| Summary: |
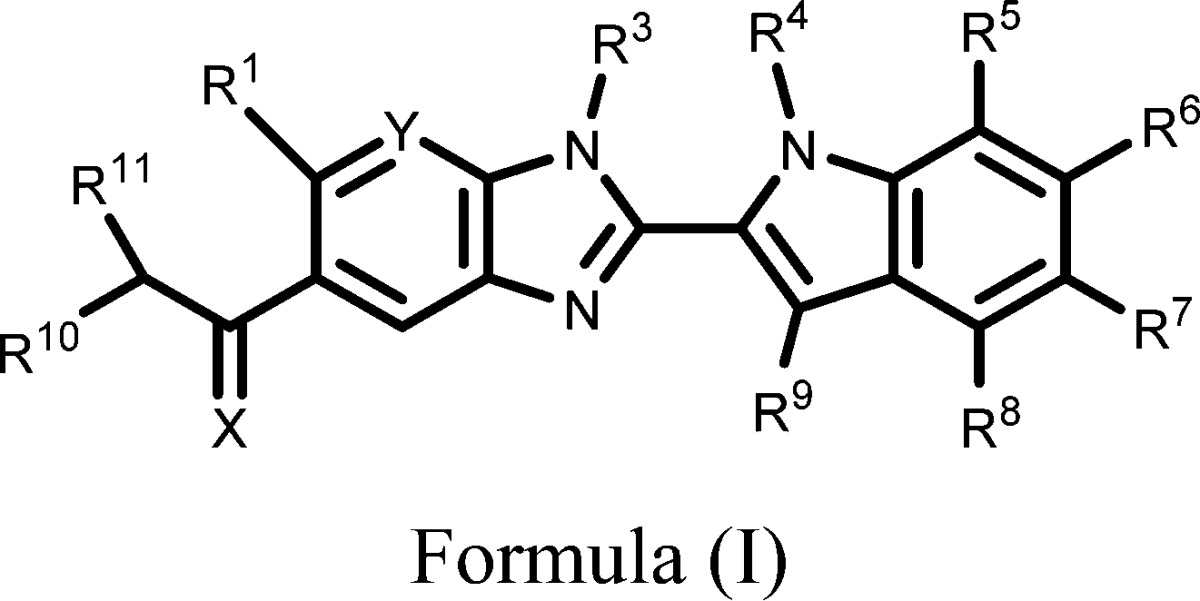
The invention in this patent application relates to
benzoimidazole derivatives represented generally by formula (I), which
are inhibitors of PAD4. These compounds may be useful for the treatment
of rheumatoid arthritis, vasculitis, systemic lupus erythematosus,
ulcerative colitis, cancer, cystic fibrosis, asthma, cutaneous lupus
erythematosis, and psoriasis. |
Peptidylarginine
deiminases (PADs) family include five members, PAD1, 2, 3, 4, and
6. They are calcium-dependent enzymes that catalyze the deimination
(or citrullination) of post-translational arginine residues within
proteins into citrulline residues. The process proceeds via the hydrolysis
of the ketimine group (C=NH) of arginine into carbonyl group
(C=O) and one molecule of ammonia as illustrated below. This
conversion changes significantly the polarity and hydrogen bonding
ability of the protein, which can affect its folding and functions.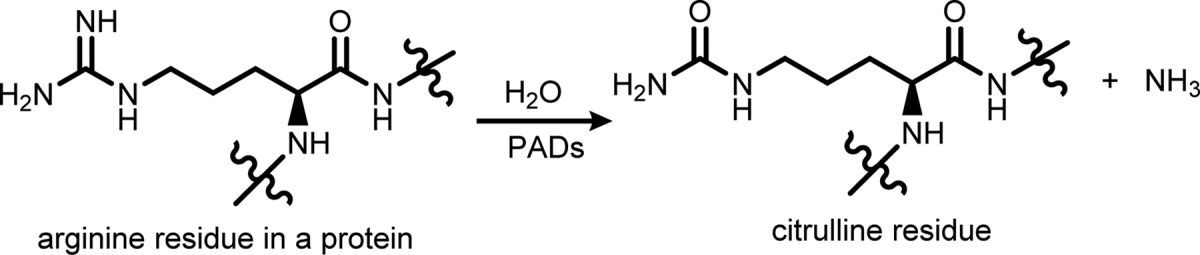
|
| Peptidylarginine deiminase 4 (PAD4) catalyzes
the citrullination
of a variety of proteins in vitro and in vivo, which causes diverse
functional responses that may lead to a variety of diseases including
rheumatoid arthritis (RA). RA is an autoimmune disease that affects
approximately 1% of the population and is characterized by inflammation
of articular joints leading to debilitating destruction of bone and
cartilage. Citrullination by PAD4 may also lead to diseases with neutrophilic
contributions to pathogenesis (for example, vasculitis, systemic lupus
erythematosus, ulcerative colitis) in addition to oncology indications. |
| PAD4 and PAD2 have been detected in synovial tissue,
and they are implicated in the citrullination of a variety of joint
proteins. This action may contribute to the pathogenesis of rheumatoid
arthritis in two different ways: |
Citrullination may trigger a response
by the immune system to form anticitrullinated protein antibodies
(ACPA). The ACPA act against citrullinated proteins in fibrinogen,
vimentin, and collagen, which can contribute to pathogenesis of RA.
The formation of ACPA can be used as a diagnostic test for RA. The increased citrullination affects the
functions of several joint and inflammatory protein mediators such
as fibrinogen, antithrombin, and multiple chemokines, which may contribute
directly to the pathogenesis of RA. In a smaller subset of RA patients,
anti-PAD4 antibodies are expressed and their level may correlate with
a more erosive form of the disease. |
| Therefore, the inhibition of PAD4 is a viable therapeutic
target that can potentially provide a treatment for rheumatoid arthritis. |
| The process of Neutrophil Extracellular Trap (NET)
formation is an innate defense mechanism in which neutrophils immobilize
and kill extracellular pathogens while minimizing damage to the host
cells. Studies suggest that NET process is associated with histone
citrullination; therefore, the inhibition of PAD4 may also provide
therapy for diseases where NET formation in tissues contributes to
local injury and disease pathology including, but not limited to,
small vessel vasculitis, systemic lupus erythematosus, cystic fibrosis,
asthma, deep vein thrombosis, periodontitis, sepsis, appendicitis,
type 2 diabetes, and stroke. Studies also provide evidence that NETs
may contribute to the pathology of some skin diseases such as cutaneous
lupus erythematosis and psoriasis. Thus, PAD4 inhibitors may additionally
treat these NET skin diseases through systemic or cutaneous administration.
PAD4 inhibitors may affect additional functions within neutrophils
and may potentially treat other neutrophilic diseases. |
| Studies have shown that PAD4 expression increases in the
tissues of many malignant tumors in numerous cancers compared to cases
of benign tumors and nontumor chronic inflammation diseases. Another
finding was that PDA4 citrullinates arginine residues in histones
at the promoters of p53-target genes such as p21, which are involved
in cell cycle arrest and induction of apoptosis. Therefore, PAD4 inhibitors
may be beneficial as antiproliferative agents. |
| PAD4 is the primary member of the PAD family that is expressed in
the nucleus as well as the cytoplasm, which may be indicative of a
larger and more general role in epigenetic regulation of gene expression.
PAD4 can indirectly decrease histone arginine methylation (and hence
epigenetic regulation associated with a particular mark) through the
depletion of the available arginine residues by converting arginine
residues into citrullines. Therefore, PAD4 inhibitors may also be
useful as epigenetic tools or therapeutics to affect the expression
of varied target genes in additional disease settings. |
| Recent studies show that PAD4-catalyzed citrullination
of arginine residues on histone H1 promoter elements can promote localized
chromatin decondensation in stem cells to regulate the pluripotent
state. Therefore, PAD4 inhibitors may be able to perform the unique
function of controlling citrullination levels and the switch between
pluripotency and differentiation in stem cells. This function may
thus be used therapeutically to affect the pluripotency status and
differentiation potential of diverse stem cells including, but not
limited to, embryonic stem cells, neural stem cells, hematopoietic
stem cells, and cancer stem cells. |
| Important Compound Classes: |
 |
| Key Structures: |
The inventors described
the structures of 124 examples of formula (I) including the following
representative compounds: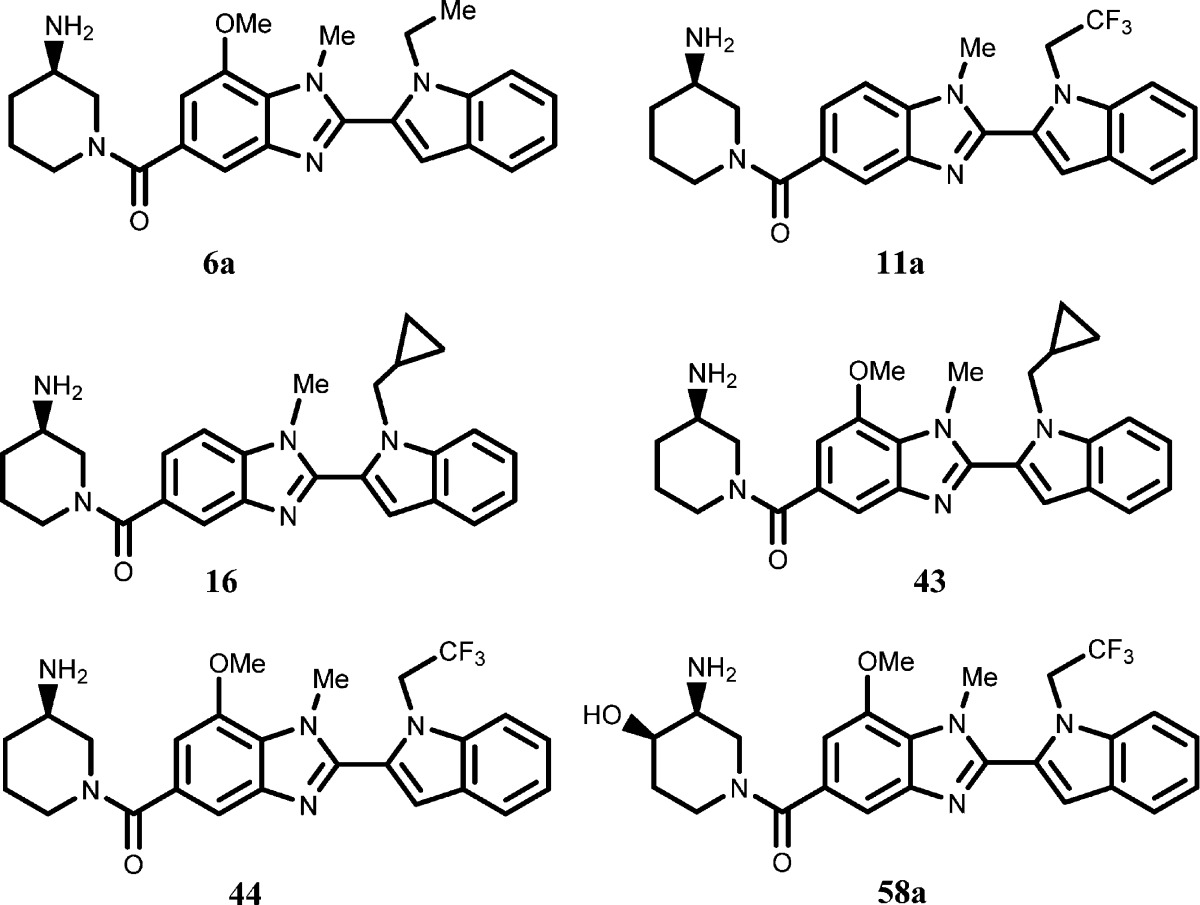
|
| Biological Assay: |
PAD4 Enzyme
Assay PAD2 Enzyme Assay |
| Biological Data: |
The mean pIC50 (−log IC50) values
obtained from testing the above representative examples in the PAD4
and PAD2 enzyme assays are listed in the following table: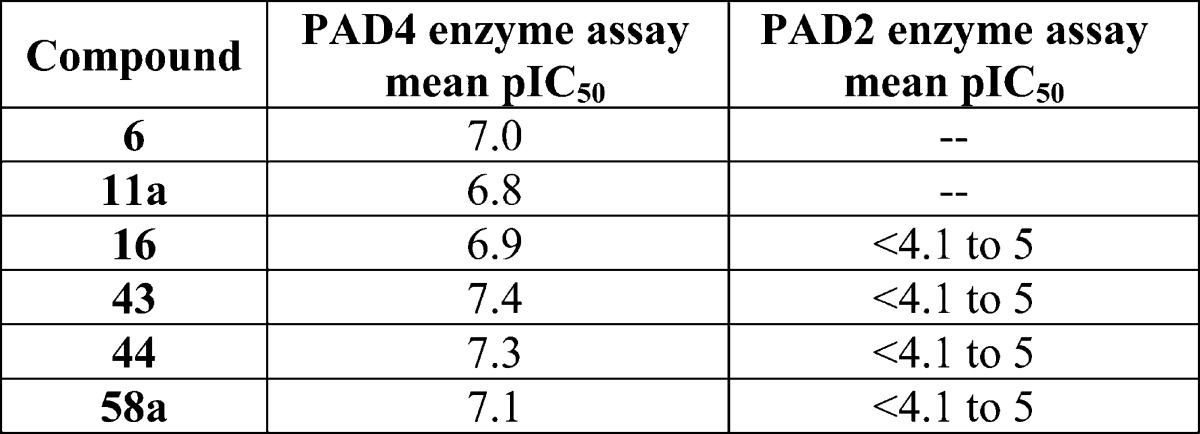
|
| Recent Review Articles: |
1. Sorensen O. E.; Borregaard N.. J. Clin. Invest. 2016, 126 ( (5), ), 1612–20. |
| 2. Slade D. J.; Subramanian V.; Thompson P. R.. Nat. Chem. Biol. 2014, 10 ( (5), ), 327–328. |
| 3. Anzilotti C.; Pratesi F.; Tommasi C.; Migliorini P.. Autoimmun. Rev. 2010, 9 ( (3), ), 158–160. |






