Abstract
Enzyme replacement therapy (ERT) can produce anti-drug antibody (ADA) responses that reduce efficacy or lead to hypersensitivity reactions. Six patients with severe mucopolysaccharidosis type I (MPS I/Hurler syndrome) who did not receive hematopoietic stem cell transplantation underwent an immunosuppression regimen prior to initiating ERT with laronidase. The primary endpoint for immune tolerance induction was the number of patients with an ADA titer ≤ 3200 after 24 weeks of laronidase at the labeled dose. Cyclosporine levels were measured weekly and doses adjusted to maintain trough levels above 400 mg/mL. A 6-week (Cohort 1) or 12-week (Cohort 2) immune tolerance induction period with cyclosporine (initial dose: 15 or 20 mg/kg/day), azathioprine (initial dose: 2.5 or 5 mg/kg/day) and low-dose laronidase infusions (0.058–0.29 mg/kg/week) was followed by an immune-challenge period with laronidase infusions at the labeled dose (0.58 mg/kg/week) for 24 weeks. Anti-laronidase IgG titers were determined following treatment. There were 147 treatment-emergent adverse events reported, most of which were mild and not related to the study treatment. While there was no evidence of immune tolerance in 3 of 3 patients in Cohort 1, there were some indications of immune tolerance induction in 2 of 3 patients in Cohort 2. Patients with lower ADA titers showed greater reductions in urinary glycosaminoglycan excretion. Routine monitoring of plasma cyclosporine parent-compound levels by high pressure liquid chromatography proved difficult for clinical practice. The evolving clinical management of MPS I and a better understanding of the clinical impact of laronidase-related immunogenicity require reassessment of immune modulation strategies in patients with MPS I receiving laronidase treatment. Clinical Trial Registration: NCT00741338.
Keywords: Mucopolysaccharidosis type I, Enzyme replacement therapy, Immune tolerance, Laronidase, Hurler syndrome, Anti-drug antibodies
1. Introduction
Mucopolysaccharidosis type I (MPS I) is an inherited metabolic disease caused by deficiency of the lysosomal enzyme α-l-iduronidase, which removes iduronic acid from the non-reducing ends of dermatan sulfate and heparan sulfate during the step-wise catabolism of glycosaminoglycans (GAGs). Accumulation of non-metabolized GAG fragments leads to a cascade of secondary pathophysiologic events that results in multisystem clinical manifestations, including coarse facies, corneal clouding, short stature, valvular heart disease, joint contractures, hepatosplenomegaly, airway obstruction, restrictive lung disease, skeletal disease, and cognitive impairment [7]. The disease occurs along a continuum of severity and is represented by three main clinical phenotypes: Hurler syndrome (severe MPS I with somatic involvement before 6 months of age, progressive cognitive impairment, and death during the first decade, if untreated), Hurler-Scheie syndrome (attenuated, intermediate MPS I with somatic involvement beginning in early childhood that can be severe and lead to early mortality), and Scheie syndrome (attenuated, least severe MPS I, with somatic involvement presenting in late childhood or adolescence, and normal life expectancy) [5].
Treatments for MPS I that address the underlying enzyme deficiency include hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT) with recombinant human α-l-iduronidase (laronidase/Aldurazyme®, EC 3.2.1.76). HSCT can prevent GAG accumulation in the CNS and is considered the treatment of choice for patients with severe MPS I who are < 2 years and have normal developmental quotients [23]. Laronidase does not cross the blood-brain barrier and is used to treat the non-neurological manifestations of MPS I [8], [15]. Laronidase is also used in the peri-transplant period as an adjunctive therapy to improve the clinical status of patients prior to HSCT and to provide a source of enzyme until bone marrow engraftment [9], [12]. In a study of MPS I patients less than age 5, most of whom had Hurler syndrome, laronidase was found to have a similar safety profile and pharmacodynamic effect as in attenuated patients [27]. Emerging long-term follow up data suggest that severe MPS I patients treated exclusively with ERT from a young age have increased overall survival compared to untreated patients [13].
Anti-drug antibodies (ADA) may develop in patient receiving ERT. > 90% of patients with MPS I develop immunoglobulin G (IgG) antibodies within the first few months of starting laronidase treatment [28], particularly patients with severe MPS I carrying 2 null mutations [27], and higher antibody titers have been associated with less urinary GAG (uGAG) reduction. Long-term immune response studies in patients with attenuated MPS I [8], [16] demonstrated that ADA titers generally decline over time, with some patients becoming seronegative indicating the development of natural immune tolerance with long-term laronidase therapy. The current study was undertaken to determine the safety and effectiveness of a prophylactic immunosuppressive regimen using cyclosporine (CsA) and azathioprine (AzA) in treatment-naïve patients with severe MPS I caused by 2 nonsense mutations, a sub-group of patients that consistently generated high antibody titers to laronidase treatment [27]. This immunosuppressive protocol was shown to successfully induce immune tolerance in a canine model of MPS I [17]. Since the completion of this study in 2008, significant changes in standard of care have occurred in patients with severe MPS I, with HSCT accepted as a first-line therapy for these patients [2], [6], including patients in less developed countries. Consequently, the study was terminated early, and the results are discussed in the context of current treatment guidelines.
2. Methods
2.1. Patients, study design, and criteria for success
This open-label, Phase 2 trial enrolled patients ≤ 5 years of age with severe MPS I who had no prior exposure to laronidase and 2 nonsense mutations in the IDUA gene. Exclusion criteria included prior HSCT, recent vaccination, homozygous thiopurine S-methyltransferase deficiency, and a history of tuberculosis. A group of 12 patients with severe MPS I meeting the same inclusion criteria and with antibody titers > 12,500 after 26 weeks of laronidase treatment [protocol ALID-014-02 [27]] served as a historical control.
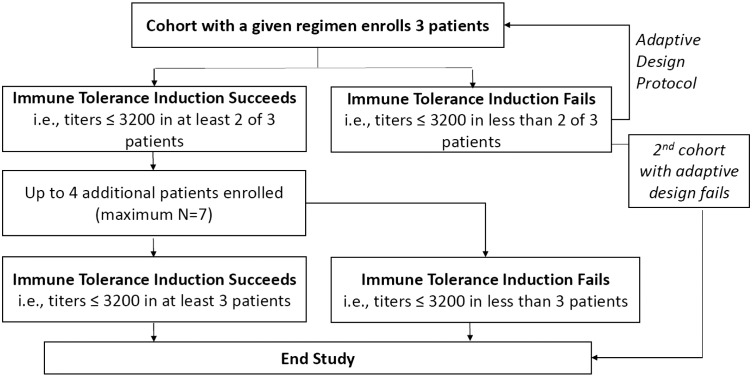
A sequential group adaptive design (Fig. 1) specified enrollment of up to 2 patient cohorts of 3 patients each, with expanded enrollment should the criterion for successful immune tolerance be met. Patients underwent a Tolerance Induction Period (TIP) of up to 19 weeks of low-dose laronidase, followed by a 24-week Immune Challenge Period (ICP) of full-dose laronidase treatment. Successful immune tolerance was established if ≥ 2 of 3 patients per cohort had antibody titers ≤ 3200 at the end of the ICP. This titer was selected since it was one dilution above the ≤ 1600 titer (assay variability is ± one dilution) that correlated with consistently robust uGAG clearance in patients from the Phase 2 clinical trial used as the historical control group [27].
Fig. 1.
Flow of patients through study per adaptive design protocol.
2.2. Treatment periods
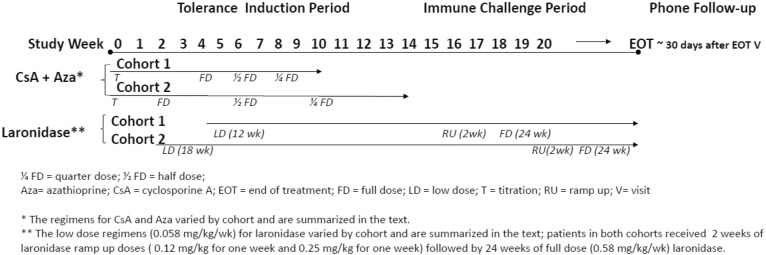
Fig. 2 shows a schematic of study treatment for both cohorts. For both cohorts, the TIP began with oral CsA (Neoral®) dose titration in order to reach the target minimum plasma trough level. During the TIP for Cohort 1, CsA and oral Aza, (Imuran®) were administered at starting doses of 15 mg/kg/day and 2.5 mg/kg/day, respectively, and titrated weekly to achieve a target trough level of at least 350 ng/mL, and preferably 400 ng/mL for CsA. For Cohort 2, starting doses were increased to 20 mg/kg/day CsA and 5 mg/kg every other day Aza. The risk of these high doses to patients was considered acceptable given the short-term exposure. Sulfisoxazole/trimethoprim (Bactrim®) was administered during the TIP for infection prophylaxis at a dose of 5 mg/kg/day on 2 consecutive days per week. To reach and maintain the target CsA concentration, patients in Cohort 1 were titrated over 4 weeks to the full dose of CsA plus Aza and remained at the full dose for 2 weeks, followed by a half-dose for 2 weeks and a quarter-dose for 2 weeks. After the CsA trough level was reached and maintained for at least 1 week, patients began low-dose laronidase infusions at 0.058 mg/kg once per week while continuing CsA. Low-dose laronidase infusions were continued during a 5-week CsA/Aza washout period. Following the adaptive design, CsA dose titration was 2 weeks in Cohort 2, and the durations of the full-, half- and quarter-dose periods were doubled from 6 weeks total for these periods in Cohort 1 to 12 weeks. The TIP duration was 12 weeks for Cohort 1 and 18 weeks for Cohort 2.
Fig. 2.
Schematic of study treatments for both cohorts.
The ICP was 26 weeks, which included a 2-week ramp-up period of 2 laronidase doses (0.12 mg/kg and 0.25 mg/kg for one week each) followed by 24 weeks of full-dose laronidase (0.58 mg/kg). The planned dose and weekly regimen for laronidase in the ICP were identical to those that had been used for the historical control group [27].
2.3. Laboratory measurements
Protocol-specified CsA levels were measured using a parent compound-specific HPLC assay in a central laboratory (ARUP Laboratories, USA). Serum samples for anti-laronidase IgG antibody (ADA) testing using ELISA [8] were obtained at baseline (i.e., TIP Week 0 for Cohorts 1 and 2) and prior to each laronidase infusion at biweekly visits throughout the period when laronidase was given. Urinary GAG levels were determined by a dye-binding assay [11].
2.4. Safety monitoring
Since the combination of CsA and Aza used in this study had not previously been used in patients with MPS I, patients were closely monitored for any immunosuppressive-related adverse events, which included regular physical exams, lab evaluations (serum creatinine, blood urea nitrogen, and blood pressure for renal function; liver function tests; and platelet count and absolute neutrophil counts for bone marrow status) and regular evaluations for possible infections.
3. Results
Five male and 2 female patients were enrolled from 2 study sites in Brazil and Russia beginning in September 2008. Six patients received treatment in the TIP and ICP stages of the study, and 5 patients completed the study. Cohort 1 consisted of 3 patients, (patients 1-1, 1-2, and 1-3) with a mean (min, max) age of 2.6 years (1.8, 3.5), all of whom completed the study. Each patient in Cohort 1 was homozygous for the W402X mutation. Four patients were enrolled in Cohort 2 (patients 2-1, 2-2, 2-3, and 2-4) and 3 received treatment. Patient 2-2 was a screen failure due to a tuberculosis infection and never received treatment. All treated patients in Cohort 2 were homozygous for the Q70X mutation. The mean (min, max) age for patients in Cohort 2 was 3.77 years (3.0, 4.2). Among the treated patients, 2 completed the study and patient 2-3 discontinued early following an infusion-associated reaction (IAR, discussed under safety).
3.1. Immunosuppressant regimens
The total duration of immunosuppressants was 10, 10, and 9 weeks, respectively, for the 3 patients in Cohort 1. Only 1 patient in Cohort 1 (Patient 1-3) maintained the required minimal CsA trough level of 350 ng/mL. Two patients in Cohort 2 maintained the target CsA trough level.
3.2. Antibody titers
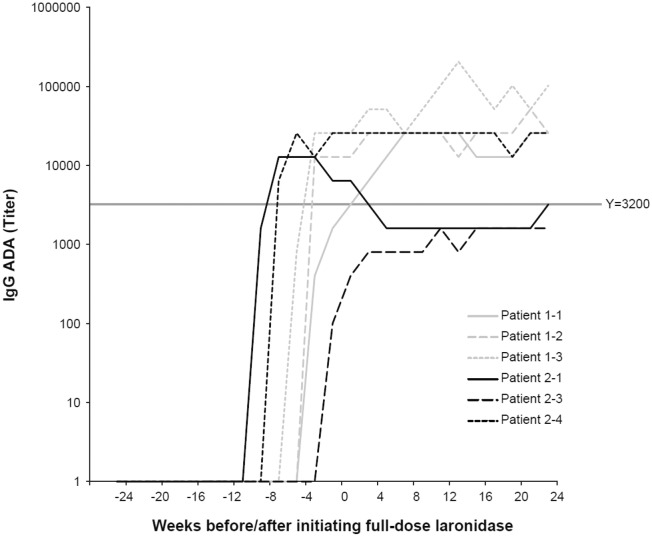
None of the patients enrolled in Cohort 1 met the success criterion for immune tolerance induction as all anti-laronidase IgG titers were > 3200 following 24 weeks of full-dose laronidase (Fig. 3). The IgG titer profiles of patients in this cohort were similar to those of the historical control group. ADAs were first detected after 10 weeks of low-dose laronidase infusions and ranged from 3200 at Week 10 to 102,400 at the end of treatment. Peak titers ranged from 25,600 to 204,800 and were reached following 20–34 weeks of laronidase treatment.
Fig. 3.
Antibody titers over time in Cohort 1 and Cohort 2 patients. Full-dose laronidase was initiated at Week 0. In Cohort 1, low-dose laronidase began at Week − 11 (Patients 1-1 and 1-3) and Week -14 (Patient 1-2); and cyclosporine A and azathioprine treatments were completed at Week − 9. In Cohort 2, low-dose laronidase began at Week − 17, and cyclosporine A and azathioprine treatments were completed at Week-7.
IgG titer profiles for the treated patients in Cohort 2 are shown in Fig. 3. Patient 2-1 had an antibody titer of 3200 at the end of treatment and was the only patient in the trial to meet the success criterion for immune tolerance induction. Anti-laronidase IgG titers were first detected in this patient at TIP visit 12, reached a peak titer of 12,800 at visit 14, and then declined and stabilized below or at a titer of 3200 throughout the remainder of the study. Patient 2-3 was on track to meet the success criterion, but discontinued treatment at Week 17 in the ICP due to an IAR (as discussed under Safety). A follow-up Week 24 titer measurement was 1600, but because of early discontinuation from the study, this patient's results were considered non-evaluable. This patient did not meet the CsA minimum trough level of 350 ng/mL until 1 week after the start of laronidase dosing. Seroconversion occurred 1 week before the ICP started. The patient had a peak titer of 1600 at the ICP Week 11 visit and titers remained low during the remainder of the ICP. Patient 2-4 met the CsA target trough level but had an end-of-treatment titer of 25,600, and thus, did not meet the immunosuppression success criterion.
3.3. uGAG levels following laronidase treatment
The uGAG levels were reduced from screening/baseline to end of treatment by 43.8%, 61.7%, 6.7%, 84.2%, 62.5%, and 72.5% for Patients 1-1, 1-2, 1-3, 2-1, 2-3, and 2-4, respectively. The greatest uGAG reductions occurred in patients with the lowest ADA titers. None of the uGAG levels reached normal levels. For Patient 1-3, uGAG values varied 10-fold between screening and baseline measurements collected before the first dose of laronidase was administered. These variations likely resulted from the testing of a dilute urine sample that was not from the first morning void. For Patient 2-3, who discontinued early, the 62.5% reduction was from a uGAG sample collected approximately 1 month after the last laronidase dose. In contrast, the uGAG reduction recorded 1 week after this patient's last laronidase infusion was 74.3%, suggesting a rebound in uGAG following the interruption.
3.4. Safety
Overall, 147 adverse events were reported in 6 patients, and most were mild and not related to any study treatments. There were no deaths or anaphylactic reactions. Twenty-nine events in 4 patients were assessed as related to laronidase and included abdominal pain (8), nausea (1), vomiting (1), rash (2), urticaria (3), alopecia (1), erythema (1), prurigo (1), pruritus (2), papular rash (1), pyrexia (4), chills (2), anxiety (1), and cough (1).
Four patients experienced 9 serious adverse events that were considered unrelated to laronidase. None of these events led to permanent treatment discontinuation, and all patients recovered without sequelae. Patient 1-2 experienced neutropenia (with an absolute neutrophil count of 490/mm3) at Week 2, central line infection at Week 10, and sinusitis at Week 34. All occurred after initiation of immunosuppressive therapy, but prior to administration of laronidase. The neutropenia triggered a treatment-stopping rule, following which the patient recovered and resumed treatment. The investigator assessed that CsA and Aza treatments were probably related to the neutropenia and possibly related to the central line infection. Patient 1-3 experienced 2 events of bronchopneumonia and 1 event of bronchospasm crisis, and Patient 2-1 had two events of viral respiratory tract infection, in all cases after initiation of laronidase treatment. Patient 2-3 was diagnosed with right-sided pneumonia 7 days after receiving the first doses of immunosuppressants. Immunosuppressant administration was temporarily interrupted and the patient was treated and recovered within 11 days. The pneumonia was assessed as unrelated to any study treatment. At ICP Week 17, this patient had IARs of moderate pruritus and urticaria, which were assessed as non-serious and probably related to laronidase. The laronidase infusion was interrupted, the patient was treated with antihistamine, and the IARs resolved completely on the same day. A sample drawn 1 week prior to the IAR tested positive for complement activation and had an elevated serum tryptase level. Samples drawn 2 days before and 4 days after the IARs were positive for laronidase-specific IgE antibodies. An allergy consultation was obtained, and skin testing recommendation was declined by the family and investigator. Therapy with laronidase was not resumed, and the patient withdrew from the study.
4. Discussion
At the time the present study began, a risk/benefit shift was taking place with regard to the clinical management of patients with severe MPS I [23]. A large case series from the 1980′s and 1990's at the University of Minnesota had reported a 5-year alive and engrafted rate for matched related donors of 53% [26], and a 2-year alive and engrafted rate for matched unrelated donors of only 18% [25]. Several technical improvements have since led to a decline in procedure-related morbidity and mortality of HSCT, such that in experienced transplant centers, the 5-year alive and engrafted rate for matched related and unrelated donors is now approximately 90% [1]. Consequently, HSCT has become more broadly used both throughout developed countries as well as in developing countries. The primary role of ERT in patients with severe MPS I has evolved into its use during the peri-transplant period to improve the clinical status of patients by reducing GAG storage prior to HSCT until engraftment [9], [12], [29].
However, HSCT is not always the primary treatment option for patients with severe MPS I. In some countries, the logistical and clinical challenges associated with HSCT remain high and families may decide not to take the risk [10]. In addition, there are individuals with severe MPS I for whom HSCT is not a treatment option due to their age, compromised physical state, or the lack of a suitable donor. Thus, patients with severe MPS I may still receive only ERT, and understanding the assessment of immune responses in these patients remains relevant.
One patient in our study showed induction of immune tolerance, while another patient was on track to meet the success criterion, but discontinued laronidase treatment prematurely. Cohort 1 patients had reductions in uGAG similar to those of the historical control group, whereas greater reductions were observed in Cohort 2 patients. Statistical analysis of the uGAG results was not performed due to the small number of patients. The immunosuppressant regimen was generally well tolerated, with no correlation of antibody titers with adverse events. The decision to terminate the study was due to changing standards of care for this patient population, and the practical difficulty of routine monitoring of plasma CsA parent compound levels in the general clinical setting. Overall, the safety profile of laronidase was consistent with the known safety and tolerability profile in the historical control group [27].
A meta-analysis of data from 73 patients with severe or attenuated MPS I who received treatment with laronidase in clinical trials [30] has provided further insight into the relationship between uGAG levels, antibody titers, and efficacy outcomes, and confirmed earlier findings that antibody titers diminish over time during laronidase ERT [8], [16]. A consistent, statistically significant inverse correlation between percent reduction in uGAG levels and both IgG antibody titer and exposure to antibodies that inhibit cellular enzyme uptake was demonstrated. However, there was no apparent relationship between IgG antibody titer and clinical outcomes (i.e., 6-minute walk test, pulmonary function as measured by percent predicted forced vital capacity, and liver volume) in attenuated patients. Patients with Hurler syndrome tended to seroconvert earlier than patients with attenuated MPS I, and ADA titers tended to be slightly higher on average, particularly in patients with 2 nonsense mutations. However, a clear relationship between ADA titers and clinical outcome or the potential for allergic reactions when classifying patients by clinical diagnosis of disease phenotype could not be confirmed. These results suggest that for patients treated with laronidase without immune tolerance induction, the risk/benefit profile over the duration of treatment is acceptable. A recent publication evaluating cross-sectional data from 2 prospective open-label studies showed that a persistent antibody response might be related to an impaired biomarker response, but as in the meta-analysis, there was no correlation with clinical outcomes [20]. On the other hand, data from sleep oximetry studies conducted in 61 patients with MPS I (44 with severe, and 17 with attenuated disease) showed a higher rate of sleep apnea/airway obstruction and need for intervention in patients receiving ERT who had a strong inhibitory antibody response, suggesting a potential benefit of immune tolerance [24].
It is not known whether immunosuppressive therapy is warranted for all ERTs, as immune responses and consequences vary widely among LSDs. In patients with MPS IVA (Morquio A syndrome) receiving ERT with elosulfase alfa (Vimizim®), higher total ADA titers and/or neutralizing antibody positivity rates were not associated with a reduced treatment effect or with an increased incidence or severity of hypersensitivity events or anaphylaxis [14]. Drug-specific IgE positivity also was not associated with anaphylaxis or treatment withdrawal. Comparable immunogenicity profiles were maintained for patients undergoing long-term ERT. In contrast, some patients with glycogen storage disease type II (Pompe disease) treated with alglucosidase alfa (Myozyme® and Lumizyme®) develop high and sustained antibody titers that diminish ERT responses [4], [19]. This is typically observed in patients with classical infantile Pompe disease who have cross-reactive immunological material (CRIM)-negative status (i.e., no endogenous enzyme), which is recognized as a poor prognostic factor for ERT. Reducing the antibody response with a targeted immune tolerance regimen (i.e., rituximab/methotrexate to eliminate alglucosidase alfa-sensitized B cells) improved ERT efficacy in a small group of ERT-naïve infants with Pome disease [21], [22]. Immunomodulation therapy has also been successful for patients who develop high ADA titers during ERT for Pompe disease using a regimen based on bortezomib in combination with rituximab, methotrexate, and intravenous immunoglobulin [3]. More recently, another strategy of prophylactic immune modulation has been reported in infantile Pompe disease using short term, low-dose methotrexate for immune tolerance induction [18]. Early data suggest that this regimen is simple, efficacious, and safe. The authors propose that a short course of this inexpensive and widely accessible regimen may have broader application to other enzyme replacement therapies. The need for active immune tolerization will require careful consideration of the disease severity and prognostic factors, the impact of ADA on ERT safety and efficacy, and the risk/benefit ratio of the immune tolerance regimen.
5. Conclusions
Novel strategies for immunosuppressive regimens that are safe in pediatric patients and that do not permanently impair the developing immune system and the response to vaccines has emerged as a clinical need for certain LSDs being treated with ERT. The use of immunosuppression regimens in patients with severe MPS I warrants consideration given the current treatment guidelines for HSCT and the use of peri-transplant laronidase treatment in patients with severe MPS I. Our study was terminated early with inconclusive results due to changing standards of care for this patient population, and due to the practical difficulty of routine monitoring plasma CsA parent compound levels in the general clinical setting. Assessment of the need of immunosuppressive therapy in pediatric patients with LSDs must be carefully considered and an individualized risk-based approach is needed for each disease and each treatment. The evolving clinical management paradigm and better understanding of the impact of laronidase-related immunogenicity requires a reassessment of immune modulation strategies in patients with severe MPS I who receive treatment with laronidase.
Details of the contributions of individual authors
YX, SR, and GC contributed to study design, execution, data analysis and interpretation, and manuscript writing. RG, TAV, MVMR, CGC, VYV, and ANS participated in the collection and analysis of data as well as in the planning and writing of the manuscript.
Competing interest statements
YX, SR, and GC were employees of Sanofi Genzyme at the time of the study completion, with stock holdings. MVMR is currently an employee of Sanofi Genzyme. RG received reimbursements for attending symposia, speaking and consulting fees, and research funds from Genzyme and BioMarin. TAV, CGC, VYV, and ANS have no competing interests to declare
Funding
This study was funded by Sanofi Genzyme and Biomarin Pharmaceutical, Inc. The external authors confirm independence from the sponsor and that the content of the article has not been influenced by the sponsor.
Ethics
Institutional Review Board or Ethics Committee at both sites approved the protocol, and all patients provided written informed consent prior to inclusion in the study.
Acknowledgements
The authors thank the patients and their families for their participation. We also wish to thank Petr Novikov (Research and Clinical Institute for Pediatrics at the Pirogov Russian National Research Medical University, Moscow, Russia) for assistance during the clinical trial, and Jonathan Fallet and Patrice Ferriola, PhD for assistance in the preparation of the manuscript.
References
- 1.Aldenhoven M., Jones S.A., Bonney D. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol Blood Marrow Transplant. 2015;21:1106–1109. doi: 10.1016/j.bbmt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Aldenhoven M., Wynn R.F., Orchard P.J. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood. 2015;125:2164–2172. doi: 10.1182/blood-2014-11-608075. [DOI] [PubMed] [Google Scholar]
- 3.Banugaria S.G., Prater S.N., McGann J.K. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med. 2013;15:123–131. doi: 10.1038/gim.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banugaria S.G., Prater S.N., Ng Y.K. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck M., Arn P., Giugliani R. The natural history of MPS I: global perspectives from the MPS I registry. Genet Med. 2014;16:759–765. doi: 10.1038/gim.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boelens J.J., Aldenhoven M., Purtill D. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke L., Heppner J. Mucopolysaccharidoses Type I. Gene Reviews. 2015 http://www.ncbi.nlm.nih.gov/books/NBK1162/?report=printable [Internet] R. Pagon, A. MP and A. HH. Seattle (WA), University of Washington, Seattle. [Google Scholar]
- 8.Clarke L.A., Wraith J.E., Beck M. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123:229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- 9.D'Aco K., Underhill L., Rangachari L. Diagnosis and treatment trends in mucopolysaccharidosis I: findings from the MPS I registry. Eur. J. Pediatr. 2012;171:911–919. doi: 10.1007/s00431-011-1644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dornelles A.D., Lapagesse de Camargo Pinto L. Enzyme replacement therapy for Mucopolysaccharidosis Type I among patients followed within the MPS Brazil Network. Genet. Mol. Biol. 2014;37:23–29. doi: 10.1590/s1415-47572014000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong J.G., Wevers R.A., Liebrand-van Sambeek R. Measuring urinary glycosaminoglycans in the presence of protein: an improved screening procedure for mucopolysaccharidoses based on dimethylmethylene blue. Clin. Chem. 1992;38:803–807. [PubMed] [Google Scholar]
- 12.de Ru M.H., Boelens J.J., Das A.M. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure. Orphanet J Rare Dis. 2011;6:55–62. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisengart J., Shapiro E.G., Rudser K. Clinical outcomes of Hurler syndrome treated exclusively with enzyme replacement therapy from a young age. Mol. Genet. Metab. 2015;114:S40. [Google Scholar]
- 14.Hendriksz C.J., Burton B., Fleming T.R. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jameson E., Jones S., Wraith J.E. Enzyme replacement therapy with laronidase (Aldurazyme) for treating mucopolysaccharidosis type I. Cochrane Database Syst. Rev. 2016;4 doi: 10.1002/14651858.CD009354.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Kakavanos R., Turner C.T., Hopwood J.J., Kakkis E.D., Brooks D.A. Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I. Lancet. 2003;361:1608–1613. doi: 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- 17.Kakkis E., Lester T., Yang R. Successful induction of immune tolerance to enzyme replacement therapy in canine mucopolysaccharidosis I. Proc. Natl. Acad. Sci. U. S. A. 2004;101:829–834. doi: 10.1073/pnas.0305480101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazi Z., Desai A., Erwin A. Prophylactic immune modulation in infantile Ρompe disease using low-dose methotrexate induction: a safe, inexpensive, widely accessible, and efficacious strategy. Mol. Genet. Metab. 2016;117:S65–S66. [Google Scholar]
- 19.Kishnani P.S., Goldenberg P.C., DeArmey S.L. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langereis E.J., van Vlies N., Church H.J. Biomarker responses correlate with antibody status in mucopolysaccharidosis type I patients on long-term enzyme replacement therapy. Mol. Genet. Metab. 2015;114:129–137. doi: 10.1016/j.ymgme.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Mendelsohn N.J., Messinger Y.H., Rosenberg A.S., Kishnani P.S. Elimination of antibodies to recombinant enzyme in Pompe's disease. N. Engl. J. Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- 22.Messinger Y.H., Mendelsohn N.J., Rhead W. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet. Med. 2012;14:135–142. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muenzer J., Wraith J.E., Clarke L.A. Mucopolysaccharidosis I: management and treatment guidelines. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 24.Pal A.R., Langereis E.J., Saif M.A. Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome. Orphanet J Rare Dis. 2015;10:42. doi: 10.1186/s13023-015-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters C., Balthazor M., Shapiro E.G. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 26.Peters C., Shapiro E.G., Anderson J. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 27.Wraith J.E., Beck M., Lane R. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human alpha-l-iduronidase (laronidase) Pediatrics. 2007;120:e37–e46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
- 28.Wraith J.E., Clarke L.A., Beck M. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-l-iduronidase (laronidase) J. Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Wynn R.F., Mercer J., Page J., Carr T.F., Jones S., Wraith J.E. Use of enzyme replacement therapy (laronidase) before hematopoietic stem cell transplantation for mucopolysaccharidosis I: experience in 18 patients. J. Pediatr. 2009;154:135–139. doi: 10.1016/j.jpeds.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Xue Y., Richards S.M., Mahmood A., Cox G.F. Effect of anti-laronidase antibodies on efficacy and safety of laronidase enzyme replacement therapy for MPS I: a comprehensive meta-analysis of pooled data from multiple studies. Mol. Genet. Metab. 2016;117:419–426. doi: 10.1016/j.ymgme.2016.02.006. [DOI] [PubMed] [Google Scholar]