Abstract
Background
For many patients, current treatments do not adequately resolve heartburn in nonerosive reflux disease (NERD).
Objective
To compare vonoprazan and placebo with respect to the frequency and severity of heartburn in patients with NERD.
Methods
This Phase III, double-blind, placebo-controlled, parallel-group, multicenter study included patients in Japan aged ≥20 years with Grade N or M NERD and recurrent acid reflux symptoms. Patients were blinded and randomized 1:1:1 to receive placebo or vonoprazan 10 mg or 20 mg. The primary efficacy outcome was the proportion of days without heartburn measured by patient scores during the 4-week treatment period.
Results
Eight hundred twenty-seven patients were randomized (placebo: n = 278, vonoprazan 10 mg: n = 278, and vonoprazan 20 mg: n = 271). Median proportion of days without heartburn was 7.4% (placebo), 10.3% (vonoprazan 10 mg), and 12.0% (vonoprazan 20 mg). Proportion of days without heartburn was not statistically significant between the vonoprazan and placebo groups (P = 0.2310 [10 mg] and P = 0.0504 [20 mg]). Mean severity of heartburn was significantly higher with placebo (median score = 1.070) than with vonoprazan 10 mg (median score = 0.990; P = 0.0440) and 20 mg (median score = 0.960; P = 0.0139). Patients whose symptoms improved at Week 2 experienced significantly increased proportion of days without heartburn and reduced mean severity of heartburn at Week 4 with vonoprazan compared with placebo (proportion of days without heartburn: P = 0.0004 [10 mg] and P = 0.0001 [20 mg] and mean severity: P < 0.0001 [10 mg] and P < 0.0001 [20 mg]). A significant difference in median proportion of days without heartburn was observed for vonoprazan 20 mg compared with placebo in patients with Grade M NERD. Incidence of treatment-emergent adverse events was 32.7% (placebo), 27.7% (vonoprazan 10 mg), and 28.0% (vonoprazan 20 mg).
Conclusions
Vonoprazan at doses of 10 mg and 20 mg are not superior to placebo with respect to proportion of days without heartburn, whereas the mean severity of heartburn is lower with vonoprazan compared with placebo in patients with NERD. ClinicalTrials.gov identifier: NCT01474369.
Key words: gastroesophageal reflux, heartburn, NERD, nonerosive gastroesophageal reflux disease, potassium-competitive acid blockers, vonoprazan
Highlights
-
•
More effective treatments are required for nonerosive reflux disease (NERD).
-
•
Vonoprazan was not superior to placebo for median proportion of days without heartburn.
-
•
The difference was significant for vonoprazan 20 mg vs placebo for NERD Grade M patients.
-
•
Patients on vonoprazan reported significantly less severe heartburn vs placebo.
-
•
Vonoprazan 20 mg may be effective for NERD, based on the per-protocol set.
Introduction
Nonerosive gastroesophageal reflux disease (NERD) negatively influences patient quality of life. NERD is characterized by the presence of the typical symptoms of gastroesophageal reflux disease without visible erosive esophagitis at endoscopy,1, 2 Proton pump inhibitors (PPIs) are currently the most effective treatment for NERD.3 However, PPIs do not completely control reflux symptoms in approximately 30% to 55% of patients.1, 2, 4
Vonoprazan is a member of a novel class of acid suppressants, the potassium-competitive acid blockers.5 Previous studies have shown that vonoprazan is noninferior to lansoprazole for the treatment of a number of acid-related disorders such as erosive esophagitis, Helicobacter pylori infection, and peptic ulcer disease.6, 7, 8 Studies in animals and healthy volunteers have shown that vonoprazan can exhibit its maximum acid-inhibitory effect in a shorter time and that this effect is longer lasting compared with lansoprazole.9, 10, 11
The aim of this study was to determine whether vonoprazan was effective in treating NERD. The primary objective was to compare vonoprazan and placebo with respect to the frequency and severity of heartburn in patients with NERD. The secondary objectives were to assess the safety of vonoprazan compared with placebo in patients with NERD, determine the recommended clinical dose, and to determine whether the response after 2 weeks of treatment with vonoprazan was predictive of the response after 4 weeks of treatment.
Patients and Methods
Study design
This study was a multicenter, randomized, parallel, double-blind, placebo-controlled trial conducted at 75 study sites in Japan between November 2011 and February 2013. The study was approved by the institutional review board at each study center and was conducted in accordance with the Declaration of Helsinki/Good Clinical Practice Guideline, and applicable local Japanese regulations. The study was registered with ClinicalTrials.gov: NCT01474369. All patients signed the informed consent form before study procedures were initiated.
Study population
Patients were eligible for inclusion if they were aged at least 20 years at the time of informed consent; had a diagnosis of Grade M or N NERD (Grade M was defined as minimal changes to the mucosa, such as erythema without sharp demarcation, whitish turbidity, and/or invisibility of vessels due to these findings; Grade N was defined as normal mucosa based on Modified Los Angeles Classification12) by endoscopy; had recurrent acid reflux symptoms on ≥2 d/wk and acid reflux symptoms of moderate or higher severity during the 3 weeks before the start of the run-in period; were compliant (≥75%) with antacid therapy during the run-in period and had heartburn on ≥2 days during the week before randomization; and provided all required information in the patient (paper) diary recorded twice daily during the run-in period. Moderate to very severe acid reflux symptoms (heartburn or regurgitation) were defined as rather painful, painful, or painful enough to affect night-time sleep or daily activities.
Patients were excluded if they had a history of surgery that affects gastroesophageal reflux; had acute upper gastrointestinal bleeding or gastric or duodenal ulcer within 30 days before the start of the run-in period; had acute gastritis (defined as epigastralgia as well as multiple gastric mucosal erosions, redness, and edema) or acute exacerbation of chronic gastritis (defined as epigastralgia as well as multiple gastric mucosal erosions, redness, and edema on the gastric mucosa with chronic gastritis or atrophy); had Zollinger-Ellison syndrome or other gastric acid hypersecretion disorders; had a history of chest pain due to cardiac diseases within 1 year or chest pain that may be caused by cardiac disease; had any other concurrent upper gastrointestinal symptoms more severe than heartburn; had surgical treatment for erosive esophagitis and NERD or any surgery affecting gastric acid secretion during the study; had a diagnosis of depression; or required treatment with any excluded medications (including atazanavir and rilpivirine hydrochloride).
Randomization, treatment, and follow-up
The randomization table was generated by designated randomization personnel and was only accessible to authorized persons. Patients were randomized 1:1:1 according to their endoscopic findings (Grade N or M) at Visit 1. Assignment of study drugs was kept blinded by using drug numbers allocated to each study site and blinding information was kept centrally until all data were collected. Serum gastrin and pepsinogen I or II levels were not disclosed during the data collection period to maintain integrity of blinding. The study treatments (vonoprazan 10 mg or 20 mg and placebo) were indistinguishable in appearance.
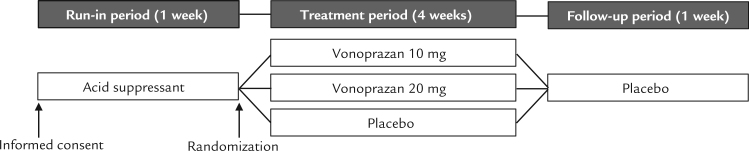
Patients were randomized to receive vonoprazan 10 mg, vonoprazan 20 mg, or placebo. All study drugs were manufactured by Takeda Pharmaceutical Company Ltd, Osaka, Japan. During the 1-week run-in period, all patients received an oral antacid (Maaredge Combination-DS for Suspension, 1.2 g, Towa Pharmaceutical Co., Ltd., Osaka, Japan) 3 times daily after each meal (Figure 1). During the 4-week treatment period, patients received a tablet of vonoprazan 10 mg, vonoprazan 20 mg, or placebo once daily after breakfast. During the 1-week follow-up period, all patients received a placebo tablet once daily after breakfast.
Fig. 1.
Study design.
All concomitant medications, including vitamin supplements, over-the-counter drugs, and herbal medicines were recorded. Excluded medications were PPIs, H2-receptor antagonists, muscarinic M3 receptor antagonists (eg, tiquizium bromide, and pirenzepine), anticholinergics, prostaglandins, antacids, antigastrin drugs, mucosal protective agents, triple therapy for H pylori eradication, atazanavir sulfate, rilpivirine hydrochloride, or any investigational or postmarketing study products. Treatment compliance was assessed using patient (paper) diaries recorded twice daily and examination of the study drug container. The investigator was permitted to withdraw a patient from the study if the compliance rate was < 50% for 2 successive visits.
Outcome measures
Efficacy outcomes
The primary efficacy outcome was the proportion of days without heartburn experienced by patients during the 4-week treatment period. Additional efficacy outcomes were the cumulative improvement rate of heartburn, and the mean severity of heartburn experienced by patients during the 4-week treatment period. The heartburn symptom was recorded twice a day by patient diary and determined by patient scores (0 = no symptoms, 1 = very mild [symptoms present but often forgotten], 2 = mild [not so painful], 3 = moderate [rather painful], 4 = severe [painful], and 5 = very severe symptoms [painful enough to affect night-time sleep or daily activity]).
Secondary outcomes were determined for the proportion of days without heartburn and the mean severity of heartburn as determined by patient scores. Patient were stratified into subgroups defined as the response to treatment at Week 2 and baseline endoscopic findings (Grade M or N). Improvement at Week 2 was defined as the patient experiencing a proportion of days without heartburn lower than that observed during the run-in period.
Safety outcomes
Treatment-emergent adverse events (TEAEs), serum chemistry, hematology, urinalysis findings, serum gastrin and pepsinogen I/II concentrations, and vital signs and electrocardiogram results were recorded. TEAEs were recorded using the Medical Dictionary for Regulatory Activities code version 16.0 and were coded by treatment group by System Organ Class and Preferred Term.
Statistical analysis
The sample size was calculated according to previous lansoprazole study results.13 Based on these results, 268 patients per treatment group were required to ensure 90% power of the 2-sample Wilcoxon rank-sum test to verify the intergroup difference with a significance level of 0.05. The required number of patients per treatment group was determined to be 280 after taking into account patient dropout after randomization.
Three analysis sets were defined in this study: the full analysis set (FAS), the safety analysis set, and the per-protocol set (PPS). The FAS and safety analysis set included all patients who were randomized to the study treatments and received at least 1 dose of the study drugs. The PPS included all patients in the FAS who had available measurements for the primary variable, who had a prespecified minimal exposure to the treatment regimen, and who had no major protocol violations.
The proportion of days without heartburn and the mean severity of heartburn during the 4-week treatment period for each treatment group were analyzed in the FAS. Descriptive statistics were used to summarize these 2 outcome measures during the 4-week treatment period by treatment group. A Hodges-Lehmann estimation was used to estimate the difference between the treatment groups and a 2-sample Wilcoxon rank-sum test was used for comparison between treatment groups.
The Kaplan-Meier method was used to estimate the cumulative improvement rate of heartburn during the 4-week treatment period for each treatment group. The event date was defined as the first confirmed date of heartburn improvement that continued until the last day of study treatment. A log-rank test was used to compare the cumulative improvement rate between the vonoprazan groups and the placebo group.
A sensitivity analysis for efficacy outcomes was performed in the PPS to confirm the robustness of the analysis. The same analyses were performed in the subgroups. Analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
Patient disposition, demographic characteristics, and baseline clinical characteristics
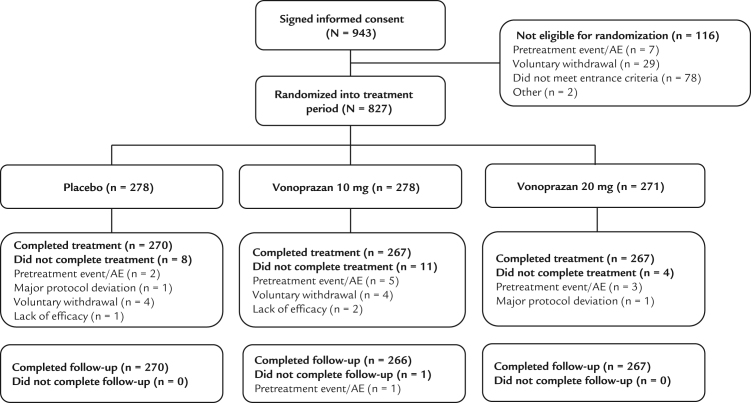
A total of 943 patients gave informed consent (Figure 2). Of these, 827 were randomized for treatment (vonoprazan 20 mg = 271 patients, vonoprazan 10 mg = 278 patients, and placebo group = 278 patients). No obvious differences were observed between the 3 treatment groups at baseline (Table I).
Fig. 2.
Patient flow. AE = adverse event.
Table I.
Patient baseline characteristics.
| Baseline characteristic | Placebo n = 278 | Vonoprazan 10 mg n = 278 | Vonoprazan 20 mg n = 271 | Total N = 827 |
|---|---|---|---|---|
| Male*, n (%) | 109 (39.2) | 116 (41.7) | 98 (36.2) | 323 (39.1) |
| Age†, y | 52.4 (14.57) | 52.9 (14.07) | 52.4 (14.35) | 52.6 (14.31) |
| Height†, cm | 161.3 (8.47) | 160.8 (10.16) | 161.3 (8.67) | 161.1 (9.13) |
| BMI† | 22.45 (3.478) | 23.15 (3.691) | 22.12 (3.243) | 22.58 (3.499) |
| Smoking* | ||||
| Never smoked | 143 (51.4) | 134 (48.2) | 158 (58.3) | 435 (52.6) |
| Current smoker | 39 (14.0) | 56 (20.1) | 36 (13.3) | 131 (15.8) |
| Previous smoker | 96 (34.5) | 88 (31.7) | 77 (28.4) | 261 (31.6) |
| Alcohol consumption* | ||||
| Every day | 59 (21.2) | 65 (23.4) | 72 (26.6) | 196 (23.7) |
| 2 or 3 d/wk | 41 (14.7) | 47 (16.9) | 48 (17.7) | 136 (16.4) |
| 2 or 3 d/mo | 76 (27.3) | 53 (19.1) | 63 (23.2) | 192 (23.2) |
| Never drink | 102 (36.7) | 113 (40.6) | 88 (32.5) | 303 (36.6) |
| Caffeine consumption* | ||||
| Yes | 219 (78.8) | 232 (83.5) | 214 (79.0) | 665 (80.4) |
| No | 59 (21.2) | 46 (16.5) | 57 (21.0) | 162 (19.6) |
| Modified LA Grade* | ||||
| N | 128 (46.0) | 134 (48.2) | 127 (46.9) | 389 (47.0) |
| M | 150 (54.0) | 144 (51.8) | 144 (53.1) | 438 (53.0) |
| Heartburn severity during the run-in period† | 1.748 (0.9288) | 1.741 (0.9805) | 1.779 (0.9289) | 1.756 (0.9455) |
| Esophageal hiatal hernia* | ||||
| Yes, ≥2 cm | 20 (7.2) | 12 (4.3) | 20 (7.4) | 52 (6.3) |
| Yes, <2 cm | 95 (34.2) | 104 (37.4) | 95 (35.1) | 294 (35.6) |
| No | 163 (58.6) | 162 (58.3) | 156 (57.6) | 481 (58.2) |
| H pylori serology* | ||||
| Positive | 77 (27.8) | 79 (28.6) | 76 (28.1) | 232 (28.2) |
| History of H pylori eradication* | ||||
| Yes, ≤1 y | 7 (2.5) | 2 (0.7) | 6 (2.2) | 15 (1.8) |
| Yes, >1 y | 50 (18.0) | 52 (18.7) | 52 (19.2) | 154 (18.6) |
| No | 221 (79.5) | 224 (80.6) | 213 (78.6) | 658 (79.6) |
| Laboratory tests | ||||
| Serum gastrin†, pg/mL | 99.2 (97.46)‡ | 107.8 (130.05) | 104.9 (118.54)§ | 103.9 (116.05)|| |
| Serum pepsinogen I/II† | 5.98 (2.093)‡ | 6.05 (2.056) | 6.14 (2.225)¶ | 6.05 (2.123)# |
BMI = body mass index; H pylori = Helicobacter pylori; LA = Los Angeles.
Values are presented as n (%).
Values are presented as mean (SD).
n = 277.
n = 270.
n = 825.
n = 269.
n = 824.
Efficacy outcome measures
In the FAS, the proportion of days without heartburn during the 4-week treatment period was not significantly different between the vonoprazan treatment groups and the placebo group (P = 0.2310 [vonoprazan 10 mg] and P = 0.0504 [vonoprazan 20 mg]) (Table II). In the sensitivity analysis comprising the PPS population, the proportion of days without heartburn was significantly higher in the vonoprazan 20 mg group compared with the placebo group (P = 0.0304).
Table II.
Proportion of days without heartburn and mean severity of heartburn during the 4-week treatment period (full analysis set).
| Measure | Median* | Q1, Q3* | P† |
|---|---|---|---|
| Proportion of days without heartburn | |||
| Placebo, n = 278 | 7.40 | 0.00, 39.30 | |
| Vonoprazan 10 mg, n = 278 | 10.30 | 0.00, 55.60 | 0.2310 |
| Vonoprazan 20 mg, n = 271 | 12.00 | 0.00, 57.10 | 0.0504 |
| Mean severity of heartburn | |||
| Placebo, n = 278 | 1.070 | 0.520, 1.950 | |
| Vonoprazan 10 mg, n = 278 | 0.990 | 0.360, 1.730 | 0.0440 |
| Vonoprazan 20 mg, n = 271 | 0.960 | 0.320, 1.700 | 0.0139 |
Q1 = quartile 1; Q3 = quartile 3.
Values are presented as %.
Wilcoxon rank-sum test calculated on the median.
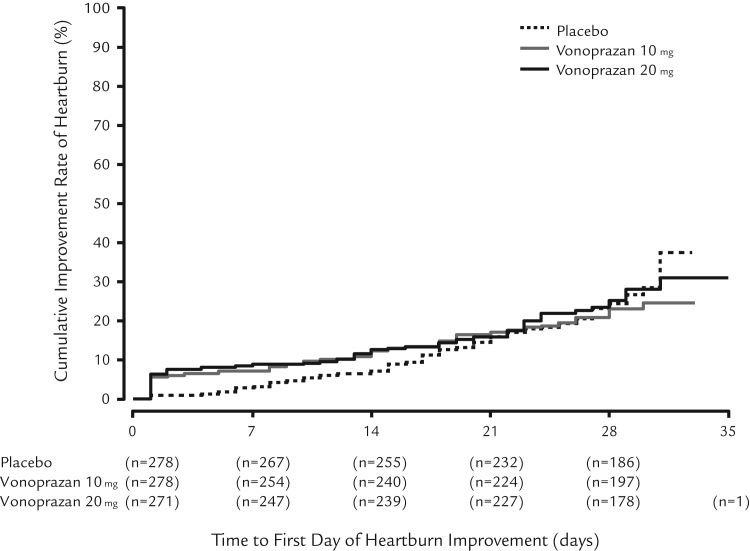
The differences in the cumulative improvement rate of heartburn were not statistically significant between the vonoprazan treatment groups and the placebo group. However, time until the event date (the first confirmed date of heartburn improvement) was shorter in the vonoprazan groups compared with the placebo group (Figure 3). In addition, the proportion of combined days without heartburn and days with very mild heartburn was significantly greater in the treatment groups compared with the placebo group (median [Quartile 1, Quartile 3]: placebo = 60.70% [14.30, 85.70], vonoprazan 10 mg = 72.40% [21.40, 96.00]; P = 0.0099, and vonoprazan 20 mg = 71.40% [20.00, 96.30]; P = 0.0025). The mean severity of heartburn was significantly lower in the vonoprazan treatment groups (median score = 0.990 [vonoprazan 10 mg] and 0.960 [vonoprazan 20 mg]) compared with the placebo group (score = 1.070) (P = 0.0440 [vonoprazan 10 mg] and P = 0.0139 [vonoprazan 20 mg]).
Fig. 3.
Improvement rate of heartburn during the treatment period, full analysis set (FAS).
Outcome measures in the subgroup analyses
In patients whose symptoms had improved at Week 2, the proportion of days without heartburn was significantly higher and the mean severity of heartburn was significantly lower in the vonoprazan treatment groups compared with the placebo group (proportion of days without heartburn: P = 0.0004 [vonoprazan 10 mg] and P = 0.0001 [vonoprazan 20 mg] and mean severity: P < 0.0001 [vonoprazan 10 mg] and P < 0.0001 [vonoprazan 20 mg]) (Table III).
Table III.
Subgroup analyses of proportion of days without heartburn and mean severity of heartburn by NERD Grade and symptom improvement at Week 2.
| Subgroup | Median (Q1, Q3) | P value* |
|---|---|---|
| Proportion of days without heartburn, % | ||
| Symptom improvement at Week 2 | ||
| Improved | ||
| Placebo, n = 104 | 44.7 (21.1, 68.5) | |
| Vonoprazan 10 mg, n = 121 | 60.7 (34.5, 88.9) | 0.0004 |
| Vonoprazan 20 mg, n = 123 | 60.0 (40.0, 85.7) | 0.0001 |
| Not improved | ||
| Placebo, n = 174 | 0.0 (0.0, 11.1) | |
| Vonoprazan 10 mg, n = 157 | 0.0 (0.0, 3.4) | 0.0358 |
| Vonoprazan 20 mg, n = 148 | 0.0 (0.0, 3.6) | 0.0884 |
| NERD Grade | ||
| Grade N | ||
| Placebo, n = 128 | 7.9 (0.0, 46.3) | |
| Vonoprazan 10 mg, n = 134 | 6.7 (0.0, 46.4) | 0.8729 |
| Vonoprazan 20 mg, n = 127 | 10.7 (0.0, 59.3) | 0.4525 |
| Grade M | ||
| Placebo, n = 150 | 7.1 (0.0, 36.0) | |
| Vonoprazan 10 mg, n = 144 | 13.8 (0.0, 60.7) | 0.0657 |
| Vonoprazan 20 mg, n = 144 | 17.0 (0.0, 57.1) | 0.0451 |
| Mean severity of heartburn, score (Q1, Q3) | ||
| Symptom improvement at Week 2 | ||
| Improved | ||
| Placebo, n = 104 | 0.54 (0.31, 0.82) | |
| Vonoprazan 10 mg, n = 121 | 0.32 (0.07, 0.64) | < 0.0001 |
| Vonoprazan 20 mg, n = 123 | 0.30 (0.12, 0.62) | < 0.0001 |
| Not improved | ||
| Placebo, n = 174 | 1.66 (1.07, 2.21) | |
| Vonoprazan 10 mg, n = 157 | 1.61 (1.07, 2.27) | 0.7834 |
| Vonoprazan 20 mg, n = 148 | 1.61 (1.17, 2.12) | 0.9230 |
| NERD Grade | ||
| Grade N | ||
| Placebo, n = 128 | 1.08 (0.51, 1.95) | |
| Vonoprazan 10 mg, n = 134 | 1.00 (0.46, 1.79) | 0.4162 |
| Vonoprazan 20 mg, n = 127 | 1.05 (0.32, 1.91) | 0.2975 |
| Grade M | ||
| Placebo, n = 150 | 1.07 (0.64, 1.95) | |
| Vonoprazan 10 mg, n = 144 | 0.96 (0.33, 1.64) | 0.0382 |
| Vonoprazan 20 mg, n = 144 | 0.91 (0.33, 1.63) | 0.0155 |
NERD = nonerosive esophageal reflux disease; Q1 = quartile 1; Q3 = quartile 3.
Wilcoxon rank-sum test for difference in median between vonoprazan and placebo.
In patients with NERD Grade M at baseline, the mean severity of heartburn was significantly lower in the vonoprazan treatment groups compared with the placebo group (vonoprazan 10 mg: P = 0.0382 and vonoprazan 20 mg: P = 0.0155). Similarly, the proportion of days without heartburn was significantly higher in the vonoprazan 20 mg treatment group compared with the placebo group in patients with NERD grade M (P = 0.0451). There was no significant difference between treatment groups in the proportion of days without heartburn or the mean severity of heartburn in patients with NERD grade N.
Safety and tolerability measures
The safety profiles of vonoprazan 10 mg and 20 mg were similar to that of placebo. The incidence of TEAEs was 27.7% in the vonoprazan 10 mg group, 28.0% in the vonoprazan 20 mg group, and 32.7% in the placebo group (Table IV). The most common TEAE was nasopharyngitis.
Table IV.
Summary of safety outcomes.
| Adverse events | Placebo (n = 278) |
Vonoprazan 10 mg (n = 278) |
Vonoprazan 20 mg (n = 271) |
|||
|---|---|---|---|---|---|---|
| Events | Patients (%) | Events | Patients (%) | Events | Patients (%) | |
| TEAEs | 118 | 91 (32.7) | 97 | 77 (27.7) | 99 | 76 (28.0) |
| Related | 23 | 18 (6.5) | 14 | 10 (3.6) | 11 | 10 (3.7) |
| Not related | 95 | 73 (26.3) | 83 | 67 (24.1) | 88 | 66 (24.4) |
| Mild | 108 | 83 (29.9) | 92 | 72 (25.9) | 94 | 71 (26.2) |
| Moderate | 10 | 8 (2.9) | 5 | 5 (1.8) | 5 | 5 (1.8) |
| Severe | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) |
| Leading to discontinuation | 1 | 1 (0.4) | 6 | 6 (2.2) | 3 | 3 (1.1) |
| SAEs | 0 | 0 (0.0) | 1 | 1 (0.4) | 3 | 3 (1.1) |
| Related | 0 | 0 (0.0) | 1 | 1 (0.4) | 0 | 0 (0.0) |
| Not related | 0 | 0 (0.0) | 0 | 0 (0.0) | 3 | 3 (1.1) |
| Leading to discontinuation | 0 | 0 (0.0) | 1 | 1 (0.4) | 0 | 0 (0.0) |
| Deaths | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) |
SAE = serious adverse event; TEAE = treatment-emergent adverse event.
Most of the reported TEAEs were mild in intensity and no severe TEAEs were reported during the study period. No deaths were reported. One serious adverse event (SAE) was reported in 1 patient in the vonoprazan 10 mg group and 3 SAEs were reported in 3 patients in the vonoprazan 20 mg group. Of these, 1 SAE (diverticulitis) in the vonoprazan 10 mg group was considered related to the study drug. No SAEs were reported by patients in the placebo group. Mean levels of serum gastrin, pepsinogen I, and pepsinogen II increased after the study drug administration in the vonoprazan 10 mg and 20 mg groups (Table V). No clinically significant changes in gastrin, pepsinogen I, or pepsinogen II were observed in the placebo group.
Table V.
Serum gastrin, pepsinogen I, and pepsinogen II (safety analysis set).
| Variable | Placebo |
Vonoprazan 10 mg |
Vonoprazan 20 mg |
|||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Gastrin, pg/mL | ||||||
| Baseline | 277 | 99.2 (97.46) | 278 | 107.8 (130.05) | 270 | 104.9 (118.54) |
| Week 4 | 268 | 90.1 (99.70) | 268 | 316.7 (295.94) | 265 | 397.0 (242.50) |
| Follow-up | 271 | 85.1 (83.17) | 269 | 102.7 (93.62) | 268 | 84.6 (59.59) |
| Pepsinogen I, ng/mL | ||||||
| Baseline | 277 | 50.49 (22.003) | 278 | 50.56 (20.755) | 269 | 52.15 (22.988) |
| Week 4 | 268 | 47.69 (20.088) | 268 | 182.38 (136.398) | 265 | 229.91 (143.751) |
| Follow-up | 271 | 47.56 (19.696) | 269 | 71.41 (82.950) | 268 | 71.27 (40.354) |
| Pepsinogen II, ng/mL | ||||||
| Baseline | 277 | 10.23 (7.406) | 278 | 10.10 (8.355) | 269 | 10.48 (8.025) |
| Week 4 | 268 | 9.86 (7.630) | 268 | 31.68 (23.121) | 265 | 42.86 (27.348) |
| Follow-up | 271 | 9.75 (7.384) | 269 | 13.38 (17.654) | 268 | 12.25 (8.819) |
Discussion
To our knowledge, this is the first randomized controlled trial to examine the effect of vonoprazan in patients with NERD. We observed that vonoprazan 10 mg and 20 mg were not superior to placebo in NERD in the FAS with respect to the proportion of days without heartburn. In the PPS, vonoprazan 20 mg was superior to placebo and the proportion of days without heartburn was higher in the vonoprazan 20 mg group. However, according to the stratified analysis at Week 2, we observed no meaningful difference between vonoprazan 10 mg and 20 mg doses.
We also found that the difference in mean severity of heartburn was significantly lower with vonoprazan 10 mg and 20 mg compared with the placebo group, although there was no significant difference in cumulative improvement rate of heartburn. Our results are consistent with previous studies of patients with NERD, which indicate that the pathology of NERD may be distinct from erosive acid-related diseases and patients with NERD may not respond as well to some treatments that are effective in GERD with erosive mucosal injury. For example, patients with NERD often respond poorly to some PPIs.12, 14, 15 However, the severity of acid reflux may correlate with response to therapy. One study suggested that severity of acid reflux in NERD is predictive of response to rabeprazole.15 Similarly, in our study, patients with some observable vague erythema and/or whitish turbidity mucosa (NERD Grade M) experienced significantly lower mean severity of heartburn with vonoprazan compared with placebo. In contrast, there was no significant difference in mean severity of heartburn between vonoprazan and placebo in patients with no observable mucosal change (NERD Grade N).
We observed that patients with NERD who responded to vonoprazan at Week 2 experienced a significantly increased proportion of days without heartburn and significantly reduced severity of heartburn at Week 4 compared with patients who did not respond at Week 2. Similarly, we observed that patients in the vonoprazan treatment groups experienced faster resolution of heartburn compared with placebo. Our results indicate that resolution of symptoms at Week 2 with vonoprazan may be predictive of response at Week 4.
The strengths of our study include its randomized, multicenter study design and the good retention of patients in the follow-up phase. In addition, the study stratified patients into Grades M and N categories; these are standard classifications used by Japanese endoscopists. The limitations of the study include the low numbers of patients in some groups for the subgroup analyses. Because the resolution of symptoms was measured by patient diary entries and was not confirmed by pH monitoring, recorded symptoms may be subjective or not related to NERD; for example, based on the grading scale, it is possible that very mild symptoms could be categorized as no symptoms.
There are a number of other factors that may have confounded the results of our study. First, we included a run-in period with antacid treatment in our study design. Participants were excluded from the study when they responded to antacids in the run-in period. Therefore, our study design allowed the possibility that appropriate patients with acid-induced heartburn may have been excluded. Second, the postobservation period with placebo in this study may have confounded the cumulative heartburn improvement rate. Third, according to the stratified analysis based on response at Week 2, many patients with possibly confounding conditions were included in this study; for example, patients with functional disorders such as functional dyspepsia and functional heartburn. Fourth, it should also be considered that patients with no response to antacid agents during the run-in phase may actually have had functional heartburn and would therefore not be expected to improve with vonoprazan at Week 2 or at Week 4. In contrast, patients who responded to antacid agents were probably more likely to have had true NERD; these patients responded to vonoprazan at Week 2 and continued to show response to vonoprazan at Week 4. Therefore, in patients with heartburn who have normal or equivocal findings at endoscopy, failure to respond to antacid may be predictive of failure to respond to acid-suppressing medicines. Finally, in the group of patients who responded at Week 2, there was no meaningful difference in efficacy between 10 mg and 20 mg vonoprazan despite the fact that the acid inhibitory effect of vonoprazan 20 mg is greater than vonoprazan 10 mg. One possible explanation for this result is that vonoprazan 10 mg is a sufficient dose for the improvement of NERD. The recommended dose of PPIs for treatment of NERD in Japanese patients is half the maximum dose for treatment of erosive esophagitis16; the maximum dose of vonoprazan for treatment of erosive esophagitis is 20 mg.
Conclusions
Results from the FAS suggest that vonoprazan 10 mg and 20 mg are not superior to placebo with respect to proportion of days without heartburn in patients with NERD. However, the results from the PPS suggest that vonoprazan 20 mg may be effective in the treatment of NERD. In addition, there was no meaningful difference observed between 10 mg and 20 mg doses of vonoprazan. To confirm the clinical influence and efficacy of vonoprazan in patients with NERD, future studies should be designed to overcome the limitations found in our study.
Conflicts of Interest
Funding for this work was provided by Takeda Pharmaceutical Company Limited, Osaka, Japan. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Funding support
This work was supported by Takeda Pharmaceutical Company Limited, manufacturer / licensee of vonoprazan. Medical writing assistance was provided by Elise Magatova, PhD, and Tania Dickson, PhD, of ProScribe - Envision Pharma Group, and was funded by Takeda Pharmaceutical Company Limited. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
Role of the sponsor
Takeda Pharmaceutical Company Limited was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Role of contributors
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. YK, KA, YS, MS, and AN were involved in the study design and interpretation. YK served as the coordinating investigator. TM served as a medical expert. EU, KI, and KA served as the Central Adjudication Committee. KK conducted the statistical analysis.
Conflict of interest statement
YS, MS, KK, and AN are employees of Takeda Pharmaceutical Company Limited. YK has received study support from Takeda Pharmaceutical Company Limited and has participated in consultancies / advisory panels and speakers' bureaus for Takeda Pharmaceutical Company Limited. YK, TM, KI, EU, and KA received consulting fees from Takeda Pharmaceutical Company Limited during the study period.
Other contributors/acknowledgments
The authors would like to thank all study patients. The authors thank Ana Oliveira, Fiona Steinkamp, Matthias Binek, Nigel Brayshaw, Richard Jenkins, Tanja Franolic, Yasunori Gotou, and Yukio Shimasaki from Takeda for reviewing this manuscript.
References
- 1.Fass R., Fennerty M.B., Vakil N. Nonerosive reflux disease--current concepts and dilemmas. Am J Gastroenterol. 2001;96(2):303–314. doi: 10.1111/j.1572-0241.2001.03511.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee E.S. Comparison of risk factors and clinical responses to proton pump inhibitors in patients with erosive oesophagitis and non-erosive reflux disease. Aliment Pharmacol Ther. 2009;30(2):154–164. doi: 10.1111/j.1365-2036.2009.04021.x. [DOI] [PubMed] [Google Scholar]
- 3.Fock K.M. Asia-Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol. 2008;23(1):8–22. doi: 10.1111/j.1440-1746.2007.05249.x. [DOI] [PubMed] [Google Scholar]
- 4.Miwa H. Efficacy of rabeprazole on heartburn symptom resolution in patients with non-erosive and erosive gastro-oesophageal reflux disease: a multicenter study from Japan. Aliment Pharmacol Ther. 2007;26(1):69–77. doi: 10.1111/j.1365-2036.2007.03350.x. [DOI] [PubMed] [Google Scholar]
- 5.Shin J.M. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin e monofumarate (TAK-438) J Pharmacol Exp Ther. 2011;339(2):412–420. doi: 10.1124/jpet.111.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashida K. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43(2):240–251. doi: 10.1111/apt.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami K. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda Takecab (vonoprazan tablets) package insert. Available at: http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/400256_2329030F1020_1_04. Accessed 28 Sep 2016.
- 9.Hori Y. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin e monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335(1):231–238. doi: 10.1124/jpet.110.170274. [DOI] [PubMed] [Google Scholar]
- 10.Hori Y. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337(3):797–804. doi: 10.1124/jpet.111.179556. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai Y. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Rising TAK-438 (Vonoprazan) Doses in Healthy Male Japanese/non-Japanese Subjects. Clin Transl Gastroenterol. 2015;6:e94. doi: 10.1038/ctg.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006;41(2):95–99. doi: 10.1007/s00535-006-1775-4. [DOI] [PubMed] [Google Scholar]
- 13.Hongo Ma.Y.H. Efficacy and safety of lansoprazole (AG-1749) 15 mg and 30 mg in Japanese patients with non-erosive reflux disease (NERD) - a phase III multicenter, double-blind, placebo-controlled trial. Jpn Pharmacol Ther. 2008;36(7):655–671. [Google Scholar]
- 14.Adachi K. A study on the efficacy of rebamipide for patients with proton pump inhibitor-refractory non-erosive reflux disease. Dig Dis Sci. 2012;57(6):1609–1617. doi: 10.1007/s10620-012-2087-6. [DOI] [PubMed] [Google Scholar]
- 15.Shimatani T. Predicting the efficacy of proton pump inhibitors in patients with non-erosive reflux disease before therapy using dual-channel 24-h esophageal pH monitoring. J Gastroenterol Hepatol. 2012;27(5):899–906. doi: 10.1111/j.1440-1746.2011.06975.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto M., Manabe N., Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med. 2010;49(15):1469–1476. doi: 10.2169/internalmedicine.49.3615. [DOI] [PubMed] [Google Scholar]