| Summary: |
The invention in this patent application
relates to novel 3-(indol-3-yl)pyridine derivatives represented generally
by Formula (I) that possess activities as TDO2 inhibitors. These compounds
may be useful for the treatment and/or prevention of cancer, neurodegenerative
disorders such as Parkinson’s disease, Alzheimer’s disease,
and Huntington’s disease, chronic viral infections such as
HCV and HIV, depression, and obesity. |
| Some
sites in the human body (e.g., the brain, the eye, the testis, and
the placenta) can tolerate the presence of foreign antigens without
causing an inflammatory immune response. This behavior is known as
immune privilege. Thus, tissue grafts, which are normally attacked
by the immune system, can survive in these immune privileged sites
for extended periods of time without being rejected. It is believed
that immune privilege occurs in these sites because their tissues
perform unique functions and cannot be regenerated if they were damaged
by a responsive immune reaction. |
|
L-Tryptophan (Trp) is an essential amino acid that performs a variety
of important functions within the cell. Besides its typical role in
protein synthesis, it is also utilized in the biosynthesis of nicotinamide
adenine dinucleotide (NAD+), the neurotransmitter serotonin,
and the hormone melatonin in the pineal gland. Earlier studies have
indicated that Trp catabolism is essential to maintaining the immune
privilege of the placenta, but recent growing evidence implicates
Trp catabolism in survival of cancer cells and immune evasion. |
| Tryptophan is catabolized in the local microenvironment
of tumors, immune-privileged sites, or sites of inflammation. The
degradation and depletion of Trp and accumulation of its metabolites
in these sites create an immunosuppressive environment that shuts
down antitumor immune responses in tumors and in tumor-draining lymph
nodes by inducing T-cell anergy and apoptosis. |
| The major Trp catabolism pathway in mammals is known as the kynurenine
pathway. The first and rate limiting step in this pathway, which converts
Trp into N-formylkynurenine is catalyzed by specific
enzymes that may vary in their expression levels or with the cell
type. These enzymes include indoleamine-2,3-dioxygenases (IDO1 and
IDO2) and tryptophan 2,3-dioxygenase (TDO2). TDO2 is a member of the
oxidoreductases family, and it is normally expressed in the liver
to regulate systemic Trp levels. Recent studies have revealed that
TDO2 is constitutively expressed in a wide variety of cancer cells,
such as bladder carcinoma, hepatocarcinoma, melanoma, mesothelioma,
neuroblastoma, sarcoma, breast carcinoma, leukemia, renal cell carcinoma,
colorectal carcinoma, head and neck carcinoma, lung carcinoma, brain
tumor, glioblastoma, astrocytoma, myeloma, and pancreatic carcinoma.
The TDO2-catalyzed catabolism of Trp in tumor cells is responsible
for immune response suppression and consequently prevention of tumor
rejection. This in turn promotes the survival, growth, invasion, and
metastasis of malignant cells. Studies using the P815 mastocytoma
tumor model provided the first clear evidence of the role of TDO2
in regulating tumor growth. These studies have shown that inhibition
of TDO2 decreases tumor growth in P815 mTDO2 implanted tumors. |
| The following observations provide additional evidence
on the role of TDO2 and Trp catabolism in immune response suppression
in different tumors and cancers: |
TDO2 is highly expressed in hepatocellular
carcinoma (HCC). The inhibition of TDO2 could lead to increased Trp
concentration and decreased downstream production of Trp metabolites
and hence may be useful for the treatment of liver diseases that can
progress to the stage of liver carcinoma. Evidence shows that increased
Trp concentration can benefit conditions such as cirrhotic livers.
Evidence also shows that increased levels of serum Trp metabolites
causes negative effects, for example, increased levels of the Trp
metabolite, quinolinic acid correlates with hepatic dysfunction in
patients with liver cirrhosis, while indole-3-lactic acid is associated
with alcohol-induced liver disease in mice. TDO2 is also expressed in neurons, microglia, and astrocytes. Overexpression
of TDO2 in the glioma cells produces the Trp metabolite kynurenine
(Kyn) that activates the aryl hydrocarbon receptor (AHR). This TDO-AHR
pathway is active in brain tumors, and it is implicated in malignant
progression and poor patient survival. In addition, tryptophan catabolism
occurs in microglia cells and causes the accumulation of quinolinic
acid, which is believed to be associated with a malignant phenotype. TDO2 mRNA is found in tumors such as breast
carcinoma, bladder, renal cell, pancreatic, colorectal, head and neck
carcinoma, lung carcinoma, and melanoma. Enhanced Trp catabolism is observed in different forms of gynecological
cancers such as ovarian carcinoma, cervical cancer, and endometrial
cancer. Inflammatory mediators, particularly
IFN-gamma, induce Trp catabolism as an endogenous mechanism to restrict
excessive immune responses and prevent immunopathology. However, there
is a mounting evidence that suppression of antitumor immune responses
in precancerous lesions and established cancers by Trp catabolism
promotes tumor growth. |
| These studies and observation provide a compelling rational that
shows the great potential and increased importance of the inhibition
of Trp catabolism as an attractive biological target for therapeutic
intervention against cancer. Therefore, a considerable research has
been directed toward achieving this goal through identifying selective
and efficient inhibitors of TDO2 that may be used to block the Trp
catabolism and possibly provide effective treatment for cancer. |
| In addition to their potential as novel cancer treatment,
TDO2 inhibitors may also provide promising and needed treatments for
neurological and brain disorders. TDO2 is expressed in neurons and
brain vasculature and additionally in astroglial cells in the case
of schizophrenia. The inhibition of the kynurenine pathway has become
a viable therapeutic target to develop medications to treat cognitive
diseases such as bipolar disorder and Tourette syndrome, neurodegenerative
disorders such as Alzheimer’s disease, and motor neuron diseases
including Amyotrophic lateral sclerosis, Multiple sclerosis, and Huntington’s
and Parkinson’s diseases. |
| HIV-associated
neurocognitive disorders (HAND) are caused by cognitive changes related
to Trp catabolism in patients infected with human immunodeficiency
virus type-1 (HIV-1). In addition, T cell hyporesponsiveness has been
recently associated with the Trp catabolic pathway in HIV-infected
patients, and this may also be associated with other chronic viral
infectious diseases such as Hepatitis C. |
| While there are several known TDO2 inhibitors, they either suffer
from limited affinity for the target or possess unfavorable pharmacokinetic
properties that make them unsuitable to be developed as drugs for
human use. |
| Therefore, there exists a need
for the discovery and development of new TDO2 inhibitors with improved
efficacy such as the compounds described in this patent application
for the treatment and/or prevention of cancer and possibly for the
treatment of several neurodegenerative disorders and chronic viral
infections such as HIV and HCV. |
| Important
Compound Classes: |
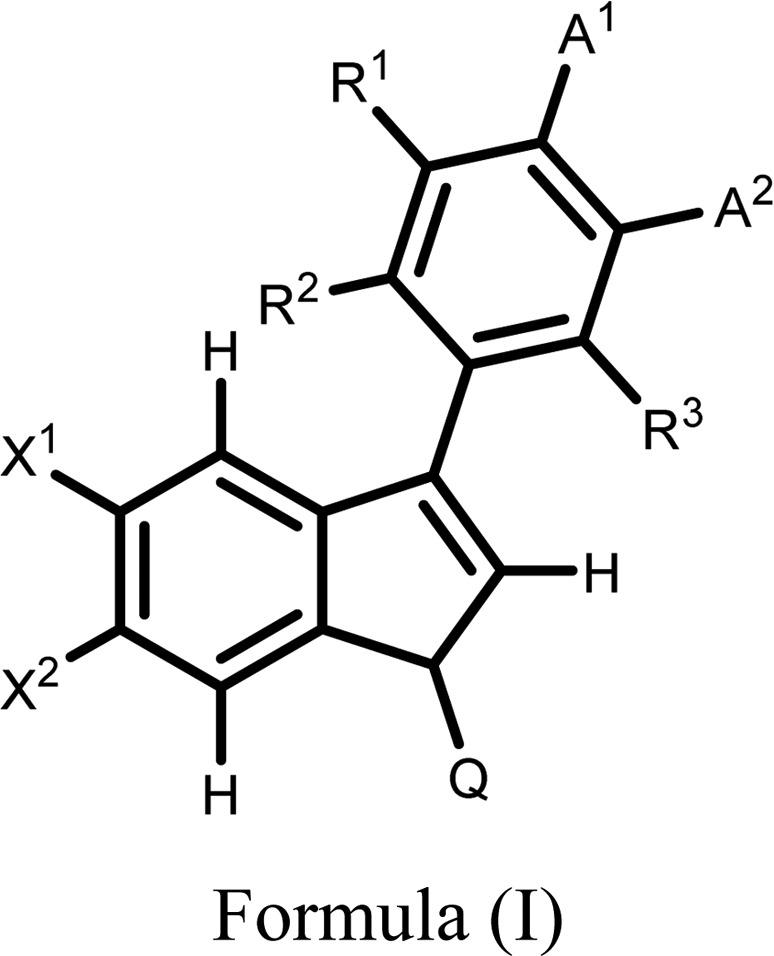 |
| Key Structures: |
The inventors described
the structures and methods of synthesis of 153 compounds of Formula
(I) including the following representative examples: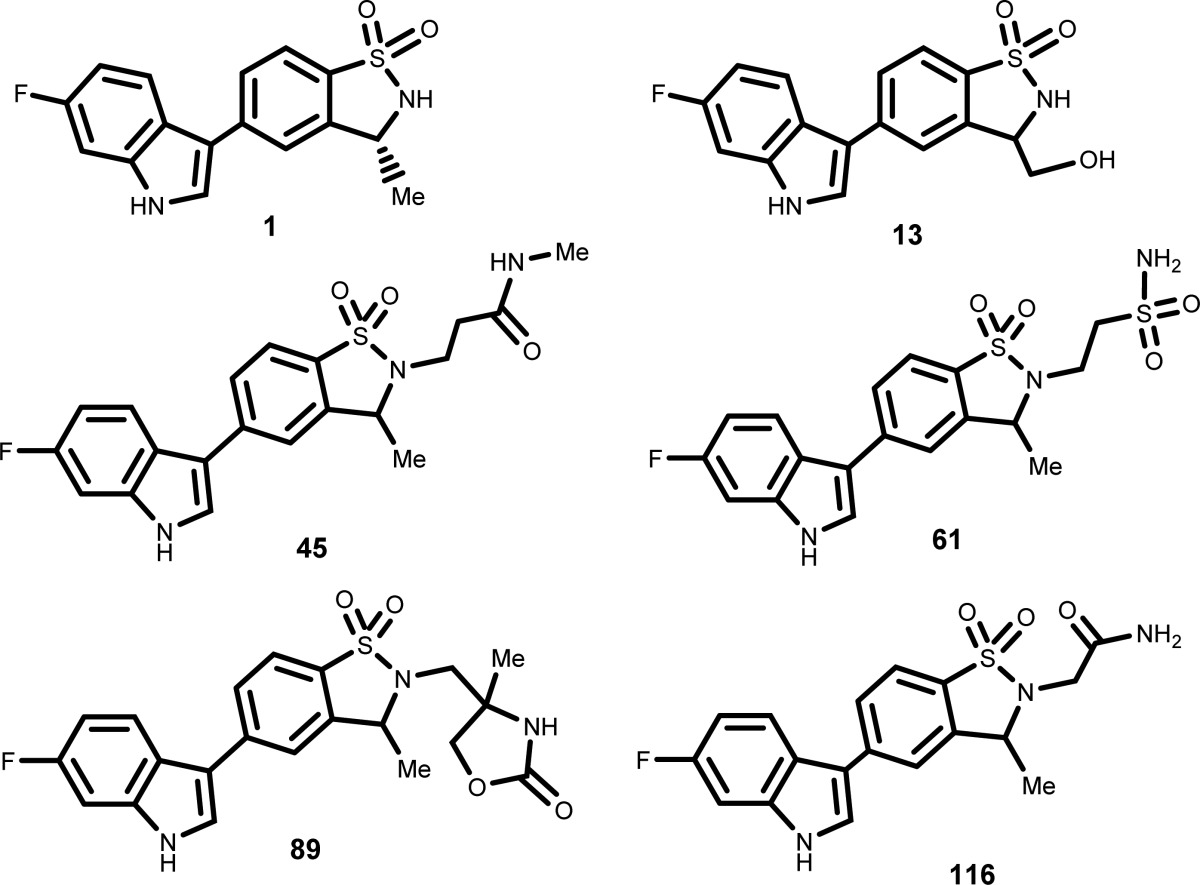
|
| Biological Assay: |
-
1.
Assay for TDO2 Enzymatic
Activity Determination
-
2.
Cellular Assay for TDO2 Activity Determination
-
3.
Pharmacodynamic Assay for TDO2 in Vivo
Activity Determination: Increase of Blood Tryptophan Levels in Mice
|
| Biological Data: |
The biological data obtained from testing the above
representative compounds in human brain glioblastoma cells using the
Cellular Assay for TDO2 Activity Determination are summarized in the
following table: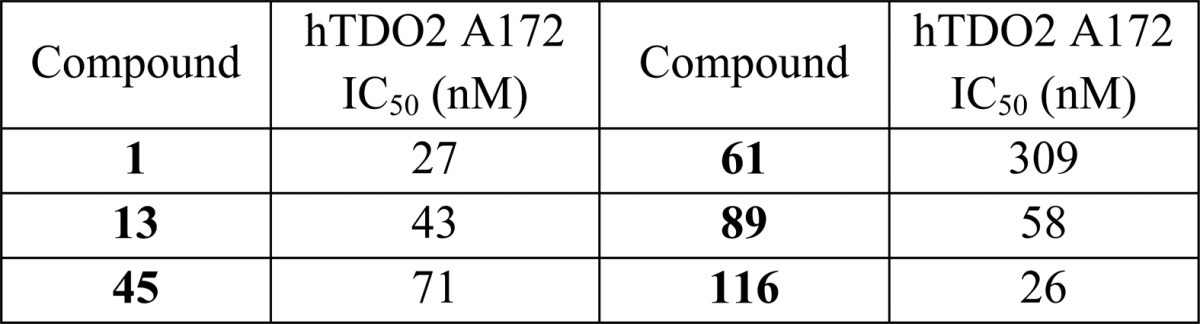
|
| Recent Review
Articles: |
Yu C.-P.; Pan Z.-Z.; Luo D.-Y.. Metab. Brain Dis. 2016, 31 ( (4), ), 737–747. |
| Zhai L.; Spranger S.; Binder D. C.; Gritsina G.; Lauing K. L.; Giles F. J.; Wainwright D. A.. Clin. Cancer Res. 2015, 21 ( (24), ), 5427–5433. |
| Austin C. J. D.; Rendina L. M.. Drug Discovery Today 2015, 20 ( (5), ), 609–617. |
| Platten M.; Wick W.; Van den Eynde B. J.. Cancer Res. 2012, 72 ( (21), ), 5435–5440. |





