Abstract
Pork is one of the major food sources of human salmonellosis worldwide, while beef products have been implicated in numerous foodborne outbreaks. As a result, effective interventions to reduce Salmonella contamination during beef and pork processing are of interest to both regulators and industry. We conducted a rapid systematic review and meta-analysis of literature investigating the efficacy of slaughter and processing interventions to control Salmonella in beef and pork. Review steps included: a comprehensive search strategy; relevance screening of abstracts; relevance confirmation of articles; data extraction; risk-of-bias assessment; meta-analysis (where appropriate); and a weight-of-evidence assessment. A total of 191 relevant experimental studies were identified. Two controlled trials indicated that hot water and steam treatments are effective at reducing the prevalence of Salmonella on beef carcasses (relative risk [RR] = 0.11, 95% confidence interval [CI]: 0.02, 0.58), while four trials found that pre-chill organic acid washes are effective at reducing Salmonella on pork carcasses (RR = 0.32, 95% CI: 0.13, 0.78), with high confidence in the estimates of effect. Four quasi-experimental studies found that post-exsanguination chemical washes were effective to reduce the prevalence of Salmonella on cattle hides, with low confidence in the specific estimate of effect; moderate confidence was found for the effect estimates of scalding (RR = 0.20, 95% CI: 0.14, 0.29) and singeing (RR = 0.34, 95% CI: 0.22, 0.52) of pork carcasses. The overall evidence supported enhanced reductions of Salmonella through a multiple-hurdle approach. In conclusion, various slaughter and processing interventions can contribute to reducing Salmonella on beef and pork carcasses, depending on the context of application; an appropriate combination should be selected, validated, and verified by establishment operators within their local conditions.
Keywords: knowledge synthesis, review, salmonellae, meat, interventions
Non-typhoidal Salmonella spp. (hereafter referred to as Salmonella) are one of the most common causes of gastroenteritis worldwide, resulting in an estimated 155,000 deaths and 80 million cases of foodborne illness annually (64). Moreover, non-typhoidal salmonellosis can lead to possible chronic sequelae such as reactive arthritis and irritable bowel syndrome (58). As a result, the disease has a substantial economic burden on society due to a number of direct (e.g. healthcare) and indirect (e.g. lost productivity) costs (55, 67). Salmonella can also have negative impacts on the agri-food industry and trade due to contamination events, associated food recalls, and loss of market access (30).
Along with poultry and eggs, pork products are frequently identified globally as one of the most common sources of non-typhoidal salmonellosis in humans (77). While beef products are less often associated with the burden of non-typhoidal salmonellosis, they have been implicated in several large outbreaks of Salmonella infections in the USA during the past 15 years, sometimes due to multi-drug resistant strains (61). In addition, recent outbreaks of non-typhoidal salmonellosis have occurred in several European countries due to the consumption of various beef products contaminated with multi-drug resistant strains (42, 70, 79).
Both pigs and cattle, along with other food-producing animals, are known reservoirs for Salmonella, which they frequently shed as asymptomatic carriers (18). Salmonella can spread between animals on the farm, during transport, and in lairage environments, contributing to the incoming level of contamination on animals at slaughter (4, 6, 18, 100). Salmonella contamination of pork and beef hides, internal organs, and viscera, as well as the abattoir environment and workers, can lead to cross-contamination of carcasses during slaughter and processing (5, 18, 32, 65, 82, 100). Processing establishments worldwide typically implement a variety and combination of decontamination interventions, along with good hygiene practices (GHP) and hazard analysis critical control point (HACCP) principles, to reduce the occurrence and levels of Salmonella and other microbial pathogens on beef and pork carcasses and meat products. The specific interventions used in the industry varies by country and setting according to the options approved for use in that region, in-plant efficacy, incoming pathogen load, and other factors such as installation and operation costs, feasibility of implementation, environmental impacts, occupational health and safety, and consumer acceptability and preferences (1, 4, 9, 100).
In 2011, the Codex Alimentarius Commission published international guidelines on the control of Salmonella and Campylobacter in chicken meat (21). In November, 2013, the Codex Committee on Food Hygiene (CCFH) agreed to develop similar guidelines for control of Salmonella in fresh beef and pork due to a recognized need for global guidance in this area (22). The CCFH later requested the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) Joint Expert Meeting on Microbiological Risk Assessment (JEMRA) to provide scientific advice to inform the new guidelines by conducting a systematic literature review and convening an expert meeting. This article reports on the process and results from the systematic review of the global literature on interventions to control Salmonella in beef and pork during slaughter and processing. This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (71).
Materials and Methods
Review approach and team
The review was conducted following recommended procedures for systematic reviews as outlined in a pre-specified protocol (52, 80), with some steps streamlined using a rapid approach given the broad review scope and limited timeframe for completion (protocol available from corresponding author upon request). The following specific steps were modified under the rapid approach: the search was targeted; detailed analyses were prioritized; and only one reviewer conducted the relevance confirmation and data extraction steps.
Review question and eligibility criteria
The initial review question was: “What is the efficacy of all possible interventions to control Salmonella in fresh pork and beef, from primary production to consumption?” Any interventions applied throughout the food chain continuum were initially considered relevant; however, post-processing interventions were later excluded from the scope as the search was not optimized to appropriately capture literature on interventions at this stage. This article only reports on relevant articles that investigated slaughter and processing interventions; studies investigating pre-harvest interventions will be reported in a separated publication. For a summary of all results, readers can refer to the full technical report (39).
The population of interest included all pigs and cattle produced for domestic meat consumption, including their carcasses and trimmings. Any outcome measure was considered relevant (including environmental samples). Manufactured (e.g. cured, dried, and fermented) products such as sausages and salamis were excluded. All available evidence published in English, French, or Spanish was considered for inclusion, including previously published systematic reviews, risk assessments and stochastic models, and primary research of any study design, as long as there was a measure of intervention efficacy reported in relation to a comparison group.
Search strategy
Comprehensive search algorithms were developed for beef and pork by extracting key words from a selection of known relevant articles, and by reviewing search strategies of previously published reviews and risk assessments. Key terms were combined using the Boolean operator OR into categories for Pathogen (Salmonella terms), Population (beef and pork terms), and Intervention (intervention terms), and the categories were combined using the AND operator. Algorithms were pre-tested in Scopus and final searches were implemented in the bibliographic databases Scopus and CAB Abstracts on February 11, 2015. For the beef search, no publication year or other restrictions were imposed. For the pork search, only literature from 2009-2015 was searched, as a citation list of potentially relevant articles covering any intervention against Salmonella in the pork chain was available from a previously conducted scoping review that included a broad search of literature published up to 2009 (102). Search verification included: reviewing relevant conference proceedings; conducting targeted searches in Google to identify grey literature (e.g. government and industry reports); and reviewing the reference lists of a sample of relevant articles. In addition, any documents received from a complementary FAO/WHO public “call for data” were reviewed for potential inclusion.
Relevance screening, confirmation, and prioritization
Each reference was assessed for relevance to the review scope at the title and abstract level using an a priori developed form. After this stage, we decided to exclude all research published prior to 1990, as evidence on interventions published prior to this period was not considered reflective of current industry conditions and practices. Relevant references were procured as full articles and confirmed for relevance using another pre-specified form. This form was used to characterize articles according to the document type, study design, and commodity (beef or pork), point in chain, and intervention categories investigated. Results from this stage were used to prioritize more detailed data synthesis according to the availability and applicability of evidence. All experimental study designs (controlled trials, challenge trials, and quasi-experimental studies) were considered for detailed data extraction and risk-of-bias assessment.
Data extraction and risk-of-bias assessment
These steps were conducted using pre-specified tools. The data extraction tool included targeted questions about intervention and population descriptions, outcomes measured, and intervention efficacy results. The risk-of-bias tool was adapted from the Cochrane Collaboration’s recommended tools for randomized and non-randomized study designs (20, 52).
Review management
Identified references were uploaded to RefWorks (Thomson ResearchSoft, Philadelphia, PA), de-duplicated, and imported into the systematic review software DistillerSR (Evidence Partners, Ottawa, ON) for relevance screening, relevance confirmation, and risk-of-bias assessment. Data extraction was conducted in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA). Review forms were pre-tested before use on a selection of relevant references and articles (30 for relevance screening and confirmation, and five for data extraction and risk-of-bias assessment). Reviewing for relevance screening proceeded only when consistent agreement was achieved between reviewers (kappa >0.8). For other steps, pre-tests were used to ensure consistent interpretation and clarity of the forms. Two independent reviewers (I.Y. and B.W.) conducted relevance screening and risk-of-bias assessment, while only one conducted relevance confirmation and data extraction. Any disagreements between reviewers were resolved through discussion. A copy of all forms used in the review and more details on the search strategy (e.g. search algorithms) are available in the full technical report (39).
Data analysis
For the purposes of this article, we focused quantitative analysis on studies conducted under commercial conditions (i.e. controlled trials and quasi-experiments). Challenge trials are summarized narratively only; quantitative log reduction summaries of these studies are available in the technical report (39). Results were stratified into comparable subgroups for meta-analysis (14, 52). Data were first stratified by point in the processing chain, then study design, then into specific intervention categories (Table 1) that were created based on insights from previous reviews (9, 100), and finally by different outcome measures (e.g. sample types). Random-effects meta-analysis was conducted in each subgroup when sufficiently reported data were available from ≥2 trials. The unit of analysis in all cases was individual trials reported within studies. All studies reported prevalence data, which were summarized using the relative risk (RR) measure (14, 52). Meta-analysis models were calculated using the restricted maximum-likelihood method in R Version 3.1.3 (78, 99).
Table 1.
Categories of processing interventions used to summarize and report efficacy data in this review.
| Intervention categoriesa | Definition |
|---|---|
| Dry heat | Non-hydrating thermal interventions such a forced-air heating. |
| Electricity | Application of electricity through, for example, electric stunners. |
| Multiple intervention combinations | Combination of two or more intervention types. |
| Non-thermal | Non-chemical and non-heat-based interventions that aim to reduce microbial contamination while preserving product quality and nutrients that can be affected by thermal treatments (100). Examples include irradiation, plasma gases, and high pressure processing. |
| Packaging-based interventions | Includes a range of interventions that can be applied to prevent spoilage and inhibit microbial growth during final product distribution and storage. |
| Standard processing steps/GHPs | |
| Bunging | Closing off the rectum during removal of intact viscera at slaughter to minimize the spread of cross-contamination on a carcass (92). |
| Chilling | Step at the end of the slaughter process and before fabrication of rapidly reducing the carcass temperature to prevent microbial growth and preserve product quality. |
| Cleaning/disinfection | The removal of dirt and organic substances from and sanitation of meat processing plant equipment and environments. |
| HACCP | An internationally recognized system that identifies, evaluates, and controls hazards that are significant for food safety. |
| Scalding | Step during pork processing of immersing pigs in hot water tanks with the primary purpose of softening hairs on the pig skin to make them easier to remove. |
| Singeing | Step during pork processing of removing remaining hairs from a pig carcass. |
| Steam vacuuming | Spot application of steam and/or hot water to loosen soil and kill bacteria, followed by a vacuum to remove contaminants. |
| Trimming | Physical removal of visible contamination from carcasses. |
| Washes/rinses/sprays | |
| Hot water/steam pasteurization | Heat treatment to destroy microbial cells. |
| Multiple wash combinations | Combinations of two or more types of washes, rinses, or sprays. |
| Natural extracts | Plant-based and other (e.g. dairy) extracts. |
| Organic acids | Antimicrobials such as lactic, acetic, and citric acid that affect microbial growth through disruptions to nutrient transport and energy generation and that can cause injury to microbial cells through their low pH (100). |
| Other chemicals | Chemicals that destroy bacteria through various actions, such as oxidation and disruption of cellular functions, or that prevent bacterial attachment to meat (100). Examples include chlorine, trisodium phosphate, acidified sodium chlorite, and ozone. |
| Protective bacterial cultures | Lactic acid bacteria to control pathogens through the production of antimicrobial compounds. |
| Water | Ambient or cold water to physically remove contamination. |
Note that categories are only shown here if they were represented by at least one study identified in the review and reported results on efficacy against Salmonella.
RR estimates were used to determine the effect of each intervention on changing a given baseline prevalence of Salmonella as a more intuitive measure of intervention efficacy (52). Within each intervention subgroup, we used the median prevalence of Salmonella in the comparison groups of included studies as a baseline measure of prevalence. This value was multiplied by the respective RR measure to obtain the corresponding change in prevalence of Salmonella due to the intervention (52).
Heterogeneity was assessed using I2, which represents the proportion of variation in effect estimates across studies that is due to heterogeneity rather than sampling error (53). For each meta-analysis subgroup, heterogeneity was considered high and average estimates of effect were not shown when I2≥60% (52, 53). In these cases, a median and range of effect estimates from individual studies in the intervention subgroup was shown instead, as presenting pooled meta-analysis estimates in the presence of high heterogeneity can be misleading (54). Meta-analysis effect estimates were considered significant if the 95% confidence intervals (CI) excluded the null. We intended to assess possible publication bias in meta-analysis subgroups using Begg’s rank correlation and Egger’s regression tests if there were ≥10 trials and if heterogeneity was not high (52); however, none of the subgroups met these criteria.
GRADE assessment
The Cochrane Collaboration’s Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to assess the confidence in the estimated measures of intervention effect for each subgroup in terms of how well they might be excepted to represent intervention effects in practice or in future studies conducted under similar conditions (48, 52). The GRADE approach classifies the confidence in findings from each subgroup into one of four levels: very low, low, moderate, or high. The approach was adapted to the needs of the topic and applicable study designs (52, 86). Due to inherent differences in strength of evidence for intervention efficacy by study design (48, 86), controlled trials started at high and quasi-experiments at a moderate rating. Five downgrading and three upgrading criteria were then assessed for each subgroup, which could lead to reducing or increasing, respectively, the GRADE levels (Table 2).
Table 2.
GRADE approach and criteria applied to sub-groups of data where quantitative intervention efficacy results were summarized.
| Criteria | GRADE rating change | Explanation |
|---|---|---|
| Downgrading criteria | ||
|
a) = -1 b) = -2 |
Rating from risk-of-bias tool |
|
a) = -1 b) = -2 |
Heterogeneity in the results is measured by I2 (only where meta-analysis was possible). |
|
a) = -1 |
For prevalence outcomes, assume at least a 10% reduction in risk (for common outcomes, ~750-800 samples, for rare outcomes, ~200-300). Actual sample size determined by inputting median control group prevalence in a sample size calculator. For concentration outcomes, total sample size is >400. For rare events, may be less. Actual sample size determined by inputting median group means and SDs in sample size calculator. |
|
a) = -1 b) = -2 |
Indirectness indicates studies do not directly measure the target parameter of interest to the review question. E.g. studies only measuring surrogate/intermediate outcomes (e.g. environmental samples) or those that deliberately contaminate carcasses with fecal material may be rated down. |
|
a) = -1 |
If meta-analysis is possible, publication bias detected in dataset via statistical tests and visual examination. If meta-analysis is not possible, publication bias suspected by other means (e.g. lack of small studies showing no treatment effect). |
| Upgrading criteria | ||
|
a) = +1 |
Large effect considered at least a 2-fold reduction in risk. |
|
a) = +1 |
E.g. the actual effect of the intervention is likely to be even greater than the data suggest. |
|
a) = +1 |
|
Results
Descriptive results
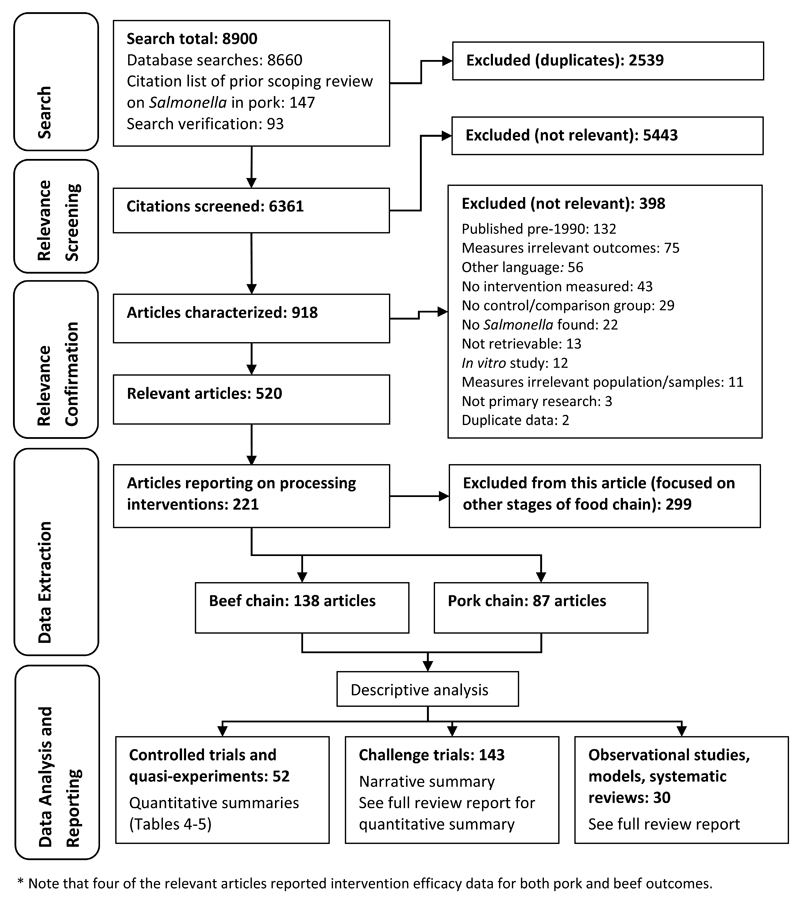
A flow chart of the review process and results is shown in Figure 1. From a total of 221 relevant articles that reported on the efficacy of slaughter and processing interventions against Salmonella, more (n=138 vs. 87) investigated beef than pork, with four articles covering both commodities. The key characteristics of the articles are shown in Table 3. Most studies (81%) investigating interventions in the beef chain were conducted in North America, while studies investigating interventions for pork had a more global distribution (Table 3). The most common study design for beef studies was challenge trials (80%), while pork studies mostly comprised challenge trials, quasi-experiments, and risk assessments and stochastic models (Table 3). Nearly 50% of studies investigating interventions in pork were conducted under commercial conditions, compared to only 20% for beef (Table 3). The most common category of interventions investigated was various washes, rinses, and sprays (66%) for beef, and processing steps and GHPs (54%) for pork (Table 3). As shown in Figure 1, only the experimental primary research studies (n=191) are summarized further in this article. A citation list of all relevant articles is available in technical report (39). More detailed characteristics, risk of bias and GRADE assessments, and results for each relevant study are available as supplementary materials: http://tinyurl.com/zba5b6x.
Figure 1.
Systematic review flow chart.
Table 3.
Key characteristics of 221 relevant articles that reported data on the efficacy of processing interventions to control Salmonella in fresh pork or beef.
| Beef (n=138) | Pork (n=87) | |||
|---|---|---|---|---|
| Article characteristic | No. | % | No. | % |
| Region: | ||||
| North America | 112 | 81.2 | 26 | 29.9 |
| Europe | 8 | 5.8 | 40 | 46.0 |
| Asia/Middle East | 8 | 5.8 | 14 | 16.1 |
| Africa | 5 | 3.6 | 0 | 0.0 |
| Central and South America/Caribbean | 3 | 2.2 | 5 | 5.7 |
| Australia/South Pacific | 2 | 1.4 | 2 | 2.3 |
| Document type: | ||||
| Journal article | 129 | 93.5 | 74 | 85.1 |
| Conference proceedings paper/abstract | 4 | 2.9 | 9 | 10.3 |
| Report | 4 | 2.9 | 3 | 3.4 |
| Thesis | 1 | 0.7 | 1 | 1.1 |
| Study design: | ||||
| Challenge trial | 111 | 80.4 | 33 | 37.9 |
| Quasi-experiment | 20 | 14.5 | 23 | 26.4 |
| Controlled trial | 3 | 2.2 | 7 | 8.0 |
| Observational study | 2 | 1.4 | 7 | 8.0 |
| Risk assessment/stochastic model | 2 | 1.4 | 17 | 19.5 |
| Systematic review/meta-analysis | 1 | 0.7 | 4 | 4.6 |
| Study conditions: | ||||
| Laboratory | 70 | 50.7 | 25 | 28.7 |
| Pilot plant | 39 | 28.3 | 4 | 4.6 |
| Commercial | 27 | 19.6 | 41 | 47.1 |
| Not reported | 0 | 0.0 | 1 | 1.1 |
| Intervention type: | ||||
| Decontamination washes/rinses/sprays | 91 | 65.9 | 36 | 41.4 |
| Packaging-based interventions | 31 | 22.5 | 8 | 9.2 |
| Processing steps/GHPs | 17 | 12.3 | 47 | 54.0 |
| Other | 11 | 8.0 | 10 | 11.5 |
Controlled trial and quasi-experiment results for beef
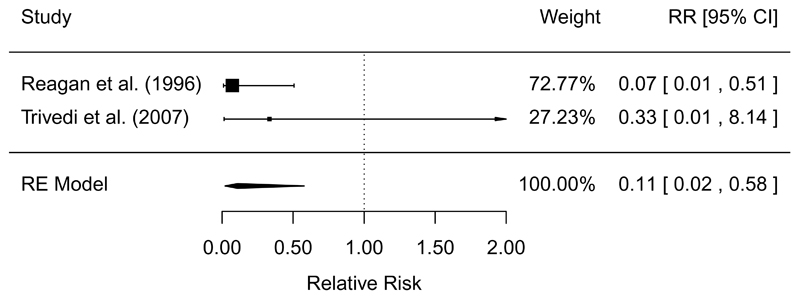
The synthesized results from controlled trial and quasi-experimental studies measuring the effect of slaughter and processing interventions to control Salmonella in beef are shown in Table 4. Only three controlled trials were identified, with interventions supported mostly by only one study each (Table 4). However, two studies found that pre-chill hot water washes and steam pasteurization were effective to reduce the prevalence of Salmonella on beef carcasses (RR = 0.11, 95% CI: 0.02, 0.58), with high GRADE confidence in the estimate of effect (Figure 2).
Table 4.
Synthesized results of controlled trials and quasi-experiments that investigated the efficacy of processing interventions to control Salmonella in beef.
| Intervention / stage in chain (comparison group) | Study design | No. trials / studies | Relative risk estimate of effect: meta-analysis/single study (95% CI) or median (range)a | I2 | Estimated change in prevalence (%) in the study population (95% CI or range)b | GRADEc | References |
|---|---|---|---|---|---|---|---|
| Water wash | |||||||
| Hide pre-slaughter (pre vs. post) | QE | 2/1 | MA = 1.22 (1.01, 1.47) | 0% | 46.7 to 56.8 (47.0, 68.6) | Very low | (69) |
| Hide post-exsanguination (pre vs. post) | QE | 2/2 | Median = 0.79 (0.28, 1.29) | 99% | 78.1 to 61.3 (21.5, 100) | Very low | (6, 88) |
| Pre-chill (vs. no treatment) | CT | 1/1 | Study = 0.30 (0.17, 0.53) | - | 30.3 to 9.0 (5.1, 16.1) | Very low | (81) |
| Pre-chill (pre vs. post) | QE | 4/4 | MA = 0.59 (0.31, 1.13) | 0% | 7.2 to 4.2 (2.2, 8.1) | Low | (31, 49, 72, 96) |
| Hot water/steam | |||||||
| Pre-chill (vs. no treatment) | CT | 2/2 | MA = 0.11 (0.02, 0.58) | 0% | 15.4 to 1.7 (0.3, 8.9) | High | (81, 97) |
| Pre-chill (pre vs. post) | QE | 2/2 | MA = 0.18 (0.02, 1.54) | 0% | 7.0 to 1.3 (0.2, 10.8) | Moderate | (74, 104) |
| Organic acid wash | |||||||
| Hide pre-slaughter (pre vs. post) | QE | 1/1 | Study = 1.04 (0.78, 1.39) | - | 50.0 to 52.2 (39.2, 69.5) | Very low | (69) |
| Hide post-exsanguination (pre vs. post) | QE | 1/1 | Study = 0.68 (0.49, 0.93) | - | 74.0 to 50.0 (36.2, 69.0) | Low | (88) |
| Post-chill (pre vs. post) | QE | 1/1 | Study = 0.45 (0.16, 1.30) | - | 1.0 to 0.5 (0.2, 1.3) | Very low | (84) |
| Other chemical wash | |||||||
| Hide pre-slaughter (pre vs. post) | QE | 1/1 | Study = 0.93 (0.72, 1.19) | - | 60.0 to 55.6 (43.3, 71.3) | Very low | (69) |
| Hide post-exsanguination (pre vs. post) | QE | 5/4 | Median = 0.70 (0.24, 0.87) | 98% | 62.3 to 43.6 (15.1, 54.3) | Low | (7, 15, 88, 90) |
| Pre-chill (vs. no treatment) | CT | 1/1 | Study = 1.18 (0.82, 1.71) | - | 30.3 to 35.8 (24.8, 51.7) | Very low | (81) |
| Trimming | |||||||
| Pre-chill (vs. no treatment) | CT | 1/1 | Study = 0.26 (0.14, 0.48) | - | 30.3 to 7.7 (4.2, 14.4) | Very low | (81) |
| Multiple interventions | |||||||
| Pre-chill (vs. no treatment) | CT | 1/1 | Study = 0.05 (0.01, 0.19) | - | 30.3 to 1.4 (0.3, 5.6) | Very low | (81) |
| Pre-chill (pre vs. post) | QE | 1/1 | Study = 0.08 (0.06, 0.12) | - | 28.1 to 2.3 (1.6, 3.5) | Low | (84) |
| Pre-evisceration to pre-chill (pre vs. post) | QE | 7/7 | Median = 0.02 (0.006, 0.62) | 97% | 28.7 to 0.5 (0.2, 17.9) | Very low | (8, 10, 16, 59, 82, 84, 91) |
| Processing steps/GHPs | |||||||
| Bunging (before vs. after pre-evisceration wash) | CT | 1/1 | Study = 0.09 (0.005, 1.61) | - | 8.3 to 0.8 (0.04, 13.4) | Low | (92) |
| Chilling (pre vs. post) | QE | 5/5 | MA = 1.50 (0.61, 3.74) | 17% | 2.0 to 3.0 (1.2, 7.4) | Low | (31, 40, 49, 91, 96) |
| HACCP (pre vs. post) | QE | 5/5d | MA = 1.13 (0.68, 1.87) | 0% | 1.2 to 1.4 (0.8, 2.2) | Moderate | (101) |
CI=confidence interval; CT=controlled trial; GHP=good hygiene practice; MA=meta-analysis; QE=quasi-experiment.
Meta-analysis estimates from random-effects models are shown if I2 was <60%. If I2 was ≥60%, a median and range of effects are shown. If only one trial from a single study was available, the estimate and 95% CI from the study are shown.
The first value is the median prevalence from the control group samples in the respective studies (baseline prevalence without intervention). The second value indicates the expected reduction (or increase) in prevalence due to the intervention, as calculated from the respective estimate of effect (see methods section for additional details). The values in brackets represent a 95% CI for the estimated change for meta-analysis and individual study estimates, or a range of values for intervention estimates with significant heterogeneity.
GRADE rating descriptions: very low=the true effect is likely to be substantially different from the measured estimate; low=the true effect may be substantially different from the measured estimate; moderate=the true effect is likely to be close to the measured estimate, but there is a possibility that it is substantially different; high=there is strong confidence that the true effect lies close to that of the measured estimate.
Individual studies were summarized in a previous systematic review-meta-analysis.
Figure 2.
Forest plot of the results of controlled trials measuring the efficacy of pre-chill hot water washes and steam pasteurization to reduce the prevalence of Salmonella on beef carcasses. RE=random-effects meta-analysis.
Quasi-experimental studies found that washes containing chemicals other than organic acids (e.g. chlorine, sodium hydroxide, hydrogen bromide) significantly reduced the prevalence of Salmonella on cattle hides when applied post-exsanguination, but with low GRADE confidence in the specific effect estimate due to significant heterogeneity among studies (Table 4). A similar effect was found in one of the studies for an organic acid hide wash applied post-exsanguination (Table 4). Several studies that measured changes in Salmonella prevalence on carcasses sampled between pre-evisceration and chilling, after a series of in-plant interventions were applied, found large reductions in prevalence (Table 4), but with very low GRADE confidence in the effect estimates due to heterogeneity across studies and potential for other confounding factors to have contributed to the findings. Inconsistent results were identified in quasi-experimental studies for the effect of live animal hide washes, pre- and post-chill washes, chilling, and HACCP (Table 4).
Controlled trial and quasi-experiment results for pork
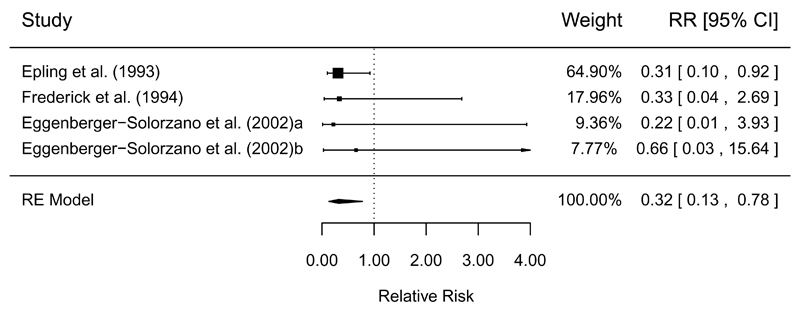
The synthesized results from controlled trial and quasi-experimental studies measuring the effect of slaughter and processing interventions to control Salmonella in pork are shown in Table 5. Three controlled trial studies found that pre-chill organic acid washes were effective to reduce the prevalence of Salmonella on pork carcasses (RR = 0.32, 95% CI: 0.13, 0.78), with high GRADE confidence in the estimate (Figure 3). High confidence was also found for the effect estimate of hot water washes and steam pasteurization, which showed a trend for reduction, but was non-significant (Table 5). Other interventions investigated in controlled trials showed inconsistent results (e.g. spray vs. air chilling) or were supported by only one study (e.g. other chemical washes) (Table 5).
Table 5.
Synthesized results of controlled trials and quasi-experiments that investigated the efficacy of processing interventions to control Salmonella in pork.
| Intervention / stage in chain (comparison group) | Study design | No. trials / studies | Relative risk estimate of effect: meta-analysis/single study (95% CI) or median (range)a | I2 | Estimated change in prevalence (%) in the study population (95% CI or range)b | GRADEc | References |
|---|---|---|---|---|---|---|---|
| Water wash (high pressure) | |||||||
| Pre-chill (pre vs. post) | QE | 1/1 | Study = 0.18 (0.05, 0.65) | - | 91.7 to 16.7 (4.6, 59.7) | Very low | (17) |
| Hot water/steam | |||||||
| Pre-chill (vs. no treatment) | CT | 4/3 | MA = 0.29 (0.08, 1.08) | 46% | 4.3 to 1.2 (0.3, 4.6) | High | (36, 50, 97) |
| Organic acid wash | |||||||
| Pre-chill (vs. no treatment) | CT | 4/3 | MA = 0.32 (0.13, 0.78) | 0% | 7.7 to 2.5 (1.0, 6.0) | High | (36, 37, 41) |
| Pre-chill (vs. water wash) | CT | 2/1 | Median = 0.26 (0.08, 0.44) | 95% | 100 to 26.0 (7.5, 44.4) | Very low | (98) |
| Pre-chill (pre vs. post) | QE | 1/1 | Study = 0.48 (0.25, 0.93) | - | 13.9 to 6.7 (3.5, 12.9) | Low | (60) |
| Other chemical wash | |||||||
| Pre-chill (vs. no treatment) | CT | 1/1 | Study = 0.44 (0.20, 0.98) | - | 16.0 to 7.0 (3.1, 15.6) | Low | (50) |
| Unspecified wash | |||||||
| Pre-chill (pre vs. post) | QE | 2/2 | MA = 0.60 (0.11, 3.17) | 31% | 5.1 to 3.1 (0.6, 16.1) | Low | (51, 65) |
| Steam vacuum | |||||||
| Pre-chill (vs. trimming) | CT | 1/1 | Study = 3.0 (0.13, 71.74) | - | 2.2 to 6.7 (0.3, 100) | Low | (83) |
| Multiple interventions | |||||||
| Pre-chill (vs. no treatment) | CT | 2/1 | MA = 0.54 (0.09, 3.22) | 0% | 4.2 to 2.3 (0.4, 13.4) | Low | (36) |
| Pre-chill (pre vs. post) | QE | 2/2 | MA = 0.95 (0.52, 1.74) | 0% | 8.6 to 8.2 (4.5, 15.0) | Moderate | (24, 25) |
| Post-exsanguination to post-chill (pre vs. post) | QE | 1/1 | Study = 0.01 (0.002, 0.08) | - | 73.0 to 0.8 (0.1, 5.8) | Very low | (94) |
| Pre-evisceration to post-chill (pre vs. post) | QE | 4/4 | Median = 0.08 (0.03, 0.19) | 92% | 14.9 to 0.9 (0.6, 2.9) | Very low | (57, 85, 89, 103) |
| Post-evisceration to post-chill (pre vs. post) | QE | 3/3 | Median = 0.71 (0.17, 2.94) | 94% | 9.4 to 9.1 (1.5, 18.8) | Very low | (3, 27, 56) |
| Processing steps/GHPs | |||||||
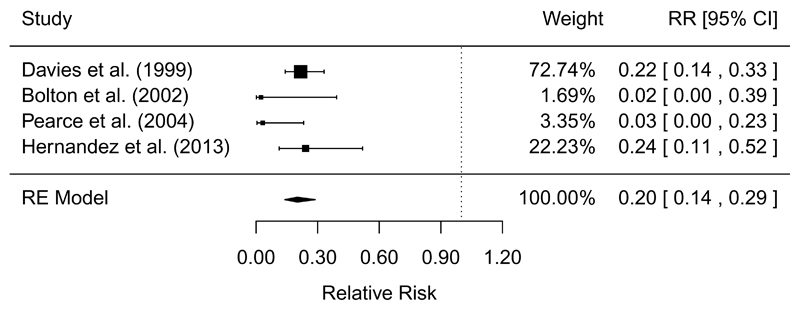
| Scalding (pre vs. post) | QE | 4/4 | MA = 0.20 (0.14, 0.29) | 0% | 34.8 to 7.0 (4.9, 10.0) | Moderate | (13, 26, 51, 76) |
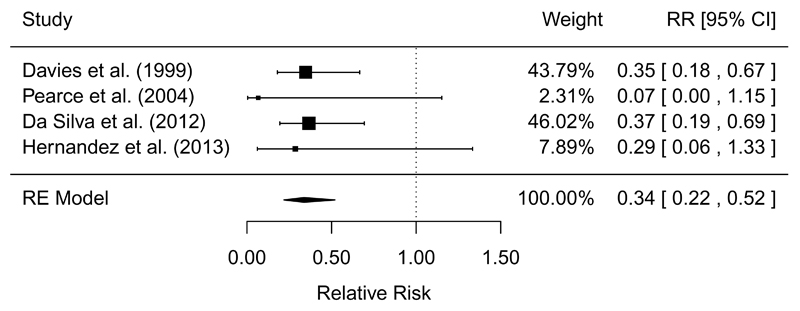
| Singeing (pre vs. post) | QE | 4/4 | MA = 0.34 (0.22, 0.52) | 0% | 18.1 to 6.1 (4.0, 9.4) | Moderate | (25, 26, 51, 76) |
| Chilling (spray vs. air) | CT | 1/1 | Study = 0.70 (0.37, 1.33) | - | 13.3 to 9.3 (4.9, 17.8) | Low | (37) |
| Chilling (pre vs. post) | QE | 13/13d | Median = 0.44 (0.16, 2.50) | 85% | 10.3 to 4.6 (1.7, 25.7) | Very low | (45) |
| HACCP (pre vs. post) | QE | 3/3d | Median = 0.94 (0.59, 1.26) | 89% | 6.9 to 6.5 (4.0, 8.7) | Low | (101) |
| Cleaning and disinfection (pre vs. post) | QE | 2/2 | MA = 0.33 (0.20, 0.52) | 0% | 22.8 to 7.5 (4.7, 12.0) | Low | (23, 43) |
CI=confidence interval; CT=controlled trial; GHP=good hygiene practice; MA=meta-analysis; QE=quasi-experiment.
Meta-analysis estimates from random-effects models are shown if I2 was <60%. If I2 was ≥60%, a median and range of effects are shown. If only one trial from a single study was available, the estimate and 95% CI from the study are shown.
The first value is the median prevalence from the control group samples in the respective studies (baseline prevalence without intervention). The second value indicates the expected reduction (or increase) in prevalence due to the intervention, as calculated from the respective estimate of effect (see methods section for additional details). The values in brackets represent a 95% CI for the estimated change for meta-analysis and individual study estimates, or a range of values for interventions estimate with significant heterogeneity.
GRADE rating descriptions: very low=the true effect is likely to be substantially different from the measured estimate; low=the true effect may be substantially different from the measured estimate; moderate=the true effect is likely to be close to the measured estimate, but there is a possibility that it is substantially different; high=there is strong confidence that the true effect lies close to that of the measured estimate.
Individual studies were summarized in a previous systematic review-meta-analysis.
Figure 3.
Forest plot of the results of controlled trials measuring the efficacy of pre-chill organic acid washes to reduce the prevalence of Salmonella on pork carcasses. RE=random-effects meta-analysis.
Quasi-experimental studies found that both scalding (RR = 0.20, 95% CI: 0.14, 0.29) and singeing (RR = 0.34, 95% CI: 0.33, 0.52) provided significant reductions in Salmonella prevalence, with moderate GRADE confidence in the effect estimates (Table 5 and Figures 4-5). Two studies found that cleaning and disinfection in the processing plant significantly reduced Salmonella prevalence, but with low GRADE confidence in the effect estimate because the studies only measured environmental samples (Table 5). Studies that measured changes in Salmonella prevalence on carcasses sampled between pre-evisceration and post-chilling, after a series of in-plant interventions were applied, found large reductions in prevalence, but inconsistent results were found in studies that measured the initial samples post-evisceration (Table 5). Inconsistent results were identified in quasi-experimental studies for the effects of pre-chill washes, chilling, and HACCP (Table 5).
Figure 4.
Forest plot of the results of quasi-experiments measuring the efficacy of scalding to reduce the prevalence of Salmonella on pork carcasses. RE=random-effects meta-analysis.
Figure 5.
Forest plot of the results of quasi-experiments measuring the efficacy of singeing to reduce the prevalence of Salmonella on pork carcasses. RE=random-effects meta-analysis.
Challenge trial results
A total of 143 challenge trial studies conducted under laboratory or pilot plant conditions were identified that evaluated a range of interventions along the processing chain for beef (n=111) and pork (n=33). Overall, the challenge trials found wide-ranging reductions in Salmonella concentrations for most investigated interventions in both commodities (ranging from almost no reduction to multiple logs). In general, challenge trials found that multiple combinations of interventions implemented concurrently or successively were the most consistently effective to reduce Salmonella concentrations on beef and pork compared with no treatment and ambient water washes.
Risk of bias
The overall results of the risk-of-bias assessment for the relevant studies are shown stratified by study design in Table 6. The main risks of bias for controlled trials related to a lack of reporting of random treatment allocation and associated risks that confounding factors other than the intervention could have contributed to the outcomes (Table 6). A limited number of challenge trials also had similar risks of bias, along with risks related to incomplete outcome data and selective reporting. The primary risk of bias for quasi-experiments related to the potential for confounding factors to have influenced the outcomes (Table 6).
Table 6.
Risk-of-bias assessment summary for 191 experimental primary research articles that investigated the efficacy of processing interventions to control Salmonella in beef or pork. Some studies reported on more than one experimental design.
| No. of studies low/unclear/high risk | |||
|---|---|---|---|
| Criteria | CT (n=9) | QE (n=43) | ChT (n=143) |
| Random sequence generation | 2/6/1 | N/Aa | 139/2/2 |
| Allocation concealment | 9/0/0 | N/Aa | 143/0/0 |
| Blinding of participants and personnel | 9/0/0 | N/Aa | 143/0/0 |
| Independence of intervention effect from confounding bias | 2/6/1 | 16/24/3 | 137/6/0 |
| Incomplete outcome data | 6/2/1 | 37/6/0 | 138/5/0 |
| Selective reporting | 9/0/0 | 43/0/0 | 135/8/0 |
| Other biases | 7/2/0 | 43/0/0 | 140/3/0 |
| Overall risk-of-bias: | 2/5/2 | 16/24/3 | 134/10/1 |
CT=controlled trials; ChT=challenge trials; QE=quasi-experiments.
N/A=These criteria were not assessed for this study design.
Discussion
Efficacy of slaughter and processing interventions
Salmonella contamination of incoming cattle hides is a major source of beef carcass cross-contamination in processing establishments (6, 10, 82). A molecular source-tracking study in the USA found that cross-contamination of hides in the lairage environment accounted for a larger proportion of cattle hide and carcass contamination with Salmonella than the initial level of contamination on cattle leaving the feedlot (6). Hide decontamination interventions can be applied to either the live animals or carcasses post-exsanguination to reduce the incoming Salmonella load. Quasi-experimental studies from the USA consistently found that post-exsanguination hide washes containing chemicals such as lactic acid, sodium hydroxide, hydrogen bromide, or chlorine were effective at reducing the prevalence of Salmonella (6, 7, 15, 88, 90), although the specific magnitude of reduction was variable. In contrast, ambient water washes without chemicals were not effective, and in some cases increased contamination, potentially because they might mobilize bacteria on the hide, which can be encapsulated in dirt, mud, and feces (69, 88). Similarly, hide washes applied to live animals showed inconsistent results, and additionally may cause animal stress or irritations (69).
Finisher pigs can harbor a wide variety of Salmonella serotypes in various tissues, which can lead to cross-contamination of carcasses during processing if GHPs are not followed (32, 34, 62). Scalding and singeing are process steps that provide opportunities to reduce Salmonella contamination of pork carcasses early along the slaughter line. The effect of both steps for reducing Salmonella prevalence was demonstrated in several quasi-experimental studies (13, 25, 26, 51, 76), as well as in observational studies (28, 62, 66). However, the efficacy of scalding and singeing depends on the procedures used, including achieving appropriate time-temperature combinations, and GHPs such as frequently changing the scald tank water and ensuring the singe flame reaches the entire carcass (34). Additionally, it is important to maintain GHPs in steps following scalding and singeing (e.g. polishing, evisceration, and splitting), including training and awareness of plant personnel, in order to prevent carcass recontamination (13, 75).
Beef and pork carcass decontamination treatments such as hot water, steam pasteurization, and organic acid washes are used by processing establishments worldwide, in accordance with local regulatory approvals (1, 44, 47, 100). For example, lactic acid was recently approved as a decontamination intervention for beef carcasses, halves, and quarters in the European Union, where previously only heat-based treatments were allowed (38).
Controlled trial studies indicated that pre-chill hot water washes and steam pasteurization were effective at reducing Salmonella contamination of beef carcasses, with a similar trend for pork carcasses, although the overall effect was not statistically significant. Controlled trials also found that organic acid washes were effective at reducing the prevalence of Salmonella on pork carcasses. In contrast, despite their wide use in practice and extensive evidence of efficacy under laboratory and pilot plant conditions (33), no evidence collected under commercial conditions was identified for their effect on beef carcasses. Inconsistent evidence from commercial settings has also been noted for the efficacy of organic acid washes for reducing populations of indicator organisms on beef carcasses, which could be due to several factors, such as potential dilution of chemical concentrations if applied on wet carcasses and difficulties in achieving uniform coverage of application across the whole carcass surface (44). The efficacy of carcass decontamination treatments may vary depending on the specific process parameters used (e.g. water temperature and pressure, treatment time, chemical concentration) as well as other factors (e.g. levels of carcass contamination and bacterial attachment to the meat surface) (44, 100). They are typically applied using automated spray cabinets, which may be cost-prohibitive for smaller establishments. It has been suggested that operators of smaller establishments could consider the use of handheld spray devices as an alternative to automated cabinets (2, 97). Additionally, decontamination interventions may cause a discoloration (e.g. cooked appearance) of the meat surface depending on the application parameters, but this change is usually temporary and may be reversible following chilling (2, 50, 100).
It has been suggested that the process of carcass chilling might contribute to a reduction in the prevalence of microbial pathogens such as Salmonella on beef and pork carcasses (45, 47); for example, through the combined effects of cold shock and desiccation (35). However, we identified inconsistent and variable evidence for chilling to reduce the prevalence of Salmonella on beef and pork carcasses, though there was more evidence suggesting a potential reduction effect in pork than in beef (Tables 4-5). The variability in the apparent effect of chilling for reducing Salmonella contamination could be due to a number of factors, such as potential issues with recovery and isolation of Salmonella from chilled carcasses, carcass cross-contamination in the chilling room, variable sampling and laboratory methods across studies, and differences in chilling methods and procedures (35, 45, 68, 84). The difference in evidence of effect for beef and pork could be due to additional processing steps in the latter, such as singeing, which might reduce attachment of Salmonella to carcass surfaces. Further research is needed to investigate if different chilling methods (e.g. spray or blast vs. conventional air chilling) might provide additional opportunities to reduce Salmonella contamination of beef and pork carcasses during processing.
Various additional interventions, such as carcass washes with chemicals other than organic acids, post-fabrication treatments, packaging-based treatments, and emerging technologies (e.g. natural extracts, non-thermal plasma), were investigated in laboratory-based challenge trials and show promise for reducing Salmonella contamination of beef and/or pork under those conditions (39). However, further research is needed to investigate their feasibility and effectiveness in commercial settings.
The overall body of evidence synthesized in this review for both beef and pork suggests that the implementation of multiple interventions concurrently or successively along the processing chain (i.e. a “multiple-hurdle” strategy) results in enhanced reductions in Salmonella compared to single interventions alone (39). However, given the wide variability of the studies synthesized in this review, the effectiveness of each intervention and combination thereof should be validated and verified within the context and conditions of each specific processing establishment. Additionally, pre-harvest control measures may also be needed as part of a holistic food-chain approach to controlling Salmonella in beef and pork (34, 63, 95). For example, research has shown that deep-tissue lymph nodes might be a more important source of Salmonella contamination in ground beef compared to fecal and hide contamination (59, 63). Processing interventions such as carcass decontamination washes are unlikely to affect Salmonella in these beef tissues, which are generally not removed prior to the grinding step (46, 59, 63). Similarly, research has shown that pig carcasses from farms with higher herd seropositivity for Salmonella are more likely to be contaminated at the abattoir than those originating from lower prevalence herds (1, 32, 62). Therefore, appropriate pre-harvest interventions may be warranted for both commodities in order to reduce the incoming burden of Salmonella in different tissues and the subsequent potential for cross-contamination of beef and pork carcasses during processing.
Review strengths and limitations
Randomized controlled trials conducted under commercial conditions provide the strongest evidence to measure the efficacy of an intervention, because the random allocation of study units to treatment and control groups balances unmeasured confounding variables and helps to ensure that measured changes in the outcome (e.g. Salmonella) are due to the intervention and not to other factors (86). In our GRADE assessment of intervention efficacy measures, quasi-experiments were automatically downgraded by one level due to inherent study design differences: specifically, these studies do not have a concurrent comparison group, and any observed intervention effects between pre- and post-intervention samples cannot be assumed to be due to the intervention (86). Some risks of bias were identified for studies of both designs related to a lack of reporting of key design features (e.g. randomization allocation) and outcomes; future research in this field should report findings in accordance with internationally recommended reporting guidelines for experimental studies of interventions (29, 87). Due to their highly controlled conditions, we did not find many risks of bias in challenge trials that we believe could have affected their outcomes. However, they were summarized only narratively in this article, because they are conducted using specific strains of Salmonella that are inoculated, often at very high doses, to portions or pieces of meat under laboratory or pilot plant settings that do not accurately reflect real-world conditions, and the strains used may not be representative of those naturally found on pork and beef in different settings.
This review used a structured and transparent approach to identify, assess, and synthesize available evidence on the efficacy of interventions to control Salmonella during beef and pork processing, which provides more credible and reliable evidence to decision-makers than traditional narrative reviews (52, 80). One limitation of this review was that the search was streamlined using a rapid approach (only two bibliographic databases were used), and given the broad scope, some important search terms could have been missed. Therefore, it is possible that we could have missed some potentially relevant literature that might have resulted in additional evidence, though a search verification strategy was implemented to attempt to mitigate this bias. Another related limitation is that only literature published in English, French, and Spanish was considered for inclusion, which could have resulted in underrepresentation of evidence from some geographic regions. Only one reviewer conducted the relevance confirmation and data extraction steps of the review, which could have led to possible misclassification of articles or data extraction errors, respectively.
The original review question aimed to include evidence on post-processing interventions, but these were later excluded from the scope of the review as the search was not optimized to reliability capture such studies. Post-processing interventions, such as temperature control, cooking, and prevention of cross-contamination, are critical steps in the food chain for ensuring the safety of beef and pork and for reducing the risk of salmonellosis among consumers (11, 35, 93). The slaughter and processing interventions summarized and reported in this review should be considered as important strategies to address in-plant Salmonella contamination of beef and pork, but appropriate post-processing controls are also needed as part of a whole food chain approach to prevent subsequent cross-contamination and Salmonella growth and survival prior to consumption.
Meta-analysis provides a useful analytical tool to combine the results of multiple primary research studies into a weighted, average estimate of intervention effect (52). However, a limitation of the analysis in this review is that study results were synthesized across relatively broad intervention categories. This was conducted for pragmatic reasons in order to facilitate summarization and presentation of intervention efficacy trends from a large body of literature. However, as a consequence, details such as intervention application parameters (e.g. dose, treatment duration) and differences in study sampling and laboratory methods were not investigated as possible sources of variation in intervention effects across studies. It is likely that these and other study factors could contribute to the heterogeneity in effects observed for many intervention categories, but it was beyond the scope of the review to investigate them in detail. Future research synthesis research focusing on more specific commodities, points in the food chain, and interventions could allow more in-depth investigation of such factors.
The meta-analysis estimates of intervention effect, along with the GRADE ratings of confidence in these effects, provide a guide for interpreting how likely one could expect a similar effect in future studies or applications. However, given that the intervention effects reported in the literature reflect the specific conditions and parameters of those studies, such interventions should always be validated under local plant settings and conditions. The GRADE weight-of-evidence approach used in this review was adapted from the healthcare sector. Further research is needed on how best to define and evaluate the specific parameters of the GRADE assessment criteria and how these should be ideally applied within the context of food safety research.
We identified very little research conducted under commercial conditions, and of those studies that were identified, most were quasi-experiments rather than controlled trials. This is likely due to pragmatic issues with evaluating various interventions in commercial processing establishments, such as infrequent in-plant Salmonella contamination of carcasses when operating under good hygiene and manufacturing practices. For example, we identified several additional controlled trials that measured Salmonella but did not isolate any positive samples (39), which precluded evaluation of intervention effect on this pathogen. We decided not to include intervention research on potential surrogate organisms (e.g. non-pathogenic Escherichia coli) within the scope of the review because it was not known to what extent these results might reflect and correspond similarly to the control of Salmonella. For example, the behavior of surrogates such as E. coli can differ when compared with different Salmonella serotypes, intervention processes, and meat products (73).
Nevertheless, we recognize that individual establishment operators will likely need to use surrogate organisms to validate and verify the effectiveness of interventions in their specific settings and local conditions. Various studies have found that aerobic plate counts (APC) and Enterobacteriaceae could be considered as suitable surrogate measures for evaluating the in-plant effectiveness of interventions to reduce Salmonella contamination of beef and pork (3, 32, 84, 103). For example, in a study that measured >5000 samples from three processing plants in the USA over an 18-month period, Ruby et al. (84) found that both APC and Enterobacteriaceae counts were significantly associated with the presence of Salmonella on beef carcasses. Similarly, in a study that measured pork carcass swab samples from 152 processing establishments across the USA, Williams et al. (103) found that log reductions in APC between pre-evisceration and post-chill samples were associated with reductions in Salmonella. In contrast, while some studies have indicated that some non-pathogenic strains of E. coli might also be useful for this purpose in specific contexts (12, 19, 73), they are not likely to be suitable for monitoring reductions after chilling given the large proportion of samples that tend to be negative after this step (103).
Conclusion
Various slaughter and processing interventions were shown to be effective to reduce the prevalence of Salmonella in beef and pork under commercial conditions in different contexts. Several other interventions were investigated only under laboratory or pilot plant conditions and showed promise in those settings, but require further research on their commercial applicability. As the studies summarized in this review were conducted under varying conditions and often found wide-ranging effects, the specific selection and implementation of different interventions in practice should be guided by the local conditions and situation. Additionally, any interventions should be validated and verified for their effectiveness in each establishment setting. If Salmonella levels are low, then monitoring should be conducted using applicable surrogate organisms, though their limitations should be recognized. GHPs and HACCP-based principles provide the basis upon which slaughter and processing interventions should be implemented, as the positive effects of an intervention can be negated if cross-contamination occurs subsequently during processing and if contamination occurs further along the chain. In addition, consideration should be given to a holistic and integrated food-chain approach to Salmonella control, with slaughter and processing interventions implemented alongside appropriate pre-harvest and post-processing control measures.
Supplementary Material
Acknowledgements
We thank CCFH working group leads and all of the participants of the JEMRA Expert Meeting on “Interventions for the Control of Nontyphoidal Salmonella spp. in Beef and Pork” for their feedback on this review. This review was funded by the FAO and WHO. The views and opinions expressed in this article are those of the authors and do not represent the official views and opinions of their organizations.
References
- 1.Alban L, Baptista FM, Møgelmose V, Sørensen LL, Christensen H, Aabo S, Dahl J. Salmonella surveillance and control for finisher pigs and pork in Denmark – a case study. Food Res Int. 2012;45:656–665. [Google Scholar]
- 2.Alban L, Sørensen LL. Hot-water decontamination is an effective way of reducing risk of Salmonella in pork. Fleischwirtschaft. 2010;90:109–113. [Google Scholar]
- 3.Algino RJ, Badtram GA, Ingham BH, Ingham SC. Factors associated with Salmonella prevalence on pork carcasses in very small abattoirs in Wisconsin. J Food Prot. 2009;72:714–721. doi: 10.4315/0362-028x-72.4.714. [DOI] [PubMed] [Google Scholar]
- 4.Arguello H, Alvarez-Ordoñez A, Carvajal A, Rubio P, Prieto M. Role of slaughtering in Salmonella spreading and control in pork production. J Food Prot. 2013;76:899–911. doi: 10.4315/0362-028X.JFP-12-404. [DOI] [PubMed] [Google Scholar]
- 5.Arguello H, Carvajal A, Collazos JA, Garcia-Feliz C, Rubio P. Prevalence and serovars of Salmonella enterica on pig carcasses, slaughtered pigs and the environment of four Spanish slaughterhouses. Food Res Int. 2012;45:905–912. [Google Scholar]
- 6.Arthur TM, Bosilevac JM, Brichta-Harhay D, Kalchayanand N, King DA, Shackelford SD, Wheeler TL, Koohmaraie M. Source tracking of Escherichia coli O157:H7 and Salmonella contamination in the lairage environment at commercial U.S. beef processing plants and identification of an effective intervention. J Food Prot. 2008;71:1752–1760. doi: 10.4315/0362-028x-71.9.1752. [DOI] [PubMed] [Google Scholar]
- 7.Arthur TM, Bosilevac JM, Brichta-Harhay D, Kalchayanand N, Shackelford SD, Wheeler TL, Koohmaraie M. Effects of a minimal hide wash cabinet on the levels and prevalence of Escherichia coli O157:H7 and Salmonella on the hides of beef cattle at slaughter. J Food Prot. 2007;70:1076–1079. doi: 10.4315/0362-028x-70.5.1076. [DOI] [PubMed] [Google Scholar]
- 8.Bacon RT, Sofos JN, Belk KE, Hyatt DR, Smith GC. Prevalence and antibiotic susceptibility of Salmonella isolated from beef animal hides and carcasses. J Food Prot. 2002;65:284–290. doi: 10.4315/0362-028x-65.2.284. [DOI] [PubMed] [Google Scholar]
- 9.Baer AA, Miller MJ, Dilger AC. Pathogens of interest to the pork industry: a review of research on interventions to assure food safety. Compr Rev Food Sci Food Saf. 2013;12:183–217. [Google Scholar]
- 10.Barkocy-Gallagher G, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M. Seasonal prevalence of shiga toxin-producing Escherichia coli, including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J Food Prot. 2003;66:1978–1986. doi: 10.4315/0362-028x-66.11.1978. [DOI] [PubMed] [Google Scholar]
- 11.Bollaerts K, Messens W, Aerts M, Dewulf J, Maes D, Grijspeerdt K, Stede Y. Evaluation of scenarios for reducing human salmonellosis through household consumption of fresh minced pork meat. Risk Anal. 2010;30:853–865. doi: 10.1111/j.1539-6924.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 12.Bollerslev A, Nauta M, Hald T, Hansen T, Aabo S. Use of a slaughter hygiene indicator (Escherichia coli) to quantify the risk of human salmonellosis related to pork in Denmark – an approach for risk based meat control?. Proceedings of the 10th International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork; Portland, ME. 2013. pp. 122–124. [Google Scholar]
- 13.Bolton DJ, Pearce RA, Sheridan JJ, Blair IS, McDowell DA, Harrington D. Washing and chilling as critical control points in pork slaughter hazard analysis and critical control point (HACCP) systems. J Appl Microbiol. 2002;92:893–902. doi: 10.1046/j.1365-2672.2002.01599.x. [DOI] [PubMed] [Google Scholar]
- 14.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons, Ltd; Chichester, UK: 2009. [Google Scholar]
- 15.Bosilevac JM, Arthur TM, Bono JL, Brichta-Harhay D, Kalchayanand N, King DA, Shackelford SD, Wheeler TL, Koohmaraie M. Prevalence and enumeration of Escherichia coli O157:H7 and Salmonella in U.S. abattoirs that process fewer than 1,000 head of cattle per day. J Food Prot. 2009;72:1272–1278. doi: 10.4315/0362-028x-72.6.1272. [DOI] [PubMed] [Google Scholar]
- 16.Brichta-Harhay D, Guerini MN, Arthur TM, Bosilevac JM, Kalchayanand N, Shackelford SD, Wheeler TL, Koohmaraie M. Salmonella and Escherichia coli O157:H7 contamination on hides and carcasses of cull cattle presented for slaughter in the United States: an evaluation of prevalence and bacterial loads by immunomagnetic separation and direct plating methods. Appl Environ Microbiol. 2008;74:6289–6297. doi: 10.1128/AEM.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brustolin JC, Pisol A, Steffens J, Toniazzo G, Valduga E, Luccio M, Cansian RL. Decontamination of pig carcasses using water pressure and lactic acid. Brazilian Arch Biol Technol. 2014;57:954–961. [Google Scholar]
- 18.Buncic S, Sofos J. Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Food Res Int. 2012;45:641–655. [Google Scholar]
- 19.Cabrera-Diaz E, Moseley TM, Lucia LM, Dickson JS, Castillo A, Acuff GR. Fluorescent protein-marked Escherichia coli biotype I strains as surrogates for enteric pathogens in validation of beef carcass interventions. J Food Prot. 2009;72:295–303. doi: 10.4315/0362-028x-72.2.295. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane Effective Practice and Organisation of Care (EPOC) Group. [Accessed 6 July 2016];Suggested risk of bias criteria for EPOC reviews. 2015 Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors.
- 21.Codex Alimentarius Commission. [Accessed 6 July 2016];Guidelines for the control of Campylobacter and Salmonella in chicken meat. CAC/GL 78-2011. 2011 Available at: http://www.fao.org/input/download/standards/11780/CXG_078e.pdf.
- 22.Codex Alimentarius Commission. [Accessed 6 July 2016];Report of the 45th session of the Codex Committee on Food Hygiene: Ha Noi Viet Nam 11-15 November 2013. REP14/FH. 2014 Available at: ftp://ftp.fao.org/codex/reports/reports_2014/REP14_FHe.pdf.
- 23.Conter M, Zanardi E, Ghidini S, Guidi E, Campanini G. Microbiological condition of carcasses and equipment in a pig slaughterhouse and evaluation of a steam decontamination system. Ital J Anim Sci. 2006;18:387–396. [Google Scholar]
- 24.Creus E, Pérez JF, Mateu E. Salmonella contamination on pork carcasses: a study of critical points. Proceedings of the 6th International Symposium on the Epidemiology and Control of Foodborne Pathogens in Pork; Rohnert Park, CA. 2005. pp. 304–306. [Google Scholar]
- 25.Da Silva LE, Dias V, Ferronatto A, Guerra P, Berno L, Triches N, Kich JD, Corbellini LG, Cardoso M. Longitudinal dissemination of Salmonella enterica clonal groups through the slaughter process of Salmonella-positive pig batches. J Food Prot. 2012;75:1580–1588. doi: 10.4315/0362-028X.JFP-11-515. [DOI] [PubMed] [Google Scholar]
- 26.Davies RH, McLaren IM, Bedford S. Distribution of Salmonella contamination in two pig abattoirs. Proceedings of the 3rd International Symposium on the Epidemiology and Control of Salmonella in Pork; Washington, DC. 1999. pp. 267–272. [Google Scholar]
- 27.De Busser EV, Maes D, Houf K, Dewulf J, Imberechts H, Bertrand S, De Zutter L. Detection and characterization of Salmonella in lairage, on pig carcasses and intestines in five slaughterhouses. Int J Food Microbiol. 2011;145:279–286. doi: 10.1016/j.ijfoodmicro.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Delhalle L, De Sadeleer L, Bollaerts K, Farnir F, Saegerman C, Korsak N, Dewulf J, De Zutter L, Daube G. Risk factors for Salmonella and hygiene indicators in the 10 largest Belgian pig slaughterhouses. J Food Prot. 2008;71:1320–1329. doi: 10.4315/0362-028x-71.7.1320. [DOI] [PubMed] [Google Scholar]
- 29.Des Jarlais DC, Lyles C, Crepaz N, Group T. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361–366. doi: 10.2105/ajph.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey M, Mayo JA, Saville D, Wolyniak C, Klontz KC. Recalls of foods due to microbiological contamination classified by the U.S. Food and Drug Administration, fiscal years 2003 through 2011. J Food Prot. 2013;76:932–938. doi: 10.4315/0362-028X.JFP-12-464. [DOI] [PubMed] [Google Scholar]
- 31.Dong P, Zhu L, Mao Y, Liang R, Niu L, Zhang Y, Li K, Luo X. Prevalence and profile of Salmonella from samples along the production line in Chinese beef processing plants. Food Control. 2014;38:54–60. [Google Scholar]
- 32.Duggan SJ, Mannion C, Prendergast DM, Leonard N, Fanning S, Gonzales-Barron U, Egan J, Butler F, Duffy G. Tracking the Salmonella status of pigs and pork from lairage through the slaughter process in the Republic of Ireland. J Food Prot. 2010;73:2148–2160. doi: 10.4315/0362-028x-73.12.2148. [DOI] [PubMed] [Google Scholar]
- 33.EFSA Panel on Biological Hazards (BIOHAZ) Scientific opinion on the evaluation of the safety and efficacy of lactic acid for the removal of microbial surface contamination of beef carcasses, cuts and trimmings. EFSA J. 2011;9:2317. [Google Scholar]
- 34.EFSA Panel on Biological Hazards (BIOHAZ) Scientific opinion on a quantitative microbiological risk assessment of Salmonella in slaughter and breeder pigs. EFSA J. 2010;8:1547. [Google Scholar]
- 35.EFSA Panel on Biological Hazards (BIOHAZ) Scientific opinion on the public health risks related to the maintenance of the cold chain during storage and transport of meat. Part 1 (meat of domestic ungulates) EFSA J. 2014;12:3601. [Google Scholar]
- 36.Eggenberger-Solorzano L, Niebuhr SE, Acuff GR, Dickson JS. Hot water and organic acid interventions to control microbiological contamination on hog carcasses during processing. J Food Prot. 2002;65:1248–1252. doi: 10.4315/0362-028x-65.8.1248. [DOI] [PubMed] [Google Scholar]
- 37.Epling LK, Carpenter JA, Blankenship LC. Prevalence of Campylobacter spp. and Salmonella spp. on pork carcasses and the reduction effected by spraying with lactic acid. J Food Prot. 1993;56:536–540. doi: 10.4315/0362-028X-56.6.536. [DOI] [PubMed] [Google Scholar]
- 38.European Commission. Commission regulation (EU) no 101/2013 of 4 February 2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcases. Off J Eur Union. 2013;L34:1–3. [Google Scholar]
- 39.FAO/WHO. Interventions for the Control of Nontyphoidal Salmonella spp. in Beef and Pork: Report of a Joint FAO/WHO Expert Meeting. Rome, Italy: 2015. [Accessed 6 July 2016]. Rapid systematic review of the efficacy of interventions to control Salmonella in pork and beef. Available at: http://tinyurl.com/h28lrg2. [Google Scholar]
- 40.Fegan N, Vanderlinde P, Higgs G, Desmarchelier P. A study of the prevalence and enumeration of Salmonella enterica in cattle and on carcasses during processing. J Food Prot. 2005;68:1147–1153. doi: 10.4315/0362-028x-68.6.1147. [DOI] [PubMed] [Google Scholar]
- 41.Frederick TL, Miller MF, Thompson LD, Ramsey CB. Microbiological properties of pork cheek meat as affected by acetic acid and temperature. J Food Sci. 1994;59:300–302. [Google Scholar]
- 42.Friesema IHM, Schimmer B, Ros JA, Ober HJ, Heck MEOC, Swaan CM, de Jager CM, Peran i Sala RM, van Pelt W. A regional Salmonella enterica serovar Typhimurium outbreak associated with raw beef products, The Netherlands, 2010. Foodborne Pathog Dis. 2012;9:102–107. doi: 10.1089/fpd.2011.0978. [DOI] [PubMed] [Google Scholar]
- 43.Gantzhorn MR, Pedersen K, Olsen JE, Thomsen LE. Biocide and antibiotic susceptibility of Salmonella isolates obtained before and after cleaning at six Danish pig slaughterhouses. Int J Food Microbiol. 2014;181:53–59. doi: 10.1016/j.ijfoodmicro.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Gill CO. Effects on the microbiological condition of product of decontaminating treatments routinely applied to carcasses at beef packing plants. J Food Prot. 2009;72:1790–1801. doi: 10.4315/0362-028x-72.8.1790. [DOI] [PubMed] [Google Scholar]
- 45.Gonzales-Barron U, Cadavez V, Sheridan JJ, Butler F. Modelling the effect of chilling on the occurrence of Salmonella on pig carcasses at study, abattoir and batch levels by meta-analysis. Int J Food Microbiol. 2013;163:101–113. doi: 10.1016/j.ijfoodmicro.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Gragg SE, Loneragan GH, Brashears MM, Arthur TM, Bosilevac JM, Kalchayanand N, Wang R, Schmidt JW, Brooks JC, Shackelford SD, Wheeler TL, et al. Cross-sectional study examining Salmonella enterica carriage in subiliac lymph nodes of cull and feedlot cattle at harvest. Foodborne Pathog Dis. 2013;10:368–374. doi: 10.1089/fpd.2012.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greig JD, Waddell L, Wilhelm B, Wilkins W, Bucher O, Parker S, Rajić A. The efficacy of interventions applied during primary processing on contamination of beef carcasses with Escherichia coli: a systematic review-meta-analysis of the published research. Food Control. 2012;27:385–397. [Google Scholar]
- 48.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Hajmeer MN, Marsden JL, Crozier-Dodson B, Basheer IA, Higgins JJ. Reduction of microbial counts at a commercial beef koshering facility. J Food Sci. 1999;64:719–723. [Google Scholar]
- 50.Hamilton D, Holds G, Lorimer M, Kiermeier A, Kidd C, Slade J, Pointon A. Slaughterfloor decontamination of pork carcases with hot water or acidified sodium chlorite – a comparison in two Australian abattoirs. Zoonoses Public Health. 2010;57(Suppl 1):16–22. doi: 10.1111/j.1863-2378.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez M, Gomez-Laguna J, Luque I, Herrera-Leon S, Maldonado A, Reguillo L, Astorga RJ. Salmonella prevalence and characterization in a free-range pig processing plant: tracking in trucks, lairage, slaughter line and quartering. Int J Food Microbiol. 2013;162:48–54. doi: 10.1016/j.ijfoodmicro.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 52.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions: Version 5.1.0. The Cochrane Collaboration; 2011. [Accessed 6 July 2016]. Available at: http://handbook.cochrane.org/ [Google Scholar]
- 53.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann S, Batz MB, Morris JG., Jr Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot. 2012;75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 56.Keelara S, Scott HM, Morrow WM, Gebreyes WA, Correa M, Nayak R, Stefanova R, Thakur S. Longitudinal study of distributions of similar antimicrobial-resistant Salmonella serovars in pigs and their environment in two distinct swine production systems. Appl Environ Microbiol. 2013;79:5167–5178. doi: 10.1128/AEM.01419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keenliside J, Gensler G, McFall M, Goonewardene L. Prevalence and relatedness of Salmonella spp. in a Canadian abattoir. Proceedings of the 6th International Symposium on the Epidemiology and Control of Foodborne Pathogens in Pork; Rohnert Park, CA. 2005. pp. 38–41. [Google Scholar]
- 58.Keithlin J, Sargeant JM, Thomas MK, Fazil A. Systematic review and meta-analysis of the proportion of non-typhoidal Salmonella cases that develop chronic sequelae. Epidemiol Infect. 2015;143:1333–1351. doi: 10.1017/S0950268814002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koohmaraie M, Scanga JA, La Zerda D, Koohmaraie B, Tapay L, Beskhlebnaya V, Mai T, Greeson K, Samadpour M. Tracking the sources of Salmonella in ground beef produced from nonfed cattle. J Food Prot. 2012;75:1464–1468. doi: 10.4315/0362-028X.JFP-11-540. [DOI] [PubMed] [Google Scholar]
- 60.Larsen ST, McKean JD, Hurd HS, Rostagno MH, Griffith RW, Wesley IV. Impact of commercial preharvest transportation and holding on the prevalence of Salmonella enterica in cull sows. J Food Prot. 2003;66:1134–1138. doi: 10.4315/0362-028x-66.7.1134. [DOI] [PubMed] [Google Scholar]
- 61.Laufer AS, Grass J, Holt K, Whichard JM, Griffin PM, Gould LH. Outbreaks of Salmonella infections attributed to beef – United States, 1973-2011. Epidemiol Infect. 2015;143:2003–2013. doi: 10.1017/S0950268814003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Letellier A, Beauchamp G, Guevremont E, D’Allaire S, Hurnik D, Quessy S. Risk factors at slaughter associated with presence of Salmonella on hog carcasses in Canada. J Food Prot. 2009;72:2326–2331. doi: 10.4315/0362-028x-72.11.2326. [DOI] [PubMed] [Google Scholar]
- 63.Li M, Malladi S, Hurd HS, Goldsmith TJ, Brichta-Harhay DM, Loneragan GH. Salmonella spp. in lymph nodes of fed and cull cattle: relative assessment of risk to ground beef. Food Control. 2015;50:423–434. [Google Scholar]
- 64.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 65.Mannion C, Fanning J, McLernon J, Lendrum L, Gutierrez M, Duggan S, Egan J. The role of transport, lairage and slaughter processes in the dissemination of Salmonella spp. in pigs in Ireland. Food Res Int. 2012;45:871–879. [Google Scholar]
- 66.Marier EA, Snow LC, Floyd T, McLaren IM, Bianchini J, Cook AJC, Davies RH. Abattoir based survey of Salmonella in finishing pigs in the United Kingdom 2006-2007. Prev Vet Med. 2014;117:542–553. doi: 10.1016/j.prevetmed.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 67.McLinden T, Sargeant JM, Thomas MK, Papadopoulos A, Fazil A. Association between component costs, study methodologies, and foodborne illness-related factors with the cost of nontyphoidal Salmonella illness. Foodborne Pathog Dis. 2014;11:718–726. doi: 10.1089/fpd.2014.1750. [DOI] [PubMed] [Google Scholar]
- 68.Mellefont LA, Kocharunchitt C, Ross T. Combined effect of chilling and desiccation on survival of Escherichia coli suggests a transient loss of culturability. Int J Food Microbiol. 2015;208:1–10. doi: 10.1016/j.ijfoodmicro.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 69.Mies PD, Covington BR, Harris KB, Lucia LM, Acuff GR, Savell JW. Decontamination of cattle hides prior to slaughter using washes with and without antimicrobial agents. J Food Prot. 2004;67:579–582. doi: 10.4315/0362-028x-67.3.579. [DOI] [PubMed] [Google Scholar]
- 70.Mindlin MJ, Lang N, Maguire H, Walsh B, Verlander NQ, Lane C, Taylor C, Bishop LA, Crook PD. Outbreak investigation and case-control study: penta-resistant Salmonella Typhimurium DT104 associated with biltong in London in 2008. Epidemiol Infect. 2013;141:1920–1927. doi: 10.1017/S0950268812002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, Clark J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narváez-Bravo C, Rodas-González A, Fuenmayor Y, Flores-Rondon C, Carruyo G, Moreno M, Perozo-Mena A, Hoet AE. Salmonella on feces, hides and carcasses in beef slaughter facilities in Venezuela. Int J Food Microbiol. 2013;166:226–230. doi: 10.1016/j.ijfoodmicro.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Niebuhr SE, Laury A, Acuff GR, Dickson JS. Evaluation of nonpathogenic surrogate bacteria as process validation indicators for Salmonella enterica for selected antimicrobial treatments, cold storage, and fermentation in meat. J Food Prot. 2008;71:714–718. doi: 10.4315/0362-028x-71.4.714. [DOI] [PubMed] [Google Scholar]
- 74.Nutsch AL, Phebus RK, Riemann MJ, Schafer DE, Boyer JE, Jr, Wilson RC, Leising JD, Kastner CL. Evaluation of a steam pasteurization process in a commercial beef processing facility. J Food Prot. 1997;60:485–492. doi: 10.4315/0362-028X-60.5.485. [DOI] [PubMed] [Google Scholar]
- 75.O’Connor AM, Wang B, Denagamage T, McKean J. Process mapping the prevalence of Salmonella contamination on pork carcass from slaughter to chilling: a systematic review approach. Foodborne Pathog Dis. 2012;9:386–395. doi: 10.1089/fpd.2011.1040. [DOI] [PubMed] [Google Scholar]
- 76.Pearce RA, Bolton DJ, Sheridan JJ, McDowell DA, Blair IS, Harrington D. Studies to determine the critical control points in pork slaughter hazard analysis and critical control point systems. Int J Food Microbiol. 2004;90:331–339. doi: 10.1016/s0168-1605(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 77.Pires SM, Vieira AR, Hald T, Cole D. Source attribution of human salmonellosis: an overview of methods and estimates. Foodborne Pathog Dis. 2014;11:667–76. doi: 10.1089/fpd.2014.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 79.Raguenaud ME, Le Hello S, Salah S, Weill FX, Brisabois A, Delmas A, Germonneau P. Epidemiological and microbiological investigation of a large outbreak of monophasic Salmonella Typhimurium 4,5,12:i:- in schools associated with imported beef in Poitiers, France, October 2010. Euro Surveill. 2012;17:20289. [PubMed] [Google Scholar]
- 80.Rajić A, Young I. Knowledge synthesis, transfer and exchange in agri-food public health: A handbook for science-to-policy professionals. University of Guelph; Guelph, Canada; 2013. [Accessed 6 July 2016]. Available at: https://atrium.lib.uoguelph.ca/xmlui/handle/10214/7293. [Google Scholar]
- 81.Reagan JO, Acuff GR, Buege DR, Buyck MJ, Dickson JS, Kastner CL, Marsden JL, Morgan JB, Nickelson R, II, Smith GC, Sofos JN. Trimming and washing of beef carcasses as a method of improving the microbiological quality of meat. J Food Prot. 1996;59:751–756. doi: 10.4315/0362-028X-59.7.751. [DOI] [PubMed] [Google Scholar]
- 82.Rivera-Betancourt M, Shackelford SD, Arthur TM, Westmoreland KE, Bellinger G, Rossman M, Reagan JO, Koohmaraie M. Prevalence of Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella in two geographically distant commercial beef processing plants in the United States. J Food Prot. 2004;67:295–302. doi: 10.4315/0362-028x-67.2.295. [DOI] [PubMed] [Google Scholar]
- 83.Le Roux A, Minvielle B, Gault E. Validation of steam-vacuum process as corrective measure for visible faecal contamination on carcasses: preliminary results. Proceedings of the 54th International Congress of Meat Science and Technology; Cape Town, South Africa. 2008. pp. 2A–12. [Google Scholar]
- 84.Ruby JR, Zhu J, Ingham SC. Using indicator bacteria and Salmonella test results from three large-scale beef abattoirs over an 18-month period to evaluate intervention system efficacy and plan carcass testing for Salmonella. J Food Prot. 2007;70:2732–2740. doi: 10.4315/0362-028x-70.12.2732. [DOI] [PubMed] [Google Scholar]
- 85.Saide-Albornoz J, Lynn Knipe C, Murano EA, Beran GW. Contamination of pork carcasses during slaughter, fabrication, and chilled storage. J Food Prot. 1995;58:993–997. doi: 10.4315/0362-028X-58.9.993. [DOI] [PubMed] [Google Scholar]
- 86.Sargeant JM, Kelton DF, O’Connor AM. Study designs and systematic reviews of interventions: building evidence across study designs. Zoonoses Public Health. 2014;61(Suppl 1):10–17. doi: 10.1111/zph.12127. [DOI] [PubMed] [Google Scholar]
- 87.Sargeant JM, O’Connor AM, Gardner IA, Dickson JS, Torrence ME, Participants CM. The REFLECT statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration. Zoonoses Public Health. 2010;57:105–136. doi: 10.1111/j.1863-2378.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- 88.Scanga JA, Buschow AW, Kauk JL, Burk TE, Koohmaraie B, La Zerda D, Motlagh AM, Samadpour M, Koohmaraie M. Localized chemical decontamination of cattle hides to reduce microbial loads and prevalence of foodborne pathogens. Food Prot Trends. 2011;31:569–574. [Google Scholar]
- 89.Schmidt JW, Brichta-Harhay D, Kalchayanand N, Bosilevac JM, Shackelford SD, Wheeler TL, Koohmaraie M. Prevalence, enumeration, serotypes, and antimicrobial resistance phenotypes of Salmonella enterica isolates from carcasses at two large United States pork processing plants. Appl Environ Microbiol. 2012;78:2716–2726. doi: 10.1128/AEM.07015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt JW, Wang R, Kalchayanand N, Wheeler TL, Koohmaraie M. Efficacy of hypobromous acid as a hide-on carcass antimicrobial intervention. J Food Prot. 2012;75:955–958. doi: 10.4315/0362-028X.JFP-11-433. [DOI] [PubMed] [Google Scholar]
- 91.Sofos JN, Kochevar SL, Reagan JO, Smith GC. Incidence of Salmonella on beef carcasses relating to the U.S. Meat and Poultry Inspection regulations. J Food Prot. 1999;62:467–473. doi: 10.4315/0362-028x-62.5.467. [DOI] [PubMed] [Google Scholar]
- 92.Stopforth JD, Lopes M, Shultz JE, Miksch RR, Samadpour M. Location of bung bagging during beef slaughter influences the potential for spreading pathogen contamination on beef carcasses. J Food Prot. 2006;69:1452–1455. doi: 10.4315/0362-028x-69.6.1452. [DOI] [PubMed] [Google Scholar]
- 93.Swart AN, van Leusden F, Nauta MJ. A QMRA model for Salmonella in pork products during preparation and consumption. Risk Anal. 2016;36:516–530. doi: 10.1111/risa.12522. [DOI] [PubMed] [Google Scholar]
- 94.Tamplin ML, Feder I, Palumbo SA, Oser A, Yoder L, Luchansky JB. Salmonella spp. and Escherichia coli biotype I on swine carcasses processed under the hazard analysis and critical control point-based inspection models project. J Food Prot. 2001;64:1305–1308. doi: 10.4315/0362-028x-64.9.1305. [DOI] [PubMed] [Google Scholar]
- 95.Teagasc. Salmonella in pork on the island of Ireland: a microbial risk assessment. Ashtown Food Research Centre; Dublin, Ireland: 2010. [Accessed 6 July 2016]. Available at: http://www.safefood.eu/SafeFood/files/e6/e6af0e48-626d-4c04-ae05-0399964185b3.pdf. [Google Scholar]
- 96.Trairatapiwan T, Lertpatarakomol R, Mitchaothai J. Evaluation of Salmonella contamination and serovar isolation from slaughtering and cutting processes of Thai indigenous beef cattle. Proceedings of the 3rd International Conference on Sustainable Animal Agriculture for Developing Countries; Nakhon Ratchasima, Thailand. 2011. pp. 829–833. [Google Scholar]
- 97.Trivedi S, Reynolds AE, Chen J. Use of a commercial household steam cleaning system to decontaminate beef and hog carcasses processed by four small or very small meat processing plants in Georgia. J Food Prot. 2007;70:635–640. doi: 10.4315/0362-028x-70.3.635. [DOI] [PubMed] [Google Scholar]
- 98.Van Netten P, Mossel DA, Huis In ’t Veld J. Lactic acid decontamination of fresh pork carcasses: a pilot plant study. Int J Food Microbiol. 1995;25:1–9. doi: 10.1016/0168-1605(94)00039-9. [DOI] [PubMed] [Google Scholar]
- 99.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 100.Wheeler TL, Kalchayanand N, Bosilevac JM. Pre- and post-harvest interventions to reduce pathogen contamination in the U.S. beef industry. Meat Sci. 2014;98:372–382. doi: 10.1016/j.meatsci.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 101.Wilhelm BJ, Rajić A, Greig JD, Waddell L, Harris J. The effect of hazard analysis critical control point programs on microbial contamination of carcasses in abattoirs: a systematic review of published data. Foodborne Pathog Dis. 2011;8:949–960. doi: 10.1089/fpd.2010.0809. [DOI] [PubMed] [Google Scholar]
- 102.Wilhelm B, Rajić A, Parker S, Waddell L, Sanchez J, Fazil A, Wilkins W, McEwen SA. Assessment of the efficacy and quality of evidence for five on-farm interventions for Salmonella reduction in grow-finish swine: a systematic review and meta-analysis. Prev Vet Med. 2012;107:1–20. doi: 10.1016/j.prevetmed.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 103.Williams MS, Ebel ED, Allender HD. Industry-level changes in microbial contamination on market hog and broiler chicken carcasses between two locations in the slaughter process. Food Control. 2015;51:361–370. [Google Scholar]
- 104.Wright KD. Validation of hot water and lactic acid sprays for the reduction of enteric pathogens on the surface of beef carcasses. Texas A&M University; College Station, TX: 2009. MSc thesis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.