Abstract
Background
The endothelial nitric oxide synthase cofactor tetrahydrobiopterin (BH4) plays a pivotal role in maintaining endothelial function in experimental vascular disease models and in humans. Augmentation of endogenous BH4 levels by oral BH4 treatment has been proposed as a potential therapeutic strategy in vascular disease states. We sought to determine the mechanisms relating exogenous BH4 to human vascular function and to determine oral BH4 pharmacokinetics in both plasma and vascular tissue in patients with coronary artery disease.
Methods and Results
Forty-nine patients with coronary artery disease were randomized to receive low-dose (400 mg/d) or high-dose (700 mg/d) BH4 or placebo for 2 to 6 weeks before coronary artery bypass surgery. Vascular function was quantified by magnetic resonance imaging before and after treatment, along with plasma BH4 levels. Vascular superoxide, endothelial function, and BH4 levels were determined in segments of saphenous vein and internal mammary artery. Oral BH4 treatment significantly augmented BH4 levels in plasma and in saphenous vein (but not internal mammary artery) but also increased levels of the oxidation product dihydrobiopterin (BH2), which lacks endothelial nitric oxide synthase cofactor activity. There was no effect of BH4 treatment on vascular function or superoxide production. Supplementation of human vessels and blood with BH4 ex vivo revealed rapid oxidation of BH4 to BH2 with predominant BH2 uptake by vascular tissue.
Conclusions
Oral BH4 treatment augments total biopterin levels in patients with established coronary artery disease but has no net effect on vascular redox state or endothelial function owing to systemic and vascular oxidation of BH4. Alternative strategies are required to target BH4-dependent endothelial function in established vascular disease states.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00423280.
Keywords: coronary artery disease; endothelium; nitric oxide synthase; 5,6,7,8,-tetrahydrobiopterin
The endothelial nitric oxide (NO) synthase (eNOS) cofactor tetrahydrobiopterin (BH4) plays a pivotal role in maintaining normal endothelial function in experimental models of vascular disease1,2 and in human blood vessels.3 When BH4 is limiting, eNOS becomes uncoupled, producing reactive oxygen species rather than NO.4,5 Under conditions of oxidative stress, BH4 can be readily oxidized to dihydrobiopterin (BH2) and eventually biopterin, which lack eNOS cofactor activity. Recently, higher BH4 levels in vascular tissue obtained from patients undergoing coronary artery bypass graft surgery (CABG) have been shown to be associated with increased NO bioavailability and decreased reactive oxygen species production.6 Furthermore, alterations in vascular redox status resulting from genetic variation in BH4 synthesis implicate a causal role for endogenous BH4 in modulating human endothelial function.7 Thus, uncoupled eNOS, resulting from diminished BH4 in vascular disease states, is an important source of reactive oxygen species production in the human vasculature.
Given the importance of endogenous BH4 levels on vascular endothelial function, administration of exogenous BH4 is a potential therapeutic strategy in vascular disease states. Indeed, a number of studies in human subjects with either overt coronary artery disease (CAD)8 or vascular risk factors9–11 have demonstrated that administration of exogenous BH4 by intra-arterial infusion leads to rapid improvements in endothelial function. However, these studies used very high BH4 doses and tested only short-term effects. In forearm plethysmography studies, plasma BH4 levels are typically elevated to between 50 and 100 μmol/L,12 >1000-fold higher than physiological plasma BH4 levels of 10 to 50 nmol/L.6 These rapid and short-term supraphysiological BH4 doses may be confounded by nonspecific effects, including direct antioxidant effects on the vessel wall. Furthermore, intra-arterial infusion is not a relevant therapeutic approach to improve endothelial function in patients with CAD.
A recent trial of long-term oral BH4 treatment demonstrated an improvement in endothelial function in subjects with hypercholesterolemia.13 However, the mechanisms relating oral BH4 treatment to changes in vascular BH4 and eNOS coupling in vascular disease states are unknown, and the applicability to patients with established CAD is uncertain. Accordingly, we conducted a randomized, placebo-controlled trial of oral BH4 treatment in patients with CAD to determine the mechanisms relating exogenous BH4 treatment to human vascular function. We determined how oral BH4 treatment modified levels of biopterins in both plasma and vascular tissue and the effect of oral BH4 treatment on vascular superoxide production and endothelial function in patients with CAD both in vivo and ex vivo.
Methods
Study Design and Subjects
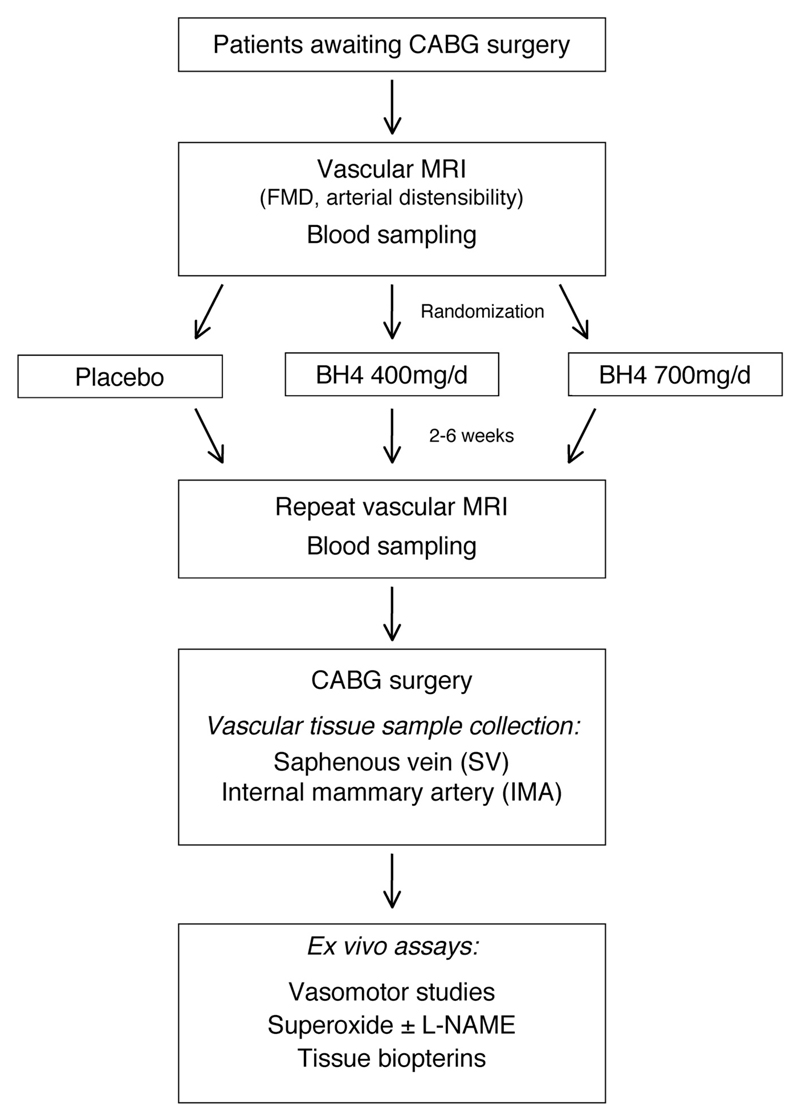
Forty-nine patients with multivessel CAD undergoing elective CABG surgery at the John Radcliffe Hospital, Oxford, UK, were randomized in a double-blind, parallel design to receive sapropterin dihydrochloride (6R-BH4) at either 700 mg/d (high dose) or 400 mg/d (low dose) or matching placebo for 2 to 6 weeks before surgery (www.clinicaltrials.gov; identifier, NCT00423280). The study drug and randomization plan were provided by BioMarin Pharmaceutical Inc, Novato, CA. Figure 1 outlines the study protocol. Study medication was taken at 10 pm, at least 1 hour after the evening meal.
Figure 1.
Study scheme. CABG indicates coronary artery bypass graft surgery; MRI, magnetic resonance imaging; FMD, flow-mediated dilatation; BH4, tetrahydrobiopterin; and L-NAME, NG-nitro-l-arginine methyl ester.
Patients >18 years of age who were scheduled for elective CABG surgery were included. Exclusion criteria were recent acute coronary syndrome (<4 weeks); heart failure requiring diuretic therapy with evidence of severe left ventricular systolic dysfunction; emergency CABG surgery; newly diagnosed diabetes mellitus (<4 weeks); body weight >130 kg; renal impairment (serum creatinine >2.0 mg/dL); hepatic impairment (serum alanine aminotransferase or aspartate aminotransferase >2 times the upper limit of normal); contraindications to magnetic resonance imaging (MRI) scanning, eg, pacemakers; pregnancy or a planned pregnancy; terminal illness; known hypersensitivity to 6R-BH4; and enrollment in another clinical trial.
In total, 247 potential subjects were screened. Forty-one subjects were excluded because they were known to fulfill at least one of the exclusion criteria. The remaining 206 subjects were invited to participate, of whom 51 agreed. Many eligible subjects could not be included for the sufficient treatment period because of the planned clinical scheduling of their CABG surgery and/or the logistic complexity of returning for MRI scans at baseline and before surgery. One patient was unable to tolerate the initial MRI scan and thus was not randomized. One patient declined CABG and therefore dropped out of the study. Thus, 49 subjects completed the study. Demographic characteristics are presented in Table 1. The study protocol was approved by the Oxfordshire Research Ethics Committee, and each participant gave written informed consent.
Table 1. Demographic Characteristics of Study Participants.
| Placebo | 6R-BH4 400 mg/d | 6R-BH4 700 mg/d | |
|---|---|---|---|
| Patients (M:F), n | 19 (16:3) | 14 (13:1) | 16 (14:2) |
| Age (SEM), y | 68 (2) | 69 (2) | 68 (2) |
| Treatment duration (SEM), d | 30 (3) | 30 (4) | 29 (3) |
| BMI (SEM), kg/m2 | 27.1 (1.0) | 29.5 (1.1) | 27.2 (0.8) |
| Systolic BP (SEM), mm Hg | 133 (4) | 141 (4) | 132 (5) |
| Diastolic BP (SEM), mm Hg | 69 (2) | 79 (3) | 70 (3) |
| Creatinine (SEM), mg/dL | 1.23 (0.05) | 1.15 (0.06) | 1.27 (0.06) |
| Cholesterol, mg/dL | |||
| Median | 135 | 170 | 147 |
| 25th–75th percentiles | 127–174 | 143–205 | 127–162 |
| HDL, mg/dL | |||
| Median | 46 | 50 | 42 |
| 25th–75th percentiles | 39–54 | 35–62 | 39–58 |
| Triglycerides, mg/dL | |||
| Median | 106 | 106 | 115 |
| 25th–75th percentiles | 88–124 | 88–212 | 80–195 |
| HbA1c, % | |||
| Median | 5.7 | 6.2 | 5.6 |
| 25th–75th percentiles | 5.6–6.7 | 5.6–6.9 | 5.3–6.4 |
| Diabetes mellitus, n (%) | 5 (26) | 5 (36) | 4 (25) |
| Hypertension, n (%) | 13 (68) | 11 (79) | 11 (69) |
| Family history, n (%) | 6 (32) | 7 (50) | 2 (13) |
| MI, n (%) | 9 (47) | 6 (43) | 3 (19) |
| Smoking status, n (%) | |||
| Current or recent smoker | 2 (11) | 2 (14) | 4 (25) |
| Ex-smoker >1 y | 13 (68) | 10 (71) | 10 (63) |
| Never smoked | 4 (21) | 2 (14) | 2 (13) |
| Extent of CAD, n (%) | |||
| 2-Vessel | 3 (16) | 1 (7) | 7 (44) |
| 3-Vessel | 16 (84) | 13 (93) | 9 (56) |
| Medication, n (%) | |||
| Aspirin | 19 (100) | 11 (79) | 14 (88) |
| Clopidogrel | 3 (16) | 4 (29) | 5 (31) |
| Statin | 18 (95) | 13 (93) | 16 (100) |
| ACE inhibitor or ARB | 16 (84) | 11 (79) | 9 (56) |
| β-blocker | 16 (84) | 10 (71) | 9 (56) |
| Calcium channel blocker | 7 (37) | 6 (43) | 6 (38) |
| Diuretic | 4 (21) | 2 (14) | 2 (13) |
| Nitrate | 6 (32) | 6 (43) | 3 (19) |
| Nicorandil | 2 (11) | 0 (0) | 2 (13) |
| Insulin | 2 (11) | 1 (7) | 0 (0) |
| Oral hypoglycemic | 3 (16) | 4 (29) | 2 (13) |
6R-BH4 indicates sapropterin dihydrochloride; BMI, body mass index; BP, blood pressure; HbA1c, hemoglobin A1c; MI, myocardial infarction; CAD, coronary artery disease; ACE, angiotensin-converting enzyme; and ARB, angiotensin receptor blocker.
MRI Quantification of Vascular Function
Vascular function was quantified by high-resolution MRI at baseline and at the end of the treatment period. Images of the aorta and carotid arteries were used to determine arterial distensibility and aortic pulse wave velocity as indexes of vascular stiffness. Arterial distensibility was determined in the ascending aorta, proximal descending aorta, distal descending aorta, and both common carotid arteries with a high-resolution gradient-echo pulse sequence on a 1.5-T MR scanner (Siemens Sonata, Erlangen, Germany), as described previously.14 A velocity-encoding gradient for phase-contrast MRI was applied to determine pulse wave velocity in the aorta.14
Brachial artery flow-mediated dilatation (FMD) was used as a measure of endothelial function. Using cross-sectional images of the brachial artery piloted in 3 dimensions, we defined flow-mediated dilatation as the maximal percentage change in luminal area from baseline after 4 minutes of forearm ischemia; endothelium-independent dilation was similarly quantified after administration of 200 μg sublingual glyceryl trinitrate.
Tissue and Plasma Samples
Paired samples of saphenous vein (SV) and internal mammary artery (IMA) were obtained from each patient at the time of CABG surgery and transferred to the laboratory within 30 minutes in ice-cold Krebs Henseleit buffer. Blood samples were obtained at baseline and immediately before surgery. Blood was centrifuged at 4000 rpm for 5 minutes; plasma was collected and stored at −80°C. Plasma samples for BH4 quantification were collected and stored in the presence of the antioxidant dithioerythritol (DTE; 1 mmol/L) to prevent oxidation.
Quantification of Plasma and Vascular Biopterins
BH4, BH2, and biopterin levels in plasma and vascular tissue were determined separately by high-performance liquid chromatography followed by electrochemical (for BH4) and fluorescent (for BH2 and biopterin) detection, as described previously.6 Levels of biopterins were expressed as picomoles per gram of tissue for vessels and nanomoles per liter for plasma.
Vasomotor Studies
Endothelium-dependent and -independent relaxations were assessed in SV rings in isometric tension studies. Four rings from each vessel were precontracted with phenylephrine (3×10−6 mol/L), after which endothelium-dependent relaxations to acetylcholine (10−10–10−6 mol/L) were quantified. Finally, relaxations to the endothelium-independent NO donor sodium nitroprusside (10−10–10−6 mol/L) were evaluated in the presence of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 100 μmol/L).
Quantification of Vascular Superoxide Production
Vascular superoxide production was measured from paired segments of intact SV and IMA with lucigenin-enhanced chemiluminescence, as validated previously.15 Vessels were opened longitudinally to expose the endothelial surface and equilibrated for 30 minutes in oxygenated (95% O2/5% CO2) Krebs-HEPES buffer (pH 7.4) at 37°C. Lucigenin-enhanced chemiluminescence was measured with low-concentration lucigenin (5 μmol/L). NOS-derived superoxide production was determined by the difference in superoxide production after 30 minutes of preincubation with L-NAME (100 μmol/L).
Ex Vivo Incubations of Vessels and Blood
To further assess the vascular uptake of exogenous biopterins, SV rings from an additional 6 CABG patients were incubated for 30 minutes in oxygenated Krebs-HEPES buffer at 37°C either in buffer alone or in the presence of BH4 (100 nmol/L, Schircks Laboratories, Jona, Switzerland), BH4 plus DTE (1 mmol/L), or BH2 (100 nmol/L, Schircks Laboratories). Samples of incubation media and vessel rings were stored at −80°C before high-performance liquid chromatography analysis for quantification of BH4, BH2, and biopterin.
To assess whether exogenous BH4 is present in the vascular endothelium, SV rings from 6 additional patients were incubated either alone (n=2 rings per patient) or with BH4 plus DTE (n=2 rings per patient) under the same reagent concentrations and conditions as the experiment above. After incubation, the endothelium was removed from 1 ring by gentle luminal abrasion, as described previously.6 Vessel rings were stored at −80°C before high-performance liquid chromatography analysis.
To further evaluate the oxidation of endogenous and exogenous BH4, whole blood and plasma (separated from whole blood after centrifugation) from 3 healthy volunteers were incubated either alone or with supplementary BH4 (50 nmol/L), BH4 plus DTE (1 mmol/L), or BH2 (50 nmol/L). The whole-blood samples were placed on rollers during incubation to prevent separation of cellular content from plasma. Samples of plasma (either incubated as plasma or after separation from whole blood) were taken at baseline and after 4 hours of incubation and stored at −80°C before high-performance liquid chromatography analysis.
Statistical Analysis
All variables were tested for normal distribution with the Kolmogorov-Smirnov test. Nonnormally distributed variables were log transformed for analyses. Normally distributed variables are presented as mean±SEM.
According to the original power calculations (19 patients per group), the study would be able to detect a 4.7% difference in the change of flow-mediated dilatation between any of the 2 active treatment groups versus placebo, with an assumed normally distributed SD of the change of 4, α of 0.025, and power of 90%. However, the study recruited 19 patients in the placebo group, 14 in the 400-mg/d group, and 16 in the 700-mg/d group. Therefore, post hoc analysis showed that the true statistical power to detect the same differences was 83% for the 400-mg/d group versus placebo and 86% for the 700-mg/d group versus placebo.
For MRI and plasma variables in the clinical trial, we used 2-factor ANOVA with a time– by–treatment group interaction to compare the effect of treatment on these variables between groups. When a significant difference was observed between groups, individual comparisons were performed with the Bonferroni post hoc test.
For tissue variables and the ex vivo experiments, we used 1- or 2-factor ANOVA (as stated in the figure legends) followed by Bonferroni post hoc test to compare variables between groups.
For the organ bath experiments, the effect of treatment on vasorelaxation in response to acetylcholine or sodium nitroprusside was evaluated with 2-way ANOVA for repeated measures (examining the effect of acetylcholine or sodium nitroprusside concentration– by–treatment group interaction on vasorelaxations) in a full factorial model.
All tests were 2 tailed, and values of P<0.05 were considered significant. All statistical tests were performed with SPSS 19.0.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Effect of Oral BH4 Treatment on Plasma Biopterin Levels
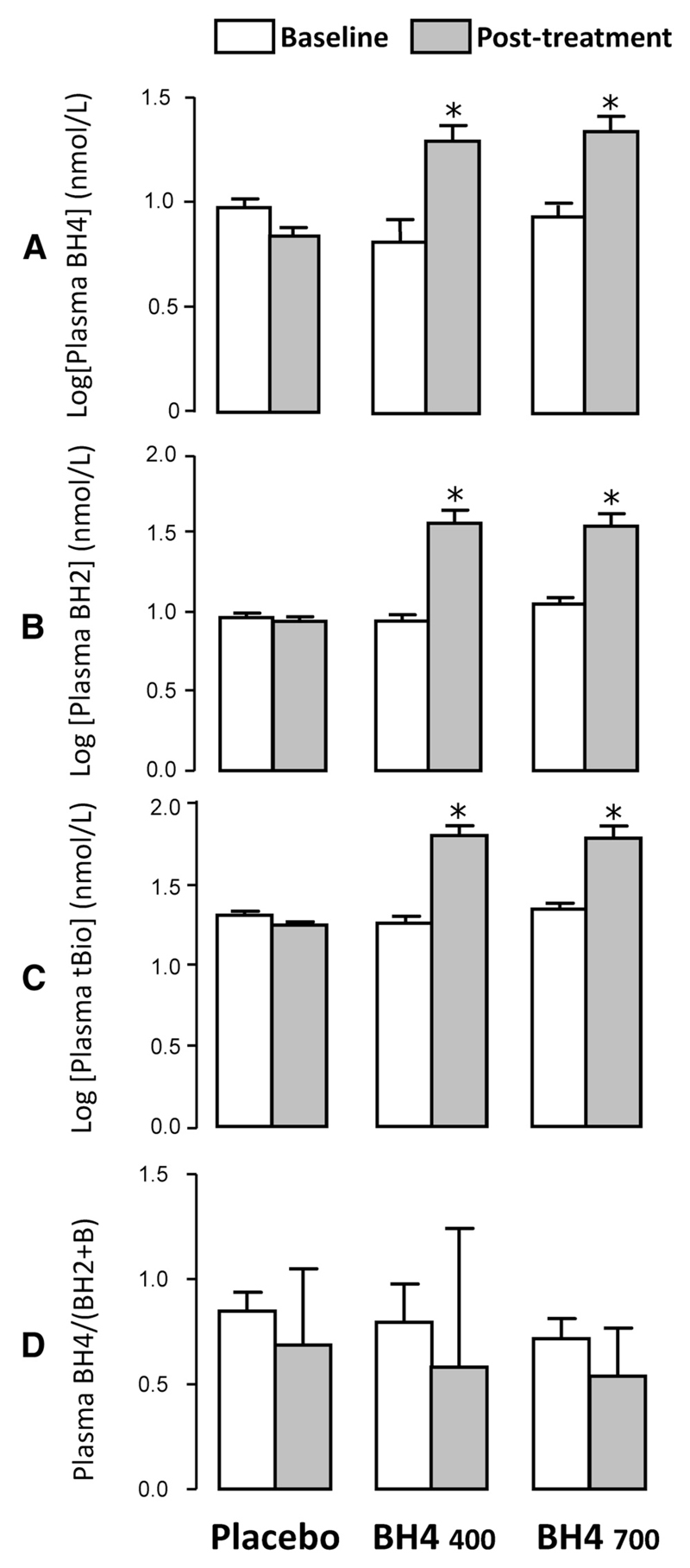
Both low- and high-dose oral BH4 treatment elevated plasma BH4 levels ≈2.5-fold compared with placebo (P<0.001; Figure 2A). There was a similar significant elevation in plasma BH2 (Figure 2B) and biopterin. Accordingly, total biopterin levels (the sum of BH4, BH2, and biopterin) were elevated by treatment (Figure 2C), but the ratio of reduced to oxidized biopterins, BH4/(BH2+biopterin), was not significantly altered by oral BH4 treatment (Figure 2D).
Figure 2.
Effect of oral tetrahydrobiopterin (BH4) treatment on plasma biopterins. Levels of plasma biopterin species were quantified at baseline and after treatment with oral BH4 or placebo. Treatment with oral BH4 400 or 700 mg/d resulted in a significant increase in plasma BH4 levels compared with placebo (A) but also a significant increase in plasma dihydrobiopterin (BH2; B) and biopterin (data not shown). Accordingly, total biopterins (tBio; the sum of BH4 and BH2 and biopterin) were significantly elevated by BH4 treatment (C), but there was no change in the ratio of reduced to oxidized biopterins, BH4/(BH2+biopterin) (D). Values are expressed as mean±SEM of log-transformed values. *P<0.001 vs placebo group for change from baseline; P values calculated with 2-factor ANOVA with time–by–treatment group interaction followed by Bonferroni post hoc test (2 comparisons per panel: BH4 400 and 700 mg/d vs placebo).
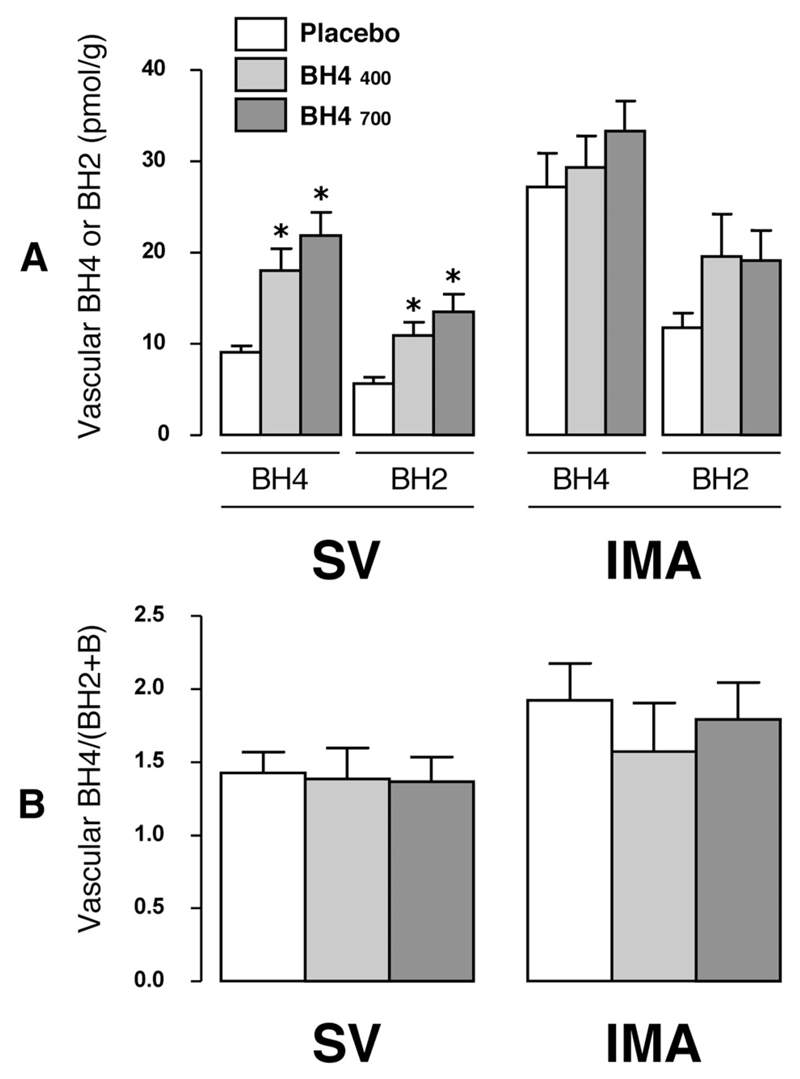
Effect of Oral BH4 Treatment on Vascular Biopterin Levels
In SV, BH4 levels were increased 2-fold by both low- and high-dose oral BH4 treatment compared with placebo (P<0.001; Figure 3A), with a similar significant increase in BH2 and biopterin. In IMA, however, there was no significant difference in BH4 (P=0.334), BH2 (P=0.134), or biopterin (P=0.082) in BH4-treated compared with placebo-treated patients. In both SV and IMA there was no difference in the BH4/(BH2+biopterin) ratio between placebo- and BH4-treated patients (Figure 3B).
Figure 3.
Effect of oral tetrahydrobiopterin (BH4) treatment on vascular biopterins. Samples of saphenous vein (SV) and internal mammary artery (IMA) were collected at the time of coronary artery bypass graft surgery for quantification of biopterin species. In SV, treatment with oral BH4 resulted in a significant increase in tissue levels of BH4 and dihydrobiopterin (BH2; A) and biopterin (data not shown) vs placebo. In IMA, there were no significant differences between treatment groups (A). In both vessel types, treatment with oral BH4 did not alter the tissue ratio of reduced to oxidized biopterins, BH4/(BH2+biopterin) (B). Values are expressed as mean±SEM. *P<0.001 vs placebo; 2-factor ANOVA showed a significant individual effect of vessel type and treatment group on both BH4 (P<0.0001 for both) and BH2 (P<0.001 for both), but there was no significant vessel type–by–treatment group interaction for either BH2 (P=0.380) or BH4 (P=0.053). This analysis was followed by Bonferroni post hoc test for individual between-group comparisons for each vessel type (SV or IMA) and each biopterin species [BH4 or BH2 or BH4/(BH2+biopterin) ratio] separately (2 comparisons per variable: BH4 400 and 700 mg/d vs placebo).
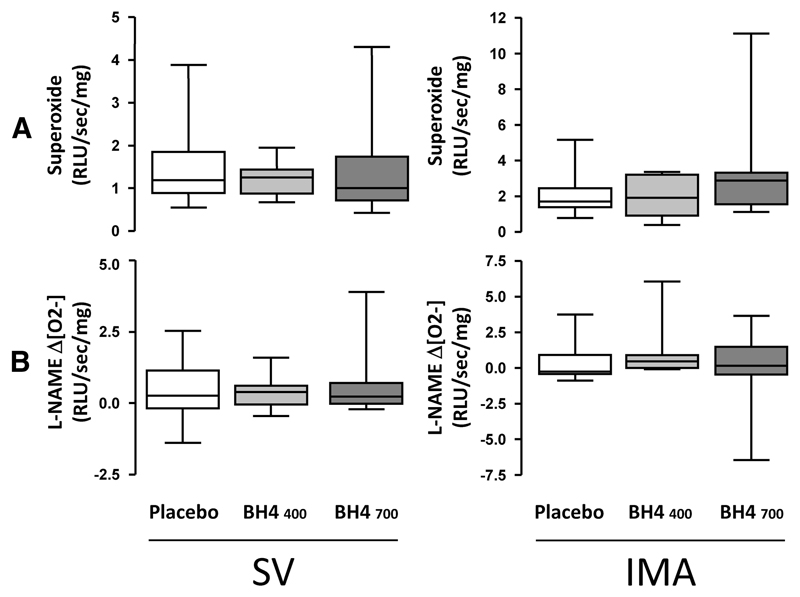
Effect of Oral BH4 Treatment on Vascular Superoxide Production, Endothelial Function, and Arterial Stiffness
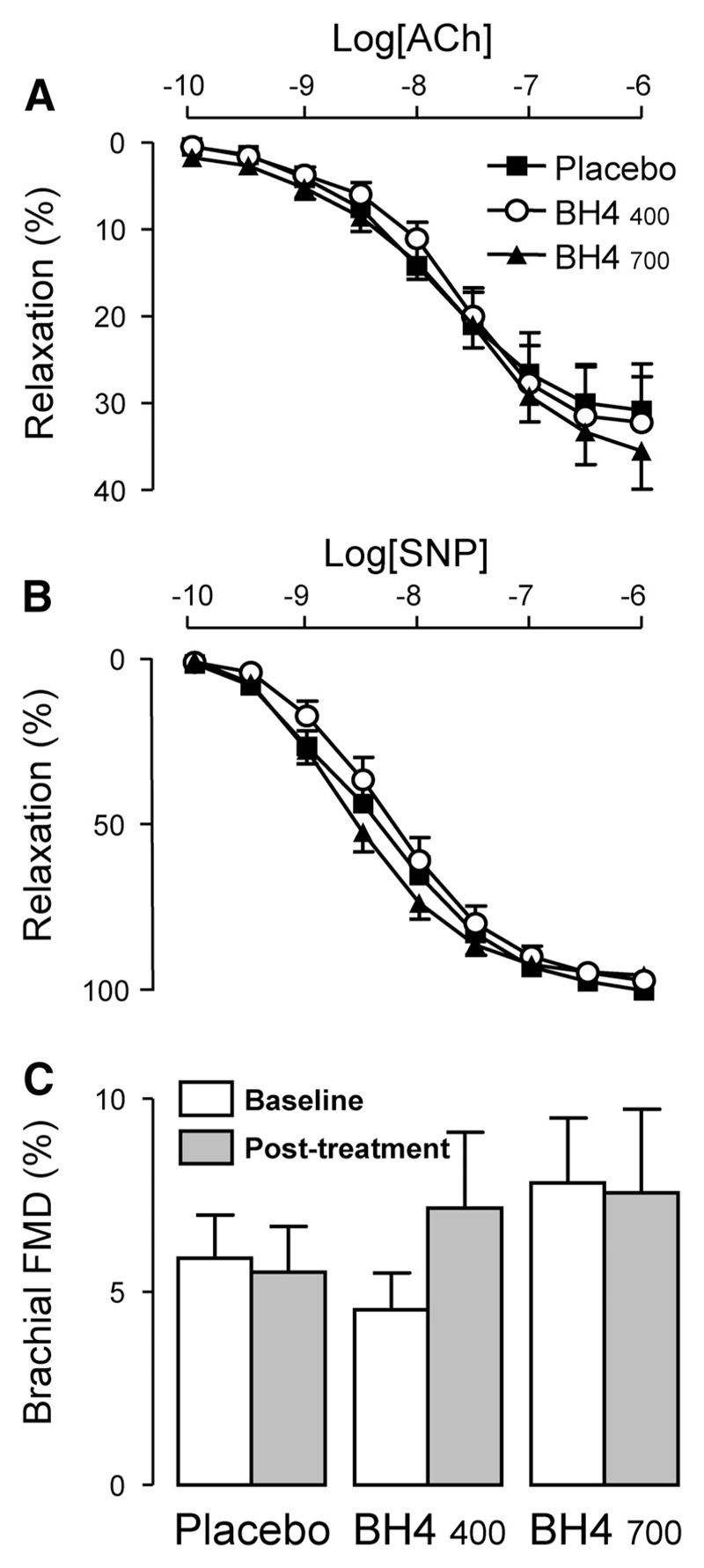
There was no significant difference in either total vascular superoxide production or NOS-derived superoxide production between placebo- and BH4-treated subjects in either SV (P=0.70) or IMA (P=0.12; Figure 4). Quantification of vascular function by MRI at baseline and after treatment with either placebo or BH4 revealed no effect of oral BH4 treatment on brachial flow-mediated dilatation (P=0.325), aortic or carotid distensibility, or aortic pulse wave velocity (Table 2). Correspondingly, samples of SV harvested at the time of CABG surgery for ex vivo organ bath studies showed no significant difference in endothelium-dependent relaxation to acetylcholine or endothelium-independent relaxation to sodium nitroprusside between treatment groups (Figure 5).
Figure 4.
Effect of oral tetrahydrobiopterin (BH4) treatment on vascular superoxide production and eNOS coupling. Samples of saphenous vein (SV) and internal mammary artery (IMA) were collected at the time of coronary artery bypass graft surgery for quantification of superoxide production by lucigenin-enhanced chemiluminescence in the presence and absence of the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (L-NAME). There was no effect of BH4 treatment on total vascular superoxide production in SV or IMA (A) and no effect on L-NAME–inhibitable superoxide (B). Values are expressed as median (25th to 75th percentiles) and range. Groups were compared by use of 1-way ANOVA of log-transformed values.
Table 2. Effect of Oral BH4 Treatment on Magnetic Resonance Imaging Indexes of Arterial Stiffness.
| Placebo |
BH4 400 mg/d |
BH4 700 mg/d |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After Treatment | Δ | Baseline | After Treatment | Δ | Baseline | After Treatment | Δ | P | |
| Distensibility, 10−3 mm Hg−1 | ||||||||||
| Ascending aorta | 1.66 (0.31) | 1.54 (0.25) | −0.23 (0.23) | 1.79 (0.22) | 2.09 (0.45) | 0.28 (0.26) | 2.04 (0.37) | 2.33 (0.38) | 0.05 (0.30) | 0.40 |
| Proximal descending aorta | 2.09 (0.33) | 2.17 (0.36) | 0.09 (0.12) | 2.47 (0.20) | 2.62 (0.34) | 0.11 (0.23) | 2.73 (0.39) | 3.01 (0.37) | 0.28 (0.29) | 0.77 |
| Abdominal aorta | 2.69 (0.42) | 2.87 (0.42) | 0.18 (0.22) | 3.00 (0.41) | 3.28 (0.43) | 0.32 (0.33) | 3.32 (0.47) | 3.18 (0.46) | −0.14 (0.36) | 0.57 |
| Right carotid artery | 3.01 (0.25) | 2.71 (0.24) | −0.39 (0.24) | 3.20 (0.36) | 4.48 (0.69) | 1.12 (0.59) | 4.05 (0.57) | 3.83 (0.37) | −0.54 (0.71) | 0.11 |
| Left carotid artery | 3.59 (0.31) | 3.26 (0.40) | −0.53 (0.33) | 3.62 (0.33) | 4.39 (0.86) | 0.68 (0.83) | 3.53 (0.42) | 3.84 (0.54) | −0.30 (0.47) | 0.27 |
| Aortic PWV, m/s | 6.57 (0.37) | 6.41 (0.37) | −0.01 (0.46) | 6.33 (0.40) | 7.19 (1.17) | 0.73 (1.21) | 7.23 (0.72) | 6.83 (0.47) | −0.40 (0.59) | 0.58 |
BH4 indicates tetrahydrobiopterin; PWV, pulse wave velocity. Aortic and carotid distensibility and aortic PWV were measured by magnetic resonance imaging at baseline and after treatment with oral BH4 or placebo. Values are expressed as mean (SEM). P values represent 2-way ANOVA for repeated measures with time– by–treatment group interaction.
Figure 5.
Effect of oral tetrahydrobiopterin (BH4) treatment on endothelial function. Isometric tension studies were performed on saphenous vein rings to quantify vasorelaxation to acetylcholine (ACh) and sodium nitroprusside (SNP) ex vivo. Oral BH4 treatment had no significant effect on endothelium-dependent relaxation to ACh (A) or endothelium-independent relaxation to SNP (B). Endothelial function in vivo was determined with magnetic resonance imaging to quantify brachial artery flow-mediated dilatation (FMD). There was no significant effect of BH4 treatment on brachial FMD (C). Values are expressed as mean±SEM. Data in A and B were analyzed with 2-way ANOVA for repeated measures (examining the effect of ACh or SNP concentration–by–treatment group interaction on vasorelaxations) in a full factorial model. The data in C were analyzed by 2-factor ANOVA (examining the effect of time–by–treatment group interaction on brachial FMD).
Oxidation and Uptake of Biopterins in Human Vessels and Blood Ex Vivo
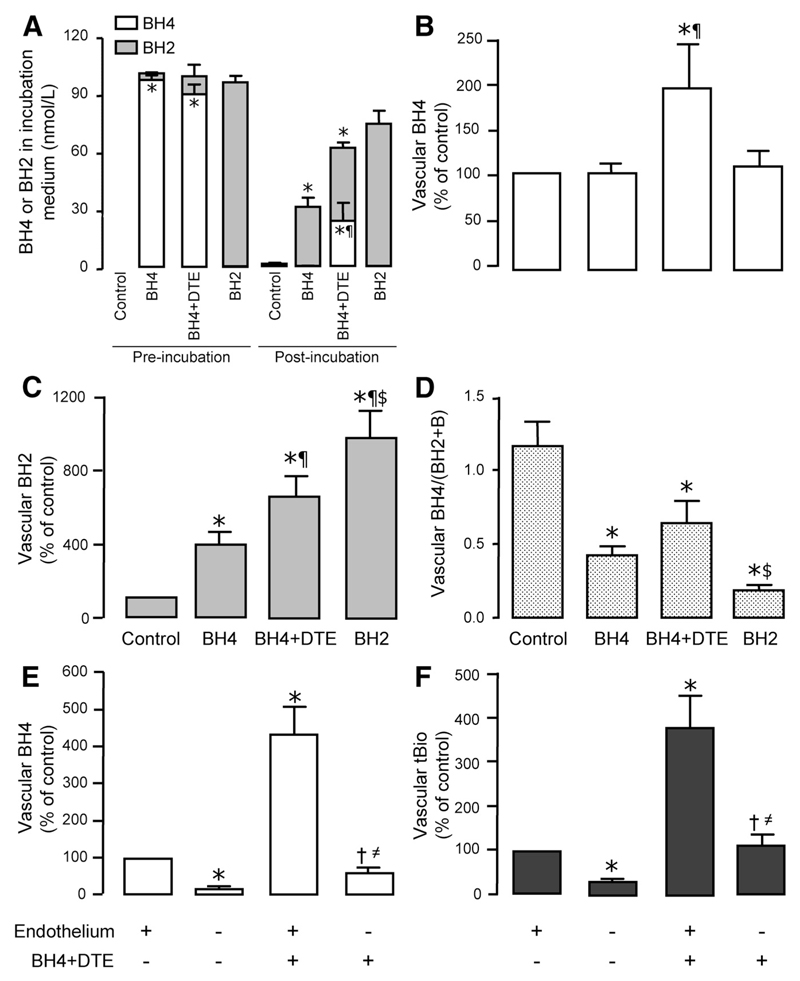
After observing that oral BH4 treatment resulted in significant increases in circulating BH4 and BH2 but no effect on biopterin redox status in either plasma or vascular tissue, we conducted ex vivo experiments to investigate the fate of exogenous BH4 in blood and the uptake of exogenous BH4 and BH2 in human vascular tissue. SV samples from an additional 6 subjects (not enrolled in the clinical trial) were collected during CABG surgery and incubated with BH4 (with or without the antioxidant DTE) or BH2. We incubated vessels in low-concentration BH4 (50 nmol/L), reflecting the plasma levels of BH4 achieved after oral BH4 therapy, rather than the very high concentrations used in previous studies.
Incubation of vessels with exogenous BH4 led to rapid and complete depletion of BH4 from the incubation medium associated with a marked accumulation of BH2. However, the addition of DTE significantly increased residual BH4 concentration (P<0.001; Figure 6A). In vascular tissue, incubation with exogenous BH4 significantly increased BH2 levels compared with control (P<0.05; Figure 6C) but significantly increased BH4 levels only in the presence of DTE (P<0.05; Figure 6B). Incubation with BH2 resulted in a larger increase in tissue BH2 but no increase in tissue BH4. Incubation in exogenous BH4 resulted in a significant reduction in tissue BH4/(BH2+biopterin) ratio compared with control (P<0.01), even in the presence of DTE (P<0.05; Figure 6D).
Figure 6.
Ex vivo incubation of saphenous vein rings with exogenous tetrahydrobiopterin (BH4) or dihydrobiopterin (BH2). A through D, Saphenous vein rings (n=6 subjects) were incubated for 30 minutes under the following experimental conditions: control, buffer alone; BH4, BH4 100 µmol/L; BH4+DTE, BH4 100 µmol/L plus dithioerythritol (antioxidant) 1 mmol/L; and BH2, BH2 100 µmol/L. Samples of incubation medium were stored at the beginning and end of the experiment. A, Exogenous BH4 in the incubation medium was totally depleted by the end of the experiment; the addition of DTE provided partial protection from oxidation. B and C, Incubation with BH4 significantly increased tissue BH2 levels compared with control but increased tissue BH4 levels only in the presence of DTE; incubation with BH2 resulted in a larger increase in tissue BH2 but no increase in tissue BH4. D, Incubation in exogenous BH4 resulted in a significant reduction in tissue BH4/(BH2+biopterin) ratio compared with control, even in the presence of DTE. *P<0.05 vs control; ¶P<0.05 vs BH4 alone; $P<0.05 vs BH4+DTE. E and F, In a further experiment, SV tissue was obtained from an additional 6 patients. Two rings from each subject were incubated with BH4 and DTE, with a further 2 control rings incubated without BH4/DTE. Endothelium was removed from one of each pair of rings. Endothelial denudation led to a reduction of vascular BH4 (E) and total biopterins (tBio; F) by ≈75%. In vessels incubated with BH4+DTE, vascular BH4 was significantly increased. After endothelium was removed from these vessels, BH4 was reduced by ≈85%, demonstrating that the majority of both endogenous and exogenous BH4 is present in the endothelium. *P<0.05 vs endo(+)/BH4(–); †P<0.05 vs endo(+)/BH4(+); ≠P<0.05 vs endo(–)/BH4(–). Values are expressed as mean±SEM. A, The levels of BH4 and BH2 in the medium before and after incubation were compared between the control, BH4, and BH4+DTE groups by use of 1-way ANOVA followed by Bonferroni post hoc test for individual between-group comparisons (3 comparisons). B through F, Variables were compared by use of 1-way ANOVA followed by Bonferroni post hoc test for individual between-group comparisons (for 6 comparisons in B–D and 4 comparisons in E and F).
In the subsequent experiment, removal of the endothelium from control vessel segments led to a reduction of vascular BH4 and total biopterins by ≈75% (Figure 6E and 6F). In vessels incubated with BH4 plus DTE, vascular BH4 was significantly increased (P<0.05). After removal of the endothelium from these vessels, BH4 was reduced by ≈85%, demonstrating that the majority of exogenous BH4 is present in the endothelium; however, as an absolute measure, BH4 remained significantly higher than in the control de-endothelialized vessel, suggesting that a significant minority of vascular biopterins measured in SV after incubation with BH4 is also due to BH4 uptake into vascular smooth muscle cell or captured in the vascular adventitia and extracellular space (Figure 6E and 6F).
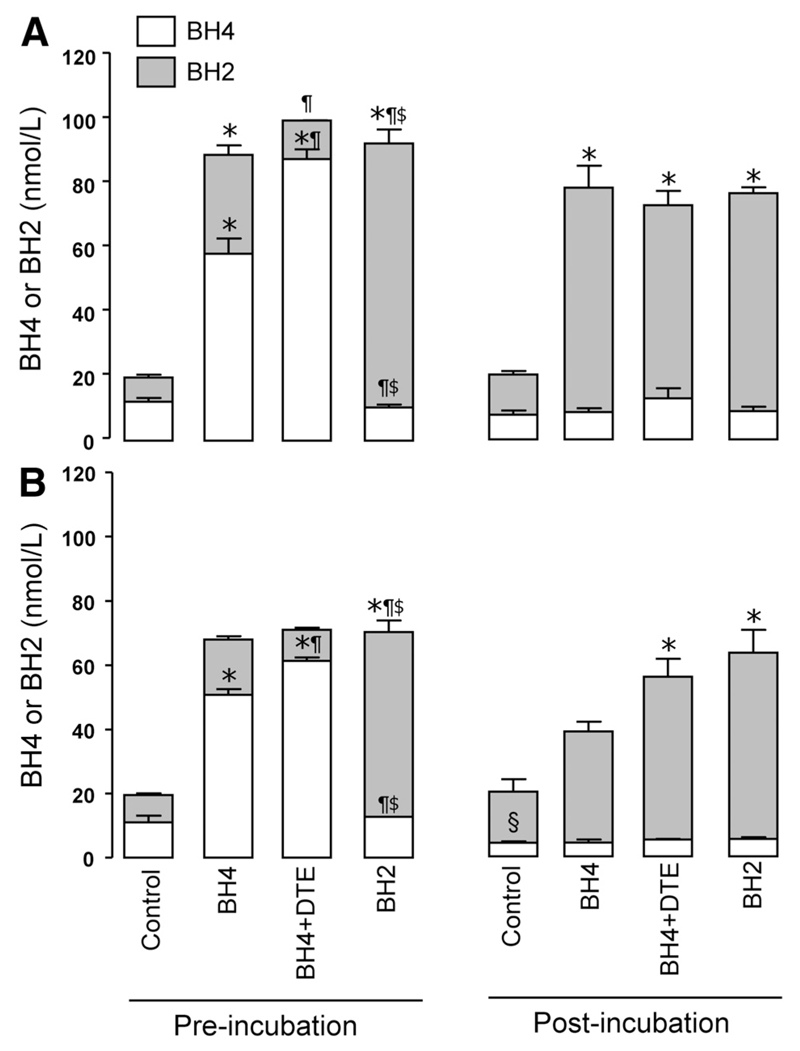
We next investigated the fate of endogenous and exogenous biopterins in whole blood and plasma supplemented with BH4 (with or without the antioxidant DTE) or BH2. Endogenous BH4 levels in whole blood remained unchanged after 4 hours of incubation. In contrast, the addition of exogenous BH4 significantly increased BH2 in human blood, even at baseline, indicating rapid BH4 oxidation (Figure 7A). Exogenous BH4 was totally depleted after 4 hours. The addition of DTE attenuated the rapid oxidation of exogenous BH4 (P<0.05) but had no effect on residual BH4 levels after 4 hours of incubation (Figure 7A).
Figure 7.
Fate of endogenous vs exogenous tetrahydrobiopterin (BH4) in whole blood and plasma. Samples of whole blood (A) and plasma (B) from healthy volunteers (n=3) were incubated for 4 hours under the following experimental conditions: control, blood or plasma alone; BH4, supplementary BH4 50 nmol/L; BH4+DTE, supplementary BH4 50 nmol/L plus dithioerythritol (antioxidant) 1 mmol/L; and BH2, supplementary dihydrobiopterin 50 nmol/L. In the whole blood samples, plasma was separated at baseline and after 4 hours. Plasma biopterins were then quantified in all samples. In whole blood, there was no significant difference in endogenous BH4 or BH2 after 4 hours of incubation, whereas in plasma alone, endogenous BH4 was oxidized to BH2. Exogenous BH4 was completely oxidized in both whole blood and plasma after 4 hours; DTE afforded protection from oxidation at baseline but not after 4 hours. *P<0.05 vs control; ¶P<0.05 vs BH4 alone; $P<0.05 vs BH4+DTE; §P<0.05 vs baseline. Both preincubation and postincubation BH4 and BH2 levels were compared separately by use of 1-way ANOVA followed by Bonferroni post hoc test for individual between-group comparisons (6 comparisons). The change in BH4 before versus after incubation was tested only in the control groups by use of a paired t test.
In contrast to whole blood, when plasma was incubated for 4 hours, there was a significant reduction in endogenous BH4 (P<0.05) with evidence of oxidation to BH2 (Figure 7B). The effect of addition of exogenous BH4 mirrored that seen in whole blood, ie, rapid oxidation to BH2 with attenuation by DTE at baseline but not after 4 hours. Taken together, these observations demonstrate that exogenous BH4 is rapidly oxidized to BH2 in human blood or blood vessels ex vivo, resulting in little or no increase in BH4 levels but an increase in BH2 and a decrease in the ratio of reduced to oxidized biopterins.
Discussion
In this study, we demonstrate that oral BH4 treatment has no effect on measures of endothelial function, arterial stiffness, or oxidative stress in patients with established CAD either in vivo or with ex vivo assays on vascular tissue obtained at the time of CABG surgery. The design of our study allows, for the first time in humans, detailed mechanistic evaluation of the pharmacokinetics and pharmacodynamics of oral BH4 treatment in both blood and vascular tissue to explain these observations. Because BH4 has been proposed as a potential treatment to improve endothelial function in vascular disease states, our results demand fundamental reconsideration of strategies to harness BH4-mediated pathways as therapeutic targets in human cardiovascular disease.
We first demonstrate that treatment with oral BH4 in subjects with CAD is indeed sufficient to significantly elevate plasma BH4 levels, despite the fact that blood samples taken at the time of CABG surgery were effectively trough levels, ie, 12 to 16 hours after the last dose. However, this elevation in plasma BH4 is tempered by similar rises in plasma BH2 and biopterin, so that the ratio of reduced to oxidized biopterins, BH4/(BH2+biopterin), in plasma remains unchanged after treatment. In vessels, there are different findings in venous and arterial tissue. Changes in SV mirror those seen in plasma, but in IMA, the smaller absolute changes and greater variability in biopterin levels did not reveal a significant effect of oral BH4 treatment. If data from the low- and high-dose BH4 groups are combined to perform an analysis of BH4 therapy versus placebo, the increase in BH2 (but not BH4) in IMA in the combined treatment group reaches statistical significance (19.3 versus 11.8 pmol/g; P=0.03). Thus, oral BH4 therapy in subjects with CAD is more effective at elevating arterial BH2 levels than BH4 levels.
The explanation for this discrepancy between venous and arterial tissue is not clear. Biopterin transport is not fully understood, especially in humans and in intact tissues. Previous work has indicated that biopterin transport is cell type dependent and that both direct uptake (as BH4) and conversion to BH2 followed by recycling via dihydrofolate reductase (DHFR) are possible mechanisms.16 The first explanation, therefore, is that there is a genuine biological difference between human veins and arteries in biopterin uptake. If BH4 enters the vessel via passive diffusion from the lumen, then slow venous blood flow in lower limb veins, compared with high-pressure arterial flow, may promote uptake. If BH4 enters the vessel via the salvage pathway, then it is possible that DHFR is regulated differently between SV and IMA.
The alternative possibility is that the difference observed between SV and IMA is not due to a different biological mechanism of transport between these tissue types. Our data show a small but statistically nonsignificant increase in a BH4 in IMA (and a slightly larger accumulation of BH2); however, the variability in BH4 was greater in IMA than in SV, so our sample size is too small to exclude a very small effect of BH4 therapy on arterial BH4 levels.
Despite the changes in BH4 seen after oral BH4 treatment, the BH4/(BH2+biopterin) ratio remains unchanged in both SV and IMA. This finding reveals important insights into the significance of BH2 levels in human vessels. Recent data from cultured endothelial cells17,18 suggest that the intracellular levels of BH2 and, more specifically, the ratio between reduced and oxidized biopterins are important in regulating eNOS coupling. Our data are consistent with the studies using purified eNOS enzyme that demonstrated that BH2 can act as a competitive inhibitor of BH4 and promote eNOS uncoupling.19 In the present study, there is no effect of oral BH4 therapy on total or NOS-derived superoxide in either SV or IMA, despite a 2-fold increase in absolute BH4 levels in SV. Furthermore, there is no effect of BH4 therapy on endothelium-dependent relaxation in SV rings. Taken together, these findings suggest that the BH4/(BH2+biopterin) ratio is a more important determinant of eNOS function than absolute BH4 levels in human vessels. Accordingly, pharmacological strategies that alter the balance between reduced and oxidized biopterins in favor of BH4 may be more effective at improving endothelial function than strategies that have either no effect, or indeed an opposite effect, on this ratio. Both folates,20 via scavenging of peroxynitrite, and vitamin C,21 via increased recycling of the trihydrobiopterin (BH3+) radical to BH4, have the potential to be coadministered with BH4 to achieve this shift in the intracellular oxidative balance. Indeed, long-term oral folic acid treatment has been shown to augment vascular BH4 levels in patients with CAD.22
The effects of oral BH4 treatment on endothelial function in patients with CAD may also be limited by uncertainty over how exogenous biopterins may enter the vascular endothelium. Studies in mice23,24 have suggested that oxidation of BH4 to BH2 and subsequent recycling back to BH4 by DHFR is necessary to augment intracellular BH4; accordingly, this elevation in BH4 can be attenuated by inhibition of DHFR with methotrexate. In the present study, we see evidence of significant oxidation of exogenous BH4 to BH2 in plasma. Thus, it is possible that coadministration of BH4 with an antioxidant (to prevent this oxidation in plasma) may result in a paradoxical reduction in drug uptake into the endothelium. Our ex vivo experiments using whole blood and vascular tissue shed further light on these important aspects of BH4 homeostasis. Using supplementary doses of exogenous BH4 to raise BH4 levels equivalent to those that are achievable through oral administration (at the doses used in the clinical trial), we show that there is a rapid oxidation of BH4 to BH2 when exogenous BH4 is added to human blood, even in healthy volunteers. Coadministration of an antioxidant (DTE) attenuates the observed oxidation in the initial stages after addition to blood but has no effect after a prolonged incubation (4 hours), during which all exogenous BH4 is totally depleted. In contrast, endogenous levels of BH4 in whole blood are unchanged after incubation, suggesting that endogenous and exogenous BH4 may be oxidized at different rates, possibly because of an interaction with the cellular content of blood.
When vessel rings are incubated in exogenous BH4 for 30 minutes, there is no increase in tissue levels of BH4, but there is a significant increase in BH2, resulting in a lower tissue BH4/(BH2+biopterin) ratio. This finding is explained by the rapid oxidation of BH4 to BH2 in the incubation media under the experimental conditions at 37°C in organ chambers, resulting in a predominance of BH2 rather than BH4 for the majority of the duration of the control experiment. Coadministration with DTE significantly attenuates the oxidation of BH4 in the incubation media. The combination of BH4 and DTE is able to increase tissue BH4 levels and, more specifically, endothelial BH4 levels but importantly also results in a greater increase in tissue BH2 compared with incubation with BH4 alone, again resulting in a lower BH4/(BH2+biopterin) ratio compared with control. Finally, incubation with BH2 alone results in a large increase in tissue BH2 but no change in tissue BH4.
Taken together, these results suggest that both exogenous BH4 and BH2 can be taken up into human vascular tissue, but accumulation of tissue BH2 does not seem to result in an increase in tissue BH4. The strategy of BH4/antioxidant coadministration, to maintain a favorable BH4/BH2 ratio in circulating blood, might be hypothesized to increase the intravascular BH4/BH2 ratio; however, our results do not support this hypothesis. Although DTE affords initial protection of exogenous BH4 from oxidation, this effect appears to be short-lived. More important, coadministration of BH4 and DTE still results in an unfavorable ratio of reduced to oxidized biopterins in tissue compared with control despite increasing absolute BH4 levels, suggesting that in vessels from patients with established CAD, there may be continued oxidation of BH4 to BH2 after uptake into the vessel wall or incomplete regeneration of BH4 from BH2 via the salvage pathway catalyzed by DHFR. Indeed, recent studies suggest an important role for DHFR in maintaining intracellular BH4 levels, especially in conditions in which BH4 levels are limiting25,26 or in response to increased oxidative stress when DHFR activity is downregulated.27,28
It is important to acknowledge that the ex vivo blood and vessel models have a number of limitations. First, in the vessel incubation experiments, it has previously been described that high-concentration BH4 can generate superoxide anion when exposed to Krebs buffer,29 potentially leading to increased BH4 oxidation. Second, exogenous BH4 in blood ex vivo may be oxidized differently compared with circulating blood in vivo; additionally, factors such as the partial pressure of oxygen in these models (no added oxygen in the blood experiments and hyperoxia in the organ chamber experiments) may exert a significant effect on the rate of BH4 oxidation. Furthermore, these models do not take into account the oxidation of exogenous BH4 before entering the bloodstream, eg, in the gut. Importantly, the concentrations of BH4 and BH2 measured in blood or plasma samples supplemented with known concentrations of exogenous BH4 or BH2 were very similar to the expected sum of endogenous levels plus the added exogenous levels, despite the samples undergoing deproteinization before high-performance liquid chromatography analysis. This would suggest that no exogenous biopterins were removed by deproteinization and that exogenous BH4 is therefore not protein bound in peripheral blood. However, the potential interaction of biopterins with plasma proteins or other biomolecules is clearly of potential importance for understanding biopterin biology and pharmacokinetics.
The findings of the present study contrast with those of the only previous study of oral BH4 treatment,13 which demonstrated an improvement in endothelial function (forearm blood flow response to acetylcholine) in middle-aged subjects with hypercholesterolemia. That study differed from our study in a number of respects. First, the dose of 400 mg BH4 was administered twice daily, potentially reducing the variation in plasma peak-trough concentrations compared with the once-daily regimen of the present study. Accordingly, vascular measurements will have been performed closer to peak plasma concentrations. Second, subjects were younger, had no evidence of cardiovascular disease, had only 1 risk factor (hypercholesterolemia), and thus were likely to have lower systemic oxidative stress than subjects in the present study. This may be important in that systemic oxidative stress may be pivotal in determining the degree of oxidation of BH4 to BH2 in plasma with a corresponding effect on vascular uptake. Third, these younger subjects without established CAD were not taking medications known to improve endothelial function, eg, statins and angiotensin-converting enzyme inhibitors; thus, there may be greater “eNOS reserve” in these subjects compared with CABG patients who already take multiple secondary prevention therapies. Indeed, statin therapy alone appears to have significant beneficial direct effects on BH4 bioavailability through upregulation of guanosine triphosphate cyclohydrolase-1 (the rate-limiting enzyme in BH4 synthesis) expression and activity and eNOS-mediated endothelial function in CAD patients independently of cholesterol lowering.30,31 Thus, the findings of the present study can be applied only to the specific population studied, which represents the most diseased end of the atherosclerosis spectrum; BH4 monotherapy may have therapeutic potential in earlier stages of endothelial dysfunction.
Conclusions
We demonstrate that oral BH4 treatment in patients with CAD significantly elevates BH4 levels in blood, but this effect is significantly limited by systemic oxidation of exogenous BH4 to BH2, which lacks eNOS cofactor activity. Accordingly, the ratio of reduced to oxidized biopterins in blood and vascular tissue is unchanged by exogenous BH4 treatment, resulting in no net effect on eNOS coupling, endothelial function, or vascular superoxide production. Targeting BH4 remains a rational therapeutic strategy in cardiovascular disease, but future studies should be directed toward interventions that can favorably alter the endogenous BH4/BH2 ratio in human vascular endothelium via a selective increase in absolute BH4 levels, prevention of BH4 oxidation, or increased BH4 recycling. In particular, the effect of antioxidant coadministration to prevent systemic and vascular oxidation of exogenous BH4 warrants further attention.
Clinical Perspective.
Tetrahydrobiopterin (BH4) is an essential cofactor for endothelial nitric oxide synthase in the vascular endothelium. Under conditions of BH4 deficiency such as the increased oxidative stress associated with atherosclerosis, endothelial nitric oxide synthase can become uncoupled, leading to the production of reactive oxygen species rather than nitric oxide, thus exacerbating oxidative stress. Accordingly, pharmacological strategies to increase BH4 levels have been proposed as therapeutic options in vascular disease states. Short-term intra-arterial infusions of BH4 have been shown to improve endothelial function; however, the effect of a long-term oral strategy in patients with coronary artery disease is unknown. In this clinical trial, we have tested the hypothesis that long-term oral BH4 treatment can increase BH4 bioavailability and improve vascular function in patients with coronary artery disease compared with placebo. Our trial results, supported by mechanistic ex vivo experiments using human blood and vascular tissue, demonstrate that exogenous BH4 is rapidly oxidized to dihydrobiopterin, which lacks endothelial nitric oxide synthase cofactor activity, and may act as a competitive inhibitor of BH4. Thus, although BH4 levels are elevated by treatment, the corresponding elevation of dihydrobiopterin levels results in no net effect of treatment on vascular redox status and hence no effect on vascular function. Therefore, future studies should address alternative therapies to target BH4-mediated vascular function or should examine strategies to ameliorate the oxidation of exogenous BH4.
Acknowledgments
Sources of Funding
BioMarin Pharmaceutical Inc supported the clinical trial (www.clinicaltrials.gov; identifier, NCT00423280) through an unrestricted educational grant to the University of Oxford. The study was an investigator-initiated trial with Dr Channon as principal investigator. BioMarin Pharmaceutical Inc provided the study drug and randomization schedule and contributed to safety monitoring and pharmacovigilance for the study. The University of Oxford was the trial sponsor and took sole responsibility for the conduct and data of the study. No author has received funding directly from or has any financial or other related interest in BioMarin Pharmaceuticals Inc. Work in the authors’ laboratory is supported by the British Heart Foundation (programme grant RG/07/003/23133) and by the National Institute for Health Research Biomedical Research Centre. Dr Choudhury is a Wellcome Trust Senior Research Fellow in Clinical Science.
Footnotes
Disclosures
None.
References
- 1.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 2.Alp NJ, Mussa S, Khoo J, Guzik TJ, Cai S, Jefferson A, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 4.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase: a Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 6.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, Ratnatunga C, et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation. 2007;116:2851–2859. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 7.Antoniades C, Shirodaria C, Van Assche T, Cunnington C, Tegeder I, Lotsch J, Guzik TJ, Leeson P, Diesch J, Tousoulis D, Stefanadis C, et al. GCH1 haplotype determines vascular and plasma biopterin availability in coronary artery disease effects on vascular superoxide production and endothelial function. J Am Coll Cardiol. 2008;52:158–165. doi: 10.1016/j.jacc.2007.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 11.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 12.Mayahi L, Heales S, Owen D, Casas JP, Harris J, MacAllister RJ, Hingorani AD. (6R)-5,6,7,8-tetrahydro-L-biopterin and its stereoisomer prevent ischemia reperfusion injury in human forearm. Arterioscler Thromb Vasc Biol. 2007;27:1334–1339. doi: 10.1161/ATVBAHA.107.142257. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino F, Hurlimann D, Delli Gatti C, Chenevard R, Blau N, Alp NJ, Channon KM, Eto M, Lerch P, Enseleit F, Ruschitzka F, et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart. 2008;94:487–492. doi: 10.1136/hrt.2007.122184. [DOI] [PubMed] [Google Scholar]
- 14.Wiesmann F, Petersen SE, Leeson PM, Francis JM, Robson MD, Wang Q, Choudhury R, Channon KM, Neubauer S. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–2064. doi: 10.1016/j.jacc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Skatchkov MP, Sperling D, Hink U, Mulsch A, Harrison DG, Sindermann I, Meinertz T, Munzel T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem Biophys Res Commun. 1999;254:319–324. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- 16.Sawabe K, Suetake Y, Nakanishi N, Wakasugi KO, Hasegawa H. Cellular accumulation of tetrahydrobiopterin following its administration is mediated by two different processes; direct uptake and indirect uptake mediated by a methotrexate-sensitive process. Mol Genet Metab. 2005;86(suppl 1):S133–S138. doi: 10.1016/j.ymgme.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 19.Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, et al. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 21.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 22.Shirodaria C, Antoniades C, Lee J, Jackson CE, Robson MD, Francis JM, Moat SJ, Ratnatunga C, Pillai R, Refsum H, Neubauer S, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115:2262–2270. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 23.Sawabe K, Wakasugi KO, Hasegawa H. Tetrahydrobiopterin uptake in supplemental administration: elevation of tissue tetrahydrobiopterin in mice following uptake of the exogenously oxidized product 7,8-dihydrobiopterin and subsequent reduction by an anti-folate-sensitive process. J Pharmacol Sci. 2004;96:124–133. doi: 10.1254/jphs.fp0040280. [DOI] [PubMed] [Google Scholar]
- 24.Sawabe K, Suetake Y, Wakasugi KO, Hasegawa H. Accumulated BH4 in mouse liver caused by administration of either 6R- or 6SBH4 consisted solely of the 6R-diastereomer: evidence of oxidation to BH2 and enzymic reduction. Mol Genet Metab. 2005;86(suppl 1):S145–S147. doi: 10.1016/j.ymgme.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284:28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011;50:1639–1646. doi: 10.1016/j.freeradbiomed.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol. 2009;47:752–760. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsutsui M, Milstien S, Katusic ZS. Effect of tetrahydrobiopterin on endothelial function in canine middle cerebral arteries. Circ Res. 1996;79:336–342. doi: 10.1161/01.res.79.2.336. [DOI] [PubMed] [Google Scholar]
- 30.Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C, Marinou K, et al. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation. 2010;122:S66–S73. doi: 10.1161/CIRCULATIONAHA.109.927376. [DOI] [PubMed] [Google Scholar]
- 31.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, et al. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124:335–345. doi: 10.1161/CIRCULATIONAHA.110.985150. [DOI] [PMC free article] [PubMed] [Google Scholar]