Abstract
BACKGROUND
Research has linked tonic and variable mood to problematic alcohol use, both between- and within-subjects. Indices of behavioral control have moderated these links, at least at the between-subjects level. The current study examines daily associations between indices of emotional functioning and alcohol involvement as a function of response inhibition.
METHODS
College student drinkers (n = 74; 58.11% female) were enrolled in a study on emotion and alcohol use. Participants completed a stop-signal task as an index of response inhibition. They then carried a personal data device for 21 days, reporting daily on mood, alcohol use, and acute alcohol use disorder symptoms. Mood instability was the mean square of successive differences from daily mood assessments.
RESULTS
There were 1,309 person days (622 drinking days) available for analysis. Pre-drinking mood instability was positively associated the likelihood of drinking and drinks consumed on drinking days. The former association was diminished among women with high response inhibition. Pre-drinking positive mood was positively associated the likelihood of drinking and drinks consumed on drinking days. The latter association was diminished among women with high response inhibition. Pre-drinking negative mood was positively associated with drinks consumed on drinking days among women with low response inhibition. Finally, pre-drinking positive mood was associated with acute alcohol use disorder symptoms among those with low response inhibition.
CONCLUSIONS
These results suggest that interventions targeting positive mood may be particularly important. Further, developing ways to improve response inhibition control may broadly influence negative drinking outcomes by affecting multiple mood-drinking associations.
Keywords: Ecological Momentary Assessment, Mood Instability, Response Inhibition, Alcohol
1. Introduction
Several theoretical models posit that mood motivates alcohol use including the tension reduction hypothesis (Conger, 1956), self-medication hypothesis (Khantzian, 1997), affective processing model of negative reinforcement (Baker et al., 2004), stressor-vulnerability model (Cooper et al., 1988), and stress-response dampening model (Sher and Levenson, 1982). However, these models focus primarily on tonic levels of mood, and most have not considered potential moderators of mood-alcohol associations.
In addition to examining positive and negative mood, recent research has demonstrated the utility of examining mood variability or instability in the context of alcohol use. Retrospective self-report studies suggest that mood instability is related to a host of alcohol-related outcomes including alcohol-related problems (Kuvaas et al., 2013; Simons, 2003; Simons et al., 2004) and dependence symptoms (Simons et al., 2009; Stevenson et al., 2015). However, these studies are limited by retrospective recall biases.
To overcome recall biases, researchers have examined mood-alcohol associations in near-real time using ecological momentary assessment (EMA; Shiffman, 2009). Several EMA studies demonstrate relationships between mood and alcohol use (see Armeli et al., 2000; Dvorak et al., 2014; Dvorak and Simons, 2014; Hussong et al., 2001; Mohr et al., 2005; Simons et al., 2010), though these studies tend to focus on level of mood rather than mood dynamics. In a notable exception, Gottfredson and Hussong (2013) used EMA to demonstrate that mood variability (operationalized using standard deviations) was related to increased drinking at both the between-subject and within-subject levels.
Mood instability represents moment-to-moment mood fluctuations, reflecting both high variability and low temporal dependency. Whereas standard deviations capture variability and autocorrelations capture temporal dependency, mood instability seems to be best captured by the mean square of successive difference (MSSD; Ebner-Priemer et al., 2009; Jahng et al., 2008; Trull and Ebner-Priemer, 2009) as it accounts for both variability and temporal dependency. In the present study, we focus on mood instability as an indicator of emotion dysregulation that is associated with alcohol-related outcomes above and beyond the effects of positive and negative mood.
Although multiple theoretical accounts justify why mood instability should relate to alcohol-related outcomes, we focus on the strength model of self-control (SMSC; Muraven and Baumeister, 2000), which considers problematic alcohol use as self-regulation failure. Based on numerous SMSC studies, regulating one’s mood is thought to use limited self-control resources (Baumeister et al., 1998). According to this model, more frequent attempts of mood regulation can lead to a state of diminished effortful resources, resulting in problematic alcohol use (i.e., heavy alcohol use and/or experiencing more AUD symptoms). Indeed, recent research has indicated it is the regulation of mood, rather than elevated mood states generally, that results in the depletion of effortful resources (Bruyneel et al., 2009). Providing evidence of the SMSC within the context of problematic alcohol use, Muraven et al. (2005) found that self-control demands during the day, which include tasks such as trying to suppress negative emotions, predicted violations of individual drinking limits. Interestingly, this depletion effect may be less pronounced among those with higher “trait” levels of behavioral control (Dvorak and Simons, 2009; Gailliot and Baumeister, 2007; Muraven et al., 2005).
Although assessed using a wide range of distinct measures, recent reviews and meta-analyses (e.g. Smith et al., 2014; Wilcox et al., 2014) have indicated that deficits in behavioral control are associated with problematic alcohol use. Furthermore, there is emerging evidence that indices related to behavioral control moderate the association between mood instability and alcohol-related problems. Using cross-sectional data, Simons, Carey, and Gaher (2004) found that the positive relationship between mood instability and alcohol-related problems was strongest among individuals with high self-report impulsivity (i.e., low behavioral control). Similarly, Stevenson et al. (2015) found the positive association between mood instability and alcohol dependence symptoms was diminished among individuals with better Stroop performance (i.e., high behavioral control). In a prospective study, Simons et al. (2009) found that mood instability was a stronger predictor of alcohol abuse symptoms six months later among those with higher self-report impulsivity (i.e., low behavioral control). Thus, there is growing evidence that associations between unstable mood and problematic use are strongest among individuals with diminished behavioral control. However, this has yet to be examined at the daily level.
Building off this research, the present study proposes that mood instability results in a depletion of effortful resources, and this effect may be less pronounced among those with better behavioral control. Overall, we expected that mood instability would predict alcohol outcomes (i.e., alcohol use and AUD symptoms) above and beyond positive and negative mood, and that response inhibition (RI), a behavioral index related to behavioral control, would moderate mood-alcohol associations such that these associations were strongest among those with lower RI (i.e., deficits in behavioral control). Finally, we explored gender as a moderator of these associations.
2. Methods
2.1. Participants
Participants (n = 74; 58.11% female) were recruited from a Midwest university for a study examining emotion and alcohol use. The sample ranged in age from 18 to 29 years (M = 21.30, SD = 2.07). Ninety-one percent of the sample was White, 1% was Black, 3% was Native American/Alaskan Native, 4% was Asian, and 1% was other.
2.2. Procedure
This study consisted of two phases. During Phase I, participants (n = 1,875) completed an online screen for Phase II (the EMA phase). Participants who met enrollment criteria (drinking 2 to 4 times per month) were invited to participate in Phase II (n = 460). The first 80 individuals who responded to the invite were scheduled for a lab appointment where they completed informed consent, baseline lab assessments of neuropsychological functioning including RI, and training in the use of the personal data device (PDD). The PDD training included: (1) a review of PDD schedule of events (i.e., random assessments and self-assessments), (2) education on a “standard” alcoholic drink using the NIAAA standard drink card, (3) discussion of acute alcohol use disorder symptoms (these were described as ‘alcohol-related problems’ – for each symptom an example was given), and (4) procedures in the event of loss, theft, or device error. Participants carried the PDD for the next 21 days. Participants were compensated $20 for the initial appointment, $0.50 for each completed random assessment and $1.00 for each completed morning assessment.
2.2.1. Ecological Momentary Assessments (EMA)
EMA participants responded to three assessments on the PDD: morning (a self-initiated assessment occurring between 8:00AM–10:00AM), random mood/drinking assessments (occurring randomly nine times per day between 8:00AM–2:00AM), and an evening assessment (not used here). Morning assessments primarily examined alcohol use variables. Random assessments primarily assessed current mood and drinks consumed (if currently drinking). Participants could set the PDD to ‘Vibrate’ and could postpone random assessments for up to 10 minutes. All assessments were date and time stamped.
2.3. Measures
2.3.1. Emotional Functioning
Emotional functioning was assessed by 18 items from subscales of the PANAS-X (Watson and Clark, 1999) and Larsen and Diener’s (1987) mood circumplex. Each item asked “How ____ are you feeling right now?” with responses on a scale of 1 (not at all) to 11 (extremely). Five facets of mood were selected. Four negative mood states - anxiety (anxious, nervous, jittery; α = .84), anger (angry, frustrated, irritated, tense; α = .89), stress (stressed, overwhelmed; α = .84), and sadness (down, blue, depressed, sad; α = .93) – were combined to form a negative mood indicator (α = .83). Five positive mood states (excited, enthusiastic, energetic, happy, joyful; α = .93) were used to form the positive mood indicator. Mood Instability was a standardized variable formed using the Mean Square of Successive Difference (MSSD) for each primary mood state above (n = 5) across random assessments (α = .70). Previous research supports the use of MSSD as a measure of mood instability (Jahng et al., 2008). The formula for computing MSSD is below:
2.3.2. Alcohol use
Alcohol use as assessed via two different strategies. Each morning participants were asked, “How many drinks did you consume last night?” which they responded to on a scale of 0 to 50. If participants reported they had been drinking during an in situ assessment, participants were asked, “How many drinks have you consumed since your last assessment?” which they responded to on a scale of 1 to 20. There was a strong positive correlation between in situ and morning drinks reported (r = .64, p < .001).
2.3.3. Acute alcohol use disorder (AUD) symptoms
AUD symptoms included symptoms indexing a loss of control over alcohol use (e.g., “drank when promised self not to”), tolerance (e.g., “had to drink more to feel same effects”), and withdrawal (“experience withdrawal symptoms”). Participants were asked, “Did any of the following occur last night or this morning?” The 10 items were shown in a scrolling list each day during the morning assessment. At the first lab appointment, occurring prior to the EMA data collection period, all items were discussed with participants to ensure they fully understood the meaning of each symptom and could accurately report on them during the self-monitoring period.
2.3.4. Response Inhibition (RI)
RI was measured via the Stop-Signal Task using mean Stop-Signal Reaction Time (SSRT) latency. During the stop signal task, a succession of left- or right-pointing arrows is presented. Participants press the corresponding key for the arrow presented. A brief tone (stop signal), indicating participants should inhibit a response, is presented on 25% of trials. Stop signals are presented between 50 and 300 milliseconds after the arrow. The delay is adjusted using a staircase approach based on the previous response to the last stop signal. Participants completed one practice block of 32 trials followed by four test blocks of 64 trials each. Previous research indicates that the Stop Signal RT (SSRT; the mean RT required to inhibit a pre-potent response) serves as an accurate measure of RI (Logan, 1994). Previous research has linked the SSRT to alcohol-related outcomes (Aragues et al., 2011). Lower scores indicate better RI.
2.4. Data preparation
The original sample had 80 participants; however, one individual completed no EMA assessments, two individuals reported no alcohol use, two individuals had extremely low compliance (i.e., <20% with no self-initiated assessments), and one individual reported alcohol use, but no daytime mood. These observations were removed, resulting in an analysis sample of n = 74.
Tonic positive and negative mood were calculated as the mean of mood assessments prior to alcohol consumption on drinking nights. Mood instability was the mean of the five mood instability indicators assessed prior to drinking initiation on drinking nights. On non-drinking nights, or nights in which alcohol was not endorsed during the evening but was reported the following morning, each individual’s mean time to drink across drinking nights was used as a stopping point for calculation of mean mood and mood instability.
Participants reported 490 drinking episodes during in situ assessments; however, 31 of these days had too few mood assessments (i.e., <2) to calculate MSSD, reducing the in situ drinking assessments to 459. Whenever possible, in situ drinks served as the primary outcome of drinks consumed. In the absence of in situ drinks (e.g., due to device failure, dead battery, device forgotten at home, etc.), the morning report was used (n = 165). There were a total of 1,574 person days; however, 95 person days contained no mood or alcohol use data. These were removed, resulting in 1,479 days. Among these, there were a total of 1,309 days with sufficient mood assessments to compute MSSD; of these, there were a total of 622 drinking days.
2.5. Analysis plan
The data were analyzed using mixed-effects multilevel modeling in Mplus 7.3 (Muthén and Muthén, 2012). At level 1, mean pre-drinking positive and negative mood, as well as pre-drinking mood instability were added as person-centered predictors of the three alcohol outcomes (drinking likelihood, drinks consumed, and acute AUD symptoms). Gender and RI were grand-mean centered and used as predictors of the level 1 intercept and mood slopes for each outcome. In addition, interactions of RI × Gender were examined as predictors of each level 1 coefficient. For all three outcomes, the model intercepts had significant variance and thus were allowed to vary randomly. The RI indicator was divided by 1000, making it a fraction of a second rather than milliseconds, in order to increase interpretability of the regression coefficients.
We first estimated a logistic model predicting whether an individual reported drinking (drinks > 0) with level 1 variables centered across all analysis days. Next, a negative binomial count model predicting the number of drinks on drinking days was estimated, with level 1 variables centered across drinking days. Finally, a negative binomial count model predicting acute AUD symptoms on drinking days was estimated. This model mirrored the previous analysis, however, alcohol use was added as a person-centered level 1 predictor. For the logistic model, we present Odds Ratios (OR); for the two count models, we present Incident Rate Ratios (IRR).
3. Results
3.1. Descriptive and compliance statistics
Table 1 displays the descriptive statistics for all study variables. Correlations of between-subjects variables are listed in Table 2. Participants carried the PDD for an average of 20.55 days (SD = 2.80; range 9–24) days. Participants had good compliance, completing 82.14% of signaled random assessments and 86.75% of morning assessments.
Table 1.
Descriptive statistics for study variables
| Variables | Mean | SD | Range |
|---|---|---|---|
|
|
|
||
| Between-Subjects | |||
| Age | 21.297 | 2.072 | 18–29 |
| Response Inhibition | 248.893 | 104.134 | 30.838–943.543 |
| Drinking Days | 9.351 | 3.943 | 1–19 |
| EMA Data | |||
| Positive Mood | 4.834 | 1.983 | 0.100–9.960 |
| Negative Mood | 2.250 | 1.788 | 0–10.333 |
| Mood Instability | 3.394 | 0.671 | 0–48.910 |
| Drinks Consumed | 3.514 | 3.837 | 0–73 |
| Acute AUD Sxs | 0.482 | 1.105 | 0–9 |
Note. Sxs = Symptoms. Response Inhibition = Stop-signal Response Time.
Between-subjects (n = 74), EMA Data (n = 1309).
Table 2.
Bivariate correlations of between-subject associations
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 1. Age | ---- | |||||||
| 2. Gender | −.10 | ---- | ||||||
| 3. Response Inhibition | .04 | .21 | ---- | |||||
| 4. Positive Mood | −.09 | .28 | −.32 | ---- | ||||
| 5. Negative Mood | .24 | −.16 | −.16 | −.32 | ---- | |||
| 6. Mood Instability | .14 | −.08 | .02 | −.16 | .55 | ---- | ||
| 7. Drinking Days | .35 | .04 | .09 | .02 | .08 | .18 | ---- | |
| 8. Drinks Consumed | .04 | .35 | −.01 | .09 | .10 | .17 | .42 | ---- |
| 9. Acute AUD Sxs | .06 | .14 | .11 | −.01 | .11 | .08 | .26 | .42 |
Note. Sxs = Symptoms. Response Inhibition = Stop-Signal Response Time. Gender coded: 0 = Women, 1 = Men
Data are based on subject means.
r ≥ |.24|, p < .05
3.2. Alcohol use likelihood
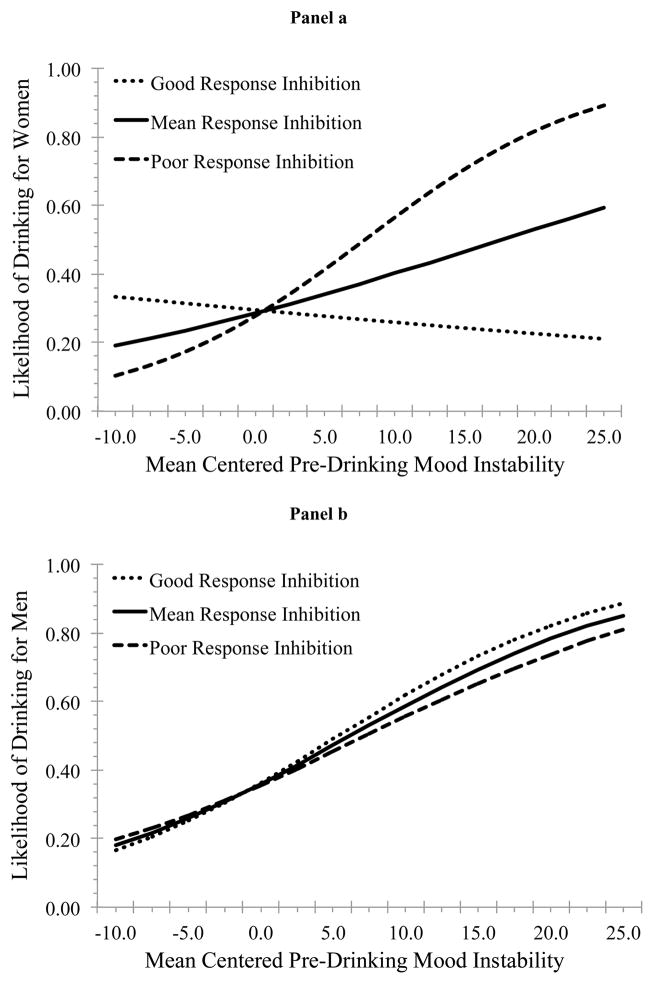
The likelihood of drinking was higher on Thursday (OR = 2.22, p = .001), Friday (OR = 6.42, p < .001), and Saturday (OR = 6.26, p < .001), relative to Sunday. Positive mood was associated with an increased likelihood of drinking. RI and gender moderated the association between mood instability and use likelihood. We calculated the simple slopes of mood instability on use likelihood at high (+1SD) and low (−1SD) levels of RI for women (Figure 1 Panel a) and men (Figure 1 Panel b). For individuals with good RI (i.e., −1SD) there was a positive association between mood instability and drinking likelihood for men (OR = 1.11, p = .015) but not women (OR = 0.98, p = .731). However, among those with poor RI (i.e., +1SD) this association was significant for both women (OR = 1.13, p = .023) and men (OR = 1.08, p = .011).
Figure 1.
Associations between mood instability and alcohol use likelihood at high and low levels of RI among women (panel a) and men (panel b)
3.3. Drinks consumed on drinking days
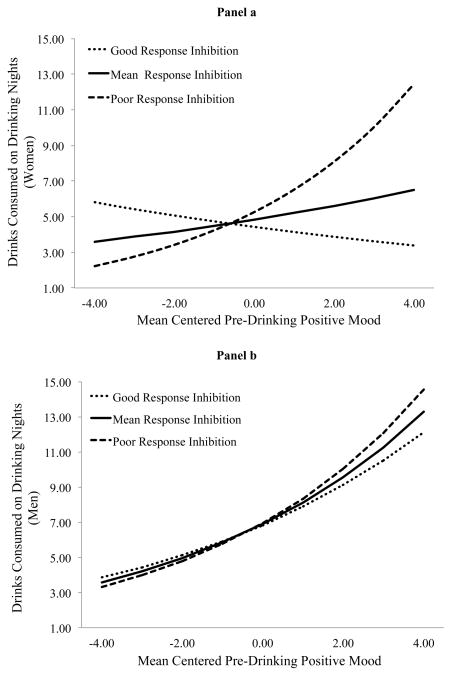
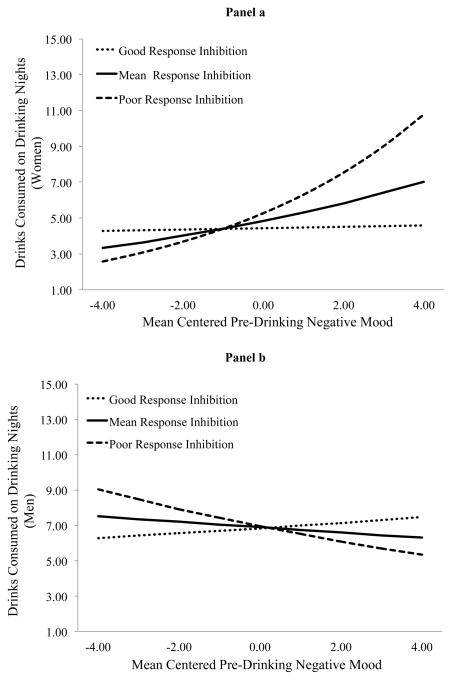
Men consumed more drinks than women on drinking days. On drinking days individuals consumed fewer drinks on Mondays (IRR = 0.67, p = .035), but more on Fridays (IRR = 1.40, p = .044) and Saturdays (IRR = 1.70, p < .001), relative to Sundays. Mood instability was positively associated with drinks consumed. RI and gender moderated the association between pre-drinking positive mood and drinks consumed as well as between pre-drinking negative mood and drinks consumed. Figure 2 depicts the cross-level interaction between positive mood and drinks consumed and Figure 3 depicts the cross-level interaction between negative mood and drinks consumed. Positive mood did not predict drinks consumed (IRR = 0.94, p = .163) for women with good RI (i.e., −1SD), but did predict drinks consumed (IRR = 1.24, p = .015) for women with poor RI (i.e., +1SD). Positive mood predicted drinks consumed for men with poor RI (IRR = 1.20, p = .001) and good RI (IRR = 1.15, p = .029), although this association was slightly attenuated among those with good RI. Negative mood did not predict drinks consumed (IRR = 1.00, p = .899) for women with good RI. Though it did predict drinks consumed (IRR = 1.20, p = .063) for women with poor RI, this did not reach conventional levels of statistical significance using a +/− 1SD approach. Negative mood did not predict drinks consumed for men with good (IRR = 1.02, p = .754) or poor (IRR = 0.94, p = .386) RI.
Figure 2.
Association between positive mood and drinks consumed on drinking nights at high and low levels of RI for women (panel a) and men (panel b).
Figure 3.
Association between negative mood and drinks consumed on drinking nights at high and low levels of RI for women (panel a) and men (panel b).
3.4. Acute alcohol use disorder symptoms on drinking days
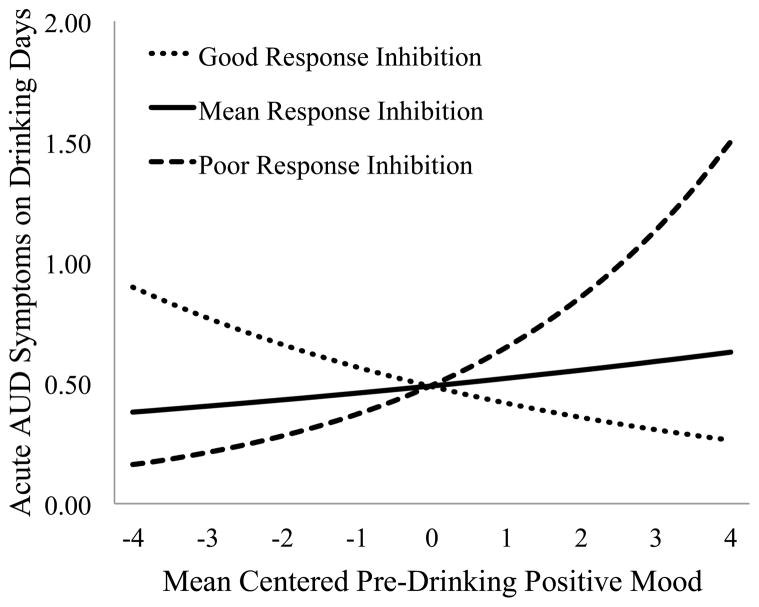
On drinking days, more acute AUD symptoms were experienced on Fridays relative to Sundays (IRR = 1.84, p = .018). Alcohol use was positively associated with acute AUD symptoms experienced on drinking days. Negative mood was also positively associated with the rate of experiencing acute AUD symptoms on drinking nights. There was a significant cross-level interaction between pre-drinking positive mood and RI in the prediction of the rate of acute AUD symptoms experienced on drinking days that did not vary by gender (see Figure 4). For those with good RI (i.e., −1SD) pre-drinking positive mood was inversely associated with acute AUD symptoms (IRR = 0.86, p = .009); however, among those with poor RI (i.e., +1SD) this association was positive (IRR = 1.36, p < .001).
Figure 4.
Association between pre-drinking positive mood and acute AUD symptoms on drinking nights at high and low levels of RI.
4. Discussion
The current study examined the association between three indices of daily emotional functioning (positive mood, negative mood, and mood instability) and alcohol involvement (alcohol use likelihood, drinks consumed on drinking days, and acute AUD symptoms on drinking days). We then examined the extent to which these associations varied as a function of RI and gender. The findings were largely consistent with hypotheses. Pre-drinking positive mood and mood instability were positively associated with both the likelihood of drinking on any given day as well as the amount consumed on drinking days. These associations varied by gender, RI, and interaction of gender and RI. In addition, negative mood predicted drinks on drinking nights, but only for women with poor RI. Finally, positive mood was associated with a higher rate of experiencing acute AUD symptoms among those with poor RI; however, this relationship was reversed among those with good RI.
Consistent with previous EMA research (Gottfredson and Hussong, 2013), mood instability was associated with increased alcohol use. Interestingly, previous research using a between-subjects approach has indicated that mood instability is seldom associated with alcohol use (see Simons, 2003; Simons et al., 2009), but is frequently associated with alcohol-related consequences (Kuvaas et al., 2013; Simons, 2003; Simons et al., 2004; Simons et al., 2009). The current study suggests that this latter finding may occur via a process of repeated exposure at the daily level. Over time individuals who tend to have more mood instability may be more likely to drink and/or drink at higher rates, subsequently resulting in more problematic use patterns.
Previous research has suggested that the association between emotional instability and alcohol involvement (primarily problems) is moderated by indices of behavioral control (Simons et al., 2004; Simons et al., 2009; Stevenson et al., 2015). Though we did not find this association for alcohol-related problems (i.e., AUD symptoms) or consumption rates on drinking days, we did find this association for alcohol use likelihood, but only for women. Further, we found a similar association between tonic negative mood and drinks consumed on drinking days as a function of RI. Both of these interactions varied by gender. Research has shown that daily mood-alcohol associations might be differentially moderated by indices of neuropsychological functioning (Sher et al., 2007) and that this may vary by gender (Dvorak and Simons, 2014). However, the current findings are quite different from those of Dvorak and Simons (2014) in which better neuropsychological functioning (i.e., sustained attention and attention shifting) increased the association between negative mood and alcohol involvement, but only for men. There are notable differences between these studies, making them difficult to compare, but on the surface they seem quite contradictory. Future research may benefit from comparing several different aspects of behavioral and cognitive control as moderators of mood-alcohol associations in near real-time.
From a dual process perspective (Wiers et al., 2007), comparing variables which seem to tap into effortful control (i.e., attention control, working memory, error monitoring, etc.) to factors which seem to be more relevant for behavioral impulse control (e.g., pre-potent stopping, RI, etc.) may be particularly relevant. Combined with previous research, the current findings seem to suggest differential moderation of mood-alcohol associations by each process as a function of gender. However, these findings are complicated by research indicating that the same task (e.g., Stop-signal) may activate different brain regions in men and women (Li et al., 2006). Li et al. (2009) have posited that this gender difference in activation (at least during the Stop-signal task) may place men at an increased risk of impulse control related psychopathology such as substance use disorders.
The most pervasive aspect of mood-alcohol associations appears to be those related to positive mood. We found that pre-drinking positive mood was associated with increased alcohol use, both likelihood and amount following initiation. Interestingly, this latter association did not vary by RI for men, but it did for women. Specifically, the link between positive mood and consumption rates on drinking nights was attenuated among women with good RI abilities. As noted above, there appear to be different brain regions associated with RI in men and women. One of these differences is higher activation in the caudate nucleus in men, relative to women (Li et al., 2009). Given the role of the caudate nucleus in both motor functioning (Boehler et al., 2010) and reward processing (Liu et al., 2011), perhaps this difference manifests in a sort of “reward override” for men, increasing the appetitive draw of alcohol use during positive mood. In contrast, motor responses and reward processing may not become “entangled” in women, who do not show the same activation pattern in the caudate nucleus. Although this is speculative, it is at least partially consistent with some research on adolescents (Bar-Haim et al., 2009). Future research is needed to fully understand this differential association.
Though positive mood was not directly related to acute AUD symptoms, after controlling for use, this association was moderated by RI and did not vary by gender, which is consistent with research indicating that impulse control may be especially relevant in reducing alcohol related consequences (Smith et al., 2014). Additionally, there is considerable research linking positive mood based rash action to alcohol-related consequences (Arbeau et al., 2011; Cyders et al., 2009; Dinc and Cooper, 2015). The current study supports these findings, and suggests that indices of behavioral control may be particularly important when it comes to regulating positive-mood induced rash action.
4.1. Treatment Implications
Regulation of negative emotion is a mainstay of many addictions treatments (Berking et al., 2011; Marlatt and Tapert, 1993; Stasiewicz et al., 2013). In addition, the new wave of mindfulness-based approaches seems particularly relevant for addressing mood instability (Britton et al., 2012). However, less attention has been paid to the regulation of positive emotion as a mechanism to prevent rash action, despite the fact that enhancement drinking has long been linked to negative outcomes (Cooper et al., 1995; Cooper et al., 1992). Perhaps research could begin to focus on ways to redirect positive mood toward more adaptive goal-driven behaviors. At the very least, psychoeducation on the risks of positive mood seem prudent. Finally, the present findings add to the literature suggesting that better behavioral control is associated with a host of beneficial outcomes (Smith et al., 2014). There have been recent attempts to retrain aspects of behavioral control (Friese et al., 2011; Houben et al., 2012; Houben et al., 2011). These have been met with some success (Manuel et al., 2013; Manuel et al., 2010; Sahdra et al., 2011; Spierer et al., 2013; Wiers et al., 2011), but there are still important issues to be resolved before we can deem these interventions efficacious (Enge et al., 2014).
4.2. Limitations
The findings of this study should be evaluated in the context of the limitations. First, this was a fairly well adjusted, predominantly white, upper Midwest, college student sample. Thus, generalization to other populations should be done with caution. The current study used a fairly crude assessment of mood and mood instability. Previous research has indicated disaggregating mood into its constituent parts is more informative than using simple “positive” and “negative” mood states (Hussong and Chassin, 1994). The same may be true for mood instability. For example, instability in anger may be more influential than instability in sadness, or vice-versa. With this small sample of 74 individuals, we did not have the statistical power to explore a number of associations for various tonic and unstable moods. Future research should strive to take a more comprehensive evaluation of these issues. As reflected in our review, there are a wide range of both self-report and behavioral measures that target aspects of behavioral control; however, we only examined one behavioral measure (i.e., Stop-signal task) that assesses pre-potent RI. Behavioral control is likely not a unitary construct, so future research examining multiple indices related to behavioral control would provide a more nuanced understanding of how these trait-like variables affect mood-alcohol associations. Relatedly, we conceptualized RI as a stable trait. However, it is quite possible that RI varies, at least to some extent, from moment to moment, which remains a question for future research.
4.3. Conclusions
Consistent with the SMSC, the current study found that mood instability led to increased likelihood of alcohol use. However, this association was attenuated among women with better RI. Similar results were found for the both pre-drinking positive and negative mood. In addition, positive mood was associated with higher rates of acute AUD symptoms among men and women with poor RI, but inversely associated with problems among those with good RI abilities. These results suggest that developing ways to improve RI may broadly influence negative drinking outcomes by affecting multiple mood-drinking associations.
Table 3.
Multilevel models predicting alcohol use likelihood, drinks consumed on drinking days, and acute alcohol use disorder symptoms experienced on drinking days.
| Predictor Variables | Level | Outcome Variables | ||
|---|---|---|---|---|
| Alcohol Use Likelihood1 | Drinks Consumed on Drinking Days2 | Acute AUD Symptoms2 | ||
|
|
|
|
||
| (OR) | (IRR) | (IRR) | ||
| Intercept | 1 | 0.460*** | 5.696*** | 0.493*** |
| Gender | 2 | 1.406 | 1.436** | 1.163 |
| Response Inhibition | 2 | 0.711 | 1.657 | 1.111 |
| Gender × Response Inhibition | 2 | 1.441 | 0.472 | 1.158 |
| Positive Mood Slope | 1 | 1.190*** | 1.119** | 1.083 |
| Gender | 2 | 1.000 | 1.093 | 0.995 |
| Response Inhibition | 2 | 0.928 | 2.397** | 8.900*** |
| Gender × Response Inhibition | 2 | 6.919 | 0.314* | 0.279 |
| Negative Mood Slope | 1 | 0.858 | 1.047 | 1.155* |
| Gender | 2 | 0.843 | 0.891 | 0.944 |
| Response Inhibition | 2 | 2.370 | 1.353 | 2.063 |
| Gender × Response Inhibition | 2 | 4.217 | 0.289* | 1.011 |
| Mood Instability Slope | 1 | 1.072** | 1.034* | 1.060 |
| Gender | 2 | 1.042 | 1.047 | 1.010 |
| Response Inhibition | 2 | 1.411 | 0.937 | 1.489 |
| Gender × Response Inhibition | 2 | 0.456* | 1.003 | 0.716 |
| Alcohol Use Slope | 1 | N/A | N/A | 1.050*** |
| Gender | 2 | N/A | N/A | 1.000 |
| Response Inhibition | 2 | N/A | N/A | 0.923 |
| Gender × Response Inhibition | 2 | N/A | N/A | 1.170 |
Note. Level 1: Within-subjects effects, centered at subject level. Level 2: Between-subjects effects, centered at grand-mean. Six day-of-week dummy coded indicators were included in all analyses, but are not depicted above.
n = 74 subjects,
n = 1309 person-days,
n = 622 person-days.
p ≤ .001;
p ≤ .01;
p ≤ .05
References
- Aragues M, Jurado R, Quinto R, Rubio G. Laboratory paradigms of impulsivity and alcohol dependence: A review. European Addiction Research. 2011;17:64–71. doi: 10.1159/000321345. [DOI] [PubMed] [Google Scholar]
- Arbeau KJ, Kuiken D, Wild TC. Drinking to enhance and to cope: A daily process study of motive specificity. Addictive Behaviors. 2011;36:1174–1183. doi: 10.1016/j.addbeh.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Armeli S, Carney MA, Tennen H, Affleck G, O’Neil T. Stress and alcohol use: A daily process examination of the stressor-vulnerability model. Journal of Personality and Social Psychology. 2000;78:979–994. doi: 10.1037//0022-3514.78.5.979. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Perez-Edgar K, Pine DS, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, Junghanns K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J Consult Clin Psychol. 2011;79:307–318. doi: 10.1037/a0023421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain—Conjunction analyses of the Stop-signal task. Neuro Image. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Shahar B, Szepsenwol O, Jacobs WJ. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: Results from a randomized controlled trial. Behavior Therapy. 2012;43:365–380. doi: 10.1016/j.beth.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyneel SD, Dewitte S, Franses PH, Dekimpe MG. I felt low and my purse feels light: Depleting mood regulation attempts affect risk decision making. Journal of Behavioral Decision Making. 2009;22:153–170. [Google Scholar]
- Conger JJ. Reinforcement theory and the dynamics of alcoholism. Quarterly Journal of Studies on Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Cooper LM, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cooper LM, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychological Assessment. 1992;4:123–132. [Google Scholar]
- Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: A test of social learning formulations. Journal of Abnormal Psychology. 1988;97:218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104:193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinc L, Cooper A. Positive affective states and alcohol consumption: The moderating role of trait positive urgency. Addictive Behaviors. 2015;47:17–21. doi: 10.1016/j.addbeh.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Dvorak RD, Pearson MR, Day AM. Ecological momentary assessment of acute alcohol use disorder symptoms: Associations with mood, motives, and use on planned drinking days. Experimental and Clinical Psychopharmacology. 2014;22:285–297. doi: 10.1037/a0037157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak RD, Simons JS. Moderation of resource depletion in the self-control strength model: Differing effects of two modes of self-control. Personality and Social Psychology Bulletin. 2009;35:572–583. doi: 10.1177/0146167208330855. [DOI] [PubMed] [Google Scholar]
- Dvorak RD, Simons JS. Daily associations between anxiety and alcohol use: Variation by sustained attention, set shifting, and gender. Psychology of Addictive Behaviors. 2014:969–979. doi: 10.1037/a0037642. [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Eid M, Kleindienst N, Stabenow S, Trull TJ. Analytic strategies for understanding affective (in)stability and other dynamic processes in psychopathology. Journal of Abnormal Psychology. 2009;118:195–202. doi: 10.1037/a0014868. [DOI] [PubMed] [Google Scholar]
- Enge S, Behnke A, Fleischhauer M, Küttler L, Kliegel M, Strobel A. No evidence for true training and transfer effects after inhibitory control training in young healthy adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2014;40:987–1001. doi: 10.1037/a0036165. [DOI] [PubMed] [Google Scholar]
- Friese M, Hofmann W, Wiers RW. On taming horses and strengthening riders: Recent developments in research on interventions to improve self-control in health behaviors. Self and Identity. 2011;10:336–351. [Google Scholar]
- Gailliot MT, Baumeister RF. Self-regulation and sexual restraint: Dispositionally and temporarily poor self-regulatory abilities contribute to failures at restraining sexual behavior. Personality and Social Psychology Bulletin. 2007;33:173–186. doi: 10.1177/0146167206293472. [DOI] [PubMed] [Google Scholar]
- Gottfredson NC, Hussong AM. Drinking to dampen affect variability: Findings from a college student sample. Journal of Studies on Alcohol and Drugs. 2013;74:576–583. doi: 10.15288/jsad.2013.74.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben K, Havermans RC, Nederkoorn C, Jansen A. Beer à no-go: Learning to stop responding to alcohol cues reduces alcohol intake via reduced affective associations rather than increased response inhibition. Addiction. 2012;107:1280–1287. doi: 10.1111/j.1360-0443.2012.03827.x. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, Wiers RW, Jansen A. Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug and Alcohol Dependence. 2011;116:132–136. doi: 10.1016/j.drugalcdep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Chassin L. The stress-negative affect model of adolescent alcohol use: Disaggregating negative affect. Journal of Studies on Alcohol. 1994;55:707–718. doi: 10.15288/jsa.1994.55.707. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Hicks RE, Levy SA, Curran PJ. Specifying the relations between affect and heavy alcohol use among young adults. Journal of Abnormal Psychology. 2001;110:449–461. doi: 10.1037//0021-843x.110.3.449. [DOI] [PubMed] [Google Scholar]
- Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychological Methods. 2008;13:354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kuvaas NJ, Dvorak RD, Pearson MR, Sargent EM. Self-regulation and alcohol use involvement: A latent class analysis. 2013 doi: 10.1016/j.addbeh.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RJ, Diener E. Affect intensity as an individual difference characteristic: A review. Journal of Research in Personality. 1987;21:1–39. [Google Scholar]
- Li CSR, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. Neuro Image. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li CSR, Zhang S, Duann J, Yan P, Sinha R, Mazure CM. Gender Differences in Cognitive Control: an Extended Investigation of the Stop Signal Task. Brain Imaging and Behavior. 2009;3:262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego, CA US: 1994. pp. 189–239. [Google Scholar]
- Manuel AL, Bernasconi F, Spierer L. Plastic modifications within inhibitory control networks induced by practicing a stop-signal task: An electrical neuroimaging study. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2013;49:1141–1147. doi: 10.1016/j.cortex.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Manuel AL, Grivel J, Bernasconi F, Murray MM, Spierer L. Brain dynamics underlying training-induced improvement in suppressing inappropriate action. J Neurosci. 2010;30:13670–13678. doi: 10.1523/JNEUROSCI.2064-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Tapert SF. Harm reduction: Reducing the risks of addictive behaviors. In: Baer JS, Marlatt GA, McMahon RJ, editors. Addictive behaviors across the life span: Prevention, treatment, and policy issues. Sage Publications, Inc; Thousand Oaks, CA US: 1993. pp. 243–273. [Google Scholar]
- Mohr CD, Armeli S, Tennen H, Temple M, Todd M, Clark J, Carney MA. Moving Beyond the Keg Party: A Daily Process Study of College Student Drinking Motivations. Psychology of Addictive Behaviors. 2005;19:392–403. doi: 10.1037/0893-164X.19.4.392. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Shiffman S, Paty JA. Daily fluctuations in self-control demands and alcohol intake. Psychology of Addictive Behaviors. 2005;19:140–147. doi: 10.1037/0893-164X.19.2.140. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus Statistical Modeling Software: Release 7.0. Muthén & Muthén; Los Angeles, CA: 2012. [Google Scholar]
- Sahdra BK, MacLean KA, Ferrer E, Shaver PR, Rosenberg EL, Jacobs TL, Zanesco AP, King BG, Aichele SR, Bridwell DA, Mangun GR, Lavy S, Wallace BA, Saron CD. Enhanced response inhibition during intensive meditation training predicts improvements in self-reported adaptive socioemotional functioning. Emotion. 2011;11:299–312. doi: 10.1037/a0022764. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, Wood MD. Stress-response-dampening effects of alcohol: Attention as a mediator and moderator. Journal of Abnormal Psychology. 2007;116:362–377. doi: 10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Levenson RW. Risk for alcoholism and individual differences in the stress-response-dampening effect of alcohol. Journal of Abnormal Psychology. 1982;91:350–367. doi: 10.1037//0021-843x.91.5.350. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS. Differential prediction of alcohol use and problems: The role of biopsychological and social-environmental variables. American Journal of Drug and Alcohol Abuse. 2003;29:861–879. doi: 10.1081/ada-120026265. [DOI] [PubMed] [Google Scholar]
- Simons JS, Carey KB, Gaher RM. Lability and Impulsivity Synergistically Increase Risk for Alcohol-Related Problems. American Journal of Drug and Alcohol Abuse. 2004;30:685–694. doi: 10.1081/ada-200032338. [DOI] [PubMed] [Google Scholar]
- Simons JS, Carey KB, Wills TA. Alcohol abuse and dependence symptoms: A multidimensional model of common and specific etiology. Psychology of Addictive Behaviors. 2009;23:415–427. doi: 10.1037/a0016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Dvorak RD, Batien BD, Wray TB. Event-level associations between affect, alcohol intoxication, and acute dependence symptoms: Effects of urgency, self-control, and drinking experience. Addictive Behaviors. 2010;35:1045–1053. doi: 10.1016/j.addbeh.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug and Alcohol Dependence. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Spierer L, Chavan CF, Manuel AL. Training-induced behavioral and brain plasticity in inhibitory control. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, Bradizza CM, Schlauch RC, Coffey SF, Gulliver SB, Gudleski GD, Bole CW. Affect regulation training (ART) for alcohol use disorders: Development of a novel intervention for negative affect drinkers. Journal of Substance Abuse Treatment. 2013;45:433–443. doi: 10.1016/j.jsat.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BL, Dvorak RD, Kuvaas NJ, Williams TJ, Spaeth DT. Cognitive Control Moderates the Association Between Emotional Instability and Alcohol Dependence Symptoms. Psychology of Addictive Behaviors. 2015 doi: 10.1037/adb0000045. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer UW. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: Introduction to the special section. Psychological Assessment. 2009;21:457–462. doi: 10.1037/a0017653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. Unpublished Manuscript. The University of Iowa; 1999. The PANAS - X: Manual for the Positive and Negative Affective Schedule. [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RCME, Sher KJ, Grenard J, Ames SL, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacology, Biochemistry and Behavior. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2011;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: Deficits and clinical relevance. Reviews in the Neurosciences. 2014;25:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]