| Summary: |
The invention in this patent application
relates to 6-pyrimidinone-4-carboxamide derivatives represented generally
by formula (I), which act as inhibitors of the phosphodiesterase 2
(PDE2) enzyme. These compounds may be useful as therapeutic agents
for the treatment of central nervous system and/or peripheral disorders
associated with PDE2. They may also treat neurological and psychiatric
disorders such as schizophrenia, psychosis, Alzheimer’s, cognitive
impairment, anxiety, depression, migraines, Huntington’s disease,
Parkinson’s disease, Parkinson’s disease dementia (PDD),
and other diseases associated with striatal hypofunction or basal
ganglia dysfunction. |
| Schizophrenia is a debilitating
mental and behavioral disorder that affects the motor functions of
the brain. It is associated with symptoms that are indicative of cognitive
impairment and functional disabilities such as hallucinations and
delusions and may cause anhedonia or social withdrawal. While there
is no cure for schizophrenia, the symptoms may be managed and reduced
primarily by the use of typical antipsychotic drugs, such as haloperidol,
or atypical antipsychotics, such as clozapine or olanzapine. However,
these drugs are unsatisfactory and can result in extremely high rate
of noncompliance or discontinuation of medication. They may lack of
efficacy and may cause intolerable and undesirable metabolic, extrapyramidal,
prolactic, and cardiac adverse effects. |
| To
determine the causes of pathogenesis of schizophrenia, researchers
have focused their studies on the dysfunction in the role of glutamate/N-methyl-d-aspartate (NMDA) receptor and the dopaminergic
receptors associated with the levels of cyclic adenosine monophosphate
(cAMP). It is believed that cAMP regulates the activity of cAMP-dependent
protein kinase (PKA), which in turn phosphorylates and regulates many
types of proteins including ion channels, enzymes, and transcription
factors. Cyclic guanosine monophosphate (cGMP) is thought to be similarly
responsible for downstream regulation of kinases and ion channels. |
| The 3′,5′-cyclic nucleotide-specific
phosphodiesterases (PDEs) superfamily includes 11 families of PDEs.
The PDE enzymes catalyze the hydrolysis of cAMP and cGMP to regulate
their intracellular concentrations. Thus, the regulation of PDEs may
affect the levels of these cyclic nucleotides. The PDE families are
subdivided according to their catalytic domain homology and substrate
specificity into three groups: |
-
1.
cAMP-specific PDEs:
include PDE4A-D, 7A, 7B, 8A, and 8B;
-
2.
cGMP-specific PDEs: include PDE5A, 6A-C, and 9A;
-
3.
Dual substrate PDEs: include
PDE1A-C, 2A, 3A, 3B, 10A, and 11A.
|
| The homology between the different PDE families ranges
from 20% to 45%; therefore, it may be possible to develop selective
inhibitors for each one of these families. |
| PDE2 is highly expressed in the brain, but it is also found in many
other tissues. It plays important roles in many functions and utilities
including but not limited to neuronal development, learning, and memory,
prolactin and aldosterone secretion, bone cell differentiation, growth,
and bone resorption, immunological response, vascular angiogenesis,
inflammatory cell transit, cardiac contraction, platelet aggregation,
female sexual arousal disorder, osteoarthritis pain, malignant melanoma,
heart failure, pulmonary hypertension, depression and anxiety, and
hypoxic pulmonary vasoconstriction. |
| Studies
using multiple preclinical models of cognitive performance have shown
that inhibition of PDE2 enhances cognitive functions such as recognition
memory, social interactions, and working memory, which are all deficient
in schizophrenia patients. It also improves cognitive deficits that
develop as a result of aging or from Alzheimer’s disease. PDE2
inhibition was also effective in preclinical models of anxiety and
depression. |
| The role of PDE2 inhibition in
cognitive disorders was further confirmed using BAY60-7550, which
is a known potent and selective inhibitor of PDE2A. It suppresses
the activity of PDE2 enzyme but showed no significant effects on other
PDEs including PDE1, 3B, 4B, 5, 7B, 8A, 9A, 10A, and 11A. It was reported
to have high clearance and limited brain penetration. |
| Increased activity of PDE2 was linked to increase in vascular
permeability. PDE2 and PDE3 can control the concentration levels of
cGMP in the endothelium to regulate endothelial permeability, which
may be associated with migraine. Cerebral vasodilation is considered
a major cause of migraine. Therefore, PDE2 inhibition may have utility
as a treatment or prophylactic of migraine. |
| The modulation of PDE2 has become an increasingly important therapeutic
target to develop treatments for multiple diseases and disorders associated
with dysregulated PDE2 such as cognitive impairment associated with
schizophrenia, depression, Alzheimer’s disease, migraines,
and many others. It is therefore desirable to identify novel selective
inhibitors of PDE2, such as the compounds described in this patent
application, which may be useful as therapeutics for a wide variety
of neurological and psychiatric disorders that may benefit from increased
levels of cAMP and/or cGMP within neurons. |
| Important Compound Classes: |
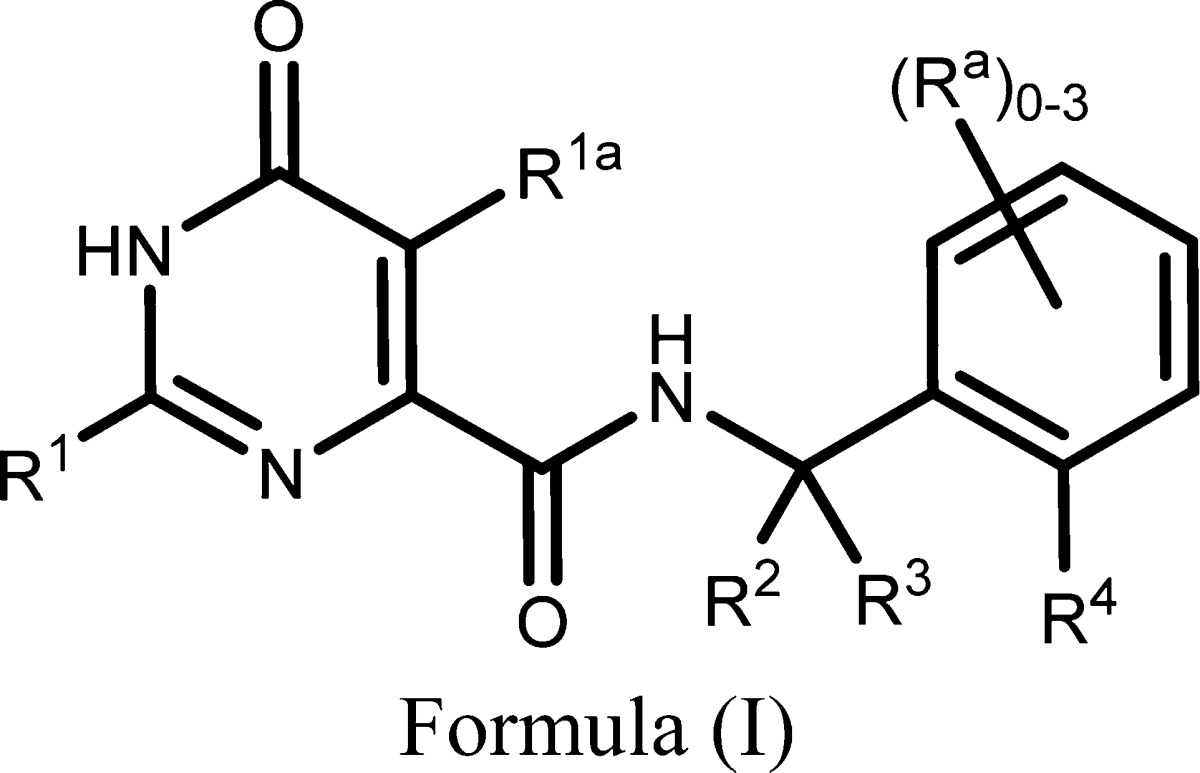 |
| Key Structures: |
The inventors described
the synthesis and structures of 212 compounds of formula (I) including
the following representative examples: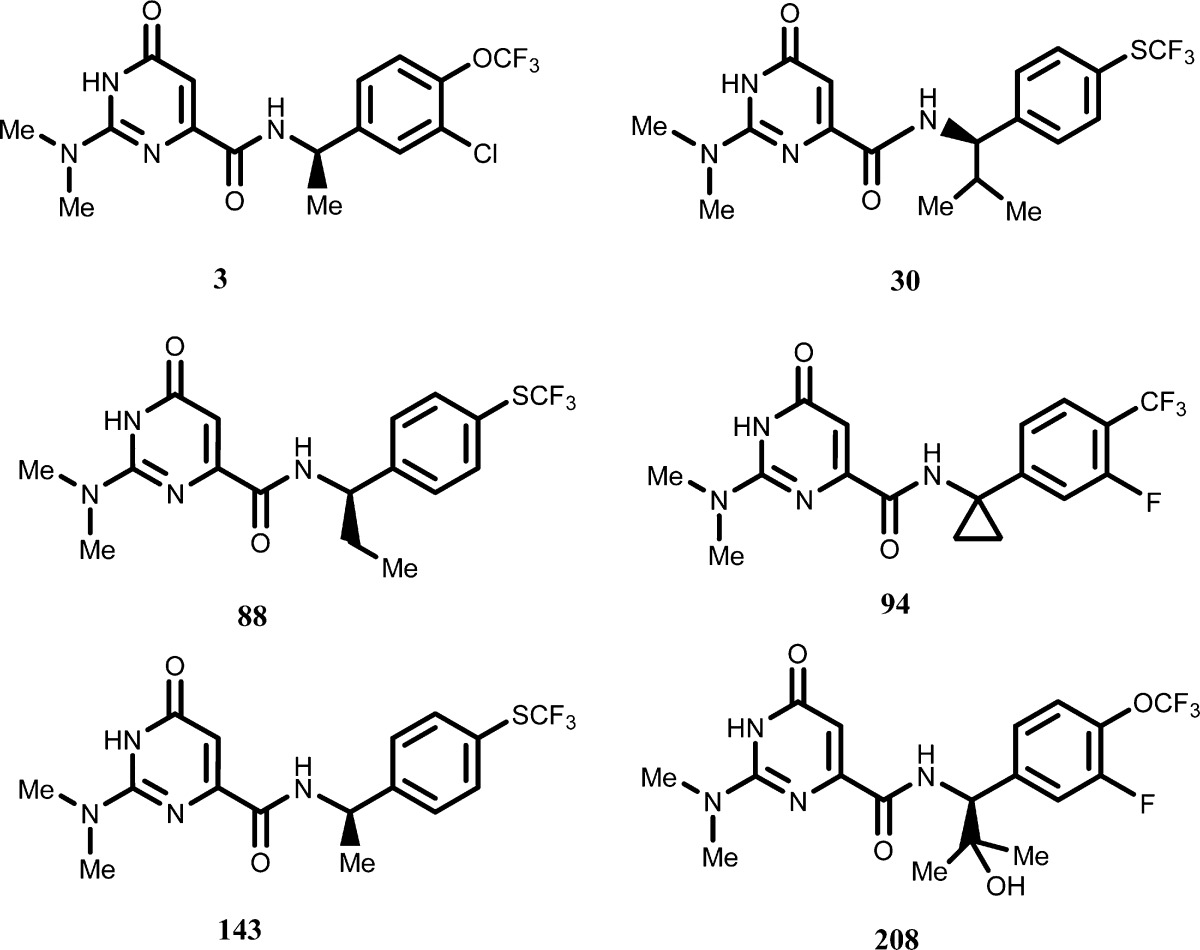
|
| Biological Assay: |
The activities
of the compounds of formula (I) as PDE2 inhibitors were determined
by their ability to inhibit the hydrolysis of the phosphate ester
bond of a cyclic nucleotide. |
| Biological
Data: |
The values of Ki (inhibitory constant) for the above examples are listed in the following
table as conducted in two laboratories (Lab A or B):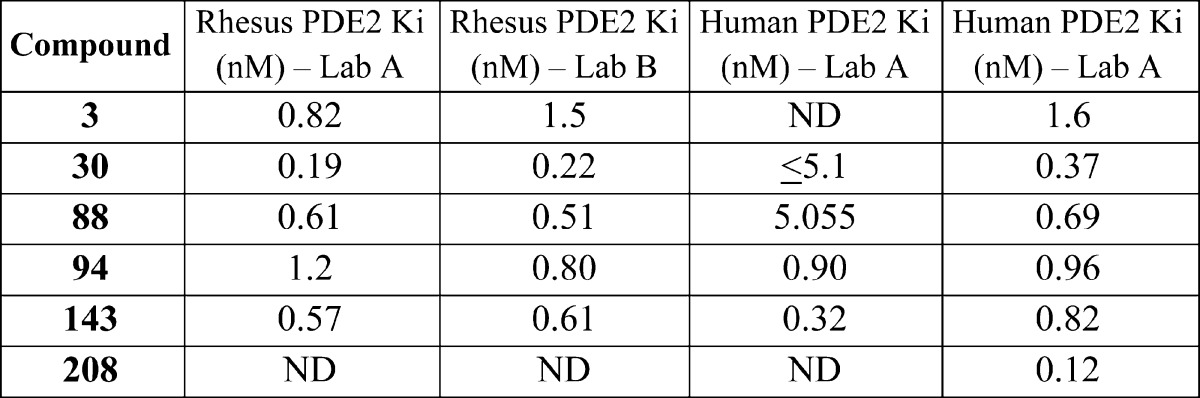
|
| Recent Review Articles: |
1. Van Duinen M.; Reneerkens O. A. H.; Lambrecht L.; Sambeth A.; Rutten B. P. F.; Van Os J.; Blokland A.; Prickaerts J.. Curr. Pharm.
Des. 2015, 21 ( (26), ), 3813–3828. |
| 2. Gomez L.; Breitenbucher J. G.. Bioorg. Med. Chem.
Lett. 2013, 23 ( (24), ), 6522–6527. |
| 3. Keravis T.; Lugnier C.. Br. J. Pharmacol. 2012, 165 ( (5), ), 1288–1305. |





