Idiopathic REM sleep behavior disorder (IRBD) is a REM sleep parasomnia comprising unpleasant dreams, dream-enacting behaviors, and loss of muscle atonia during REM sleep. Longitudinal studies have demonstrated that because most patients with IRBD develop with time Parkinson disease (PD) and other synucleinopathies including dementia with Lewy bodies (DLB) or multiple system atrophy (MSA), IRBD represents a prodromal stage of these diseases.1 In PD, 5%–10% of cases are caused by nonfrequent mendelian mutations segregating with disease in pedigrees, but the vast majority of cases are sporadic. Unbiased genome-wide association studies in sporadic PD (sPD) have shown that single nucleotide polymorphisms (SNPs) in the α-synuclein (SNCA) and microtubule-associated protein tau (MAPT) genes modulate disease susceptibility.2 Given that IRBD often antedates sPD and that, akin to PD, familial clustering of IRBD is rare, we hypothesized that genetically shared variation at SNCA and MAPT may influence disease susceptibility to both conditions. Accordingly, we genotyped PD-associated genetic variants in SNCA and MAPT in a cohort of Spanish patients with IRBD.
Methods.
The SNPs rs356219 (A/G) in SNCA and rs1800547 (H1/H2) in MAPT have been consistently associated with sPD in the Spanish population.3,4 Here, we genotyped these markers in a Spanish Caucasian sample of 121 patients with IRBD and 175 healthy controls. This population was studied in a previous report.5 The IRBD group was recruited at the Multidisciplinary Sleep Unit of the Hospital Clínic de Barcelona, had a polysomnography-confirmed diagnosis, and consisted of 21/100 women/men (17.4/82.6%), with age at IRBD onset of 68.3 ± 6.2 years and age at sample collection of 71.2 ± 6.4 years. Patients fulfilled the diagnosis of IRBD and were free of parkinsonism, mild cognitive impairment, and dementia at the time of sample collection. After 6.8 ± 4.1 years of follow-up from IRBD diagnosis, 38 patients (31.4%) were clinically diagnosed with a synucleinopathy (DLB in 19 patients, PD in 18, and MSA in 1). Sex-, age-, and demographic-matched controls were recruited at the Multidisciplinary Sleep Unit of the Hospital Clínic de Barcelona (n = 45) and the Clínica Universitaria de Navarra (n = 129), and consisted of 21/154 women/men (13.6/86.4%) with age at sample collection of 71.7 ± 6.4 years. DNA was extracted from the peripheral blood using standard procedures. Genotyping was performed using the predesigned TaqMan assays C-1020193-10 (SNCA rs356219) and C-7563692-10 (MAPT rs1800547) in a StepOnePlus Real-time PCR System (Applied Biosystems, Foster City, CA). Allelic and genotypic frequencies were compared using SNPstats software (bioinfo.iconcologia.net/SNPstats).
Standard protocol approvals, registrations, and patient consents.
Samples were collected after patient's signed consent, and the local ethics committee approved the study.
Results.
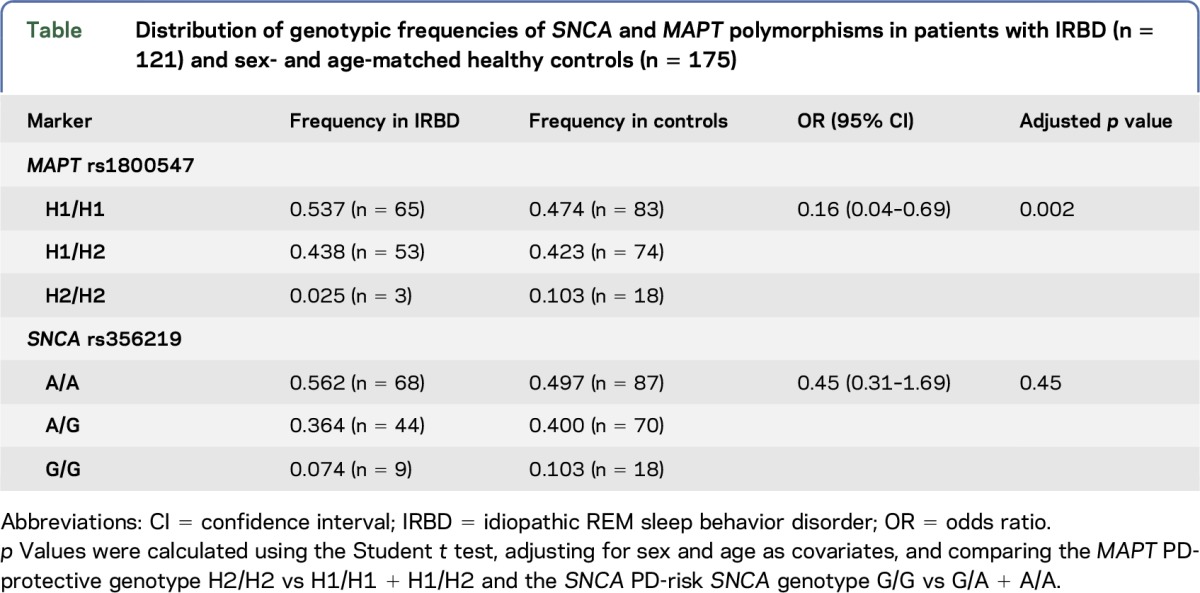
We found no allelic or genotypic distribution differences of the SNCA marker rs356219 in patients with IRBD and in controls. However, we found an association of the MAPT polymorphism rs1800547 with IRBD. More specifically, we found that the frequency of the MAPT H2/H2 genotype which is protective for PD3,4 was underrepresented in our patients with IRBD. This association held statistically significant after adjusting for sex and age as covariates (odds ratio = 0.16; 95% confidence interval = 0.04–0.69; p = 0.002; table). Moreover, we observed that MAPT genotypes in patients with IRBD, but not in controls, were not in the Hardy-Weinberg equilibrium (p = 0.049), a finding which is consistent with previous observations for disease-linked loci in disease-affected individuals.6
Table.
Distribution of genotypic frequencies of SNCA and MAPT polymorphisms in patients with IRBD (n = 121) and sex- and age-matched healthy controls (n = 175)
Discussion.
We found that the MAPT marker rs1800547 is associated with IRBD. Consistently, we detected that the H2 variant which is protective for PD was underrepresented in our patients with IRBD. On the contrary, we did not find an association of the SNCA polymorphism rs356219 with IRBD. Altogether, these results are in agreement with another IRBD study reporting association for MAPT but not for SNCA polymorphisms.7 Yet, markers in both studies were different. These data raise the question whether IRBD shares with PD the same genetic risk factors or whether, alternatively, IRBD represents a specific endophenotype of PD with only certain genetic risk factors shared in common by both conditions. The later might be a plausible explanation because, although IRBD is considered a prodrome of sPD, not all patients with sPD develop RBD prior to parkinsonism. Supporting this view, we have recently reported that pathogenic mutations in the leucine-rich repeat kinase 2 gene (LRRK2), which are the most frequent genetic cause of PD, are absent in our IRBD cohort.5 One limitation of our study is the reduced number of patients with IRBD. Thus, future studies in larger cohorts are warranted to validate the association of MAPT with IRBD and also to further elucidate whether or not SNCA polymorphisms play a role in disease. We found that one polymorphism in MAPT which is associated with PD also modulates the propensity to IRBD. These results point toward an at least in part overlapping genetic susceptibility to both conditions in our Spanish population.
Acknowledgments
Acknowledgment: The authors thank the patients and their relatives who participated in the study. This study was conducted at the Centre de Recerca Biomèdica Cellex, Barcelona, Spain. The authors also thank the CERCA from the Generalitat de Catalunya and the European FEDER Programmes to IDIBAPS.
Footnotes
Author contributions: R.F.-S., A.I., and M.E. codirected the study and wrote the first draft of the manuscript. A.I, C.G, P.P., M.S., E.T., and J.S. diagnosed and followed up the patients, and recruited the controls. R.F.-S., M.F., P.P., and M.E. performed the genotyping. All authors interpreted the data and revised the manuscript.
Study funding: This work was supported by funds from the Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED) to the Movement Disorders Unit of the Neurology Service from the Hospital Clínic de Barcelona (grant PRI-16-207 to R.F.-S., A.I., C.G, M.S., M.F., E.T., J.S., and M.E.), and from the Spanish Ministry of Economy and Competitivity (MINECO) (grant SAF2013-47939-R to P.P.). R.F.-S. was supported by a Marie Skłodowska-Curie contract of the European Commission (EC) and Juan-de-la-Cierva contract of the Spanish Ministry of Economy and Competitiveness (MINECO), and M.E. by a Miguel Servet contract of the Instituto de Salud Carlos III (ISCIII).
Disclosure: Dr. Fernández-Santiago, Dr. Iranzo, Dr. Gaig, Ms. Serradell, Mr. Fernández, and Dr. Pastor report no disclosures. Dr. Tolosa has received speaker honoraria from UCB, Boeringer Ingelheim, Novartis, Abbot, Medtronic, Solvay, GSK, and Teva; has served on the editorial board of Neurological Sciences; holds a patent for Method for the subclassification of patients suffering from Parkinson disease; and has been a consultant for the Michael J. Fox Foundation for Parkinson's Disease, Novartis, UCB, Abbot, and Teva. Dr. Santamaría has received research support from Fundació La Marató TV3. Dr. Ezquerra reports no disclosures. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by CIBERNED.
References
- 1.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
- 2.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet 2009;41:1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastor P, Ezquerra M, Munoz E, et al. Significant association between the tau gene A0/A0 genotype and Parkinson's disease. Ann Neurol 2000;47:242–245. [PubMed] [Google Scholar]
- 4.Botta-Orfila T, Ezquerra M, Rios J, et al. Lack of interaction of SNCA and MAPT genotypes in Parkinson's disease. Eur J Neurol 2011;18:e32. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Santiago R, Iranzo A, Gaig C, et al. Absence of LRRK2 mutations in a cohort of patients with idiopathic REM sleep behavior disorder. Neurology 2016;86:1072–1073. [DOI] [PubMed] [Google Scholar]
- 6.Lee WC. Searching for disease-susceptibility loci by testing for Hardy-Weinberg disequilibrium in a gene bank of affected individuals. Am J Epidemiol 2003;158:397–400. [DOI] [PubMed] [Google Scholar]
- 7.Gan-Or Z, Girard SL, Noreau A, et al. Parkinson's disease genetic loci in rapid eye movement sleep behavior disorder. J Mol Neurosci 2015;56:617–622. [DOI] [PubMed] [Google Scholar]


