food preservation
Research into the utility of essential oils in the preservation of food commodities in order to enhance shelf-life has been successfully carried out in recent years. Various investigators have used essential oils, either in pure or formulation forms, to enhance the shelf-life of food commodities in different storage containers such as those made of cardboard, tin, glass, polyethylene, or natural fabrics and have observed significant enhancement of shelf-life (Tripathi and Kumar, 2007; Pandey et al., 2014a). An earlier study reported that some essential oil constituents such as citral, citronella, citronellol, eugenol, farnesol, and nerol could protect chili seeds and fruits from fungal infection for up to 6 months (Tripathi et al., 1984). Essential oil from Ageratum conyzoides successfully controlled rotting of mandarins by blue mold and increased mandarin shelf-life by up to 30 days (Dixit et al., 1995). Anthony et al. (2003) investigated essential oils from Cymbopogon nardus, C. flexuosus, and Ocimum basilicum and observed that they could significantly control anthracnose in banana and increased banana shelf-life by up to 21 days. Cymbopogon flexuosus essential oil (20 μL/mL) is capable of protecting against rotting of Malus pumilo fruits for up to 3 weeks (Shahi et al., 2003). An fumigant application of essential oils from Putranjiva roxburghii was effective against A. flavus and A. niger infecting groundnuts during storage and enhanced the shelf-life of groundnut from fungal biodeterioration for up to 6 months (Tripathi and Kumar, 2007). The use of Cymbopogon pendulous essential oil as a fumigant increased groundnut shelf-life by 6–12 months (Shukla, 2009), thus proving to be more effective than P. roxburghii essential oil. These differences in efficacy of essential oils may be related to the use of oils from different plant species, as well as to their chemical composition, dose level, and storage container type.
Thyme (Thymus capitata) (0.1%) and maxican lime (Citrus aurantifolia) (0.5%) oil reduced disease incidence in papaya fruit (Bosquez-Molina et al., 2010), while cinnamon (0.3%) oil extended the storage life of banana by up to 28 days and reduced fungal disease incidence in banana (Maqbool et al., 2010). Seed dressing and fumigation of Ocimum cannum oil (1 μL/mL) enhanced the self-life of Bhuchanania (Singh et al., 2011). Clausena pentaphylla and Chenopodium ambrosioides oils, when used as fumigants in glass containers and natural fabric bags were able to protect pigeon pea seeds from A. flavus, A. niger, A. ochraceus, and A. terreus infection for up to 6 months (Pandey et al., 2013a,b). Powder-based formulations of C. pentaphylla and C. ambrosioides oils were also able to preserve pigeon pea seeds for up to 6 months (Pandey et al., 2014c). Artemisia nilagirica oil as a fumigant in cardboard improved the shelf-life of table grapes by up to 9 days (Sonker et al., 2015). Similarly, Lippia alba oil when used as an air dosage treatment in glass containers inhibited fungal proliferation and aflatoxin production in green gram (Vigna radiata) and enhanced its shelf-life by up to 6 months (Pandey et al., 2016).
Conclusion and future prospects
Worldwide investigations carried out on essential oils have motivated researchers to focus their interest toward the study of botanical antimicrobials. It is apparent that the use of essential oils and their derivatives has been widely described, and essential oils have been used against a wide range of pathogens. Accordingly, this review provides a brief overview of essential oils, their active constituents, and their potential as sources of antibacterials, antifungals, and food preservatives. The relevant literature summary shows that essential oils exhibit a diverse range of antimicrobial properties, and indicates their natural sustainability when used as potential biocontrol agents against fungal and bacterial pathogens. Hence, we conclude from this review that essential oils are potential sources of biocontrol products that should be further explored due to their potential to protect food commodities. Also, an essential oil-based fumigant having antimicrobial activity should have a promising GRAS status in mammalian systems. The LD50 values of some botanicals like azadirachtin and carvone are found to be high in rat and are reportedly nontoxic for human consumers. Additionally, several essential oils and their constituents (e.g., carvone, carvacrol, cinnamaldehyde, thymol, linalool, citral, limonene, eugenol, limonene, and menthol) are reported by the United States Food and Drug Administration to have a GRAS status and are approved as flavor or food additives.
Essential oil applications are evolving as a means of integrating pathogens into food containers; for example, fumigants that can be useful in natural fabric and cardboard containers, and even containers made of wooden boards. Some oils can be used as light sprays and integrated as a fumigant into the commodity itself. Many essential oils and their active constituents are active against bacteria and fungi, and they can be produced from commonly available raw materials; perhaps in many cases right at the site of use so as to be rather low-cost treatments. Based on this review, it can be summarized that it is possible to develop techniques for food commodity protection without the use, or with reduced use, of commercial bactericides and fungicides. Although the available literature indicates that essential oils are host specific, biodegradable, have limited effect on non-target organisms, have low levels of mammalian toxicity. There, sustainable and commercial uses have some drawbacks, such as their cost effectiveness. Regardless, there are innumerable potential uses of essential oils and more research is needed to meet the needs of a food industry shifting toward the use of green technology.
Author contributions
AP, PS, and NT conceived and designed the experiments. AP performed the experiments. AP and PK write the manuscript and PK and VB did the editing. All the authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors (AP, PS, and NT) would like to thanks the Head, Department of Botany, DDU Gorakhpur University, Gorakhpur for providing the necessary facilities. AP is grateful to CST UP, Lucknow for financial assistance (Grant no. CST/AAS, D-09, April 3, 2007). PK thankful to Director and Head, Department of Forestry, NERIST, Nirjuli, Arunachal Pradesh, India. VB sincerely thankful to Yeungnam University, Republic of Korea.
References
- Abdollahi A., Hassani A., Ghosta Y., Bernousi I., Meshkatalsadat M. H. (2010). Study on the potential use of essential oils for decay control and quality preservation of Tabarzeh table grapes. J. Plant Prot. Res. 50, 45–52. 10.2478/v10045-010-0008-2 [DOI] [Google Scholar]
- Abdollahi M., Karimpour H., Monsef-Esfehani H. R. (2003). Antinociceptive effects of Teucrium polium L. total extract and essential oil in mouse writhing test. Pharmacol. Res. 48, 31–35. 10.1016/s1043-6618(03)00059-8 [DOI] [PubMed] [Google Scholar]
- Agrios G. N. (2005). Plant Pathology, 5th Edn. Oxford: Elsevier Academic Press. [Google Scholar]
- Anthony S., Abeywickrama K., Wijeratnam S. W. (2003). The effect of spraying essential oils Cymbopogon nardus, C. flexuosus and Ocimum basilicum on post-harvest diseases and storage life of Embul banana. J. Hort. Sci. Biotech. 78, 780–785. 10.1080/14620316.2003.11511699 [DOI] [Google Scholar]
- Arora R., Pandey G. N. (1977). The application of essential oils and their isolates for blue mould decay control in Citrus reticulata. J. Food Sci. Technol. 14, 14–16. [Google Scholar]
- Arras G., Usai M. (2001). Fungitoxic activity of 12 essential oils against four post-harvest Citrus pathogens, chemical analysis of Thymus capitatus oil and its effect in sub-atmospheric pressure conditions. J. Food Protect. 64, 1025–1029. 10.4315/0362-028X-64.7.1025 [DOI] [PubMed] [Google Scholar]
- Asthana A., Chandra H., Dikshit A., Dixit S. N. (1982). Volatile fungitoxicants from leaves of some higher plants against Helminthosporium oryzae. Z. Pflanzenkr. Pflanzenschutz 89, 475–479. [Google Scholar]
- Asthana A., Tripathi N. N., Dixit S. N. (1986). Fungitoxic and phytotoxic studies with essential oil of Ocimum adscendens. J. Phytopathol. 117, 152–159. 10.1111/j.1439-0434.1986.tb00639.x [DOI] [Google Scholar]
- Azizi M., Farzad S., Jafarpour B., Rastegar M. F., Jahanbakhsh V. (2006). Inhibitory effect of some medicinal plants essential oils on post-harvest fungal disease of Citrus fruits. Acta. Hortic. 768, 279–286. [Google Scholar]
- Bachir R. G., Benali M. (2012). Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2, 739–742. 10.1016/S2221-1691(12)60220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai V. K., Cho M. J., Kang S. C. (2010a). Control of plant pathogenic bacteria of Xanthomonas spp. by the essential oil and extract of Metasequoia glyptostroboides Miki ex Hu In vitro and In vivo. J. Phytopathol. 158, 479–486. 10.1111/j.1439-0434.2009.01646.x [DOI] [Google Scholar]
- Bajpai V. K., Dung N. T., Suh H., Kang S. C. (2010b). Antibacterial activity of essential oil and extract of Cleistocalyx operculatus buds against the bacteria of Xanthomonas spp. J. Am. Oil. Chem. Soc. 87, 1341–1349. [Google Scholar]
- Banerjee A., Nigam S. S. (1978). Antimicrobial efficacy of essential oil of Curcuma longa. Ind. J. Med. Res. 68, 864–866. [PubMed] [Google Scholar]
- Baruah P., Sharma R. K., Singh R. S., Ghosh A. C. (1996). Fungicidal activity of some naturally occurring essential oils against Fusarium moniliforme. J. Essent. Oil. Res. 8, 411–414. 10.1080/10412905.1996.9700649 [DOI] [Google Scholar]
- Basim E., Basim H. (2003). Antibacterial activity of Rosa damascena essential oil. Fitoterapia 74, 394–396. 10.1016/S0367-326X(03)00044-3 [DOI] [PubMed] [Google Scholar]
- Basim E., Basim H. (2004). Evaluation of antibacterial activity of essential oil of Rosa damascena on Erwinia amylovora. Phytoparasitica 32, 409–412. 10.1007/BF02979853 [DOI] [Google Scholar]
- Basim H., Yegen O., Zeller W. (2000). Antibacterial effect of essential oil of Thymbra spicata L. var. spicata on some plant pathogenic bacteria. Zeitschrift fur Pflanzenkr. Pflanzenschutz 107, 279–284. [Google Scholar]
- Bedi S., Tanuja, Vyas S. P. (2008). A Hand Book of Aromatic and Essential Oil Plants Cultivation, Chemistry, Processing and Uses. Jodhpur: AGROBIOS Publishers. [Google Scholar]
- Behravan J., Ramenzani M., Hassanzadeh M. K., Eliaspour N., Zahra S. (2006). Cytotoxic and antimicrobial activities of essential oil of Artemisia turanica Krasch from Iran. J. Essent. Oil Bear. Plants 9, 196–203. 10.1080/0972060X.2006.10643492 [DOI] [Google Scholar]
- Bishop C. D., Thornton L. B. (1997). Evaluation of antifungal activity of the essential oils of Monarda citriodora var. citriodora and Melaleuca alternifolia on post-harvest pathogens. J. Essent. Oil Res. 9, 77–82. 10.1080/10412905.1997.9700718 [DOI] [Google Scholar]
- Bosquez-Molina E., Jesus E. R., Bautista-Banos S., Verde-Calvo J. R., Morales-Lopez J. (2010). Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruits and their possible application in coatings. Postharvest Biol. Technol. 57, 132–137. 10.1016/j.postharvbio.2010.03.008 [DOI] [Google Scholar]
- Burt S. (2004). Essential oils: their antibacterial properties and potential applications in food – A review. Int. J. Food Microbiol. 94, 223–253. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Cantore P. L., Iacobellis N. S., Marco A. D., Capasso F., Senatore F. (2004). Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare var. vulgare (Miller) essential oils. J. Agric. Food Chem. 52, 7862–7866. 10.1021/jf0493122 [DOI] [PubMed] [Google Scholar]
- Chandra H., Dixit A. (1981). Volatile fungitoxicant from the leaves of Ageratum conyzoides against Colletotrichum capsici and Penicillium italicum. J. Ind. Bot. Soc. 60(Suppl.):13. [Google Scholar]
- Chebli B., Hmamouchi M., Achouri M., Hassani L. M. I. (2004). Composition and in-vitro fungitoxic activity of 19 essential oils against two post-harvest pathogens. J. Essent. Oil. Res. 16, 507–511. 10.1080/10412905.2004.9698783 [DOI] [Google Scholar]
- Chorianopoulos N. G., Giaouris E. D., Skandamis P. N., Haroutounian S. A., Nychas G. J. E. (2008). Disinfectant test against monoculture and mixed-culture biofilm composed of technological, spoilage and pathogenic bacteria: bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with acid-base sanitizers. J. Appl. Microbiol. 104, 1586–1596. 10.1111/j.1365-2672.2007.03694.x [DOI] [PubMed] [Google Scholar]
- Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12, 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S. D., Mann C. M. I., Markham J. L., Bell H. C., Gustafson J. E., Warmington J. R., et al. (2000). The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88, 170–175. 10.1046/j.1365-2672.2000.00943.x [DOI] [PubMed] [Google Scholar]
- Dhaliwal H. J. S., Thind T. S., Mohan C. (2004). Relative activity of essential oils from plants against Penicillium digitatum causing post-harvest fruit rot of kinnow mandarin. Plant Dis. Res. 19, 140–143. [Google Scholar]
- Dhingra O. D., Mizubuti E. S. G., Napoleao I. T., Jham G. (2001). Free fatty acid accumulation and quality loss of stored soybean seeds invaded by Aspergillus ruber. Seed Sci. Technol. 29, 193–203. [Google Scholar]
- Dikbas N., Kotan R., Dadasoglu F., Sahin F. (2008). Control of Aspergillus flavus with essential oil and methanol extract of Satureja hortensis. Int. J. Food Microbiol. 124, 179–182. 10.1016/j.ijfoodmicro.2008.03.034 [DOI] [PubMed] [Google Scholar]
- Dixit S. N., Chandra H., Tiwari R., Dixit V. (1995). Development of botanical fungicide against blue mould of mandarins. J. Stored Prod. Res. 31, 165–172. 10.1016/0022-474X(94)00041-Q [DOI] [Google Scholar]
- Dixit V., Shukla K. (1992). Evaluation of essential oil of Ocimum gratissimum against storage fungi. Ind. Perf. 36, 277–283. [Google Scholar]
- Dordevic S., Petrovic S., Dobric S., Milenkovic M., Vucicevic D., Zizic S., et al. (2007). Antimicrobial, anti-inflammatory, anti-ulcer and antioxidant activities of Carlina acanthifolia root essential oil. J. Ethnopharmacol. 109, 458–463. 10.1016/j.jep.2006.08.021 [DOI] [PubMed] [Google Scholar]
- Dorman H. J. D., Deans S. G. (2000). Antibacterial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88, 308–316. 10.1046/j.1365-2672.2000.00969.x [DOI] [PubMed] [Google Scholar]
- Dubey A. K., Kumar N., Tripathi N. N. (2002). Caesulia axillaris-Volatile herbal fungitoxicants. J. Basic. Appl. Mycol. 1, 68–73. [Google Scholar]
- Dubey N. K., Bhargava K. S., Dixit S. N. (1983). Protection of some stored food commodities from fungi by essential oils of Ocimum canum and Citrus medica. Int. J. Trop. Plant Dis. 1, 177–179. [Google Scholar]
- Dubey N. K., Tripathi P., Singh H. B. (2000). Prospects of some essential oils as antifungal agents. J. Med. Aroma. Plant. Sci. 22, 354. [Google Scholar]
- Dwivedi S. K., Pandey V. N., Dubey N. K. (1991). Effect of essential oils of some higher plants on Aspergillus flavus, infesting stored seeds of gaur (Cyamopsis tetragonoloba L.). Flavour Frag. J. 6, 295–297. 10.1002/ffj.2730060410 [DOI] [Google Scholar]
- El-Kamali H. H., Ahmed A. H., Mohammed A. S., Yahia A. A. M., El-Tayeb I. H., Ali A. A. (1998). Antibacterial properties of essential oils from Nigella sativa seeds, Cymbopogon citratus leaves and Pulicaria undulata aerial parts. Fitoterapia. 69, 7–12. [Google Scholar]
- Farag R. S., Daw Z. Y., Abo-Raya S. H. (1989). Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxin in a synthetic medium. J. Food Sci. 54, 74–76. 10.1111/j.1365-2621.1989.tb08571.x [DOI] [Google Scholar]
- Fico G., Panizzi L., Flamini G., Braca A., Morelli I., Tome F., et al. (2004). Biological screening of Nigella elamascena for antimicrobial and molluscicidal activities. Phytother. Res. 18, 468–470. 10.1002/ptr.1454 [DOI] [PubMed] [Google Scholar]
- Ghelardini C., Galeotti N., Mazzanti G. (2001). Local anesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta Med. 67, 564–566. 10.1055/s-2001-16475 [DOI] [PubMed] [Google Scholar]
- Gormez A., Bozari S., Yanmis D., Gulluce M., Agar G., Sahin F. (2016). The use of essential oils of Origanum rotundifolium as antimicrobial agent against plant pathogenic bacteria. J. Essent. Oil Bear. Plants 19, 656–663. 10.1080/0972060X.2014.935052 [DOI] [Google Scholar]
- Gulluce M., Sokmen M., Daferera D., Agar G., Ozakan H., Kartal N., et al. (2003). In-vitro antibacterial, antifungal and antioxidant activities of the essential oil and methanol extract of herbal parts and callus culture of Satureja hortensis L. J. Agric. Food. Chem. 51, 3958–3965. 10.1021/jf0340308 [DOI] [PubMed] [Google Scholar]
- Haider S. Z., Mohan M., Pandey A. K., Singh P. (2015). Repellent and fumigant activities of Tanacetum nubigenum Wallich. Ex DC essential oils against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Oleo Sci. 64, 895–903. 10.5650/jos.ess15094 [DOI] [PubMed] [Google Scholar]
- Hyldgaard M., Mygind T., Meyer R. L. (2012). Essentials oils in food preservation: mode of action, synergies and interactions with food matrix components. Front. Microbiol. 3:12. 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis N. S., Cantore P. L., Caepasso F., Senatore F. (2005). Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food. Chem. 53, 57–61. 10.1021/jf0487351 [DOI] [PubMed] [Google Scholar]
- Iacobellis N. S., Cantore P. L., Marco A. D., Caepasso F., Senatore F. (2004). Antibacterial activity of some essential oils. Management of plant diseases and arthropod pests by BCAs IOBC/wprs. Bulletin 27, 223–226. [Google Scholar]
- Inouye S., Takizawa T., Yamaguchi H. (2001). Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 47, 565–573. 10.1093/jac/47.5.565 [DOI] [PubMed] [Google Scholar]
- Iscan G., Demirci F., Kurkcuoglu M., Kivanc M., Baser K. H. C. (2003). The bioactive essential oil of Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Z. Naturforsch. 58, 195–200. [PubMed] [Google Scholar]
- Isman M. B. (2006). Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. 10.1146/annurev.ento.51.110104.151146 [DOI] [PubMed] [Google Scholar]
- Isman M. B., Koul O., Luezynski N. (1990). Insecticidal and antifeedant bioactivity of neem oil and their relationship to azadirachtin content. J. Agric. Food Chem. 38, 1406–1411. 10.1021/jf00096a024 [DOI] [Google Scholar]
- Jangwan J. S., Painuly A. M., Joshi V. P., Chamoli R. P. (2007). Chemical composition and antimicrobial activity of essential oil of Rosa brunonii L. Ind. Perf. 51, 53–55. [Google Scholar]
- Jantan I., Ping W. O., Visuvalingam S. D., Ahmad N. W. (2003). Larvicidal activity of the essential oils and methanolic extracts of Malaysian plants on Aedes aegypti. Pharma. Biol. 41, 234–236. 10.1076/phbi.41.4.234.15665 [DOI] [Google Scholar]
- Ji P., Momol M. T., Olson S. M., Pradhanang P. M., Jones J. B. (2005). Evaluation of Thymol as biofumigant for control of bacterial wilt of tomato under field conditions. Plant Dis. 89, 497–500. 10.1094/PD-89-0497 [DOI] [PubMed] [Google Scholar]
- Kala P. K., Tripathi R. K., Gupta K. C., Singh A. K. (1984). Effect of some essential oils on growth and aflatoxin production by Aspergillus parasiticus in stored grains. Pesticides 18, 43–46. [Google Scholar]
- Karami-Osboo R., Khodaverdi M., Ali-Akbari F. (2010). Antibacterial effect of effective compounds of Satureja hortensis and Thymus vulgaris essential oils against Erwinia amylovora. J. Agr. Sci. Technol. 12, 35–45 [Google Scholar]
- Kher A., Chaurasia S. C. (1978). Antifungal activity of essential oils of three medicinal plants. Ind. Drugs 14, 41–42. [Google Scholar]
- Kizil S., Uyar F. (2006). Antimicrobial activities of some Thymes Thymus, Satureja, Origanum and Thymbra species against important plant pathogens. Asian J. Chem. 18, 1455–1461. [Google Scholar]
- Kokoskova B., Pouvova D., Pavela R. (2011). Effectiveness of plant essential oils against Erwinia amylovora, Pseudomonas syringae pv. syringae and associated saprophytic bacteria on/in host plants. J. Plant Pathol. 93, 133–139. [Google Scholar]
- Kordali S., Kotan R., Mavi A., Cakir A., Ala A., Yildirim A. (2005). Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. draunculus, A. santonicum and A. spicigera essential oils. J. Agric. Food Chem. 53, 9452–9458. 10.1021/jf0516538 [DOI] [PubMed] [Google Scholar]
- Kotan R., Dadasǧolu F., Karagoz K., Cakir A., Ozer H., Kordali S., et al. (2013). Antibacterial activity of the essential oil and extracts of Satureja hortensis against plant pathogenic bacteria and their potential use as seed disinfectants. Sci. Hortic. 153, 34–41. 10.1016/j.scienta.2013.01.027 [DOI] [Google Scholar]
- Kotan R., Dadasoglu F., Kordali S., Cakir A., Dikbas N., Cakmaker R. (2007). Antibacterial activity of essential oils extracted from some medicinal plants, carvacrol and thymol on Xanthomonas axonopodis pv. vesicatoria (Doidge) Dye causes bacterial spot disease on pepper and tomato. J. Agric. Technol. 3, 299–306. [Google Scholar]
- Koul O., Walia S., Dhaliwal G. S. (2008). Essential oils as green pesticides: potential and constraints. Biopestic. Int. 4, 63–84. [Google Scholar]
- Kumar A., Shukla R., Singh P., Singh A. K., Dubey N. K. (2009). Use of essential oil from Mentha arvensis L. to control storage moulds and insects in stored chickpea. J. Sci. Food Agric. 89, 2643–2649. 10.1002/jsfa.3768 [DOI] [Google Scholar]
- Kumar N., Tripathi N. N. (2002). Fungal infestation in groundnut seeds during storage and their control by essential oil of Putranjiva roxburghii Wall. J. Indian Bot. Soc. 81, 127–132. [Google Scholar]
- Kumar R., Dubey N. K., Tiwari O. P., Tripathi Y. B., Sinha K. K. (2007). Evaluation of some essential oils as botanical fungitoxicants for the protection of stored food commodities from fungal infestation. J. Sci. Food Agric. 87, 1737–1742. 10.1002/jsfa.2906 [DOI] [Google Scholar]
- Lachowicz K. J., Jones G. P., Briggs D. R., Bienvenu F. E., Wan J., Wilcock A., et al. (1998). The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) acid tolerant food microflora. Lett. Appl. Microbiol. 26, 209–214. 10.1046/j.1472-765X.1998.00321.x [DOI] [PubMed] [Google Scholar]
- Lalitha V., Raveesha K. A. (2006). Fungitoxicity of some essential oils against important seed-borne pathogens of paddy. Plant Dis. Res. 21, 155–157. [Google Scholar]
- Mangena T., Muyima N. Y. O. (1999). Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected bacteria and yeast strains. Lett. Appl. Microbiol. 28, 291–296. 10.1046/j.1365-2672.1999.00525.x [DOI] [PubMed] [Google Scholar]
- Maqbool M., Ali A., Alderson P. G. (2010). Effect of cinnamon oil on incidence of anthracnose disease and post-harvest quality of bananas during storage. Int. J. Agric. Biol. 12, 516–520. [Google Scholar]
- Maurya S., Marimuthu P., Singh A., Rao G. P., Singh G. (2005). Antiviral activity of essential oils and acetone extracts of medicinal plants against papaya ring spot virus. J. Essent. Oil Bear. Plants 8, 233–238. 10.1080/0972060x.2005.10643452 [DOI] [Google Scholar]
- Mediratta P. K., Sharma K. K., Singh S. (2002). Evaluation of immunomodulatory potential of Ocimum sanctum seeds oil and its possible mechanism of action. J. Ethnopharmacol. 80, 15–20. 10.1016/S0378-8741(01)00373-7 [DOI] [PubMed] [Google Scholar]
- Mihajilov-Krstev T., Radnovic D., Kitic D., Stojanovic-Radic Z., Zlatkovic B. (2009). Antimicrobial activity of Satureja hortensis L. essential oil against pathogenic microbial strains. Biotechnol. Biotechnol. 4, 1492–1496. 10.2478/V10133-009-0018-2 [DOI] [Google Scholar]
- Mimica-Dukic N., Bozin B., Sokovic M., Mihajlovic B., Matavulj M. (2003). Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 69, 413–419. 10.1055/s-2003-39704 [DOI] [PubMed] [Google Scholar]
- Mishra A. K., Dubey N. K. (1990). Fungitoxicity of essential oil of Amomum subulatum against Aspergillus flavus. Econ. Bot. 44, 530–533. [Google Scholar]
- Mishra A. K., Dubey N. K. (1994). Evaluation of some essential oils for their toxicity against fungi causing deterioration of stored food commodities. Appl. Environ. Microbiol. 60, 1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A. K., Dwivedi S. K., Kishore N., Dubey N. K. (1991). Fungistatic properties of essential oil of Cinnamomum camphora. Int. J. Pharmacogn. 29, 259–262. [Google Scholar]
- Mishra D., Chaturvedi R. V., Tripathi S. C. (1995). The fungitoxic effect of essential oil of the herb Nardostachys jatamansi DC. Trop. Agric. 72, 48–52. [Google Scholar]
- Mishra D., Samuel C. O., Tripathi S. C. (1993). Synergistic antifungal activity of essential oils of Apium graveolens and Cuminum cyminum. Ind. Perf. 37, 134–140. [Google Scholar]
- Mishra D., Samuel C. O., Tripathi S. C. (2003). Evaluation of some essential oils against seed borne pathogens of rice. Ind. Phytopathol. 56, 212–213. [Google Scholar]
- Nedorostova L., Kloucek P., Kokoska L., Stolcova M., Pulkrabek J. (2009). Antimicrobial properties of selected essential oils in vapour phase against food borne bacteria. Food Control 20, 157–160. 10.1016/j.foodcont.2008.03.007 [DOI] [Google Scholar]
- Nehrash A. K. (1961). The antimicrobial properties of cultivated reddish I. the antimicrobial activity of extracts and essential oils from cultivated and wild radish. J. Microbiol. Kiev. 23, 32–37. [Google Scholar]
- Neri F., Mari M., Brigati S. (2005). Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 55, 100–105. 10.1111/j.1365-3059.2005.01312.x [DOI] [Google Scholar]
- Nguefack J., Somda I., Mortensen C. N., Zollo P. H. A. (2005). Evaluation of five essential oils from aromatic plants of Cameroon for controlling seed-borne bacteria of rice (Oryza sativa). Seed Sci. Technol. 33, 397–407. 10.15258/sst.2005.33.2.12 [DOI] [Google Scholar]
- Nikaido H. (1994). Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264, 382–388. 10.1126/science.8153625 [DOI] [PubMed] [Google Scholar]
- Nikaido H. (2003). Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656. 10.1128/MMBR.67.4.593-656.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk S., Ercisli S. (2006). The chemical composition of essential oil and in vitro antibacterial activities of essential oil and methanol extract of Ziziphora persica Bunge. J. Ethnopharmacol. 106, 372–376. 10.1016/j.jep.2006.01.014 [DOI] [PubMed] [Google Scholar]
- Pandey A. K., Mohan M., Singh P., Palni U. T., Tripathi N. N. (2014b). Chemical composition, antibacterial and antioxidant activity of essential oil of Eupatorium adenophorum Spreng from Eastern Uttar Pradesh, India. Food Biosci. 7, 80–87. 10.1016/j.fbio.2014.06.001 [DOI] [Google Scholar]
- Pandey A. K., Palni U. T., Tripathi N. N. (2013a). Evaluation of Clausena pentaphylla (Roxb.) DC oil as fungitoxicant against storage mycoflora of pigeon pea seeds). J. Sci. Food Agric. 93, 1680–1686. 10.1002/jsfa.5949 [DOI] [PubMed] [Google Scholar]
- Pandey A. K., Palni U. T., Tripathi N. N. (2014a). Repellent activity of some essential oils against two stored product beetles Callosobruchus chinensis L. and C. maculates F. (Coleoptera: Bruchidae) with reference to Chenopodium ambrosioides L. for the safety of pigeon pea seeds). J. Food Sci. Technol. 51, 4066–4071. 10.1007/s13197-012-0896-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A. K., Singh P., Mohan M., Tripathi N. N. (2015). Chemical composition and antimicrobial activity of Nepeta hindostana (Roth) Haines from India. Rec. Nat. Prod. 9, 224–233. [Google Scholar]
- Pandey A. K., Singh P., Palni U. T., Tripathi N. N. (2012). In-vitro antibacterial activity of essential oils of aromatic plants against Erwinia herbicola (Lohnis) and Pseudomonas putida (Krish Hamilton). J. Serb. Chem. Soc. 77, 313–323. 10.2298/JSC110524192P [DOI] [Google Scholar]
- Pandey A. K., Singh P., Palni U. T., Tripathi N. N. (2011a). Use of essential oils of aromatic plants for the management of pigeon pea infestation by pulse bruchids during storage. Int. J. Agric. Technol. 7, 1615–1624. [Google Scholar]
- Pandey A. K., Singh P., Palni U. T., Tripathi N. N. (2013b). Application of Chenopodium ambrosioides Linn. essential oil as botanical fungicide for the management of fungal deterioration in pulse. Biol. Agric. Hortic. 29,197–208. 10.1080/01448765.2013.822828 [DOI] [Google Scholar]
- Pandey A. K., Singh P., Palni U. T., Tripathi N. N. (2014c). In vivo evaluation of two essential oil based botanical formulations (EOBBF) for the use against stored product pests, Aspergillus and Callosobruchus (Coleoptera: Bruchidae) species. J. Stored Prod. Res. 59, 285–291. 10.1016/j.jspr.2014.09.001 [DOI] [Google Scholar]
- Pandey A. K., Singh P., Tripathi N. N. (2011b). Impact of essential oils on eggs hatchability and feeding activity of pulse beetles. J. Entomol. Res. 35, 221–225. [Google Scholar]
- Pandey A. K., Sonker N., Singh P. (2016). Efficacy of some essential oils against Aspergillus flavus with special reference to Lippia alba oil an inhibitor of fungal proliferation and aflatoxin b1 production in green gram seeds during storage. J. Food Sci. 81, 928–934. 10.1111/1750-3841.13254 [DOI] [PubMed] [Google Scholar]
- Pandey A. K., Tripathi N. N. (2011). Post- harvest fungal and insect deterioration of pigeon pea seeds and their management by plant volatiles. J. Ind. Bot. Soc. 90, 326–331. [Google Scholar]
- Pandey D. K., Asthana A., Tripathi N. N., Dixit S. N. (1981). Volatile plant products vis-à-vis potato pathogenic bacteria. Ind. Perf. 25, 10–14. [Google Scholar]
- Paranagama P. A., Abeysekera K. H. T., Abeywickrama K., Nugaliyadde L. (2003). Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett. Appl. Microbiol. 37, 86–90. 10.1046/j.1472-765X.2003.01351.x [DOI] [PubMed] [Google Scholar]
- Perricone M., Arace E., Corbo M. R., Sinigaglia M., Bevilacqu A. (2015). Bioactivity of essential oils: a review on their interaction with food components. Front. Microbiol. 6:76. 10.3389/fmicb.2015.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadzki P., Alotaibi A., Ernst E. (2012). Adverse effects of aromatherapy: a systematic review of case reports and case series. Int. J. Risk Saf. Med. 24, 147–161. 10.3233/JRS-2012-0568 [DOI] [PubMed] [Google Scholar]
- Pouvova D., Kokoskova B., Pavela R., Rysanek P. (2008). Effectivity of plant essential oils against Clavibacter michiganensis, in-vitro. Zemdirbyste- Agric. 95, 440–446. [Google Scholar]
- Pradhanang P. M., Momol M. T., Olson S. M., Jones J. B. (2003). Effect of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis. 87, 423–427. 10.1094/PDIS.2003.87.4.423 [DOI] [PubMed] [Google Scholar]
- Ranasinghe L., Jayawardena B., Abeywickrama K. P. (2002). Fungicidal activity of essential oils of Cinnomomum zeylanicum L. and Syzygium aromaticum (L.) Merr et M Perry against crown rot and anthracnose pathogens isolated form banana. Lett. Appl. Microbiol. 35, 208–211. 10.1046/j.1472-765X.2002.01165.x [DOI] [PubMed] [Google Scholar]
- Rao B. G. V. N., Joseph P. L. (1971). Activity of some essential oils towards phytopathogenic fungi. Riechest Aromas Koer. 21, 405–410. [Google Scholar]
- Reddy B. M. V., Angers P., Gosselin A., Arul J. (1998). Characterization and uses of essential oil from Thymus vulgaris against Botrytis cinerea and Rhizopus stolonifer in strawberry fruits. Phytochemistry 47, 1515–1520. 10.1016/S0031-9422(97)00795-4 [DOI] [Google Scholar]
- Regnier T., DuPlooy W., Combrinck S., Botha B. (2008). Fungitoxicity of Lippia scaberrima essential oil and selected terpenoid components on two mango post-harvest spoilage pathogens. Postharvest Biol. Technol. 48, 254–258. 10.1016/j.postharvbio.2007.10.011 [DOI] [Google Scholar]
- Renu K., Tripathi R. D., Dixit S. N. (1980). Fungitoxic properties of Cestrum diurnum. Naturwissenschaften 67, 150–151. 10.1007/BF01073626 [DOI] [Google Scholar]
- Saad R. E., Mohamed A. R., Shady A. E. M., Sheb M. S. (2008). Antibacterial screening of some essential oils, monoterpenoids and novel N-methyl carbamates based on monoterpenoids against Agrobacterium tumefaciens and Erwinia carotovora. Arc. Phytopathol. Plant Protect. 41, 451–461. 10.1080/03235400600833696 [DOI] [Google Scholar]
- Scortichini M., Rossi M. P. (1989). In vitro activity of some essential oils towards Erwinia amylovora (Burill) Winslow et al. Acta. Phytopathol. Entomol. Hung. 4, 423–431. [Google Scholar]
- Shahi S. K., Patra M., Shukla A. C., Dikshit A. (2003). Use of essential oil as botanical-pesticide against post-harvest spoilage in Malus pumilo fruits. BioControl. 48, 223–232. 10.1023/A:1022662130614 [DOI] [Google Scholar]
- Sharma N., Tripathi A. (2006). Fungitoxicity of the essential oil of Citrus sinensis on post harvest pathogens. World J. Microbiol. Biotechnol. 22, 587–593. 10.1007/s11274-005-9075-3 [DOI] [Google Scholar]
- Sharma S. K., Singh V. P. (1979). Antifungal activity of essential oils. Ind. Drugs Pharm. Ind. 14, 3–6. [Google Scholar]
- Shirurkar D. D., Wahegaonkar N. K. (2012). Antifungal activity of selected plant derived oils and some fungicides against seed borne fungi of maize. Eur. J. Exp. Biol. 2, 1693–1696. [Google Scholar]
- Shukla A. C. (2009). Volatile oil of Cymbopogon pendulus as an effective fumigant pesticide for the management of storage-pests of food commodities. Nat. Acad. Sci. Lett. 32, 51–59. [Google Scholar]
- Singh G., Kapoor I. P. S., Pandey S. K., Singh O. P. (2003). Curcuma longa-Chemical, antifungal and antibacterial investigation of rhizome oil. Ind. Perf. 47, 173–178. [Google Scholar]
- Singh G., Upadhyay R. K., Rao G. P. (1992). Fungitoxic activity of the volatile oil of Hyptis suaveolens. Fitoterapia 63, 462–465. [Google Scholar]
- Singh J., Tripathi N. N. (1999). Inhibition of storage fungi of black gram (Vigna mungo L) by some essential oils. Flavour. Frag. J. 14, 1–4. [DOI] [Google Scholar]
- Singh P., Pandey A. K., Sonker N., Tripathi N. N. (2011). Preservation of Buchnania lanzan Spreng. seeds by Ocimum canum Sims. Essential oil. Ann. Plant Protect. 19, 407–410. [Google Scholar]
- Singh S., Majumdar D. K., Rehan H. M. S. (1996). Evaluation of anti-inflammatory potential of fixed oil of Ocimum sanctum (Holybasil) and its possible mechanism of action. J. Ethnopharmacol. 54, 19–26. 10.1016/0378-8741(96)83992-4 [DOI] [PubMed] [Google Scholar]
- Slavenas J., Razinskaite D. (1962). Some studies on phytocidal substances of Juniperus oil from common Juniper. Leit TSR Moks Acad. Darbai Ser. 4, 63–64. [Google Scholar]
- Sonker N., Pandey A. K., Singh P. (2015). Efficiency of Artemisia nilagirica (Clarke) Pamp essential oil as a mycotoxicant against postharvest mycobiota of table grapes. J. Sci. Food Agric. 95, 1932–1939. 10.1002/jsfa.6901 [DOI] [PubMed] [Google Scholar]
- Sonker N., Pandey A. K., Singh P., Tripathi N. N. (2014). Assessment of Cymbopogon citratus (DC.) Stapf essential oil as herbal preservatives based on antifungal, antiaflatoxin and antiochratoxin activities and in vivo efficacy during storage. J. Food Sci. 79, M628–M634. 10.1111/1750-3841.12390 [DOI] [PubMed] [Google Scholar]
- Sylvestre M., Pichette A., Lavoie S., Longtin A., Legault J. (2007). Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrine (L). Coulter. Phytother. Res. 21, 536–540. 10.1002/ptr.2095 [DOI] [PubMed] [Google Scholar]
- Tan M., Zhou L., Qin M., Li D., Jiang W., Wang Y., et al. (2007). Chemical composition and antimicrobial activity of the flower oil of Russowia sogdiana (Bunge) B. Fedtsch. (Asteraceae) from China. J. Essent. Oil Res. 19, 197–200. 10.1080/10412905.2007.9699258 [DOI] [Google Scholar]
- Tiwari R., Dixit V., Dixit S. N. (1994). Studies on fungitoxic properties of essential oil of Cinnamomum zeylanicum Breyn. Ind. Perf. 38, 98–104. [Google Scholar]
- Tiwari R., Mishra D. N., Upadhyay P. S. (1988). Efficacy of some plant volatiles for the control of black mould of onion caused by Aspergillus niger Van.Teigh during storage. Nat. Acad. Sci. Lett. 11, 345–347. [Google Scholar]
- Tripathi N. N., Asthana A., Dixit S. N. (1984). Toxicity of some terpenoids against fungi infesting fruits and seeds of Capsicum annum L. during storage. Phytopathol. Z. 110, 328–335. 10.1111/j.1439-0434.1984.tb00072.x [DOI] [Google Scholar]
- Tripathi N. N., Dubey N. K., Dixit A., Tripathi R. D., Dixit S. N. (1983). Fungitoxic properties of Alpinia galanga oil. Trop. Plant. Sci. Res. 1, 49–52. [Google Scholar]
- Tripathi N. N., Kumar N. (2007). Putranjiva roxburghii oil- A potential herbal preservative for peanuts during storage. J. Stored Prod. Res. 43, 435–442. 10.1016/j.jspr.2006.11.005 [DOI] [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M. G., Venuti V., Cristani M., Daniele C., et al. (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49, 2474–2478. 10.1128/AAC.49.6.2474-2478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukeh D. A., Mordue A. J. (2009). Plant based repellents for the control of stored product insect pests. Biopestic. Int. 5, 1–23. [Google Scholar]
- Upadhyay P. S., Dixit K., Asthana A. (1985). Fungitoxic and phytotoxic properties of leaves of Citrus aurantifolia. Res. J. Plant Environ. 3, 17–19. [Google Scholar]
- Upadhyay P. S., Mall H. V., Renu, Tripathi N. N. (1987). Fungitoxic and phytotoxic properties of essential oil of Anisomeles indica, in Proceedings 72nd Indian Science Congress Association, 110–111. [Google Scholar]
- Vanneste J. L., Boyd R. J. (2002). Inhibition of Erwinia amylovora and potential antagonistic bacteria by essential oils and natural compounds. Acta Hortic. 590, 315–317. 10.17660/ActaHortic.2002.590.46 [DOI] [Google Scholar]
- Vasinauskiene M., Radusiene J., Zitikaite I., Surviliene E. (2006). Antibacterial activities of essential oils from aromatic and medicinal plants against growth of phytopathogenic bacteria. Agron. Res. 4, 437–440. [Google Scholar]
- Vukovic N., Milosevic T., Sukdolak S., Solujic S. (2008). The chemical composition of the essential oil and the antibacterial activities of the essential oil and methanol extract of Teucrium montanum. J. Serb. Chem. Soc. 73, 299–305. 10.2298/JSC0803299V [DOI] [Google Scholar]
- Wagacha J. M., Muthomi J. W. (2008). Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 124, 1–12. 10.1016/j.ijfoodmicro.2008.01.008 [DOI] [PubMed] [Google Scholar]