Abstract
Background
Uterine Tumors Resembling Ovarian Sex Cord Tumors (UTROSCT) are rare tumors of low malignancy. In the past, these tumors were mainly treated by hysterectomy. More recently, some authors have proposed conservative surgical management for women wishing to preserve fertility. This article is the first to report on organ-preserving treatment in the case of recurrence or disease persistence.
Cases
We report on three patients with UTROSCT, two of them young, not having completed family planning. One even gave birth to a healthy child after fertility-preserving treatment of a persistent UTROSCT. To our knowledge, this is the first pregnancy reported after surgical treatment of a persistent UTROSCT so far.
Conclusion
A fertility-sparing approach should always be considered in young women with UTROSCT who wish to preserve their fertility, also in cases of recurrence or disease persistence.
Keywords: UTROSCT, Recurrence, Treatment, Fertility, Pregnancy
Highlights
-
•
First report on organ-preserving treatment in case of UTROSCT recurrence
-
•
First pregnancy reported after surgical treatment of a persistent UTROSCT
-
•
Fertility-preserving approach also in cases of recurrence or disease persistence
1. Introduction
The occurrence of Uterine Tumors Resembling Ovarian Sex Cord Tumors (UTROSCT) was first described in 1945 (Morehead and Bowman, 1945). In 1976, a series of 14 cases was added (Clement and Scully, 1976). To date, less than 100 cases of UTROSCT have been published (Morehead and Bowman, 1945, Clement and Scully, 1976, O'Meara et al., 2009, Blake et al., 2014, Jeong et al., 2015, De Franciscis et al., 2016, Berretta et al., 2009, Giordano et al., 2010, Anastasakis et al., 2008, Hillard et al., 2004, Garuti et al., 2009, De Leval et al., 2010, Biermann et al., 2008, Gomes et al., 2015, Lantta et al., 1984). According to the WHO, UTROSCT are classified in the group of endometrial stromal and related tumors. The entity is defined as a “neoplasm resembling ovarian sex cord tumors without a component of recognizable endometrial stroma” (I.A.R.C. 2014, 4th Ed). The classification of UTROSCT found in literature is sometimes unspecified and a distinction between the more aggressive ESTSCLE (endometrial stromal tumors with sex cord-like elements) and UTROSCT is not made. However, this distinction is highly important because of the different behavior of these tumors.
UTROSCT are tumors of low malignant potential. They usually behave in a benign fashion; however, some may recur. The patients typically present with a bleeding disorder and/or a uterine mass.
These tumors are usually well-demarcated myometrial nodules with sharp or infiltrating borders. Some grow as polyps. They are smoother, fleshier and yellow to tan compared to leiomyoma. They may present different histological patterns such as trabecular, glandular, solid, diffuse or mixed. The cytoplasm can be scant or more abundant, often rich in lipid. The nuclei are small, inconspicuous and mitoses are very rare.
The immunohistochemical profile is variable. Using a marker panel (De Leval et al., 2010) is useful with sex cord markers (inhibin, calretinin, WT-1), one or more smooth muscle markers (desmin, h-caldesmon, smooth muscle actin), CD 10 and an epithelial marker (AE1/AE3 cytokeratin).
Due to potential recurrence and limited experience, in the past, UTROSCT were mainly treated by hysterectomy. More recently, conservative surgical management for women wishing to preserve fertility has been proposed (O'Meara et al., 2009, Blake et al., 2014, Jeong et al., 2015, De Franciscis et al., 2016, Berretta et al., 2009, Giordano et al., 2010, Anastasakis et al., 2008, Hillard et al., 2004, Garuti et al., 2009).
In this article, we report on three patients with UTROSCT, two of them young, not having completed family planning. One of them even gave birth to a healthy child after a second extensive fertility-sparing surgical treatment. To our knowledge, this is the first pregnancy reported under these conditions so far (Blake et al., 2014, Jeong et al., 2015, De Franciscis et al., 2016).
2. Case reports
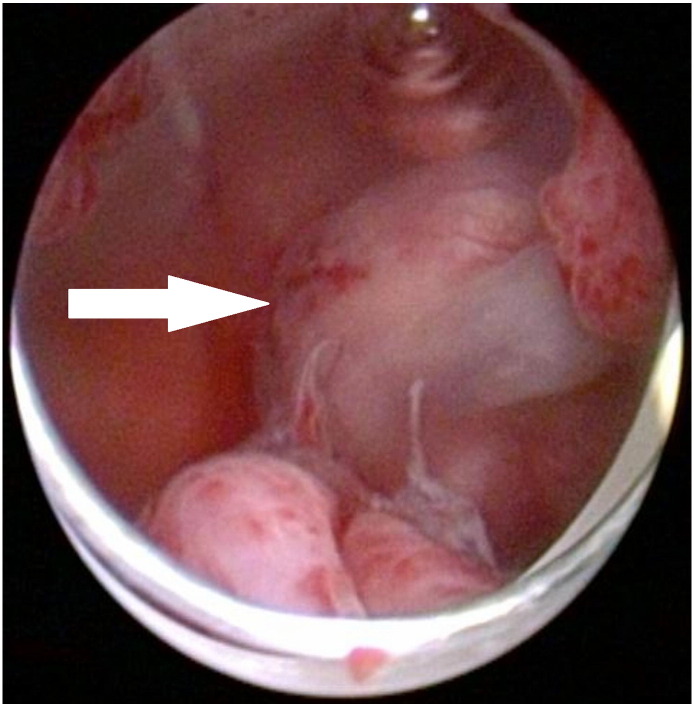
The first patient, a 24-year-old woman, suffered from abnormal uterine bleeding (hypermenorrhea) and secondary dysmenorrhea. Ultrasound examination revealed a persistent submucosal mass resembling a leiomyoma in the fundal anterior wall of the uterine corpus. Over a two-year period, the size remained stable. Due to increasing symptoms a hysteroscopy (Fig. 1) with resection of the submucosal tumor was performed. The histological diagnosis was UTROSCT with expression of calretinin, one of the smooth muscle markers, WT1 and AE1/AE3 cytokeratin. It did not express inhibin. Three months later a fundal lesion of 8 mm was visible in a pelvic MRI. The patient strongly desired fertility-preserving treatment, so a repeat hysteroscopy with biopsies was performed, showing no histological evidence of UTROSCT. We agreed on regular clinical surveillance visits with imaging by ultrasound or additionally MRI. Six months later, a pelvic MRI suggested disease recurrence with a uterine mass of 15 mm. The patient strongly desired another fertility-preserving surgery. The diagnostic hysteroscopy showed no abnormalities. In the concurrent open abdominal surgery, the intramural tumor was located by palpation and completely resected. Pathological results were consistent with UTROSCT. The margins were free of tumor and the peritoneal lavage did not exhibit any tumor cells. The patient is under clinical as well as radiological surveillance since the last surgery and has now been disease free for 56 months.
Fig. 1.
UTROSCT of the first patient in hysteroscopy.
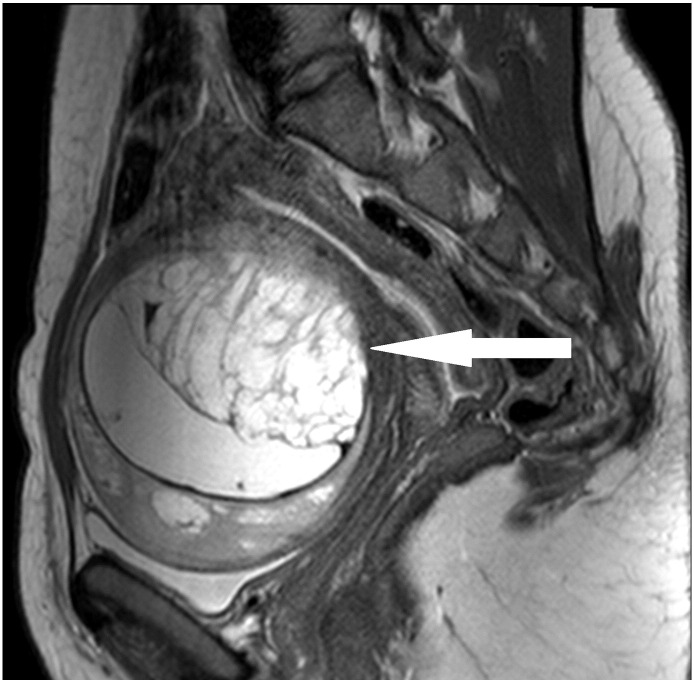
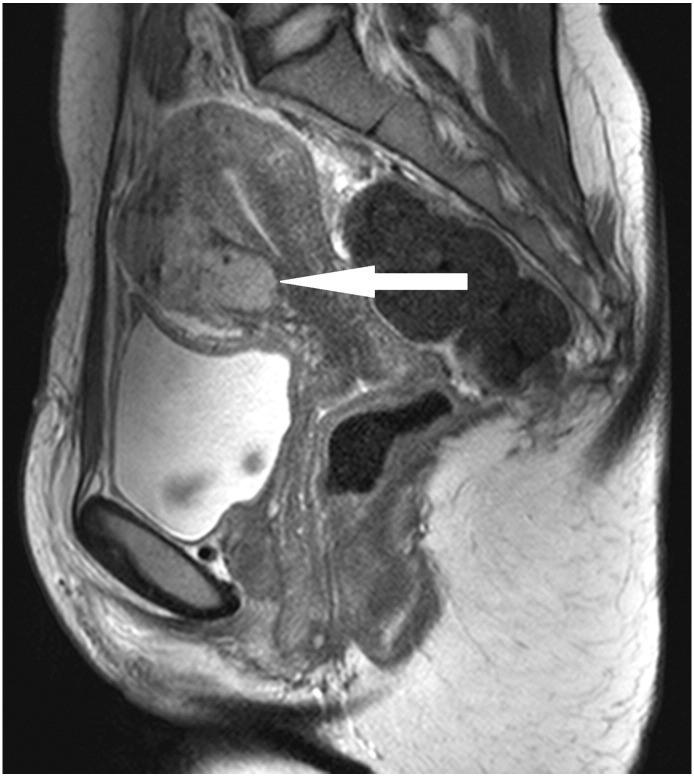
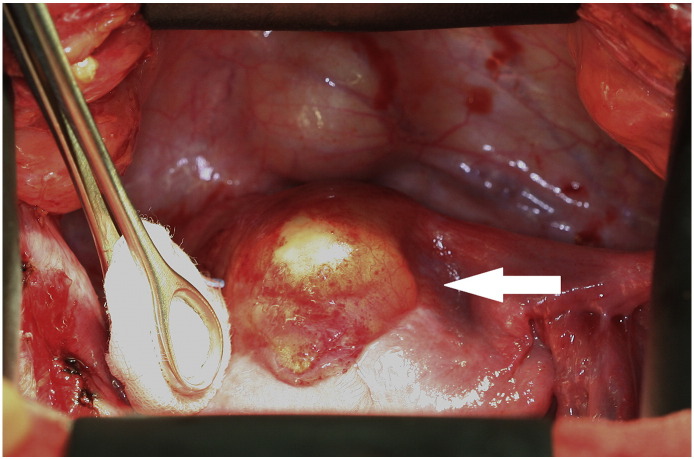
The second patient, a 28-year-old woman, was referred to us after the operation of a symptomatic, slowly growing, cystic-solid tumor of 10 cm in the uterine anterior wall (Fig. 2) performed at another hospital. The tumor had been completely resected macroscopically through a lower abdominal incision. However, the surgery was complicated by strong bleeding and unintended opening of the tumor. The histological diagnosis was UTROSCT. Because of the possibility of recurrence, the hospital, where the initial surgery was performed, recommended a subsequent hysterectomy. As the patient did not want to undergo another surgery, regular clinical surveillance visits with additional imaging by MRI were performed. In the surveillance visit two months after surgery, the MRI indicated disease persistence with a tumor mass of 3 × 4 × 5 cm in the anterior uterine wall (Fig. 3). As family planning was not completed, the patient did not wish to undergo a hysterectomy. She was referred to us for a second opinion and requested fertility-preserving surgery. There was no evidence of a macroscopic spread of the disease during open abdominal surgery. A round, yellowish mass the size of 3 × 3 cm (Fig. 4) was found on the anterior wall of the uterus. In contrast to the first case, the palpated texture was identical to the rest of the myometrium. The whole tumor was macro- and microscopically removed. The immunohistochemistry showed positivity for calretinin, one of the smooth muscle markers, WT1 and AE1/AE3 cytokeratin. It was negative for inhibin.
Fig. 2.
Original tumor of the second patient in MRI.
Fig. 3.
Recurrence of UTROSCT in the second patient (MRI).
Fig. 4.
Yellowish mass of UTROSCT in the second patient.
The patient conceived without any problems and gave birth to a healthy child by cesarean section 19 months after the last surgery. Due to completed family planning, an abdominal hysterectomy with simultaneous removal of the distal part of the fallopian tubes on both sides was performed directly after the cesarean section. The entire tissue was free of tumor.
We agreed on the same procedure for oncological surveillance visits as in the first case. In one of the following surveillance visits, 20 months later, sonography revealed a 7 cm-large, polycystic tumor in the small pelvis. MRI indicated the same finding as well as strong activity of the contrast agent. Because of strong suspicion of recurrence, a third laparotomy was performed. The lower abdomen showed peritoneal carcinomatosis. The polycystic tumor originated from the right adnexa, infiltrating a part of the vaginal wall. The tumor, both adnexa, part of the vaginal wall as well as the affected peritoneum were removed. The final pathological report showed an UTROSCT infiltrating the peritoneum, the right fallopian tube, both ovaries and the vaginal wall. A complete resection was achieved.
Due to a lack of guidelines for adjuvant treatment, chemotherapy with 3 cycles of bleomycin, etoposid and cisplatin (BEP) according to the adjuvant treatment of Sertoli-Leydig-cell tumors was considered. However, the patient declined chemotherapy. Because of the estrogen and progesterone positivity of this UTROSCT, an endocrine treatment with the aromatase inhibitor anastrozole was initiated as an alternative.
The patient is still being monitored clinically and by ultrasound every three months and by MRI every six months. She is currently disease free 34 months after the last surgery.
The third patient was a 72-year-old woman with a one-time postmenopausal bleeding. Sonography only showed an atrophied endometrial layer. Diagnostic hysteroscopy with curettage was performed and was unsuspicious. Pathology showed an UTROSCT infiltrating the endometrium. The tumor was positive for calretinin, one of the smooth muscle markers, AE1/AE3 cytokeratin and also for inhibin. A total hysterectomy with adnexectomy was recommended. An MRI of the pelvic region before surgery showed no uterine tumor or infiltration of other organs. No macroscopic tumor was found during surgery and pathology also indicated no further signs of a tumor. The patient is still being monitored clinically and radiologically and is disease free 46 months after the last surgery.
3. Discussion
A fertility-preserving option for younger women with UTROSCT has only recently been suggested by some authors (O'Meara et al., 2009, Blake et al., 2014, Jeong et al., 2015, De Franciscis et al., 2016, Berretta et al., 2009, Giordano et al., 2010, Anastasakis et al., 2008, Hillard et al., 2004, Garuti et al., 2009). The goal of this article is to show that tumor resection alone, instead of hysterectomy, may be an option for treating UTROSCT. This is viable even in cases of recurrence or disease persistence. However, a complete resection of the whole tumor without harming the external layer is vital, as is generally the case in oncological surgery.
To the best of our knowledge, this article is the first to report on organ-preserving treatment in cases of recurrence or disease persistence. The recurrence of UTROSCT in the first case may have been caused by incomplete resection during the first surgery. The persistent/recurring UTROSCT in the second case was most likely caused by incomplete resection during the first operation and the subsequent spread of tumor cells into the abdominal cavity. Due to the presence of tumors in both ovaries, a hematologic pathway for metastasis could also be discussed.
Follow-up surveillance visits in the reported cases were done regularly, clinically and with imaging by ultrasound or additionally by MRI. The shortest disease-free interval was six months in the first case mentioned in this report. To the best of our knowledge, there are only two cases of recurrent UTROSCT described in literature (O'Meara et al., 2009, Biermann et al., 2008). In these cases, recurrence after three and four years were documented (O'Meara et al., 2009, Biermann et al., 2008). Based on these data, regular and frequent long-term follow-up controls may be recommended, according to the standard gynecological tumor aftercare programs.
Four successful pregnancies following uterus-sparing treatment of UTROSCT (Blake et al., 2014, Jeong et al., 2015, De Franciscis et al., 2016) have been described in the medical literature since 1945. The second case mentioned in this article would be the fifth. However, our case is the first pregnancy described after surgical treatment of a persistent and extensive UTROSCT. Nonetheless, due to the possibility of late local recurrences and the lack of experience with UTROSCT, hysterectomy should be performed after completion of family planning.
According to the literature, chemotherapy with bleomycin, etoposide and cisplatin seems to be an option for adjuvant treatment (O'Meara et al., 2009, Gomes et al., 2015). A (follow up) treatment with anastrozole in tumors with estrogen-/progesterone-receptor positivity should also be considered. Lantta et al. (1984) suggested in 1984 already that UTROSCT should be tested for steroid receptor expression to evaluate a possible hormone treatment. However, due to a lack of cases and data, general recommendations on the type or duration of adjuvant treatment with chemotherapy cannot be made.
In conclusion, based on the cases described here, as well as on the published evidence available, a fertility-sparing approach should always be considered in women with UTROSCT who wish to preserve their fertility. If a complete resection of the tumor is achieved, recurring and persistent UTROSCT can also be treated by uterus-preserving surgery. The resection of the whole tumor as a complete mass is vital to avoid the spreading of tumor cells into the abdominal cavity and thus to reduce the risk of recurrence. Further case reports are needed to prove the safety of organ-preserving treatment in UTROSCT and to establish a treatment protocol.
The authors declare no conflicting interests. Written, informed consent was given by all three patients.
Disclosure
None of the authors have a conflict of interest.
Financial support
None.
References
- Anastasakis E., Magos A.L., Mould T., Economides D.L. Uterine tumor rembling ovarian sex cord tumors treated by hysteroscopy. Int. J. Gynecol. Obstet. 2008;101:194–195. doi: 10.1016/j.ijgo.2007.09.029. (May) [DOI] [PubMed] [Google Scholar]
- Berretta R., Patrelli T.S., Fadda G.M., Merisio C., Gramellini D., Nardelli G.B. Uterine Tumors Resembling Ovarian Sex Cord Tumors. A case report of conservative management in young women. Int. J. Gynecol. Cancer. 2009;19(4):808–810. doi: 10.1111/IGC.0b013e3181a417b4. (May) [DOI] [PubMed] [Google Scholar]
- Biermann K., Heukamp L.C., Büttner R., Zhou H. Uterine tumors resembling an ovarian sex cord tumor associated with metastasis. Int. J. Gynecol. Pathol. 2008;27(1):58–60. doi: 10.1097/pgp.0b013e318057faf5. [DOI] [PubMed] [Google Scholar]
- Blake E.A., Sheridan T.B., Wang K.L., Takiuchi T., Kodama M., Sawada K. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;181C:163–170. doi: 10.1016/j.ejogrb.2014.07.050. (Aug 8) [DOI] [PubMed] [Google Scholar]
- Clement P.B., Scully R.E. Uterine tumors resembling ovarian sex cord tumors. A clinicopathologic analysis of fourteen cases. Am. J. Clin. Pathol. 1976;66:512. doi: 10.1093/ajcp/66.3.512. [DOI] [PubMed] [Google Scholar]
- De Franciscis P., Grauso F., Ambrosio D., Torella M., Messalli E.M., Colacurci N. Conservative resectoscopic surgery, successful delivery, and 60 months of follow-up in a patient with endometrial stromal tumor with sex-cord-like differentiation. Case Rep. Obstet. Gynecol. 2016:5736865. doi: 10.1155/2016/5736865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leval L., Lim G.S.D., Waltregny D., Oliva E. Diverse phenotypic profile of uterine tumors resembling ovarian sex cord tumors. Am. J. Surg. Pathol. 2010;34(12):1749–1761. doi: 10.1097/PAS.0b013e3181f8120c. (Dec) [DOI] [PubMed] [Google Scholar]
- Garuti G., Gonfiantini C., Mirra M., Galli C., Luerti M. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J. Minim. Invasive Gynecol. 2009;16(2):236–240. doi: 10.1016/j.jmig.2008.12.006. (Mar-Apr) [DOI] [PubMed] [Google Scholar]
- Giordano G., Lombardi M., Brigati F., Mancini C., Silini E.M. Clinicopathologic features of 2 new cases of Uterine Tumors Resembling Ovarian Sex Cord Tumors. Int. J. Gynecol. Pathol. 2010;29(5):459–467. doi: 10.1097/PGP.0b013e3181dfcfdc. (Sep) [DOI] [PubMed] [Google Scholar]
- Gomes J.R., Carvalho F.M., Abrao M., Maluf F.C. Uterine tumors resembling ovarian sex-cord tumor: a case-report and a review of literature. Gynecol. Oncol. Rep. 2015;15:22–24. doi: 10.1016/j.gore.2015.11.003. (Nov 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard J.B., Malpica A., Ramirez P.T. Conservative management of a uterine tumor resembling an ovarian sex cord-stromal tumor. Gynecol. Oncol. 2004;92:347–352. doi: 10.1016/j.ygyno.2003.09.011. (Jan) [DOI] [PubMed] [Google Scholar]
- Jeong K.H., Lee H.N., Kim M.K., Kim M.L., Seong S.J., Shin E. Successful delivery after conservative resectoscopic surgery in a patient with a uterine tumor resembling ovarian sex cord tumor with myometrial invasion. Obstet. Gynecol. Sci. 2015;58(5):418–422. doi: 10.5468/ogs.2015.58.5.418. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantta M., Khanpää K., Kärkkäinen J., Lehtovirta P., Wahlström T., Widholm O. Estradiol and progesterone receptors in two cases of endometrial stromal sarcoma. Gynecol. Oncol. 1984;18(2):233–239. doi: 10.1016/0090-8258(84)90031-3. [DOI] [PubMed] [Google Scholar]
- Morehead R.P., Bowman M.C. Heterologous mesenchymal tumors of the uterus. Report of a neoplasm resembling a granulosa cell tumor. Am. J. Pathol. 1945;21:53–61. [PMC free article] [PubMed] [Google Scholar]
- O'Meara A.C., Giger O.T., Kurrer M., Schaer G. Case report: recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol. Oncol. 2009;114:140–142. doi: 10.1016/j.ygyno.2009.03.021. [DOI] [PubMed] [Google Scholar]