Abstract
Tetrahydrobiopterin (BH4) is an essential cofactor for endothelial nitric oxide synthase (eNOS) function and NO generation. Augmentation of BH4 levels can prevent eNOS uncoupling and can improve endothelial dysfunction in vascular disease states. However, the physiological requirement for de novo endothelial cell BH4 biosynthesis in eNOS function remains unclear. We generated a novel mouse model with endothelial cell–specific deletion of GCH1, encoding GTP cyclohydrolase 1, an essential enzyme for BH4 biosynthesis, to test the cell-autonomous requirement for endothelial BH4 biosynthesis in vivo. Mice with a floxed GCH1 allele (GCH1fl/fl) were crossed with Tie2cre mice to delete GCH1 in endothelial cells. GCH1fl/flTie2cre mice demonstrated virtually absent endothelial NO bioactivity and significantly greater O2·- production. GCH1fl/flTie2cre aortas and mesenteric arteries had enhanced vasoconstriction to phenylephrine and impaired endothelium-dependent vasodilatations to acetylcholine and SLIGRL. Endothelium-dependent vasodilatations in GCH1fl/flTie2cre aortas were, in part, mediated by eNOS-derived hydrogen peroxide (H2O2), which mediated vasodilatation through soluble guanylate cyclase. Ex vivo supplementation of aortic rings with the BH4 analogue sepiapterin restored normal endothelial function and abolished eNOS-derived H2O2 production in GCH1fl/flTie2cre aortas. GCH1fl/flTie2cre mice had higher systemic blood pressure than wild-type littermates, which was normalized by NOS inhibitor, NG-nitro-L-arginine methyl ester. Taken together, these studies reveal an endothelial cell-autonomous requirement for GCH1 and BH4 in regulation of vascular tone and blood pressure and identify endothelial cell BH4 as a pivotal regulator of NO versus H2O2 as alternative eNOS-derived endothelial-derived relaxing factors.
Keywords: 5,6,7,8-tetrahydrobiopterin; eNOS enzyme; hypertension
Tetrahydrobiopterin (BH4) is an essential cofactor for the nitric oxide synthase (NOS) enzymes.1 When BH4 availability is limiting, NO generation becomes uncoupled from l-arginine oxidation, resulting in superoxide radical (O2·-) rather than NO production.1 Substantial evidence suggests that reduced vascular BH4 bioavailability contributes to the pathogenesis of endothelial dysfunction.1 For example, BH4 levels in vascular tissue from diabetic mice, apolipoprotein E knockout mice hyper-cholesterolemic rabbits, and patients with diabetes mellitus are significantly reduced and associated with reduced NO-mediated endothelial function.2–5 Pharmacological supplementation with the BH4 precursor sepiapterin can improve endothelial function in various vascular disease states.3,6 Biosynthesis of BH4 is catalyzed by GTP cyclohydrolase I (GTPCH), encoded by GCH1. Recent studies have shown that GCH1 expression is a key determinant of endothelial cell BH4 levels and endothelial NOS (eNOS) regulation.7,8 Transgenic overexpression of GCH1 in mice is able to improve endothelial function in vascular disease states, such as pulmonary hypertension,9 diabetes mellitus,10 and atherosclerosis.11 Although these studies implicate reduced or augmented BH4 in vascular disease pathogenesis, the physiological requirement for endothelial BH4 in the regulation of eNOS-derived vascular NO generation, or other eNOS-derived signaling mechanisms, remains unanswered. Furthermore, whether cell-autonomous endothelial cell BH4 synthesis by GTPCH is necessary for physiological eNOS function, as distinct from endothelial cell uptake from plasma or other sources, is important in guiding therapeutic strategies that target eNOS function in vascular disease states, including hypertension.
Previous studies of the importance of BH4 biosynthesis have used systemic pharmacological inhibitors of GTPCH,12,13 or the hph-1 hyperphenylalaninemic mouse, generated by N-ethyl-N-nitrosourea mutagenesis, that has moderate systemic BH4 deficiency because of reduced gene expression from the GCH1 locus.6,9 However, in the hph-1 mouse, global BH4 deficiency has systemic effects in multiple cell types, such as neurotransmitter synthesis and autonomic function,14,15 thus confounding the inter-pretation of endothelial cell BH4 effects on vascular function and blood pressure control by eNOS. We hypothesized that endothelial cell GCH1 expression, and BH4 synthesis, is cell-autonomous requirements for eNOS regulation in vivo. To test this hypothesis, we generated a novel mouse model with endothelial cell–specific deletion of GCH1 and tested the effects on eNOS enzymatic coupling, vasomotor function, and blood pressure regulation.
Methods
For detailed Methods, see online-only Data Supplement.
Generation of Endothelial Cell–Targeted GCH1 Knockout Mice
We generated a novel mouse model of endothelial cell–specific BH4 deficiency, the GCH1fl/flTie2cre mouse. Exons 2 and 3 of GCH1, encoding for the active site of GTPCH, were flanked by loxP sites in a targeting construct that was used to produce GCH1fl/fl mice after homologous recombination in embryonic stem cells (Figure 1A). These mice were crossed with Tie2cre transgenic mice16 to produce GCH1fl/flTie2cre mice where GCH1 is deleted specifically in endothelial cells (Figure 1B), generating an endothelial cell BH4-deficient mouse. Mice were genotyped by polymerase chain reactions using DNA prepared from ear biopsies. Mice were housed in ventilated cages with a 12-hour light/dark cycle and controlled temperature (20–22°C), and fed normal chow and water ad libitum. Male GCH1fl/flTie2cre mice and their GCH1fl/fl littermates were used for all experiments at 12 to 22 weeks. All studies were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 (HMSO, London, United Kingdom).
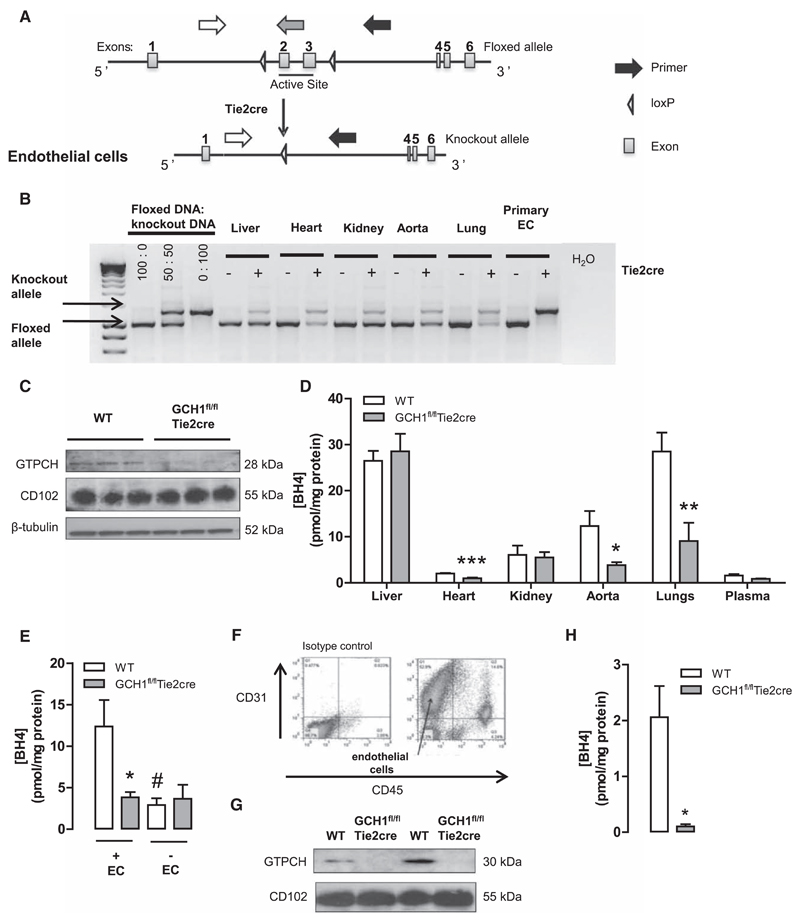
Figure 1. Generation and characterization of GCH1fl/flTie2cre mice.
A, Schematic showing the targeting of the mouse GCH1 locus with loxP sites flanking the exons (2 and 3) encoding the active site of the GTP cyclohydrolase I (GTPCH) protein. Arrows show polymerase chain reaction primers for the GCH1 floxed and Tie2cre-excised allele, GCH1 floxed allele (white and grey arrows), and deleted allele (white and black arrows). B, Evaluation of Tie2cre-mediated excision of the loxP flanked DNA in tissues and primary cells derived from GCH1fl/flTie2cre and GCH1fl/fl (wild-type [WT]) mice. The predicted 1030-bp product was detected in WT mice. In the presence of the Tie2cre transgene a 1392 bp knockout allele was detected, with efficient excision in primary endothelial cells (ECs). C, Representative Western blots for GTPCH proteins in aortas from WT and GCH1fl/flTie2cre mice. CD102 protein expression was used as endothelium-specific marker, and β-tubulin was used as loading control. D, BH4 levels were reduced in EC-rich tissues, such as heart, lung, and aorta (*P<0.05, **P<0.01, and ***P<0.001; n>6 animals per group). E, Contribution of endothelium to vascular BH4 in mouse aortas. Vascular BH4 levels were significantly decreased in WT denuded aortas but not in GCH1fl/flTie2cre denuded aortas (*P<0.05 comparing genotype, #P<0.05 comparing treatment; n=5–6 animals per group). F, Flow cytometry data of primary ECs costained with anti-CD45 and anti-CD31 antibody to show CD31+ and CD45– ECs. G, Representative immunoblot of GTPCH levels in primary ECs from WT and GCH1fl/flTie2cre mice. H, BH4 levels in primary ECs from GCH1fl/flTie2cre and WT mice (*P<0.05; n=4 animals per group).
Results
Endothelial Cell–Targeted GCH1 Deletion Reveals a Cell-Autonomous Requirement for Endothelial Cell BH4 Biosynthesis
We generated matched litters of GCH1fl/flTie2cre and GCH1fl/fl mice (hereafter referred to as wild type) by crossing male GCH1fl/flTie2cre and female GCH1fl/fl mice. Body weights between the groups were similar (30.9±1.13 g in wild-type and 30.8±1.31 g in GCH1fl/flTie2cre; n>10 per group). Genomic polymerase chain reaction demonstrated efficient excision of the floxed GCH1 allele in isolated primary endothelial cells from GCH1fl/flTie2cre mice (Figure 1B).
Western blot analysis confirmed that GTPCH protein was significantly reduced in aortas from GCH1fl/flTie2cre mice when compared with that from wild-type controls (P<0.01; Figure 1C). In GCH1fl/flTie2cre mice, BH4 levels in endothelial-rich tissues, such as lung, heart, and aorta, were significantly reduced when compared with wild-type controls (Figure 1D). Furthermore, BH4 levels were reduced by ≈75% in endothelial-denuded aortas from wild-type mice. In contrast, removal of endothelium in GCH1fl/flTie2cre aortas did not significantly reduce BH4 levels (P<0.05; Figure 1E). In non–endothelial cell-rich tissues, such as liver and kidney, there was no difference in BH4 levels between wild-type and GCH1fl/fl Tie2cre mice. Importantly, plasma BH4 levels were similar between the groups, indicating that endothelial cell BH4 biosynthesis by GTPCH does not contribute significantly to plasma BH4 levels. Despite marked BH4 deficiency, absolute BH2 levels in aortas, lung, and heart were comparable between the genotypes, such that the BH4/BH2 and biopterin ratio was significantly reduced in aortas heart and lung in GCH1fl/fl Tie2cre mice (Figure S1 in the online-only Data Supplement).
To test the endothelial cell specificity of GCH1 deletion further, and the effects of endothelial cell loss of BH4 biosynthesis on BH4 levels, we isolated primary mouse endothelial cells using immunomagnetic bead selection. Primary endothelial cells were confirmed as CD31+ CD45– by flow cytometry (Figure 1F). In endothelial cells from GCH1fl/flTie2cre mice, GTPCH protein was not detectable by Western blotting, whereas GTPCH was readily detected in wild-type endothelial cells, and the endothelial cell-surface marker CD102 was present equally in endothelial cells from both GCH1fl/flTie2cre and wild-type mice (Figure 1G). Measurement of biopterins by high-performance liquid chromatography revealed barely detectable levels of BH4 in primary endothelial cells from GCH1fl/flTie2cre mice (Figure 1H). Taken together, these results demonstrate endothelial cell–specific GCH1 knockout in GCH1fl/flTie2cre mice, leading to endothelial cell–specific BH4 deficiency. Furthermore, the finding of marked BH4 deficiency in GCH1fl/flTie2cre endothelial cells, despite normal circulating plasma levels, reveals a cell-autonomous requirement for de novo BH4 synthesis in maintenance of endothelial cell BH4 levels.
Endothelial Cell BH4 Deficiency Leads to eNOS Uncoupling With Increased Superoxide Production and Loss of Endothelial NO Generation
We next determined the effects of endothelial cell–specific BH4 deficiency on eNOS function. We first measured basal O2·- and other reactive oxygen species (ROS) production in isolated primary endothelial cells by quantification of 2-hydroxyethidium and ethidium production from dihydroethidine, using high-performance liquid chromatography. Both 2-hydroxyethidium and ethidium production were significantly elevated in primary endothelial cells from GCH1fl/fl Tie2cre mice when compared with wild-type controls (P<0.05; Figure 2A and 2B). Furthermore, there was a significant inhibition of 2-hydroxyethidium and ethidium production in GCH1fl/flTie2cre mice by the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME) (100 μmol/L), whereas no effect was observed in wild-type endothelial cells, suggesting that eNOS is a source of O2·- production in GCH1fl/flTie2cre endothelial cells.
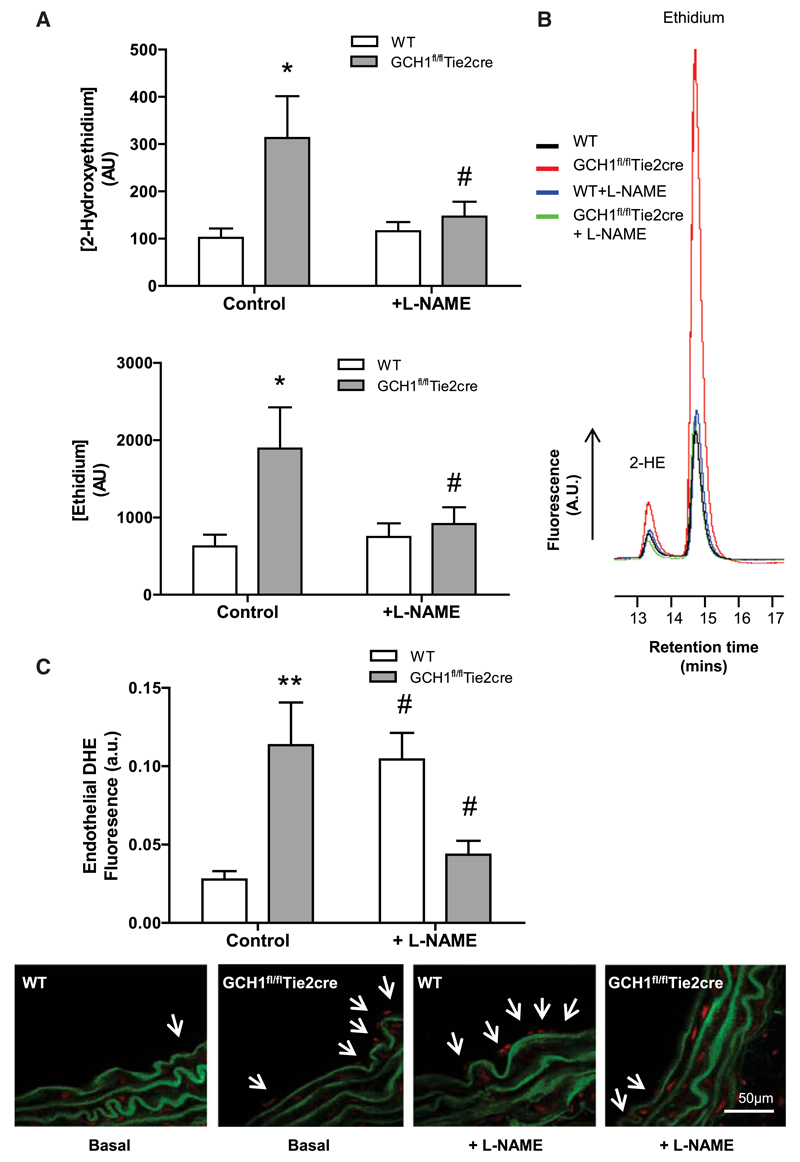
Figure 2. Quantification of superoxide production in GCH1fl/flTie2cre and wild-type (WT) mice.
A, Superoxide and other reactive oxygen species (ROS) productions detected by dihydroethidine (DHE) high-performance liquid chromatography (HPLC). Superoxide and other ROS productions as measured by 2-hydroxyethidium (2-HE) and ethidium, respectively, in primary endothelial cells isolated from GCH1fl/flTie2cre and WT mice. Preincubation of endothelial cells with NG-nitro-L-arginine methyl ester (L-NAME) inhibited the 2-HE and ethidium peaks in both WT and GCH1fl/flTie2cre endothelial cells (*P<0.05 comparing genotype, #P<0.05 comparing treatment; n=6–7 animals per group). B, Representative trances of 2-HE and ethidium peaks in primary endothelial cells isolated from WT and GCH1fl/flTie2cre mice detected by DHE HPLC in the presence and absence of L-NAME. C, Endothelium-derived superoxide production was measured by dihydroethidium staining in aortic sections from WT and GCH1fl/flTie2cre mice. Endothelium-derived superoxide, quantified in arbitrary units as area of luminal red staining/length of luminal surface, in the absence and presence of 100 μmol/L L-NAME (**P<0.01 comparing genotype, #P<0.05 comparing treatment; n=6 animals per group).
We determined the spatial distribution of ROS production in aortic tissue sections using dihydroxyethidium fluorescence microtopography. Under basal conditions, GCH1fl/flTie2cre aortas generated 4-fold more endothelium-derived ROS production than wild-type controls (P<0.01; Figure 2C). In the presence of L-NAME, the level of endothelium-derived ROS production in wild-type aortas was significantly increased when compared with untreated wild-type aortas, suggesting a tonic scavenging effect of NO on O2·- (P<0.05). In contrast, endothelial ROS production in GCH1fl/flTie2cre aortas in the presence of L-NAME was significantlty decreased when compared with untreated GCH1fl/flTie2cre aortas (P<0.05), indicating a major contribution from eNOS-derived ROS.
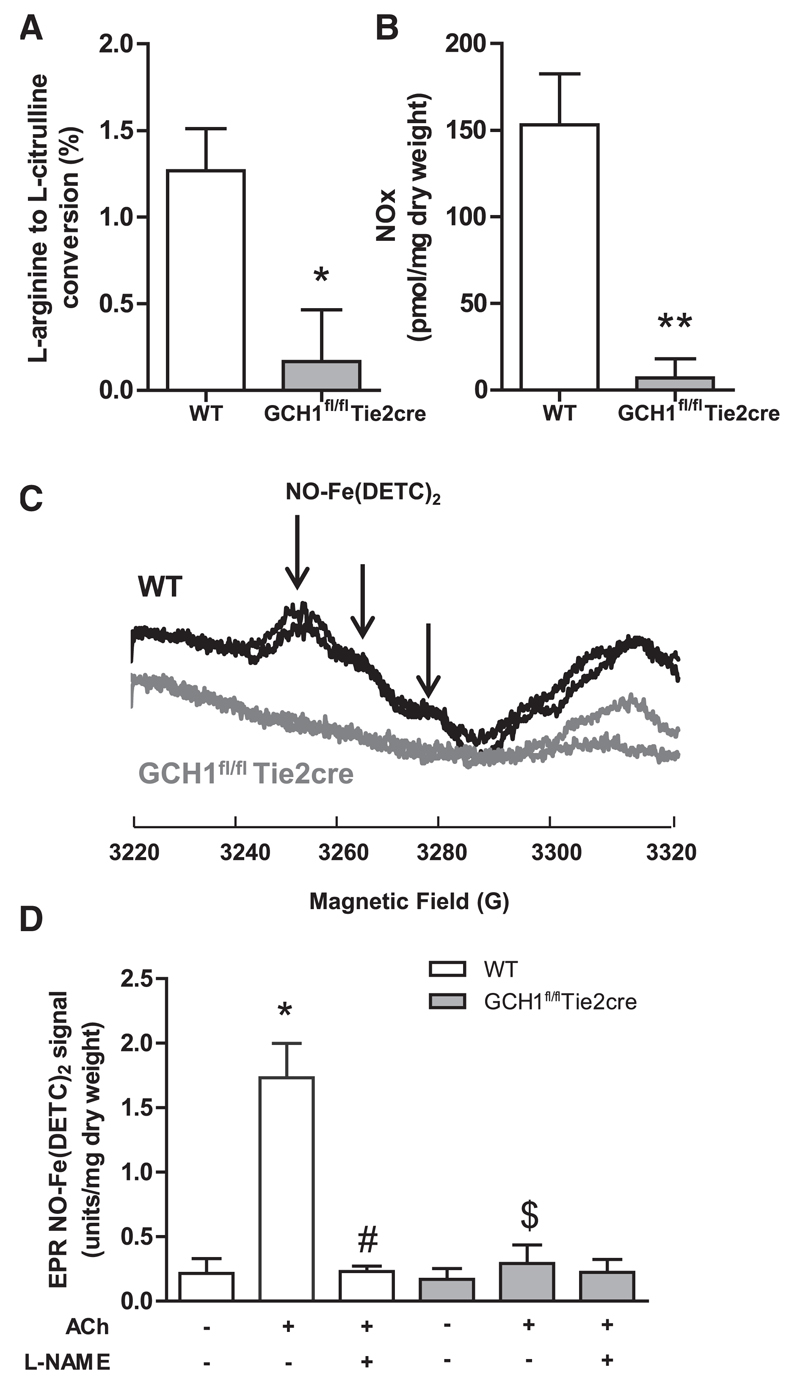
To determine the effects of endothelial cell BH4 deficiency on NO bioactivity, we measured NO bioactivity by 3 different methods. First, we measured l-arginine to l-citrulline conversion using high-performance liquid chromatography with online scintillation detection, to enhance sensitivity and specificity of citrulline detection. We found that eNOS activity was significantly decreased in endothelial cells from GCH1fl/flTie2cre mice when compared with that from wild-type littermates (P<0.05; Figure 3A). Second, nitrite and nitrate production by aortas was also reduced in GCH1fl/flTie2cre mice when compared with that in wild-type controls, to levels that were barely detectable above baseline (P<0.05; Figure 3B). Finally, we measured specific NO generation from aortas using EPR detection of NO using the spin trap colloid Fe(DETC)2. The characteristic NO-Fe(DETC)2 EPR triplet was increased (≈6-fold) by stimulation with acetylcholine in wild-type mice, and abolished by incubation with L-NAME, but was not detectable in GCH1fl/flTie2cre mice aortas (Figure 3C and 3D).
Figure 3. NO bioactivity in GCH1fl/flTie2cre and wild-type (WT) mice.
A, Conversion of 14C arginine to 14C citrulline was used as a measure of endothelial nitric oxide synthase activity (eNOS). eNOS activity (NG-methyl-L-arginine [L-NMA] inhibitable) was greatly reduced in primary endothelial cells isolated from GCH1fl/flTie2cre mice when compared with that from the WT controls (*P<0.05; n=4 per group). B, Nitrite/nitrate production in isolated fresh aorta stimulated with 1 μmol/L acetylcholine (ACh) for 30 minutes. Nitrite/nitrate production (L-NMA inhibitable) in stimulated aortas from GCH1fl/flTie2cre mice was significantly decreased when compared with that from WT controls (**P<0.01; n=7–9 animals per group). C, Representative EPR spectra of mouse aortas stimulated with 1 μmol/L ACh for 90 minutes at 37°C with colloid Fe(DETC)2 (g values ≈2.04). The characteristic triplet peaks associated with NO-Fe(DETC)2 signal are shown by the vertical arrows. D, Quantification of NO-Fe(DETC)2 signal from GCH1fl/fl Tie2cre and WT aortas with or without stimulated with 1 μmol/L ACh in the presence and absence of 1 mmol/L NG-nitro-L-arginine methyl ester (L-NAME) (*P<0.01 comparing ACh treatment in WT, #P<0.01 comparing L-NAME treatment in ACh WT, $P<0.01 comparing genotype; n=4–6 animals per group).
Taken together, these data demonstrate that endothelial cell GCH1 deletion and BH4 deficiency dramatically reduce vascular NO bioactivity.
Endothelial Cell–Specific BH4 Deficiency Leads to Enhanced Vasoconstriction and Impaired Vasodilatation
Next, we investigated the requirement for endothelial cell-specific BH4 synthesis in normal vascular function. Isometric tension studies in isolated aortas demonstrated that the maximal vasoconstriction to 60 mmol/L KCl was comparable between genotypes (6.78±0.27 mN in wild-type and 6.34±0.29 mN in GCH1fl/flTie2cre). However, vasoconstriction to phenylephrine was significantly enhanced in GCH1fl/flTie2cre aortas when compared with that in wild-type aortas (P<0.05; Figure 4A). This difference was normalized in the presence of L-NAME, indicating that the increased constrictor response in GCH1fl/flTie2cre aortas is mediated by tonic eNOS-derived vasodilatation.
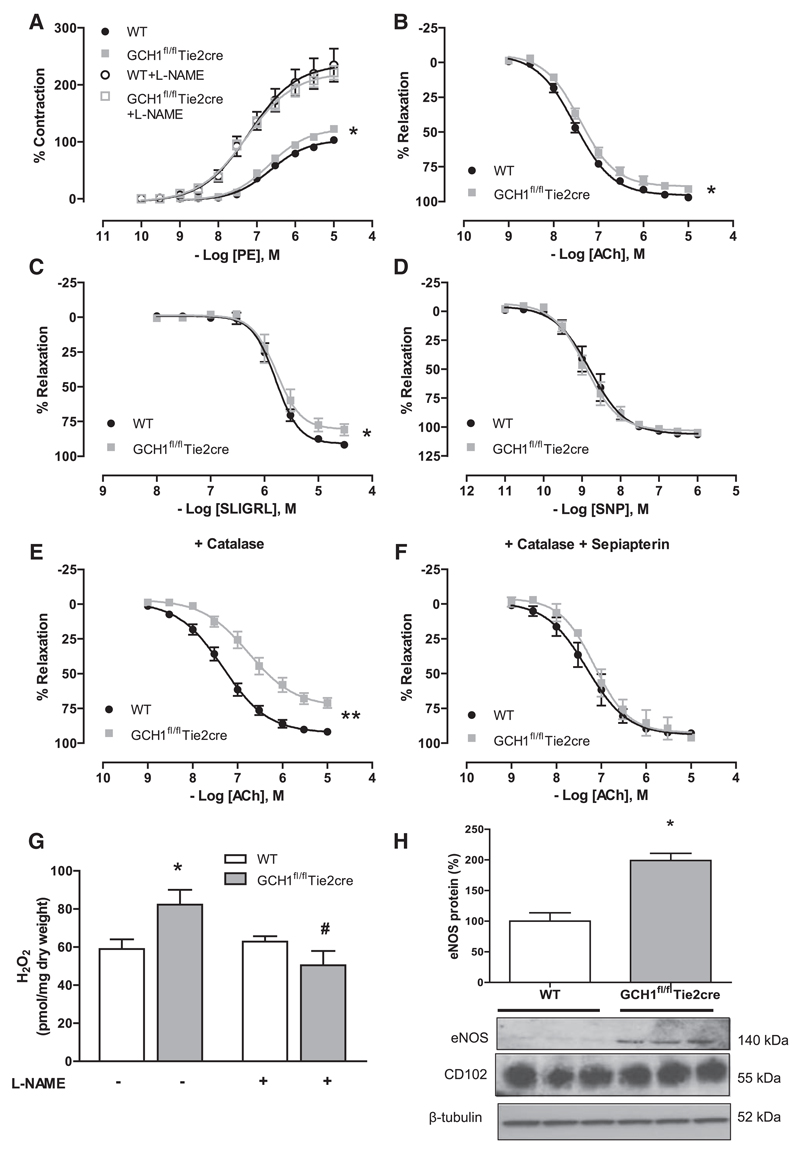
Figure 4. Vasomotor functions in isolated aortas from GCH1fl/flTie2cre and wild-type (WT) littermates.
A, Vasoconstriction to phenylephrine (PE) was enhanced in GCH1fl/flTie2cre aortas when compared with wild-type (WT) aortas (*P<0.05; n=13–15 animals per group). The difference was normalized in the presence of 100 μmol/L NG-nitro-L-arginine methyl ester (L-NAME), which yielded a significantly greater EC50 and Emax to that of untreated GCH1fl/flTie2cre and WT aortas. B, Endothelial-dependent vasodilatation to acetylcholine (ACh; *P<0.01; n=13–15 animals per group) and (C) SLIGRL in GCH1fl/flTie2cre aortas and WT aortas. D, Endothelium-independent vasodilatation in response to sodium nitroprusside (SNP). E, Endothelial-dependent vasodilatation to ACh in the presence of polyethylene glycol (PEG)-catalase (400 U/mL; **P<0.001; n=8–10 animals per group) and F, Sepiapterin (10 μmol/L) with PEG-catalase (*P<0.01; n=4–6 animals per group). G, H2O2 production in aortas from GCH1fl/flTie2cre mice and WT mice was determined by Amplex red assay in the presence of 1 μmol/L ACh with or without treatment of 1 mmol/L L-NAME (*P<0.05 comparing genotype, #P<0.05 comparing between treatment; n=4–6 animals per group). H, Representative immunoblots with corresponding quantitative data above showing endothelial nitric oxide synthase protein in WT and GCH1fl/flTie2cre aortas. Corresponding immunoblots for CD102 (endothelial cell marker) and β-tubulin (*P<0.01; n=6 animals per group).
Endothelial-dependent vasodilatation to acetylcholine was modestly impaired but statistically significant in GCH1fl/flTie2cre aortas when compared with that in wild-type aortas (P<0.01; Figure 4B). Endothelium-dependent vasodilatation in response to the protease activated receptor 2 agonist, SLIGRL (Figure 4C), was also impaired in GCH1fl/flTie2cre aortas, suggesting that the impaired vasorelaxation was not because of specific alteration of receptor signaling on endothelial cells of GCH1fl/flTie2cre mice. Endothelium-dependent vasodilatation to acetylcholine in both wild-type and GCH1fl/flTie2cre aortas was abolished in the presence of L-NAME (Figure S2A), indicating that eNOS is the major source of vasodilators in mouse aortas. Endothelium-independent vasodilatation in response to sodium nitroprusside was similar between the groups (Figure 4D).
Endothelial BH4 Deficiency Results in H2O2-Mediated Vasodilatation
We next investigated the mechanisms underlying the vasodilator responses in GCH1fl/flTie2cre aortas, given the loss of NO generation and increased ROS production. In the presence of the H2O2 scavenger, catalase-polyethylene glycol (PEG-catalase; 400 U/mL), endothelial-dependent vasodilatations to acetylcholine were significantly inhibited in GCH1fl/flTie2cre aortas but not in wild-type aortas (Figure 4E). PEG-catalase treatment had no effect on the basal vascular tone of either GCH1fl/flTie2cre or wild-type aortas. In the presence of L-NAME, vasodilatation to acetylcholine was abolished in both groups, and further addition of PEG-catalase did not alter the response in either group (Figure S2A and S2B), suggesting that eNOS is a predominant source of H2O2. Indeed, direct quantification of H2O2 in stimulated aortic rings with acetylcholine, using the Amplex red assay, revealed increased vascular H2O2 production in GCH1fl/flTie2cre aortas, which was inhibited in the presence of L-NAME (Figure 4G). We next investigated whether H2O2 production might lead to changes in eNOS or antioxidant protein expression. In aortas, the protein level of eNOS in GCH1fl/flTie2cre mice was significantly increased (≈2-fold) than that of wild-type controls (P<0.001; Figure 4H). However, the protein levels of antioxidant enzymes, catalase, Mn superoxide dismutase (SOD), extracellular SOD, and Cu/ZnSOD, were similar between the groups (Figure S3A–S3D). Furthermore, the level of phosphorylation of eNOS at Thr495, relative to the total eNOS protein content, was comparable between the groups. Phosphorylation of eNOS at Ser1177 was significantly reduced in GCH1fl/flTie2cre aortas when indexed to the total eNOS protein content (Figure S4).
To test the specificity of BH4 deficiency, we rescued BH4 levels using sepiapterin that augments BH4 levels via the salvage pathway, independent of de novo BH4 biosynthesis by GTPCH. Ex vivo incubation of GCH1fl/flTie2cre aortas with sepiapterin (10 μmol/L) for 30 minutes restored endothelial vasomotor responses and abolished the effect of PEG-catalase on vasodilatation in GCH1fl/flTie2cre aortas (Figure 4F).
Mechanism of H2O2-Mediated Vasodilatation in GCH1fl/flTie2cre Aortas
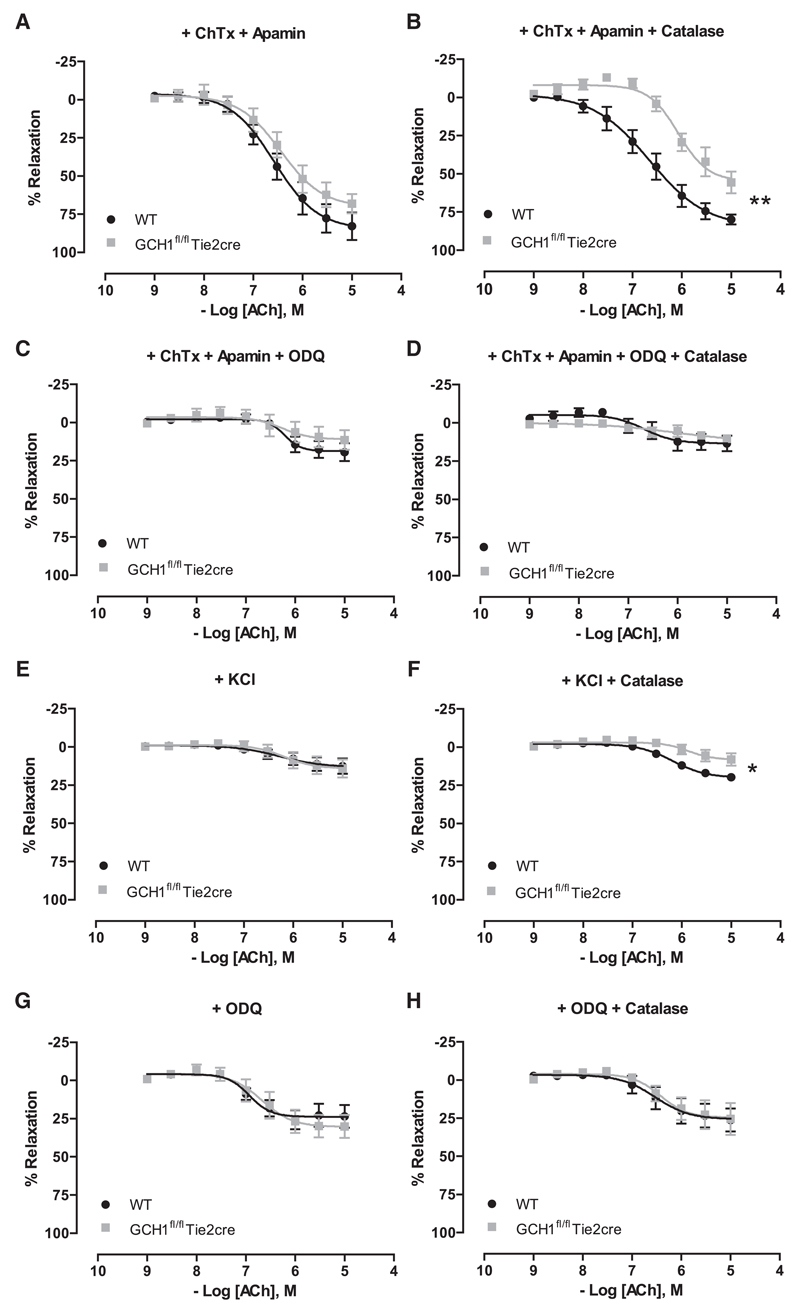
We next investigated the mechanism underlying eNOS-derived H2O2-mediated vasodilatation in GCH1fl/flTie2cre aortas. A combination of potassium channels blockers, apamin (small-conductance Ca2+-activated K+ channel blocker) and charybdotoxin (nonselective intermediate and large-conductance Ca2+-activated K+ channels blocker), was used to test K+ channel–mediated endothelium-derived hyperpolarizing factor responses.17,18 Endothelium-dependent vasodilatation to acetylcholine in GCH1fl/flTie2cre aortas in the presence of apamin and charybdotoxin was similar to wild-type controls but was significantly inhibited in the presence of apamin, charybdotoxin with PEG-catalase (Figure 5A and 5B). This difference was normalized in the presence of the soluble guanylate cyclase (sGC) inhibitor, 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one, 1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (Figure 5C and 5D), indicating the involvement of a sGC-dependent pathway. To test the specificity of this finding, vasodilatation to acetylcholine was assessed in the presence of 30 mmol/L extracellular K+, to inhibit the activity of K+ channels and thus endothelium-derived hyperpolarizing factor responses.18 In the presence of 30 mmol/L extracellular K+, vasodilatation to acetylcholine in GCH1fl/flTie2cre aortas was similar to wild-type aortas but remained significantly inhibited by PEG-catalase (Figure 5E and 5F). In the presence of ODQ, vasodilatation to acetylcholine in GCH1fl/flTie2cre aortas was inhibited to a similar extent as that observed in the presence of ODQ with PEG-catalase (Figure 5G and 5H). These findings indicate that eNOS-derived, H2O2-mediated vasodilatation in GCH1fl/flTie2cre aortas occurs through a sGC-dependent signaling pathway and not through the activation of endothelium-derived hyperpolarizing factor-sensitive components. Consistent with this finding, the levels of Ser239 phospho-vasodilator-stimulated phosphoprotein (VASP) were measured as readout of protein kinase G (PKG) activity. There was no significant difference in Ser239 phospho-VASP to total VASP protein between wild-type and GCH1fl/flTie2cre aortas, which suggests that H2O2-derived from eNOS uncoupling in GCH1fl/flTie2cre aortas mediated vasodilatation by increasing phosphorylation of VASP at Ser239 (Figure S4C). Responses to exogenous H2O2-induced vasodilatation in wild-type and GCH1fl/flTie2cre aortas were similar and were inhibited in the presence of ODQ and abolished in the presence of ODQ and PEG-catalase (Figure S2C and S2D). The combination of apamin and charybdotoxin had little effect on H2O2-induced vasodilatation (Figure S2E).
Figure 5. Mechanism of H2O2-mediated vasodilatation in GCH1fl/flTie2cre aortas.
Endothelium-dependent vasodilatation to acetylcholine (ACh) was assessed in the presence of the following agents: A, charybdotoxin (ChTx) and apamin, (B) ChTX, apamin, and polyethylene glycol (PEG)-catalase, (C) ChTX, apamin, and 1H-(1,2,4)oxadiazolo[4,3-a] quinoxalin-1-one (ODQ), (D) ChTX, apamin, ODQ, and PEG-catalase, (E) KCl, (F) KCl and PEG-catalase, (G) ODQ, and (H) ODQ and PEG-catalase (*P<0.05, **P<0.01 comparing log EC50; n=6–9 animals per group).
Impaired Vascular Function in Small Mesenteric Artery in GCH1fl/flTie2cre Mice
We next investigated the requirement for endothelial cell BH4 in vasomotor function in resistance vasculature. Using second-order mesenteric arteries in wire myograph studies, we found increased vasoconstrictions in GCH1fl/flTie2cre mice in response to U46619 (thromboxane A2 receptor agonist) and phenylephrine (data not shown) when compared with wild-type controls. This difference was normalized in the presence of L-NAME (Figure S5A). Furthermore, endothelium-dependent vasodilatations to acetylcholine and SLIGRL were significantly impaired in mesenteric arteries from GCH1fl/flTie2cre mice (Figure S5B and S5C), whereas endothelium-independent vasodilatations to sodium nitroprusside were not altered (Figure S5D). In mesenteric vessels, the protein levels of antioxidant enzymes were comparable between GCH1fl/flTie2cre and wild-type controls (Figure S3E–S3H).
Endothelial Cell–Specific GCH1 Deletion and BH4 Deficiency Increase Arterial Blood Pressure
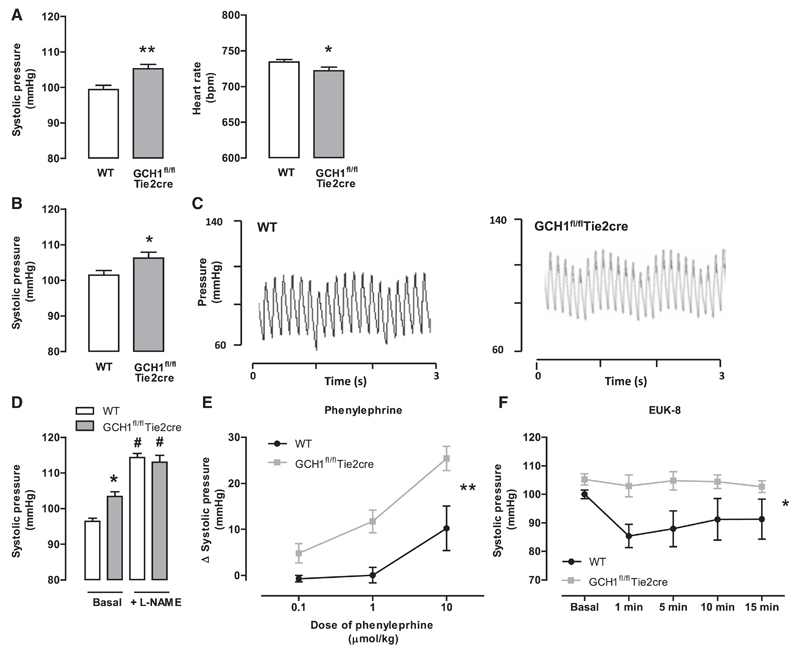
Given the striking changes in eNOS coupling and vasomotor function in GCH1fl/flTie2cre mice, we next determined the requirement for endothelial cell BH4 synthesis in blood pressure regulation, by measuring arterial blood pressure in conscious mice using tail-cuff plethysmography. GCH1fl/flTie2cre mice had significantly higher systolic blood pressure than wild-type littermates (P<0.001; 103.4±1.3 mm Hg in GCH1fl/flTie2cre versus 96.4±0.8 mm Hg in wild type). Heart rate was significantly lower in GCH1fl/flTie2cre mice when compared with that in wild-type littermates (Figure 6A). In a further cohort of mice, we measure blood pressure invasively using a Millar catheter. We again found a greater systolic blood pressure in GCH1fl/flTie2cre mice when compared with that in wild-type littermates (Figure 6B and 6C). To test the dependence of these blood pressure changes on NOS, we treated mice with L-NAME, added in the drinking water, for 7 days. L-NAME treatment increased blood pressure to a greater extent in GCH1fl/flTie2cre mice, such that blood pressures were no longer different between GCH1fl/flTie2cre mice (113.4±1.8 mm Hg) and wild-type (114.3±1.1 mm Hg), suggesting that increased blood pressure in GCH1fl/flTie2cre mice is NOS mediated (Figure 6D).
Figure 6. Hemodynamic response in GCH1fl/flTie2cre and wild-type (WT) littermates.
A, Systolic blood pressure and heart rate measured by tail-cuff method (*P<0.05, **P<0.001; n=36–38 animals per group). B, Systolic blood pressure measured in anaesthetized mice using a Millar catheter (*P<0.05; n=14–18 animals per group). C, Representative traces of systolic blood pressure in WT (left) and GCH1fl/flTie2cre (right) mice using a Millar catheter. D, Systolic blood pressure before and after treatment of 1 mg/mL NG-nitro-L-arginine methyl ester (L-NAME) in drinking water for 7 days (*P<0.05 comparing genotypes, #P<0.05 comparing treatment; n=6 animals per group). E, The change in systolic blood pressure, measured using a Millar catheter, after doses of phenylephrine administrations (**P<0.01; n=6 animals per group). F, Systolic blood pressure, measured using a Millar catheter, after a single dose of EUK-8 (25 mg/kg IP) administration (*P<0.05; n=7–9 animals per group).
We next measured changes in arterial blood pressure in response to the vasoconstrictor phenylephrine in anaesthetized mice, using a Millar catheter. Phenylephrine caused a significantly greater increase in systolic blood pressure in GCH1fl/flTie2cre mice when compared with that in wild-type controls (Figure 6E). To investigate the role of ROS from uncoupled eNOS on hemodynamic response in vivo, we administered the salen-manganese–based SOD/catalase mimetic, EUK-8 (25 mg/kg IP) in anaesthetized mice. EUK-8 significantly reduced systolic blood pressure in wild-type mice, whereas blood pressure was unaltered in GCH1fl/flTie2cre mice (Figure 6F).
Discussion
We have created a novel mouse model of endothelial cell– targeted GCH1 deletion to test the endothelial cell–specific requirements for BH4 in vascular function and hemodynamic regulation. This novel mouse model reveals several new and important findings. First, endothelial cell–targeted GCH1 deletion leads to endothelial cell BH4 deficiency, despite no significant change in plasma BH4, thus identifying a cell-autonomous requirement for endothelial cell GCH1 and de novo BH4 biosynthesis. Second, endothelial cell–specific BH4 deficiency leads to eNOS uncoupling, with loss of NO bioactivity and increased O2·- production, resulting in vascular dysfunction with eNOS-dependent vasorelaxation mediated by eNOS-derived H2O2, acting through the sGC-dependent signaling pathway. Third, the endothelial cell–specific BH4 deficiency resulting from GCH1 deletion leads to hypertension. Taken together, these studies reveal an endothelial cell-autonomous requirement for GCH1 and BH4 in regulation of vascular tone and blood pressure and identify endothelial cell BH4 as a pivotal regulator of NO versus H2O2 as alternative eNOS-derived endothelium-derived relaxing factors.
We have shown that endothelial cell–targeted GCH1 deletion abolishes GTPCH protein expression and de novo BH4 synthesis in primary endothelial cells and leads to substantial reductions in GTPCH and BH4 in aortas, demonstrating that the majority of vascular BH4 is contributed by the endothelium and confirmed by removal of vascular endothelium that reduced BH4 levels in wild-type but not in GCH1fl/flTie2cre aortas. Crucially, there was no difference in plasma BH4 levels between wild-type and GCH1fl/flTie2cre mice, revealing for the first time that the vascular endothelium is not a significant source of circulating biopterins. Furthermore, the maintenance of plasma BH4 levels in GCH1fl/flTie2cre mice was not sufficient to rescue the loss of de novo BH4 synthesis in endothelial cells. This novel observation identifies a cell-autonomous requirement for de novo BH4 synthesis in the maintenance of endothelial cell BH4 levels in vivo. This finding suggests that physiological regulation of endothelial cell BH4 is not influenced by uptake from plasma, despite previous observations that pharmacological administration of high doses of BH4 can augment BH4 levels in some tissues. Although Tie2cre may lead to cre-mediated gene deletion in hematopoietic cells, the persistence of endothelial cell BH4 deficiency, despite normal plasma BH4, argues against other nonselective effects of GCH1 deletion.
The physiological requirement for endothelial cell BH4 biosynthesis is reflected in the effect on eNOS function.19 We have previously shown that BH4 bioavailability is a determinant of eNOS function and NO production,7,8 and loss of BH4 by oxidation leads to eNOS uncoupling and vascular dysfunction in hypertension.3 However, the physiological requirement for endothelial cell BH4 in these settings remains unclear because loss of vascular BH4 could be a cause of endothelial dysfunction or could be the consequence of vascular disease. We have now shown that deficiency in endothelial cell BH4 is alone sufficient to cause eNOS uncoupling, even in the absence of vascular diseases and in the presence of normal plasma BH4 levels.
The absolute dependence of endothelial NO production on endothelial cell BH4 availability was demonstrated by 3 complementary methods, each indicating deficient, or absent endothelial NO bioactivity in GCH1fl/flTie2cre mice when compared with that in wild-type littermates. These include radiolabeled l-arginine to l-citrulline conversion using high-performance liquid chromatography detection of l-citrulline, nitrite/nitrate accumulation, and EPR detection of NO using Fe(DETC)2 as spin trap. Despite this clear evidence of loss of endothelial NO generation in GCH1fl/flTie2cre mice, endothelium-dependent vasodilatation to acetylcholine was only minimally impaired, suggesting that other eNOS-derived vasodilators are able to mediate vasodilatation when BH4 is limiting. We found that acetylcholine-induced vasodilatation in GCH1fl/flTie2cre aortas is mediated by H2O2 because PEG-catalase substantially inhibited vasodilatation in GCH1fl/flTie2cre aortas and increased vascular H2O2 production was quantified in GCH1fl/flTie2cre aortas that was inhibited by L-NAME. These observations are consistent with the studies in aortas of mice with deoxycorticosterone acetate-salt hypertension3 and in hph-1 mice with moderate systemic BH4 deficiency6 although our new findings in GCH1fl/flTie2cre mice overcome the lack of cell specificity in these previous models, and the potentially confounding effects of systemic changes, such as effects of BH4 deficiency on heart rate and sympathetic function in the hph-1 mouse.14
Emerging evidence suggests that H2O2 can mediate endothelium-dependent and endothelium-independent vasodilatation in several vascular beds in multiple species, including humans.20–22 H2O2 could mediate vasodilatation by endothelium-dependent smooth muscle hyperpolarization and vasodilatation,17 or by activating sGC, leading to increased cGMP, or by directly activating PKG, and vasodilatation.23–25 The mechanisms of H2O2-induced vasodilatation are varied depending on vascular beds and species. Our data suggest that eNOS-derived H2O2 production from uncoupled eNOS in GCH1fl/flTie2cre aortas induces vasodilatation through sGC/PKG rather than through an endothelium-derived hyperpolarizing factor–independent pathway, as evidenced by the lack of inhibition by apamin and charybdotoxin,18 or by 30 mmol/L extracellular K+, to inhibit the activity of Ca2+-activated K+ channels. In contrast, the sGC inhibitor, ODQ, inhibited vasodilatation to acetylcholine in GCH1fl/flTie2cre aortas to a similar extent as that observed in the presence of ODQ with PEG-catalase.
Previous evidence suggests that H2O2 can induce eNOS expression.3,26,27 Indeed, we found significantly greater eNOS protein in GCH1fl/flTie2cre aortas, despite impaired vascular responses. These observations are consistent with reduced H2O2 production in Nox4−/− mice, which reduced eNOS expression by 50%.28 An alternative explanation for increased endothelial-derived O2·- production may be a decrease in the protein levels of antioxidant enzymes (catalase, MnSOD, Cu/ZnSOD, and extracellular SOD). However, we found that the levels of these antioxidant enzymes were identical in both aortas and mesenteric vessels of GCH1fl/flTie2cre and wild-type mice. Thus, the GCH1fl/flTie2cre mouse reveals a new role for physiological BH4 levels in regulating eNOS-derived H2O2 and in turn eNOS expression.
Although H2O2 becomes a physiological mediator of eNOS-mediated vasorelaxations in the absence of BH4, this is not sufficient to compensate for loss of NO fully. Isometric tension studies in aortas and mesenteric arteries revealed increased responsiveness to phenylephrine that were equalized in the presence of L-NAME. Correspondingly, systolic blood pressure, measured both in conscious mice by tail-cuff plethysmography and in unconscious mice by Millar catheter, was higher in GCH1fl/flTie2cre mice but was equalized by L-NAME treatment in drinking water. Furthermore, administration of phenylephrine under anesthesia caused a greater increase in systolic blood pressure in GCH1fl/flTie2cre mice when compared with that in wild-type controls.
Acute administration of the SOD mimetic EUK-8 in anesthetized mice decreased blood pressure in wild-type mice but had little effect in GCH1fl/flTie2cre mice. EUK-8 may act to reduce blood pressure in wild-type mice, where BH4 bioavailability is not limited, by scavenging basal ROS production, which in turn increases NO bioavailability. In contrast, GCH1fl/flTie2cre have little endothelial NO bioactivity and vasorelaxations that are already dependent on eNOS-derived H2O2, such that the SOD mimetic drug alone has no significant effect on blood pressure.
To test the specificity of the observed phenotype for biochemical BH4 deficiency, we performed a rescue experiment using a BH4 precursor, sepiapterin. Ex vivo supplementation of GCH1fl/flTie2cre aortas with sepiapterin restored endothelial function, abolishing eNOS-derived H2O2-mediated vasodilatation. This result is consistent with previous studies, demonstrating that supplementation with sepiapterin in hph-1 mice,6 hypertensive mice,3 chronic smokers,29 and patients with diabetes mellitus30 improved NO bioavailability and restored endothelial function. Previous studies of high-level pharmacological supplementation of exogenous BH4 have been criticized for nonspecific antioxidant effects. However, the present studies suggest that exogenous supplementation of BH4, using sepiapterin, can augment endothelial cell BH4 levels, at least in healthy animals. Targeted cell-specific mouse models of GCH1 deletion will be useful in future studies to test the effectiveness of in vivo strategies to restore or to supplement cellular BH4 levels.
In summary, we describe for the first time that selective deficiency in endothelial cell BH4 biosynthesis, by targeted GCH1 deletion, is alone sufficient to cause eNOS uncoupling, increased O2·- production and reduced NO bioavailability, even in the absence of vascular disease and in the presence of normal plasma and systemic BH4 levels. This endothelial cell-autonomous requirement for BH4 biosynthesis is pivotal in maintaining eNOS-mediated NO versus H2O2 signaling in vascular physiology and regulates systemic arterial blood pressure. The cell-autonomous requirement for endothelial cell BH4 has important implications for vascular disease states and therapeutic strategies aimed at augmenting vascular BH4.
Perspectives
The loss of BH4 in vascular wall is observed in patients and experimental models of hypertension and other vascular diseases, resulting in loss of NO and increased ROS production. Augmenting BH4 levels can prevent eNOS uncoupling and can improve endothelial dysfunction in vascular disease states. The present study now reveals that deficiency in endothelial cell BH4 biosynthesis is alone sufficient to cause eNOS uncoupling, endothelial dysfunction, and hypertension. Furthermore, normal plasma BH4 levels are not sufficient to rescue the loss of de novo BH4 biosynthesis in endothelial cells, revealing a cell-autonomous requirement for endothelial cell BH4 synthesis in the maintenance of BH4 levels in vivo. These findings suggest that targeting endothelial cell BH4 biosynthesis rather than systemic BH4 levels may be more relevant to the development and treatment of vascular diseases.
Supplementary Material
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.114.03089/-/DC1.
Novelty and Significance.
What Is New?
Endothelial cell–targeted GCH1 deletion leads to endothelial cell BH4 deficiency, despite no alteration in plasma BH4, revealing a cell-autonomous requirement for endothelial cell GCH1 and de novo BH4 biosynthesis in vivo.
Endothelial cell BH4 plays a pivotal role in maintaining normal vascular function and in determining the formation of nitric oxide (NO) versus H2O2 as alternative endothelial NO synthase (eNOS)–derived vasodilators.
Endothelial cell–specific BH4 deficiency leads to hypertension.
What Is Relevant?
GCH1 expression is a key determinant of endothelial cell BH4 levels, which are alone sufficient to regulate physiological eNOS function and blood pressure.
Targeting endothelial cell BH4 biosynthesis rather than systemic BH4 levels may be more relevant to the development and treatment of hypertension.
Summary
Deficiency in endothelial cell BH4 biosynthesis, by targeted GCH1 deletion, is alone sufficient to cause eNOS uncoupling, increased O2·- production, and reduced NO bioavailability, even in the absence of vascular disease and in the presence of normal plasma and systemic BH4 levels. This endothelial cell-autonomous requirement for BH4 biosynthesis is pivotal in maintaining eNOS-mediated NO versus H2O2 signaling in vascular physiology and determines systemic arterial blood pressure. The cell-autonomous requirement for endothelial cell BH4 has important implications for vascular disease states and therapeutic strategies aimed at augmenting vascular BH4.
Sources of Funding
S. Chuaiphichai is funded by the British Heart Foundation (BHF). Also supported by the BHF Centre of Research Excellence (RE/08/004), British Heart Foundation grants, (PG/05/141/20098, RG/07/003/23133, and FS/11/50/29038), and Wellcome Trust Core grant (090532/Z/09/Z). Wellcome Trust Centre for Human Genetics.
Footnotes
Disclosures
None.
References
- 1.Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med. 2004;14:323–327. doi: 10.1016/j.tcm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 5.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Lüscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoi-chiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 8.Tatham AL, Crabtree MJ, Warrick N, Cai S, Alp NJ, Channon KM. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of GTP cyclohydrolase feedback regulatory protein expression. J Biol Chem. 2009;284:13660–13668. doi: 10.1074/jbc.M807959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–2133. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 10.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 12.Bune AJ, Brand MP, Heales SJ, Shergill JK, Cammack R, Cook HT. Inhibition of tetrahydrobiopterin synthesis reduces in vivo nitric oxide production in experimental endotoxic shock. Biochem Biophys Res Commun. 1996;220:13–19. doi: 10.1006/bbrc.1996.0348. [DOI] [PubMed] [Google Scholar]
- 13.Pickert G, Myrczek T, Rückert S, Weigert A, Häussler A, Ferreirós N, Brüne B, Lötsch J, Tegeder I. Inhibition of GTP cyclohydrolase reduces cancer pain in mice and enhances analgesic effects of morphine. J Mol Med (Berl) 2012;90:1473–1486. doi: 10.1007/s00109-012-0927-7. [DOI] [PubMed] [Google Scholar]
- 14.Adlam D, Herring N, Douglas G, De Bono JP, Li D, Danson EJ, Tatham A, Lu CJ, Jennings KA, Cragg SJ, Casadei B, et al. Regulation of β-adrenergic control of heart rate by GTP-cyclohydrolase 1 (GCH1) and tetrahydrobiopterin. Cardiovasc Res. 2012;93:694–701. doi: 10.1093/cvr/cvs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand MP, Heales SJ, Land JM, Clark JB. Tetrahydrobiopterin deficiency and brain nitric oxide synthase in the hph1 mouse. J Inherit Metab Dis. 1995;18:33–39. doi: 10.1007/BF00711370. [DOI] [PubMed] [Google Scholar]
- 16.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 17.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 18.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 19.Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 20.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- 22.Thomas G, Ramwell P. Induction of vascular relaxation by hydroperoxides. Biochem Biophys Res Commun. 1986;139:102–108. doi: 10.1016/s0006-291x(86)80085-7. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto S, Mori M, Tsushima H. Mechanisms underlying the hydrogen peroxide-induced, endothelium-independent relaxation of the norepinephrine-contraction in guinea-pig aorta. Eur J Pharmacol. 2003;459:65–73. doi: 10.1016/s0014-2999(02)02825-x. [DOI] [PubMed] [Google Scholar]
- 24.Wolin MS, Burke TM. Hydrogen peroxide elicits activation of bovine pulmonary arterial soluble guanylate cyclase by a mechanism associated with its metabolism by catalase. Biochem Biophys Res Commun. 1987;143:20–25. doi: 10.1016/0006-291x(87)90623-1. [DOI] [PubMed] [Google Scholar]
- 25.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 26.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Youn JY, Wang T, Blair J, Laude KM, Oak JH, McCann LA, Harrison DG, Cai H. Endothelium-specific sepiapterin reductase deficiency in DOCA-salt hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H2243–H2249. doi: 10.1152/ajpheart.00835.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 29.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.