Abstract
Purpose
NEO-102 is a novel chimeric IgG1 monoclonal antibody which recognizes a variant form of MUC5AC expressed specifically by human pancreatic and colorectal tumors. Preclinical models have demonstrated encouraging signs of antitumor activity through antibody-dependent cell-mediated cytotoxicity.
Methods
This is a Phase I, dose escalation trial of NEO-102 (Ensituximab) for patients with refractory pancreatic and colorectal cancer. The primary objective was to determine safety and tolerability of escalating doses of NEO-102. Secondary objectives were to assess pharmacokinetics, clinical benefit and biologic correlates. Patients whose tumors express NPC-1 antigen were eligible. Dose escalation was performed in a 3+3 design at doses of 1.5mg/kg, 2mg/kg, 3mg/kg and 4mg/kg.
Results
A total of 19 patients (4 pancreatic and 15 colon cancer) were enrolled at participating institutions in the treatment phase. Most common treatment-related adverse events (AEs) included anemia, fatigue, fevers, chills and flushing. Grade 3/4 AEs were hyperbiliruibinemia and anemia. There was no detectable hemolysis. Of twelve patients evaluable for disease response, the response rate at week 8 included 4 patients with stable disease and 8 patients with progressive disease (PD). The maximum tolerated dose was 3mg/kg. Of 74 patients who underwent tissue screening, positive NPC-1 expression was 47% in colon and 59% in pancreatic cancer
Conclusions
Treatment with the NEO-102, in this first-in-human study, is well tolerated with a manageable safety profile. A maximum tolerated dose of 3 mg/kg has been established. Toxicity profile is typical for this therapeutic class and allows for combination with conventional cytotoxic therapies.
Keywords: pancreatic cancer, colon cancer, immunotherapy, monoclonal antibody, clinical trial
Introduction
Cancer of the colon and pancreas represent two of the top four causes of cancer deaths among men and women in the United States[1]. NEO-102 (NPC-1C, Ensituximab; Precision Biologics, Inc.) is a novel chimeric IgG1 monoclonal antibody developed as a biological treatment for patients with pancreatic and colorectal cancers.
NEO-102 was one of several antibodies raised against an allogeneic colorectal cancer vaccine that had previously been tested in human clinical trials in the United States and not derived from MUC5AC antigen [2]. This original vaccine was screened from 79 patients with various stages of colon cancer whose tumor membranes fractions were pooled, separated by HPLC, and tested for delayed type hypersensitivity. The component that produced a strong DTH response upon screening was selected as the original vaccine and used in clinical trials. Results of that preliminary study with the vaccine in patients with refractory colorectal cancer revealed clinical benefits that correlated directly with patients developing IgG responses against the vaccine [2]. This formed the rationale for the vaccine to screen antibodies for sensitivity, specificity and anti-tumor function against colon cancer. NEO-102 was the first of 3 antibodies identified that met these criteria. Although unknown at the time of drug discovery, through protein purification and mass spectroscopy the homologous sequence to MUC5AC (NPC-1) was identified as the target for this compound. Preclinical studies demonstrated that NEO-102 antibody binds specifically to a novel MUC5AC-related antigen and directs antibody-dependent cellular cytotoxicity (ADCC) in the presence of normal human peripheral blood mononuclear cells (PBMCs)[3]. Additionally, antitumor efficacy was noted in preclinical pancreatic and colon cancer tumor xenograft models [3].
MUC5AC is a member of the mucin gene family and involves a carbohydrate structure on the amino acid backbone of a large (~100kDa) heavily glycosylated protein. The MUC5AC gene has been reported to be expressed mainly on the surface epithelium of normal gastric mucosa and normal airway epithelium [4]. MUC5AC is found in the GI tract and is preferentially expressed on colon and pancreatic cancer cells [5]. MUC5AC is predominantly expressed in the respiratory tract, in inflammatory conditions such as cystic fibrosis and COPD, producing increase levels of mucous production. Whereas MUC5AC expression has been demonstrated in fetal and pre-cancerous colonic mucosa, it is absent in normal adult colon [6,7]. Unlike the inflammatory conditions where MUC5AC is heavily glycosylated, in pancreas and colon tumors, MUC5AC is aberrantly glycosylated [8–11]. NEO-102 antibody can therefore discriminate between the native MUC5AC and the aberrantly glycosylate, NPC-1, variant of MUC5AC in tumors imparting tumor selectivity, which is exploited in this therapeutic strategy. As a companion diagnostic tool, an immunohistochemistry (IHC) based assay has been developed in parallel. This work provided the foundation for exploring NEO-102 as a therapeutic strategy for the management of pancreatic and colon cancer.
Patients and Methods
Study design and patient selection
This is a Phase 1 open label, multi-institution, dose escalation clinical trial of the therapeutic monoclonal antibody, NEO-102. Eligible patients had histologically confirmed colorectal cancer that had progressed on at least two lines of systemic therapy or advanced adenocarcinoma of the pancreas that had progressed on at least one line of systemic therapy. Patients were preselected based upon IHC testing for NPC-1 antigen expression performed on archival formalin fixed paraffin embedded tissue (FFPE). A minimum of 20% of tumor tissue staining positive at ≥ 2 + intensity was required for eligibility. Patients were required to have good performance status (ECOG performance status ≤ 2), evidence of measureable disease per Response Evaluation Criteria in Solid Tumor (RECIST criteria v1.1 [12]), adequate hematologic (hemoglobin > 8.5g/dL, absolute neutrophil count ≥ 1500/mm3 and platelets ≥ 50,000/mm3), hepatic (total bilirubin <2.0, alanine transaminase and aspartate transaminase less than 3 times the upper limit of normal or 5 times the upper limit of normal in presence of liver metastasis) and renal (serum creatinine ≤ 1.5mg/dL, creatinine clearance of > 40mL/min/1.73 m2) function. Exclusion criteria included disseminated or uncontrolled brain metastases, ascites with clinically identifiable abdominal distention, major surgery within 4 weeks of enrollment, concomitant uncontrolled illness, concurrent antineoplastic systemic therapy, uncontrolled diabetes, history of grade 2 or above allergic reaction to cetuximab, prior hemolytic anemia, concurrent warfarin use, and anticipated life expectancy of less than 8 weeks.
Patients were screened for inclusion in two phases
IHC screening and treatment screening. Informed consents for both screening phases were performed separately. Patients with positive expression of NPC-1PC-1 target antigen by IHC were eligible to initiate the treatment screening phase.
The primary objective of this study was to determine the safety and tolerability of escalating doses of NEO-102 monoclonal antibody. Secondary objectives were determination of pharmacokinetics at each dose level, as well as clinical benefit as measured by overall survival (OS) and RECIST criteria v1.1[12]. Patients were enrolled at three participating institutions with approval from the ethics committees at respective institutions and regulatory authorities. The study followed the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The study was supported by Precision Biologics, Inc., and registered at Clinicaltrials.gov (NCT01040000). All patients signed a written informed consent prior to starting study specific procedures.
NEO-102 Immunohistochemistry
Formalin-fixed paraffin embedded sections at 5 µM were obtained, placed on glass slides and stained with hematoxylin/eosin using an automated H/E stainer. IHC for NEO-102 was performed on formalin fixed paraffin embedded sections at 4 µM placed on positively charged slides. Following deparaffinization the antigen retrieval was performed at 115°C in a decloaking chamber. The endogenous peroxidase was blocked by incubating with 3% H2O2 for 10 mins. The slides were then loaded on to a (DAKO) Autostainer followed by endogenous biotin blocking. Following a brief protein blocking step, the sections were incubated for 60 minutes at room temperature with the NEO-102 antibody at a 1:200 dilution (Precision Biologics). Detection was performed using a commercially available Streptavidin-HRP antibody conjugate by incubating for 30 minutes.
Treatment
NEO-102 was administered intravenously (IV) every 14 days. Three to six patients were treated at each of the following dose levels: 1.5 mg/kg, 2 mg/kg, 3 mg/kg and 4 mg/kg. NEO-102 was initially started at a rate of 0.5 mg/min and the rate increased as tolerated in 0.5 mg/min increments every 30 minutes to a maximum rate of 4 mg/min. Patients were monitored as inpatient for 24 hours after the first infusion and remaining infusions were administered as an outpatient. Premedication with dexamethasone (10 mg IV), ranitidine (50 mg IV) and diphenhydramine (25–50 mg IV) was administered prior to each dose. Additional treatment could be offered in the absence of dose limiting or unacceptable toxicity, progressive disease, or per investigator discretion.
Adverse events were graded for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0). Dose limiting toxicity (DLT) was defined as any one of the following in the first 30 days: Any grade 3 or 4 hematologic/non-hematologic toxicity or severe infusion related reaction/allergic reaction/hypersensitivity to NEO-102. Transient toxicity related to infusion including fatigue, infusion reactions, flu-like symptoms, fever, and headache that recover to grade 1 or less within 8 hours after standard supportive treatment was not considered a DLT. A standard three-plus-three dose escalation design was followed for dose escalation. [13] The starting dose of 1.5 mg/kg for this trial was based on clinical experience with an earlier version of ensituximab (NEO-101).[14] Hemoglobin levels were checked 24–48 hours after completion of infusion to assess for hemolysis. Patients who experienced greater than 1 gm/dL drop in hemoglobin after dosing with NEO-102 underwent additional testing to rule out possible hemolysis including the following tests: direct coombs, haptoglobin, fibrinogen, D-Dimer, thrombin time or peripheral blood smear review, as indicated.
Tumor assessments were performed by conventional CT scans at baseline and then every 8 weeks. Response assessment was performed based on RECIST criteria v1.1[12]. Blood for pharmacokinetic (PK) analysis was collected at the following time points: prior to the start of infusion, at the end of infusion (EOI), and 1, 4, 24, 72 and 168 hours after the end of the first infusion. Pre-treatment and EOI blood samples were also were drawn for doses 2–4. Where possible, a sample was also collected 14 days after the fourth dose. PK analysis was performed using quantitative self-sandwich ELISA assay for NEO-102 PK developed by using anti-NPC-1C idiotype antibody (4B6). Individual concentration-time profiles were constructed for each patient for the first cycle. Peak and trough concentrations for each dose were reported as the concentration of NEO-102 within three minutes after the end of infusion and the NEO-102 concentration immediately prior to the next treatment (approximately 14 days later). Additional samples were drawn on day 1, 4, 15 and 57 for human antichimeric antibody (HACA) and cytokines. These samples were batched and testing was performed at BioReliance Corporation (Rockville, MD, USA) using a commercially available multiplex 96-well enzyme-linked immunoabsorbent assay kit (MS6000 Human Pro-Inflammatory 9-Plex Ultra-Sensitive Kit K11007; Meso Scale Diagnostics, Gaithersburg, MD, USA) on a Sector Imager 6000 according to the manufacturer's recommendation (Meso Scale Diagnostics). Pro-inflammatory cytokines (IL1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, TNF-α, INF-γ, GM-CSF) were evaluated at baseline (BL), day 4, day 15 (prior to dose 2), and day 57, when available. Serum samples were stored at −70°C. The standards and serum samples were run in triplicate with 4 time points of each subject in the same plate to avoid plate variation.
Statistical Analysis
Demographic and baseline disease characteristics for study patients were summarized by means, standard deviations (SD), medians, and ranges for continuous variables and counts, and proportions for categorical variables. Unless otherwise noted, all statistical testing was 2-sided and was carried out at the 0.05 significance level. All analyses and tabulations were carried out by SAS (version 8.2 or higher; SAS Institute) on a PC platform.
Results
A total of 19 patients received at least one dose of NEO-102. Fifteen had colorectal cancer and 4 had pancreatic cancer. Median age was 58 years (32–69 years), 58% were male, and 68% had an ECOG performance status of 1. A total of 47% of patients had received four or more prior lines of systemic therapy.
Safety
All patients who received at least one dose of NEO-102 and were eligible for safety assessment. Adverse events (AEs) attributed to being related to NEO-102 are summarized in table 2. Most common AEs were anemia (32%), fatigue (32%), fever (21%), chills (16%), flushing (16%), increased bilirubin (16%), congestion (16%). Of these, the following were grade 3/4 AEs: anemia in 4 patients (21%), increased bilirubin in 2 patients (11%) and transient hypoxia (grade 3) in one patient (5%). Hypoxia was accompanied by confusion, and radiologic evidence of new groundglass opacities. Pathology after bronchoscopy revealed diffuse alveolar damage, many neutrophils and negative for malignant cells and hypoxia resolved on oral prednisone and 1 L oxygen by nasal cannula.
Table 2.
NEO-102 Adverse Events of patients receiving therapy with NEO-102 (n=19)
| Event | NPC-1C 1.5 mg/kg (n=3) | NPC-1C 2 mg/kg (n=3) |
NPC-1C 3 mg/kg (n=6) |
NPC-1C 4 mg/kg (n=7) |
NPC-1C (n=19) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GR1/2 | %** | GR3/4 | % | GR 1/ 2 |
% | GR 3/ 4 |
% | GR 1/ 2 |
% | G R 3 / 4 |
% | GR 1/ 2 |
% | G R 3 / 4 |
% | All Gra des |
% | |||
| Anemia | 1 | 33 | 0 | 0 | 1 | 33 | 1 | 5 | 1 | 17 | 0 | 0 | 0 | 0 | 2 | 29 | 6 | 3 2 |
||
| Fatigue | 1 | 33 | 0 | 0 | 2 | 66 | 0 | 0 | 1 | 17 | 0 | 0 | 2 | 29 | 0 | 0 | 6 | 3 2 |
||
| Fever | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 17 | 0 | 0 | 3 | 43 | 0 | 0 | 4 | 2 1 |
||
| Chills | 0 | 0 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 29 | 0 | 0 | 3 | 1 6 |
||
| Flushing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 33 | 0 | 0 | 1 | 14 | 0 | 0 | 3 | 1 6 |
||
| Increased Bilirubin |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 2 | 29 | 3 | 1 6 |
||
| Upper Respiratory Congestion |
1 | 33 | 0 | 0 | 2 | 66 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 6 |
||
| Diarrhea | 0 | 0 | 0 | 0 | 1 | 33 | 0 | 0 | 1 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 1 |
||
| Abdominal Pain | 1 | 33 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 1 |
||
| Mucositis | 1 | 33 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 1 |
||
| Pruritus | 1 | 33 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 1 |
||
| Back Pain | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | ||
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 1 | 5 | ||
| Headache | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 1 | 5 | ||
| Insomnia | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | ||
| Nausea | 0 | 0 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | ||
| Weight Loss | 0 | 0 | 0 | 0 | 1 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | ||
| Hypoxia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 7 |
0 | 0 | 0 | 0 | 1 | 5 | ||
| Infusion Reaction |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 1 | 5 | ||
| Tachycardia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 1 | 5 | |||
at least possibly related
Dose titration to 4 mg/kg was completed with no observed dose limiting toxicity in the first 3 patients and preplanned expansion cohort at 4 mg/kg began. The sixth and seventh patients at 4 mg/kg experienced grade 3 anemia and transient asymptomatic grade 3 hyperbilirubinemia, respectively as mentioned above. Additionally, the fifth patient at this dose experienced a transient asymptomatic grade 3 hyperbilirubinemia after the third dose of NEO-102. Although these events occurred after completion of dose escalation phase of the clinical trial, the study cohort review committee decided to de-escalate to 3 mg/kg to ensure safety of subsequent patients. Three additional patients were then treated at the 3 mg/kg dose level with 1 out of 6 patients experiencing grade 3 reversible hypoxia (deemed to be a DLT). Therefore 3 mg/kg was established as the maximum tolerated dose (MTD).
Efficacy/ Response
Patients who received at least two doses of NEO-102 were considered evaluable for response. Sixteen patients were eligible for tumor assessment measurements. There were 5 patients with stable disease and 6 patients with PD at week 8. Five patients were removed from treatment prior to week 8 evaluation (2 for symptoms of clinical progression, 2 for treatment-related toxicity, 1 for unrelated toxicity). As of September 1, 2015 10 patients died secondary to progressive disease (7 colorectal). Patients with colorectal cancer had an overall median survival of 51 weeks (12.0 months) (range 6–108+ weeks); patients with pancreatic cancer had an overall median survival of 20 weeks (5 months) (range 10–33 weeks) in this phase I study.
Pharmacokinetics
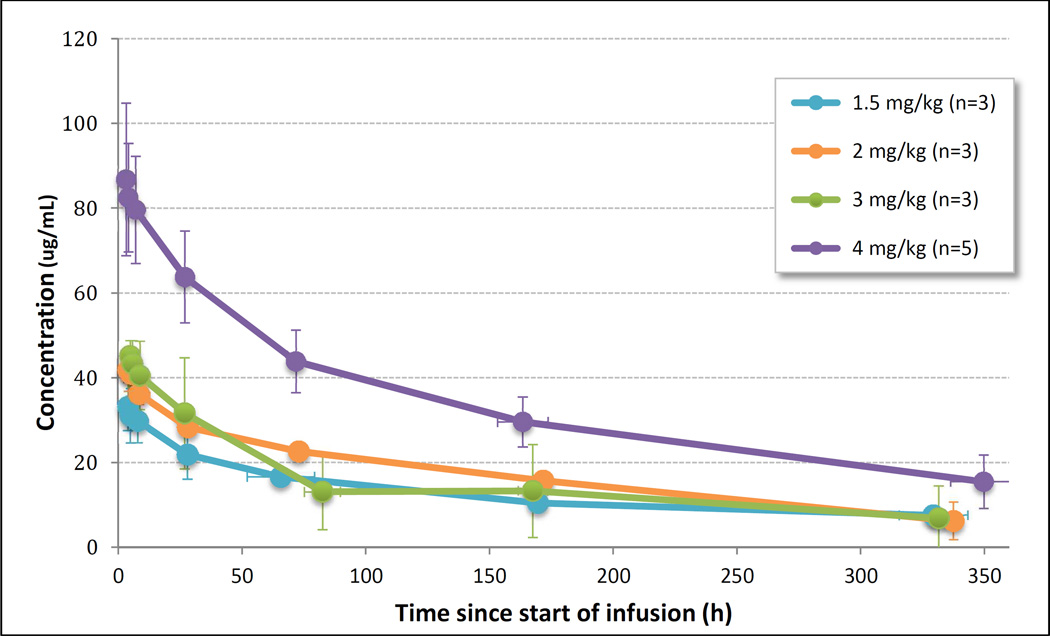
A total of 14 patients were evaluable for pharmacokinetics. First doses ranged from 104 to 531 mg (1.5 to 4.0 mg/kg), administered over 2.0–6.33 h. Mean dose and duration at the MTD of 3 mg/kg was 240.8 mg infused over 4.17 hours. Mean concentration-time profile by dose level following the first dose of NEO-102 for the four dose levels assessed are shown in Figure 2. Maximal serum concentrations of NEO-102 were typically observed at the end of infusion, with a rapid decline in concentrations over the first 24–48 hours, followed by a slower terminal elimination phase. Pharmacokinetic parameters for NEO-102 are shown in Table 3. Drug remained detectable in all patients fourteen days after the first drug administration, resulting in some drug accumulation. At the MTD of 3 mg/kg, serum concentration immediately prior to the second dose ranged from 1.79 to 15.62 ug/mL (n = 4). The mean accumulation ratio observed for each dose level ranged from 1.08 to 1.51 (not shown). The pharmacokinetics of NEO-102 appear to be non-linear with a less than dose proportional increase in exposure with increasing doses.
Figure 2. Mean serum NEO-102 concentration by dose level following the first infusion.
Error bars represent standard deviation of sampling time (horizontal) and measured concentration (vertical).
Table 3.
Pharmacokinetic Parameters following first dose of NEO-102
| Cmax (ug/mL) | Tmax (h) | AUCinf (hr*ug/mL) | t1/2 (h) | Clearance (mL/h) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Level (mg/kg) |
n | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1.5 | 3 | 33.14 | 5.64 | 4.04 | 0.61 | 6893.06^ | 1122.31 | 237.57^ | 19.49 | 18.28^ | 2.83 |
| 2 | 3 | 41.73 | 4.99 | 4.23 | 1.17 | 8415.87 | 827.31 | 195.66 | 43.72 | 13.20 | 0.12 |
| 3 | 3 | 45.41 | 3.01 | 5.77 | 2.04 | 7656.78 | 4947.73 | 152.73 | 51.72 | 44.75 | 30.28 |
| 4 | 5 | 87.07 | 17.80 | 4.00 | 1.36 | 16858.41 | 5927.30 | 197.21 | 79.82 | 21.51 | 8.18 |
n = 2
There were no elevations from baseline of serum IL-1β, IL-2, IL-10, IL-12p70, GMCSF, IFNγ, and TNFα in 13 subjects at D4, D15 and D57 samples. Increased serum levels of IL-6 were observed at D57 in two subjects, D4 in one subject, and at D15 and D57 in one subject. Three-fold higher serum concentrations of IL-8 were observed at D57 compared to baseline in two subjects. There was no evidence of clinical cytokine storm phenomena in any patients treated. HACA concentrations were less than the assay lower limit of detection (LOD) of 3.9 ng/mL in 12 patients evaluated.
IHC assay
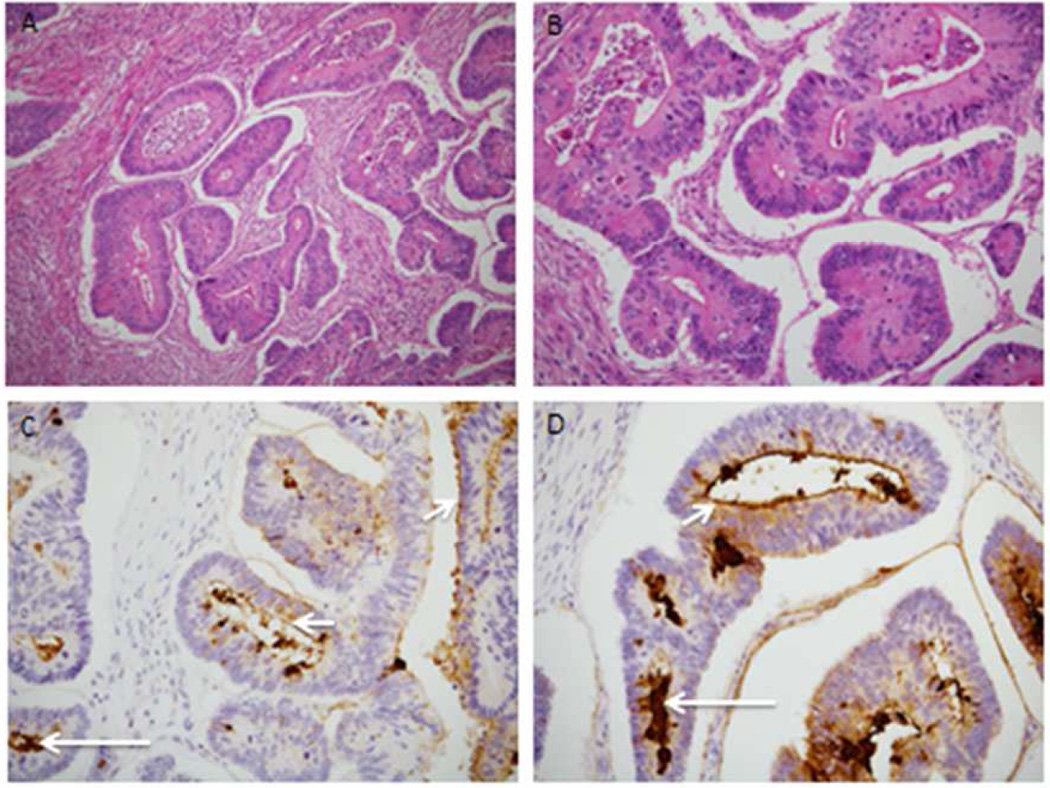
Up to four unstained formalin-fixed paraffin embedded (FFPE) tumor tissue slides from 74 potential subjects were tested using biotinylated NEO-102 antibody and a streptavidin-HRP detection system to determine IHC of the NPC-1 antigen. Figure 1 depicts tumor immunostains with NEO-102 on patient samples from the clinical trial demonstrating membrane and luminal signal. Rate of positive NPC-1 IHC (n=74) screening was 47% for colon cancer (21 of 45 tested) and 59% for pancreatic cancer (17 of 29 tested).
Figure 1.
H/E-stained sections (Panel A-100X and Panel B-200X) reveal a well differentiated and invasive colonic adenocarcinoma with infiltration into the muscularis propria and minimal mononuclear inflammation. Sections immunolabeled with the Neo-102 antibody membrane-associated positive signal (Panel C and Pane; D, small arrows, 200X) and luminal signal (Panel C and Panel D, long arrows).
Discussion
In this phase 1 study of patients preselected for target antigen expression, an aberrantly glycosylated MUC5AC-related antigen, treatment with NEO-102 is well tolerated with an encouraging safety profile. A maximum tolerated dose of 3 mg/kg has been established. Commonly experienced adverse events were mild and well-tolerated. For patients treated at the 1.5 mg/kg, 2 mg/kg and 3 mg/kg dose levels, grade 3 or 4 adverse events were anemia and one case of hypoxia. Other adverse events including fatigue, diarrhea, nausea, mucositis, weight loss and abdominal pain were mild and partly reflect characteristics of the tumors being evaluated. NEO-102 infusion reactions with standard premedications were rare (1 out 19 patients).
Two cases of grade 3 hyperbilirubinemia in patients with concomitant liver metastasis were noted at the 4mg/kg dose level. In one case, bilirubin elevation was deemed to be from biliary obstruction which improved with biliary stenting. The other patient following the 2nd dose of NEO-102 had a total bilirubin increase from 1.4 mg/dL to 4.0 mg/dL occur 24hrs after treatment, which resolved to grade 1. A 3rd dose was administered at a lower dose, but the patient developed grade 3 hyperbilirubinemia and was removed from study according to per-protocol criteria. Notably, no events of elevated bilirubin were seen at lower dose levels including the MTD of 3 mg/kg. All patients that developed anemia tested negative for hemolysis. One patient with grade 3 anemia was determined to have anemia of chronic disease combined with possible myelosuppression from multiple prior cytotoxic therapy. One case of hypoxia occurred in a patient with colon cancer with extensive lung metastases at the 2mg/kg dose level. The patient was admitted 5 days following the 1st dose of NEO-102 with hypoxia and shortness of breath and condition improved following medical management. Patient proceeded to receive a second dose of NEO-102 but was subequently taken off treatment due to declining functional status. Due to the temporal relationship with drug infusion and increasing recognition of pneumonitis related to immunological agents, hypoxia was deemed to be possibly drug related.
Overall NEO-102 demonstrated a favorable toxicity profile at the 3 mg/kg dose level. This has allowed for combination of NEO-102 with other chemotherapeutic agents in ongoing and planned trials.
The NPC-1C antibody exhibits cell-specific binding and ADCC activity against human colorectal and pancreatic tumor cells, but not against control tumor cell lines which do not express this variant of MUC5AC [15]. In vivo, the anti-tumor efficacy of NPC-1C was tested using pre-established subcutaneous tumor xenograft models [3]. Data showed significant and reproducible anti-tumor activity which provided the foundation for human studies. No partial responses were seen in the patients treated in this phase 1 study, which enrolled a heavily pretreated group of patients. This likely reflects the patient population being treated as the currently FDA approved agents for refractory colon cancer (regorafenib and TAS-102) have also show very low overall response rate[16] [17]. For agents that impart their effect through immunomodulation, survival end points are largely considered more reliable as opposed to tumor shrinkage/response rates. Although the overall survival of patients treated on this study is encouraging, no definitive conclusions can be drawn due to the small sample size. Positive selection of patients who potentially had indolent disease to begin with and an effect of additional lines of therapy received by these patients may be favorably skew results. Details on additional lines of therapy are not available for assessment. An immunohistochemistry (IHC) based companion diagnostic assay has been developed as an eligibility selection criteria to ensure that patients’ tumors express the NPC-1 target, which correlated preclinically to anti-tumor responses. In this assay, the NEO-102 antibody is biotinylated and tested for the ability to detect the MUC5AC Tumor Associated Antigen (TAA) expressed in normal and malignant human tissues. The rate of expression of target antigen in this clinical trial is 47% for colon cancer and 59% of pancreatic cancers.
Following infusion of NEO-102, maximal serum concentrations are observed and distribution appears to be rapid. Cytokine evaluation demonstrated elevations in IL-6 and IL-8 post NEO-102 infusion. In contrast, no elevations in the other proinflammatory serum cytokines IL-1β, IL-2, IL-10, IL-12p70, GMCSF, IFN-γ, and TNFα were observed. This correlated with the antibody being well tolerated post infusion without clinical evidence of cytokine release syndrome.
In summary, NEO-102 is well tolerated with a predictable pharmacokinetic profile. Current treatment strategies for colon and pancreas cancer lack predictive biomarkers and clinical toxicities limit the prospect of potential combination strategies [18–20]. The favorable toxicity profile of NEO102 as observed in this study, has allowed the exploration of the role of NEO-102 for the treatment of NPC-1 positive colon and pancreatic cancer as monotherapy, as well as in combination with cytotoxic chemotherapy. A maximum tolerated dose of 3 mg/kg has been established as the recommended phase 2 dose.
Table 1.
Baseline characteristics
| All treated patients (n=19) | |
|---|---|
| Characteristics | No. of patients (%) |
| Colon cancer | 15 (79) |
| Male | 11 (58) |
| Median Age, years | 58 (32–69) |
| ECOG Performance status |
|
| 0 | 6 (32) |
| 1 | 13 (68) |
| Prior number of chemotherapy |
|
| >5 | 4 (21) |
| 4 | 5 (26) |
| 3 | 7 (37) |
| 2 | 3 (16) |
Acknowledgments
This study was funded by Precision Biologics Inc
MSB: Grants from Precision Biologics, MIRNA therapeutics, Celgene; Personal fees from Celgene, Bayer, Ipsen
SPP: Grant Support: MedImmune, Pfizer, Amgen, Genentech. Personal fees: Boehringer Ingelheim, Eli Lilly
XPW: Employee, Precision Biologics
MB: Employee, Precision Biologics
SM: Employee, Precision Biologics
PA: Employee, Precision Biologics
Support: UL1TR001105 (MSB)
Footnotes
Prior Presentations: ‘A Phase I/IIA multicenter clinical trial of the chimeric monoclonal antibody NEO102 (NPC-1C) in adults with refractory pancreatic and colorectal cancer’ AACR 2014 Annual Meeting
Disclaimers: None
Conflicts of Interest:
NA: The authors declare that they have no conflict of interest.
JT: The authors declare that they have no conflict of interest.
MM: The author declares that they have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Hollinshead A, Elias EG, Arlen M, Buda B, Mosley M, Scherrer J. Specific active immunotherapy in patients with adenocarcinoma of the colon utilizing tumor-associated antigens (TAA). A phase I clinical trial. Cancer. 1985;56(3):480–489. doi: 10.1002/1097-0142(19850801)56:3<480::aid-cncr2820560312>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Patel SP, Bristol A, Saric O, Wang XP, Dubeykovskiy A, Arlen PM, Morse MA. Anti-tumor activity of a novel monoclonal antibody, NPC-1C, optimized for recognition of tumor antigen MUC5AC variant in preclinical models. Cancer Immunol Immunother. 2013;62(6):1011–1019. doi: 10.1007/s00262-013-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86(1):245–278. doi: 10.1152/physrev.00010.2005. doi:86/1/245 [pii] 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 5.Luka J, Arlen PM, Bristol A. Development of a serum biomarker assay that differentiates tumor-associated MUC5AC (NPC-1C ANTIGEN) from normal MUC5AC. J Biomed Biotechnol. 2011;2011:934757. doi: 10.1155/2011/934757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bara J, Chastre E, Mahiou J, Singh RL, Forgue-Lafitte ME, Hollande E, Godeau F. Gastric M1 mucin, an early oncofetal marker of colon carcinogenesis, is encoded by the MUC5AC gene. Int J Cancer. 1998;75(5):767–773. doi: 10.1002/(sici)1097-0215(19980302)75:5<767::aid-ijc17>3.0.co;2-3. doi:10.1002/(SICI)1097-0215(19980302)75:5<767::AID-IJC17>3.0.CO;2-3 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Bara J, Gautier R, Mouradian P, Decaens C, Daher N. Oncofetal mucin M1 epitope family: characterization and expression during colonic carcinogenesis. Int J Cancer. 1991;47(2):304–310. doi: 10.1002/ijc.2910470222. [DOI] [PubMed] [Google Scholar]
- 8.de Bolos C, Real FX, Lopez-Ferrer A. Regulation of mucin and glycoconjugate expression: from normal epithelium to gastric tumors. Front Biosci. 2001;6:D1256–D1263. doi: 10.2741/bolos. [DOI] [PubMed] [Google Scholar]
- 9.Kocer B, McKolanis J, Soran A. Humoral immune response to MUC5AC in patients with colorectal polyps and colorectal carcinoma. BMC Gastroenterol. 2006;6:4. doi: 10.1186/1471-230X-6-4. doi:1471-230X-6-4 [pii] 10.1186/1471-230X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80(2):210–218. doi: 10.1002/(sici)1097-0215(19990118)80:2<210::aid-ijc9>3.0.co;2-u. doi:10.1002/(SICI)1097-0215(19990118)80:2<210::AID-IJC9>3.0.CO;2-U [pii] [DOI] [PubMed] [Google Scholar]
- 11.Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122(1):61–69. doi: 10.1309/9R66-73QE-C06D-86Y4. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. Journal of the National Cancer Institute. 1997;89(15):1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 14.Patel SP, Morse MA, Diaz LA, Azad NS, Haley S, Arlen PM. A phase I safety study of NPC-1C, a novel, therapeutic antibody to treat pancreas and colorectal cancers. Cancer Res. 2013;73(8) [Google Scholar]
- 15.Bristol AKJ, Luka J, Stafford L, Gupta R, Arlen P. Pre-Clinical Studies of a Novel Antibody to Treat Pancreatic and Colorectal Tumors[abstract] Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research. 2011;4584 [Google Scholar]
- 16.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A, Group RS. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 17.Grothey A. Regorafenib in metastatic colorectal cancer. Clin Adv Hematol Oncol. 2012;10(5):324–325. [PubMed] [Google Scholar]
- 18.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of U, Intergroup P. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 19.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 20.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]