Abstract
The photoreceptor cells in the retina have a highly specialized sensory cilium, the outer segment, which is important for detecting light. Mutations in cilia related genes often result in retinal degeneration. The ability to reprogram human cells into induced pluripotent stem cells (iPSCs) and then differentiate them into a wide range of different cell types has revolutionized our ability to study human disease. To date, however, the challenge of producing fully differentiated photoreceptors in vitro has limited the application of this technology in studying retinal degeneration. In this review we will discuss recent advances in stem cell technology and photoreceptor differentiation. In particular, the development of photoreceptors with rudimentary outer segments that can be used to understand disease mechanisms and as an important model to test potential new therapies for inherited retinal ciliopathies.
Introduction: the retina and specialized cilia
The retina is responsible for transducing light into an electrochemical signal and transmitting that signal to the brain. It is comprised of around 55 neuronal subtypes, which all have different functions to allow the propagation of the light signal [1]. The cells in the retina that detect light are the photoreceptors, consisting of two types: cones and rods. Cones are responsible for daytime and colour vision in humans, whereas rod cells detect much lower levels of light and are therefore important for night vision. Photoreceptors possess a highly specialised sensory cilium known as the outer segment (OS). The OS consists of tightly packed membranous discs laden with photoresponsive opsin proteins as well as other components of the phototransduction machinery. The OS is joined to the biosynthetic inner segment (IS), which contains the endoplasmic reticulum (ER), by the connecting cilium (CC), a specialized cilia transition zone. The apical 10% of rod OS discs are shed daily and phagocytosed by the overlying retinal pigment epithelium (RPE) [2]. All the components needed for the assembly, maintenance and continuous turnover of the OS are synthesised in the IS and transported through the CC via the intraflagellar transport (IFT) machinery [3].
Cilia are microtubule-based organelles that project from the surface of eukaryotic cells. They are present from the earliest stages of development through to highly differentiated cells in adult tissues. Once thought to be a vestigial organelle they are now known to have an important function as sensors relaying information from the extracellular environment. Ciliopathies are a relatively recently defined class of inherited disorders that involve aberrant ciliary function [4]. The clinical manifestations of this dysfunction range from single organ involvement, such as retinitis pigmentosa (RP) to multi-organ involvement and syndromic disease, affecting a broad range of organs including the central nervous system, kidneys and adipose tissue [5]. Ciliopathy genes encode structural or functional components of the cilia or their basal bodies. Perhaps as a consequence of the high protein-trafficking load through the photoreceptor CC, the retina is possibly the most commonly affected organ [6].
Photoreceptor degeneration is a major cause of blindness. There are over 250 different causative genes identified to date (RetNet; https://sph.uth.edu/retnet/). Forms of inherited retinal dystrophy include RP and Leber congenital amaurosis (LCA). RP is a group of heterogeneous retinal dystrophies that usually involves progressive rod cell degeneration with secondary cone death. RP affects around 1:4000 individuals worldwide, leading to 1.5 million affected patients [7]. RP often does not present until the second decade; however X-linked forms of the disease can result in early onset degeneration. For example, mutations in the cilia-associated gene RP2 (retinitis pigmentosa 2; OMIM 312600) result in X-linked RP [8]. In addition to the rod degeneration usually seen in RP patients, some RP2 patients may also have a macular atrophy leading to decreased visual acuity in childhood [9]. LCA is an early onset retinal degeneration characterised by profound visual impairment or loss in early childhood [10]. LCA accounts for 5% of all inherited retinal dystrophies and affects up to 1:30000 patients [10]. LCA is also highly heterogeneous; there are currently 13 known genetic loci for LCA (RetNet) affecting different retinal functions. One of the most commonly mutated genes is CEP290 (centrosomal protein of 290kDa; OMIM 611755), accounting for around 15-25% of LCA cases [11]. Here, we will mainly focus on RP2 and CEP290 retinal ciliopathies.
RP2 and CEP290
RP2 is a GTPase activating protein (GAP) for the essential cilia-related GTPase, Arl3 [12,13]. RP2 is a ubiquitously expressed protein; in the retina it is predominantly localized at the plasma membrane [14]. However there is a pool of RP2 that localizes to the basal body of the cilia in photoreceptors, suggesting a role in cilia protein trafficking [14,15]. Mutations in RP2 account for approximately 15% of X-linked RP cases [8]. In particular, nonsense mutations are relatively frequent in RP2 patients, such as the R120X mutation [16].
Animal models of RP2 have reported conflicting phenotypes. For example, morpholino knockdown of RP2 in zebrafish produces a range of severe developmental eye defects, from small eyes to delayed/defective retinal lamination and photoreceptors with no outer segments and multiple systemic developmental defects [17–19]. However, an RP2 knockout zebrafish had mild retinal degeneration associated with disruption of GRK1 and rod transducin, but displayed no overt systemic phenotype [20]. RP2 knockout mouse models have produced some conflicting retinal phenotypes as well; cone opsin mis-localisation was observed in one model [21], whereas normal cone opsin traffic but abnormal traffic of prenylated phototransduction proteins PDE6-γ and GRK1 was observed in another [22]. Given the range of conflicting phenotypes reported in animal models, investigating actual human RP2 mutations in retinal cells derived from patients provides a new opportunity to gain insight into human disease mechanisms.
CEP290 is a protein found in all ciliated cells at the transition zone between the basal body and axoneme, and is essential for ciliogenesis [23,24]. A recent study utilising super-resolution microscopy located CEP290 as a hub on which other transition zone proteins assemble and postulated that these are involved in the formation of Y-links bridging the ciliary membrane and the axoneme [25]. Mutations in CEP290 are associated with numerous syndromic ciliopathies, yet non-syndromic LCA is the most common outcome [26,27]. The majority of CEP290 LCA patients (86%) have at least one copy of the most common CEP290 mutation, a deep intronic c.2991+1665A>G change which leads to mis-splicing and inclusion of a stop-codon containing cryptic exon in CEP290 mRNA [27,28]. There is still some correct splicing of exons 26 to 27, such that this intronic mutation leads to reduced CEP290 protein levels and behaves as a hypomorph.
The rd16 mouse model contains an inframe deletion in Cep290 that produces a truncated protein. rd16 mice have considerable deterioration of rod and cone function at p18 a reduced outer nuclear layer and OS degeneration [23]. A Cep290 knock out (null) mouse has no OS formation and no surviving photoreceptors at p28, the majority of these knockouts died of hydrocephalus [6]. A humanised mouse model of the CEP290 deep intronic mutation resulted in the creation of a separate, distinct cryptic exon not seen in human cells [29], highlighting the difficulties of modelling some mutations in mice to investigate human disease mechanisms. Therefore, differentiating patient induced pluripotent stem cells (iPSCs) to photoreceptors may be the only route by which to recapitulate the mechanisms of CEP290 LCA.
Using iPSCs to model retinal degeneration
Much recent work has focused on the role of iPSCs as a model of disease and as a potential route for therapy. Stem cells are an appealing approach to disease modelling, due to their pluripotent nature that allows them to differentiate into multiple cell types, coupled to the presence of the disease associated changes in the correct genomic and cellular context [30]. iPSCs were first described in 2006 and have since been used as models in a plethora of diseases [31,32]. Indeed, the generation of iPSCs and subsequent differentiation into retinal cell types from patients allows the study of mutations causing retinal degeneration in a human cell and mutation-specific paradigm [33]. Initial methods focused on creating pure, single cell-type cultures; for example, many studies have concentrated on differentiating iPSCs into RPE cells, which can be achieved in a two-dimensional culture system [34–36]. This approach has successfully resulted in insights into disorders affecting the RPE; for example, RPE cells have been differentiated from patients with mutations in MERTK [37], RPE65 [38], BEST1 [39], and OAT1 [40]. Retinal-progenitor and rod-like cells have been created from RP patients with mutations in the rhodopsin gene that were reported to recapitulate the disease phenotype; however, these progenitor cells were developmentally too immature to have OS containing rhodopsin [41,42].
3D ‘organoids’
Increasingly researchers are using three-dimensional organoid models derived from iPSCs to model disease tissue [43]. Organoid culture from iPSCs allows the creation of multiple cell-types in a more natural, organised cellular environment [44,45]. The retina has been successfully modelled in this way [46,47]. Multiple groups have achieved the generation of self-organising three-dimensional optic cup structures derived from human embryonic stem cells (ESCs) and iPSCs that develop cells with all the characteristics of photoreceptor precursors [48–50]. This technology will allow greater insight into the consequence of retinal-dystrophy mutations in highly specific cellular and genetic context. However, these studies did not produce photoreceptors with morphologically distinct OS, limiting their application to study retinal ciliopathies.
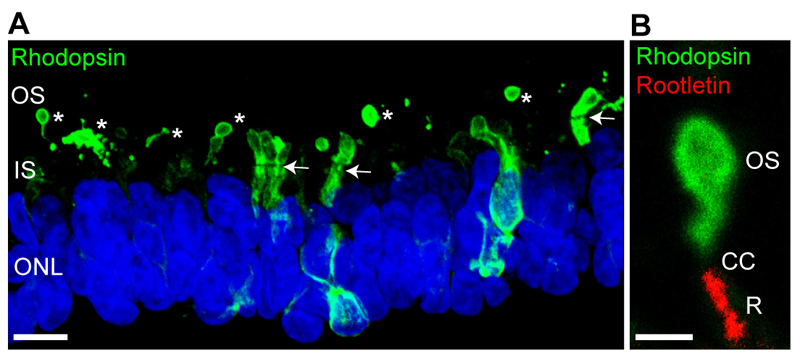
Transplantation into a host retinal context appears to enhance the differentiation of these organoid produced photoreceptor progenitors, as shown with mouse ESC [51] or human iPSC [52]. Patient iPSC-derived optic cup-like structures and their transplantation were used to probe a novel intronic mutation in USH2A that causes RP [52]. Recently, we have used these methods of 3D optic cup differentiation to produce photoreceptors with morphologically distinct rudimentary OS in vitro, without the need for transplantation [53]. These iPSC-derived OS take up to 20 weeks to develop and express rod and cone opsins ([53]; Figure 1). Furthermore, the CC can be visualised with a variety of cilia markers. Therefore, we can now use these 3D optic cups to probe retinal ciliopathy disease mechanisms and test potential therapies.
Figure 1. Rudimentary outer segments in iPSC 3D organoid optic cups.
(A) Rhodopsin localization (green) in 145 day old control iPSC derived optic cups. Rhodopsin immunoreactivity is most intense in the rudimentary outer segments (OS, and *). The inner segments (IS and separated from the outer nuclear layer stained with DAPI (blue) by the outer limiting membrane (OLM; highlighted with arrows). Immature photoreceptors have still have strong immunoreactivity in the IS and ONL. Note the rudimentary OS tend to break during processing. (B) Magnified OS stained with rhodopsin (green), the base of the cilium is highlighted with rootletin (red, R) highlighting the connecting cilum (CC) between the two. Scale bars = 10μm (A) and 2μm (B)
Retinal disease mechanisms and therapeutic development using iPSCs
Advances in gene-editing technology, such as the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas system, could allow for the correction of gene defects before organoid culture and potentially individual cell replacement therapy in the ultimate personalised medicine [54]. iPSC-derived disease cells can also be used to test small molecule based approaches that are either mutation or gene specific that cannot easily be tested in animal models. For example, translational read-through inducing drugs (TRIDs) that read-though premature termination mutations in a range of genes, or antisense oligonucleotides (AONs) to correct mis-splicing events require the specific gene and cellular context that iPSC-derived cells and organoids can provide.
We have used X-linked RP patient-iPSCs to produce RPE cells with the RP2 R120X mutation, which results in a premature stop and loss of RP2 protein from the basal body [55]. The loss of RP2 had no effect on cilia length or incidence, but IFT20 was dispersed in the RP2 patient RPE, suggesting RP2 may be involved with the cilia-based trafficking of a specific group of cilia proteins. Indeed, the traffic and membrane association of the beta subunit of transducin (Gβ1) was also disrupted [55,56]. Importantly, we were able to reverse these phenotypes using TRIDs, by restoring the RP2 protein levels up to 20% of normal in the RPE cells [55].
More recently, we used patient-derived iPSCs to model CEP290-LCA. The intronic c.2291+1665A>G mutation leads to a premature stop codon resulting in reduction in protein levels, although crucially there are residual amounts of wild-type mRNA leading to some full length protein expression [27]. The mis-splicing of CEP290 leads to reduced ciliation of cells and those cilia that are still present are shorter, even in fibroblasts, despite the expression of 40-50% of the correct wild-type exon 26-27 transcript [27,53,57]. Nevertheless, why a reduction in CEP290 of approximately 50% (the same that is present in carriers of null mutations that have no retinal phenotype) leads to LCA is unclear. To resolve this paradox we differentiated homozygous c.2291+1665A>G patient iPSC into RPE and 3D optic cups and compared cryptic splicing levels between cell types. The CEP290-LCA iPSC had reduced cilia incidence, but this did not affect their differentiation, which is expected given the phenotype of LCA but perhaps surprising given the importance of cilia for signalling during development. The comparison of the different cell types showed that optic cups had the highest levels of aberrant splicing and cilia defects, indicating that the retina has a much greater reduction of CEP290 levels than other tissues, including RPE, suggesting this is the underlying reason for LCA and not syndromic disease [53]. Interestingly, the increase in mis-spliced CEP290 coincided with photoreceptor differentiation and the splicing of other photoreceptor specific exons. RNA-Seq of human retina suggests that photoreceptors have exceptionally high levels of alternative exon usage [58] and the increased aberrant splicing in CEP290 might be a direct consequence of this attempt to maximise their genome.
CEP290-LCA iPSC retinal progenitors have also been used to test the potential of a gene replacement approach for CEP290 [59]. This study used lentivirus, as it has a higher packaging capacity compared to adeno-associated virus (AAV), which cannot package a large gene like CEP290 (8kb). The transduction of patient retinal progenitor cells with full-length-CEP290 lentivirus restored CEP290 mRNA expression, suggesting this approach might be potentially beneficial for patients in the future [59].
AON technology has previously been shown to reduce aberrant splicing in CEP290-LCA fibroblasts, leading to an increase in cilia incidence and cilia length in patient cells [57,60]. Moreover, a recent study assessed the AAV-mediated delivery of AON to the humanized mouse model of CEP290-LCA [61]. The AAV-CEP290 AON resulted in corrected splicing and decrease in the cryptic exon formation in the mouse retina [61].
We tested an AON approach in CEP290-LCA optic cups using an antisense morpholino to the cryptic splice site in CEP290 c.2291+1665A>G (CEP290-MO). Treatment with CEP290-MO reduced mis-splicing and increased the level of normal transcripts to around 50%, which led to increased ciliation, cilia length and improvement of the cilia trafficking of essential photoreceptor cilia proteins, such as RPGR [53]. This study highlights further the potential of using 3D organoids to produce human retinal cells in an appropriate cellular environment to model the effects of gene-specific mutations.
Collectively, these data and experimental models highlight that iPSC derived retinal cells offer a new paradigm to study retinal ciliopathy disease mechanisms and test potential therapies. Moreover, given that these proof of concept studies can show efficacy and safety in the correct human target cells they also provide the hope of a rapid translation to the clinic.
Acknowledgements
This research is supported by The Guide Dogs for the Blind, Fight for Sight, Moorfields Eye Charity, the Rosetrees Trust, the London Project to Cure Blindness, the Special Trustees of Moorfields Eye Hospital, The Wellcome Trust and National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
References
- [1].Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–80. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–5. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- [4].Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet. 2008;45:257–67. doi: 10.1136/jmg.2007.054999. [DOI] [PubMed] [Google Scholar]
- [5].Mockel A, Perdomo Y, Stutzmann F, Letsch J, Marion V, Dollfus H. Retinal dystrophy in Bardet-Biedl syndrome and related syndromic ciliopathies. Prog Retin Eye Res. 2011;30:258–74. doi: 10.1016/j.preteyeres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [6].Rachel RA, et al. CEP290 alleles in mice disrupt tissue-specific cilia biogenesis and recapitulate features of syndromic ciliopathies. Hum Mol Genet. 2015;24:3775–91. doi: 10.1093/hmg/ddv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Athanasiou D, Aguila M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME. The cell stress machinery and retinal degeneration. FEBS Lett. 2013;587:2008–17. doi: 10.1016/j.febslet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hardcastle AJ, et al. Mutations in the RP2 gene cause disease in 10% of families with familial X-linked retinitis pigmentosa assessed in this study. Am J Hum Genet. 1999;64:1210–5. doi: 10.1086/302325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dandekar SS, et al. An atypical phenotype of macular and peripapillary retinal atrophy caused by a mutation in the RP2 gene. Br J Ophthalmol. 2004;88:528–32. doi: 10.1136/bjo.2003.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004;49:379–98. doi: 10.1016/j.survophthal.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [11].den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [12].Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol. 2008;15:373–80. doi: 10.1038/nsmb.1396. [DOI] [PubMed] [Google Scholar]
- [13].Schwarz N, Hardcastle AJ, Cheetham ME. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vision Res. 2012;75:2–4. doi: 10.1016/j.visres.2012.07.016. [DOI] [PubMed] [Google Scholar]
- [14].Grayson C, et al. Localization in the human retina of the X-linked retinitis pigmentosa protein RP2, its homologue cofactor C and the RP2 interacting protein Arl3. Hum Mol Genet. 2002;11:3065–74. doi: 10.1093/hmg/11.24.3065. [DOI] [PubMed] [Google Scholar]
- [15].Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet. 2010;19:1358–67. doi: 10.1093/hmg/ddq012. [DOI] [PubMed] [Google Scholar]
- [16].Grayson C, Chapple JP, Willison KR, Webster AR, Hardcastle AJ, Cheetham ME. In vitro analysis of aminoglycoside therapy for the Arg120stop nonsense mutation in RP2 patients. J Med Genet. 2002;39:62–7. doi: 10.1136/jmg.39.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hurd T, et al. The retinitis pigmentosa protein RP2 interacts with polycystin 2 and regulates cilia-mediated vertebrate development. Hum Mol Genet. 2010;19:4330–44. doi: 10.1093/hmg/ddq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shu X, Zeng Z, Gautier P, Lennon A, Gakovic M, Cheetham ME, Patton EE, Wright AF. Knockdown of the zebrafish ortholog of the retinitis pigmentosa 2 (RP2) gene results in retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52:2960–6. doi: 10.1167/iovs.10-6800. [DOI] [PubMed] [Google Scholar]
- [19].Patil SB, Hurd TW, Ghosh AK, Murga-Zamalloa CA, Khanna H. Functional analysis of retinitis pigmentosa 2 (RP2) protein reveals variable pathogenic potential of disease-associated missense variants. PLoS One. 2011;6:e21379. doi: 10.1371/journal.pone.0021379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu F, et al. Knockout of RP2 decreases GRK1 and rod transducin subunits and leads to photoreceptor degeneration in zebrafish. Hum Mol Genet. 2015;24:4648–59. doi: 10.1093/hmg/ddv197. [DOI] [PubMed] [Google Scholar]
- [21].Li L, et al. Ablation of the X-linked retinitis pigmentosa 2 (Rp2) gene in mice results in opsin mislocalization and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2013;54:4503–11. doi: 10.1167/iovs.13-12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang H, et al. Mistrafficking of prenylated proteins causes retinitis pigmentosa 2. FASEB J. 2015;29:932–42. doi: 10.1096/fj.14-257915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chang B, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–57. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–97. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang TT, Su J, Wang WJ, Craige B, Witman GB, Tsou MF, Liao JC. Superresolution Pattern Recognition Reveals the Architectural Map of the Ciliary Transition Zone. Sci Rep. 2015;5:14096. doi: 10.1038/srep14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Hum Mutat. 2010;31:1097–108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- [27].Drivas TG, Wojno AP, Tucker BA, Stone EM, Bennett J. Basal exon skipping and genetic pleiotropy: A predictive model of disease pathogenesis. Sci Transl Med. 2015;7:291ra97. doi: 10.1126/scitranslmed.aaa5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–61. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garanto A, van Beersum SE, Peters TA, Roepman R, Cremers FP, Collin RW. Unexpected CEP290 mRNA splicing in a humanized knock-in mouse model for Leber congenital amaurosis. PLoS One. 2013;8:e79369. doi: 10.1371/journal.pone.0079369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tucker BA, Mullins RF, Stone EM. Stem cells for investigation and treatment of inherited retinal disease. Hum Mol Genet. 2014;23:R9–R16. doi: 10.1093/hmg/ddu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [32].Trounson A, Shepard KA, DeWitt ND. Human disease modeling with induced pluripotent stem cells. Curr Opin Genet Dev. 2012;22:509–16. doi: 10.1016/j.gde.2012.07.004. [DOI] [PubMed] [Google Scholar]
- [33].Giacalone JC, Wiley LA, Burnight ER, Songstad AE, Mullins RF, Stone EM, Tucker BA. Concise Review: Patient-Specific Stem Cells to Interrogate Inherited Eye Disease. Stem Cells Transl Med. 2016;5:132–40. doi: 10.5966/sctm.2015-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vugler A, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. 2008;214:347–61. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- [35].Carr AJ, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carr AJ, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–95. [PMC free article] [PubMed] [Google Scholar]
- [37].Lukovic D, et al. Human iPSC derived disease model of MERTK-associated retinitis pigmentosa. Sci Rep. 2015;5:12910. doi: 10.1038/srep12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tucker BA, Cranston CM, Anfinson KA, Shrestha S, Streb LM, Leon A, Mullins RF, Stone EM. Using patient-specific induced pluripotent stem cells to interrogate the pathogenicity of a novel retinal pigment epithelium-specific 65 kDa cryptic splice site mutation and confirm eligibility for enrollment into a clinical gene augmentation trial. Transl Res. 2015;166:740–749 e1. doi: 10.1016/j.trsl.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Johnson AA, et al. Autosomal Recessive Bestrophinopathy Is Not Associated With the Loss of Bestrophin-1 Anion Channel Function in a Patient With a Novel BEST1 Mutation. Invest Ophthalmol Vis Sci. 2015;56:4619–30. doi: 10.1167/iovs.15-16910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meyer JS, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–18. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jin ZB, Okamoto S, Xiang P, Takahashi M. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Transl Med. 2012;1:503–9. doi: 10.5966/sctm.2012-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yoshida T, et al. The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Mol Brain. 2014;7:45. doi: 10.1186/1756-6606-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–30. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- [44].Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- [45].Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering Stem Cell Organoids. Cell Stem Cell. 2016;18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nakano T, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- [49].Zhong X, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Reichman S, et al. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A. 2014;111:8518–23. doi: 10.1073/pnas.1324212111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gonzalez-Cordero A, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–7. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tucker BA, et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Parfitt DA, et al. Identification and Correction of Mechanisms Underlying Inherited Blindness in Human iPSC-Derived Optic Cups. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hockemeyer D, Jaenisch R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell. 2016;18:573–86. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schwarz N, et al. Translational read-through of the RP2 Arg120stop mutation in patient iPSC-derived retinal pigment epithelium cells. Hum Mol Genet. 2015;24:972–86. doi: 10.1093/hmg/ddu509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schwarz N, Novoselova TV, Wait R, Hardcastle AJ, Cheetham ME. The X-linked retinitis pigmentosa protein RP2 facilitates G protein traffic. Hum Mol Genet. 2012;21:863–73. doi: 10.1093/hmg/ddr520. [DOI] [PubMed] [Google Scholar]
- [57].Collin RW, den Hollander AI, van der Velde-Visser SD, Bennicelli J, Bennett J, Cremers FP. Antisense Oligonucleotide (AON)-based Therapy for Leber Congenital Amaurosis Caused by a Frequent Mutation in CEP290. Mol Ther Nucleic Acids. 2012;1:e14. doi: 10.1038/mtna.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Farkas MH, Grant GR, White JA, Sousa ME, Consugar MB, Pierce EA. Transcriptome analyses of the human retina identify unprecedented transcript diversity and 3.5 Mb of novel transcribed sequence via significant alternative splicing and novel genes. BMC Genomics. 2013;14:486. doi: 10.1186/1471-2164-14-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Burnight ER, et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21:662–72. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gerard X, et al. AON-mediated Exon Skipping Restores Ciliation in Fibroblasts Harboring the Common Leber Congenital Amaurosis CEP290 Mutation. Mol Ther Nucleic Acids. 2012;1:e29. doi: 10.1038/mtna.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Garanto A, et al. In vitro and in vivo rescue of aberrant splicing in CEP290-associated LCA by antisense oligonucleotide delivery. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw118. [DOI] [PMC free article] [PubMed] [Google Scholar]