Abstract
Background
Gastric cancer is among the most common types of cancer, with high morbidity and mortality. MicroRNAs (miRNAs) play vital roles in the tumorigenesis and biology of gastric cancer. This study aimed to reveal the role of miR-340 in gastric cancer cell proliferation and apoptosis and to elucidate the potential mechanisms.
Material/Methods
Human gastric cancer cells SGC-7901 were used in this study for cell transfection with miR-340 mimic or inhibitor. After transfection, cell viability, proliferation, and apoptosis were examined by MTT, BrdU, and flow cytometry assays, respectively. The protein level changes of p27, p21, Caspase 3 (CASP3), B cell lymphoma 2 (BCL2), BCL2-associated X protein (BAX), and v-AKT murine thymoma viral oncogene (AKT) were detected by Western blot.
Results
Overexpression of miR-340 significantly reduced cell viability and proliferation (P<0.01), and induced cell apoptosis (P<0.01) of SGC-7901. miR-340 elevated the protein level of cell cycle inhibitor p27, but did not affect the level of p21. Apoptosis-related factors pro-CASP3, cleaved-CASP3, and BAX were promoted, and BCL2 was inhibited by miR-340. miR-340 also suppressed the phosphorylation of AKT. Opposite effects were detected when SGC-7901 cells were transfected with miR-340 inhibitor.
Conclusions
These results indicate that miR-340 can inhibit proliferation and induce apoptosis of SGC-7901 cells, suggesting its roles in protecting against gastric cancer. The roles of miR-340 in gastric cancer cells may be associated with its regulation of the AKT pathway. Thus, miR-340 may be a potential therapeutic strategy for gastric cancer treatment.
MeSH Keywords: Apoptosis, Cell Proliferation, MicroRNAs, Signal Transduction, Stomach Neoplasms
Background
Gastric cancer is among the most common types of human malignant cancer, with high morbidity and mortality [1]. It is usually asymptomatic or with only non-specific symptoms in earlier stages, adding difficulties to timely diagnosis [2]. The mechanism for the development of gastric cancer is still largely unclear in spite of extensive clinical and basic research efforts. Currently, surgery with the assistance of chemotherapy and chemoradiation remains the only effective method for gastric cancer treatment [3]. Regulation of key factors in gastric cancer development is showing promising application values in controlling this disease [4,5]. Therefore, to understand the molecular mechanisms involved in gastric cancer will be of great significance for providing new knowledge regarding the diagnosis and treatment of gastric cancer.
MicroRNAs (miRNAs) are a group of small non-coding RNAs that play pivotal roles in various biological processes by regulating gene expression at post-transcriptional levels [6,7]. It has been well demonstrated that miRNAs participate in the tumorigenesis and biology of diverse diseases, including gastric cancer, through affecting cell cycle, proliferation, apoptosis, migration, and invasion [8]. The expression profiles of a number of miRNAs are disrupted in gastric cancer [9], some of them providing specificity and sensitivity for early diagnosis [10], and others serving as potential therapeutic targets [11]. For example, miR-198 is down-regulated in gastric cancer tissues and may be an independent prognostic marker for gastric cancer [12]. miR-211 can inhibit gastric cancer cell proliferation and invasion, partially by down-regulating SOX4 [13]. Thus, studies on miRNAs are of great significance for developing a deeper understanding of their regulatory mechanism, as well as for the improvement in gastric cancer treatment.
Previous studies suggested the aberrant expression of miR-340 in several kinds of cancers, such as non-small cell lung cancer [14], glioblastoma multiforme [15], and breast cancer [16]. Moreover, miR-340 has been revealed to be aberrantly expressed in gastric cancer tissues [17]. Thus, miR-340 appears to participate in gastric cancer modulation, but the underlying mechanisms remain elusive.
This study aimed to investigate the effect of miR-340 on gastric cancer cell proliferation and apoptosis and the potential mechanisms. Gastric cancer SGC-7901 cells were used in this study. Cell viability, proliferation, and apoptosis were assessed by MTT, BrdU, and flow cytometry methods, respectively, after the transfection of miR-340 mimic or inhibitor. The expression of cell cycle and apoptosis-related proteins were detected to reveal the potential mechanisms. This study shows the important roles of miR-340 in gastric cancer and provides a potential therapeutic strategy for gastric cancer treatment.
Material and Methods
Cells
Human gastric cancer SGC-7901 cells (ATCC, Manassas, VA) were maintained in minimal Roswell Parker Memorial Institute (RPMI)-1640 medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Gibco) in a humidified atmosphere of 5% CO2 at 37°C. Cells were passaged at a ratio of 1: 3.
Cell transfection
SGC-7901 cells were transfected with miR-340 mimic (50 nM), inhibitor (100 nM) or scramble miRNA as a control (Sangon Biotech, Shanghai, China) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Briefly, 2×105 cells were seeded in 24-well plates with antibiotic-free medium 1 day before transfection to reach a confluence of 90% at the time of transfection. The transfection complex was prepared according to the manufacturer’s instructions and added to the cells, and the plates were incubated in a humidified atmosphere with 5% CO2 at 37°C. The medium was replaced at 6 h post-transfection. Cell samples were collected at 0, 1, 2, 3, and 4 days after transfection, for further analysis.
MTT assay
SGC-7901 cell viability was analyzed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Briefly, cells were cultured in 96-well culture plates (5×104 cells per well) for 12 h. Then, 20 μL of 5 mg/mL MTT (Sigma-Aldrich, Shanghai, China) was added to each well for incubation of 4 h at 37°C, after which the medium was discarded and dimethylsulfoxide (Sigma-Aldrich) was added. The plates were shaken gently to fully dissolve crystals. The optical density at 590 nm was detected with a Multiskan Go microplate reader (Thermo Scientific, Carlsbad, CA).
BrdU assays
The cell proliferation ability of SGC-7901 was monitored at 3 days post-transfection with 5-bromo-2-deoxyuridine (BrdU) Cell Proliferation Assay Kit (BioVision, Milpitas, CA). Cells were seeded in 24-well plates (2×104/well) and cultured to reach a confluency of about 60%, after which the cells were stained with BrdU, fixed, and washed according to the manufacturer’s instruction. The horseradish peroxidase (HRP)-conjugated antibody was added (1:2000) and the cells were incubated at 4°C overnight. After washing in PBS, the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 0.5 μg/mL, Sigma-Aldrich). Fluorescent signals were observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Cell apoptosis analysis
Cells apoptosis was detected at 3 days post-transfection by use of the Annexin V-FITC Apoptosis Detection Kit I (Univ-bio, Shanghai, China) according to the manufacturer’s instructions. Briefly, SGC-7901 cells were seeded in 6-well plates (1×105 cells/well). Then, Annexin V-FITC and propidium iodide (PI) were added to each well and the cells were incubated in the dark at room temperature for 15 min. Thereafter, the binding buffer was added, and the apoptotic cells were detected using a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). FITC-positive and PI-negative cells were counted as apoptotic cells.
Western blot
Transfected SGC-7901 cells were lysed in lysis buffer (Roche, Basel, Switzerland) for protein extraction at 3 days post-transfection. Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Maibio, Shanghai, China). After blocking in 5% skim milk for 2 h at room temperature, the membranes were incubated with the specific primary antibodies for p27Kip1 (ab32034), p21Cip1 (ab109520), pro-CASP3 (ab2171), cleaved-CASP3 (ab2302), BCL2 (ab32124), BAX (ab32503), AKT (ab8805), and phosphorylated AKT (p-AKT, ab38449, Abcam, Cambridge, UK) at 4°C overnight. β-actin (ab8227) was used as an internal control. The membranes were washed in PBS 3 times and incubated in HRP-conjugated secondary antibodies for 1 h at room temperature. Positive signals were developed by ECL Plus Western Blotting Substrate (Thermo Scientific) and analyzed by ImageJ 1.49 (National Institutes of Health, Bethesda, MD).
Statistical analysis
All experiments were repeated 3 times. Results are represented as the mean ± standard deviation. Data were analyzed by t test to calculate the difference between groups using the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA). A statistically significant difference was defined as P<0.05.
Results
miR-340 reduced SGC-7901 cell proliferation
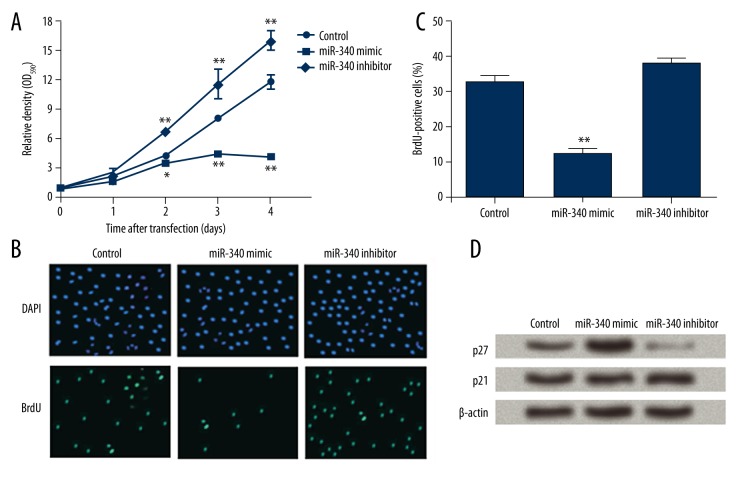
The effect of miR-340 on SGC-7901 cell proliferation was detected. MTT assay results showed that the viability of SGC-7901 cells transfected with miR-340 was strongly inhibited, while miR-340 inhibitor significantly increased cell viability (Figure 1A). Significant differences were detected at 2 days and at later time points post-transfection (P<0.05 or P<0.01). Thus, miR-430 was likely to inhibit SGC-7901 cell viability. BrdU assay showed that miR-430 mimic decreased the number of BrdU-positive cells compared to controls (Figure 1B). Counting of BrdU-positive cells indicated a significant difference was detected between the 2 groups (P<0.01, Figure 1C), but miR-340 inhibitor did not have an obvious influence on the BrdU-positive cell number (P>0.05). Taken together, our results show that miR-430 was capable of inhibiting SGC-7901 cell proliferation, suggesting its role in modulating gastric cancer cells.
Figure 1.
miR-340 inhibits SGC-7901 cell viability and proliferation. control, cells transfection with scramble miRNA as a control. miR-340 mimic, cells overexpressing miR-340. miR-340 inhibitor, cells with miR-340 down-regulation. (A) Relative optical density (OD) at 590 nm showing cell viability of the 3 groups at 0, 1, 2, 3, and 4 days post-transfection. (B) Cells counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and 5-bromo-2-deoxyuridine (BrdU) at 3 days post-transfection. (C) Percent of BrdU-positive cells that indicate cells with proliferative activity. (D) Western blot showing the protein levels of p27 and p21 in transfected cells at 3 days post-transfection. β-actin was used as an internal control. * P<0.05; ** P<0.01 compared with control.
p27 and p21, 2 factors involved in cell cycle regulation, were detected by Western blot, and p27 protein level was found to be increased by miR-340 mimic and decreased by miR-340 inhibitor (Figure 1D), but p21 protein levels remained almost unchanged in the 3 cell groups. Thus, miR-340 might regulate p27 rather than p21 in SGC-7901 cells, which is possibly associated with its effect on cell proliferation.
miR-340 induced SGC-7901 cell apoptosis
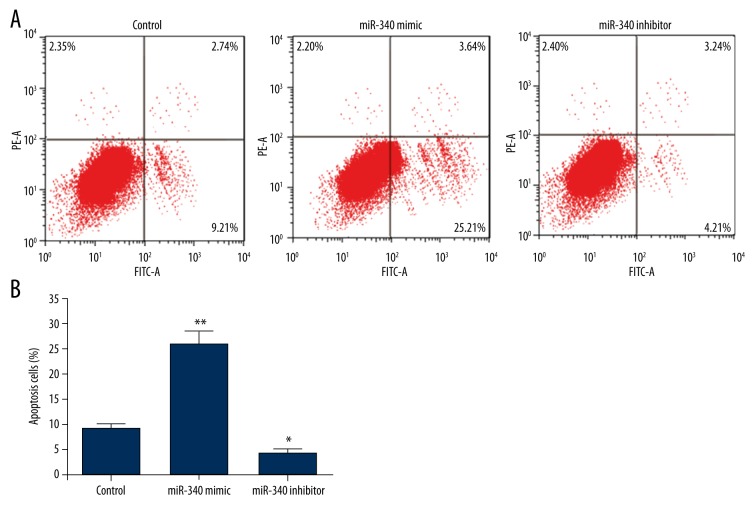
Cell apoptosis assay was carried out by flow cytometry (Figure 2A). The apoptotic cell percent was obviously higher when miR-340 was overexpressed (P<0.01, Figure 2B), and was markedly lower when miR-340 was suppressed (P<0.05). These results suggest that miR-340 might induce the apoptosis of SGC-7901 cells.
Figure 2.
miR-340 induces SGC-7901 cell apoptosis. Control, cells transfection with scramble miRNA as a control. miR-340 mimic, cells overexpressing miR-340. miR-340 inhibitor, cells with miR-340 down-regulation. (A) Flow cytometry performed at 3 days post-transfection showing cells with FITC or PI signals. (B) Histogram showing the percent of FITC-positive and PI-negative cells that indicates apoptotic cells. * P<0.05; ** P<0.01 compared with control.
miR-340 regulates apoptosis-related factors and inhibits AKT activation
Several apoptosis-related factors, including CASP3, BCL2, and BAX, as well as AKT levels, were detected by Western blot to reveal the possible mechanism of miR-340 in controlling SGC-7901 cells (Figure 3). Results showed that pro-CASP3, cleaved-CASP3, and BAX protein levels were promoted by miR-340 overexpression and inhibited by miR-340 inhibitor. BCL2 was decreased by the overexpressed miR-340 and increased by the suppression of miR-340. Thus, miR-340 was capable of regulating these apoptosis-related factors, which might be related to its promotion of SGC-7901 cell apoptosis.
Figure 3.
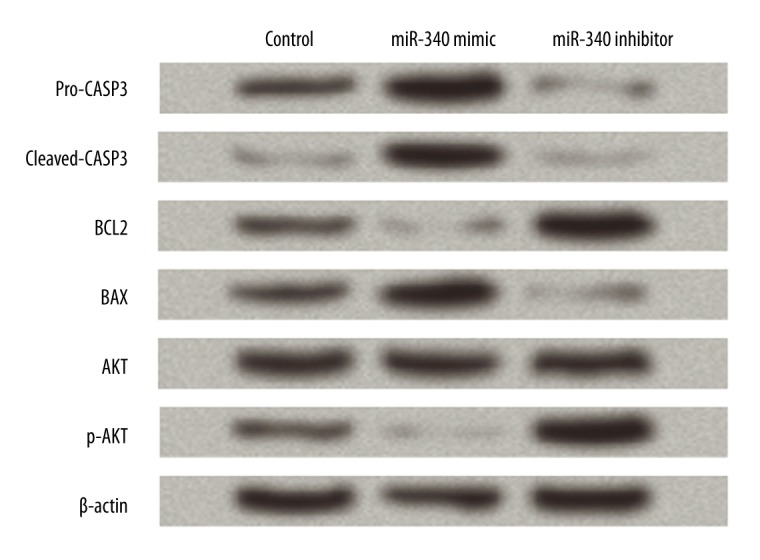
miR-340 regulates apoptosis-related proteins and AKT activation in SGC-7901 cells. Control, cells transfection with scramble miRNA as a control. miR-340 mimic, cells overexpressing miR-340. miR-340 inhibitor, cells with miR-340 down-regulation. Western blot was performed at 3 days post-transfection. β-actin is used as an internal control. CASP3 – caspase 3; BCL2 – B cell lymphoma 2; BAX – BCL2-associated X protein; AKT – v-AKT murine thymoma viral oncogene; p-AKT – phosphorylated AKT.
The AKT pathway is related to cell growth and apoptosis [18,19]; therefore, the level of AKT proteins was detected to investigate the potential mechanism of miR-340. p-AKT was suppressed in the miR-340 mimic group and elevated in the miR-340 inhibitor group, but the total AKT level was mostly unaffected by miR-340. These result suggest that miR-340 can inhibit the activation of AKT, which is likely associated with its regulation of SGC-7901 cells.
Discussion
Accumulating evidence has revealed that miRNAs play pivotal roles in developmental and pathological processes of many human malignant cancers, some of them being considered as tumor suppressor genes, while others are recognized as carcinogenic genes [8,20,21]. Nevertheless, the functions of miRNA-340 in gastric cancer and the underlying mechanisms are not fully understood. This study showed that the overexpression of miR-340 significantly inhibited proliferation and induced apoptosis of human gastric cancer SGC-7901 cells. Moreover, miR-430 regulated the expression of apoptosis-related factors, including CASP3, BCL2, and BAX, and affected the levels of p27 and AKT.
Gastric cancer development and progression is a multi-factor and multi-step process involving abnormal cell proliferation, apoptosis, invasion, and metastasis. In this study, MTT and BrdU assays showed that the SGC-7901 cell viability and proliferation were significantly suppressed by overexpression of miR-340, suggesting the suppressive role of miR-340 in cell proliferation. Previous studies demonstrated that uncontrolled gastric cancer cell proliferation is due to disruption of G1/S and G2/M checkpoints [22,23]. As a cyclin-dependent kinase inhibitor, p27 blocks uncontrolled cell cycle progression through restraining G1 phase initiation and G1/S transition [24], and many miRNAs have been reported to participate in cancer cell arrest by targeting p27 [14,25,26]. As revealed in this study, the protein level of p27 was significantly increased by miR-340, indicating that miR-340 can also regulate p27, which may be a mechanism for its suppressive role in gastric cancer proliferation. Although both p27 and p21 are inactivated by AKT [27], p21 was little changed by miR-340. We speculate that the differentiated changing pattern of p21 and p27 may be associated with the overlapping functions of these 2 factors [28,29]. Further investigation is necessary to explain this phenomenon.
This study also analyzed the effect of miR-340 on SGC-7901 cell apoptosis, and showed that miR-340 overexpression dramatically induced cell apoptosis in vitro. Additionally, the miR-340 overexpression was shown to affect the levels of 4 apoptotic factors – pro-CASP3, cleaved CASP3, BCL2, and BAX – which possess the corresponding changes of promoted cell apoptosis. The 4 factors are widely used as indicators for cell apoptosis in numerous studies; BCL2 is an anti-apoptosis factor, and CASP3 and BAX are pro-apoptosis factors [30,31]. The up-regulated pro-CASP3, cleaved-CASP3, and BAX, as well as the down-regulation of BCL2 by miR-340 further confirmed the induced apoptosis in miR-340-transfected SGC-7901 cells. Thus, miR-340 may induce apoptosis of gastric cancer cells.
A previous study in the SGC-7901 cell line indicated the pro-proliferation and anti-apoptosis roles of miR-340, without detecting the potential regulatory mechanism [17]. In this study, the opposite functions of miR-340 were found in the same gastric cancer cell line, together with its corresponding regulation of cell cycle and apoptosis factors. The potential targets of miR-340 were predicted by use of the online database TargetScanHuman 7.0 [32], and results implied that BCL2 mRNA was likely to be targeted by miR-340 by the sequence UUUAUAA in its 3′ untranslated region. Thus, it is reasonable to speculate that the anti-apoptosis role of miR-340 may be associated with the direct inhibition on BCL2 mRNA, but this needs further investigation. In addition, the reference study analyzed the role of miR-340 via transfecting miR-340 plasmid, which was different from this study using miR-340 inhibitor, and might cause different responses in the downstream pathways or targets of miR-340. This may be a possible reason for the disparities in results of the 2 studies, and may help us to explain the mechanism of miR-340 more comprehensively in future research.
The AKT pathway has been widely reported to be associated with cell growth, proliferation, apoptosis, and migration [33,34]. It is usually aberrantly activated in human cancers, making it an attractive therapeutic target [35]. Inhibitors of this pathway have been revealed to induce apoptosis of various cancer cells, such as lymphoma, myeloma, and leukemia cells [36,37]. Also, numerous studies have demonstrated that the AKT pathway is one of the most critical signaling pathways involved in gastric cancer cell apoptosis [33,38]. In our study, the overexpression of miR-340 in gastric cancer cells suppressed the phosphorylation of AKT, indicating that miR-340 can inhibit the activation of AKT and the AKT pathway. Together with the role of AKT in cell proliferation and apoptosis, we deduced that the anti-proliferative and pro-apoptotic roles of miR-340 in SGC-7901 cells may be associated with its inhibition of the AKT pathway. More conclusive evidence needs to be uncovered in further mechanism studies.
Conclusions
We found that miR-340 may function as a tumor suppressor of gastric cancer by controlling cell proliferation and inducing cell apoptosis, possibly via inhibiting activation of the AKT pathway. This study provides a potential therapeutic alternative focusing on miR-340 for gastric cancer treatment. Further studies are still necessary for a comprehensive understanding of the mechanism involved.
Footnotes
Conflicts of interest
There are no conflicts of interest
Source of support: Departmental sources
References
- 1.Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–44. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Yan JY, Tian FM, Hu WN, et al. Apoptosis of human gastric cancer cells line SGC 7901 induced by garlic-derived compound S-allylmercaptocysteine (SAMC) Eur Rev Med Pharmacol Sci. 2013;17:745–51. [PubMed] [Google Scholar]
- 3.Orditura M, Galizia G, Sforza V, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–49. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Jin Y, Pan S, et al. TCEA3 attenuates gastric cancer growth by apoptosis induction. Med Sci Monit. 2015;21:3241–46. doi: 10.12659/MSM.895860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konturek PC, Kania J, Konturek JW, et al. H. pylori infection, atrophic gastritis, cytokines, gastrin, COX-2, PPAR gamma and impaired apoptosis in gastric carcinogenesis. Med Sci Monit. 2003;9(7):SR53–66. [PubMed] [Google Scholar]
- 6.Bueno M, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7:3143–48. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 8.Visone R, Croce C. MiRNAs and cancer. Am J Pathol. 2009;174:1131–38. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katada T, Ishiguro H, Kuwabara Y, et al. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–42. [PubMed] [Google Scholar]
- 10.Liu R, Zhang C, Hu Z, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47:784–91. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Xie SS, Jin J. Emerging roles of non-coding RNAs in gastric cancer: Pathogenesis and clinical implications. World J Gastroenterol. 2016;22:1213–23. doi: 10.3748/wjg.v22.i3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Z, Zheng X, Kong D. Decreased miR-198 expression and its prognostic significance in human gastric cancer. World J Surg Oncol. 2015;14:33. doi: 10.1186/s12957-016-0784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CY, Hua L, Sun J, et al. MiR-211 inhibits cell proliferation and invasion of gastric cancer by down-regulating SOX4. Int J Clin Exp Pathol. 2018;8:14013–20. [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez S, Risolino M, Mandia N, et al. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene. 2015;34:3240–50. doi: 10.1038/onc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, Qiu S, Ge R, et al. miR-340 suppresses glioblastoma multiforme. Oncotarget. 2015;6:9257–70. doi: 10.18632/oncotarget.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CP, Sun ZL, Lu X, et al. miR-340 suppresses cell migration and invasion by targeting MYO10 in breast cancer. Onco Rep. 2016;35:709–16. doi: 10.3892/or.2015.4411. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Qiao H. Effect of miR-340 on gastric cancer cell proliferation and apoptosis. Int J Clin Exp Pathol. 2015;8:13108–13. [PMC free article] [PubMed] [Google Scholar]
- 18.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–65. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 19.Franke TF, Hornik CP, Segev L, et al. PI3K/Akt and apoptosis: Size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 20.Dong H, Luo L, Hong S, et al. Integrated analysis of mutations, miRNA and mRNA expression in glioblastoma. BMC Syst Biol. 2010;4:163. doi: 10.1186/1752-0509-4-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda T, Okumura H, Shimizu M, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M, Zhong J, Zhao M, et al. Overexpression of nuclear apoptosis-inducing factor 1 altered the proteomic profile of human gastric cancer cell MKN45 and induced cell cycle arrest at G1/S phase. PLoS One. 2014;9:e100216. doi: 10.1371/journal.pone.0100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Lee HW, Baek JH, et al. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35:251–60. doi: 10.1038/onc.2015.80. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Hou D, Luo Z, et al. The novel protective role of P27 in MLN4924-treated gastric cancer cells. Cell Death Dis. 2015;6:e1867. doi: 10.1038/cddis.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sha M, Ye J, Luan ZY, et al. Celastrol induces cell cycle arrest by MicroRNA-21-mTOR-mediated inhibition p27 protein degradation in gastric cancer. Cancer Cell Int. 2015;15:101. doi: 10.1186/s12935-015-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seviour EG, Sehgal V, Lu Y, et al. Functional proteomics identifies miRNAs to target a p27/Myc/phospho-Rb signature in breast and ovarian cancer. Oncogene. 2016;35:691–701. doi: 10.1038/onc.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2014;1:391–93. doi: 10.4161/cc.1.6.262. [DOI] [PubMed] [Google Scholar]
- 28.Schönherr E, Levkau B, Schaefer L, et al. Decorin-mediated signal transduction in endothelial cells: Involvement of Akt/protein kinase B in up-regulation of p21(WAF1/CIP1) but not p27(KIP1) J Biol Chem. 2001;276:40687–92. doi: 10.1074/jbc.M105426200. [DOI] [PubMed] [Google Scholar]
- 29.Fujio Y, Guo K, Mano T, et al. Cell Cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol. 1999;19:5073–82. doi: 10.1128/mcb.19.7.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jergens A, Young J, Moore D, et al. Bcl-2/caspase 3 mucosal imbalance favors T cell resistance to apoptosis in dogs with inflammatory bowel disease. Vet Immunol Immunopathol. 2014;158:167–74. doi: 10.1016/j.vetimm.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Renault TT, Floros KV, Elkholi R, et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol Cell. 2015;57:69–82. doi: 10.1016/j.molcel.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie HX, Xu ZY, Tang JN, et al. Effect of Huaier on the proliferation and apoptosis of human gastric cancer cells through modulation of the PI3K/AKT signaling pathway. Exp Ther Med. 2015;10:1212–18. doi: 10.3892/etm.2015.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng W, Zhang Y, Gu L, et al. Heat shock protein 27 downstream of P38-PI3K/Akt signaling antagonizes melatonin-induced apoptosis of SGC-7901 gastric cancer cells. Cancer Cell Int. 2016;16:5. doi: 10.1186/s12935-016-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chin Y, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21:470–76. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi H, Hu J, Zheng J, et al. Down-regulation of the PI3K Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cell by Baicalin. J Exp Clin Cancer Res. 2012;31:1–9. doi: 10.1186/1756-9966-31-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu C, Fruman D. Target of rapamycin signaling in leukemia and lymphoma. Clin Cancer Res. 2010;16:5374–80. doi: 10.1158/1078-0432.CCR-10-0480. [DOI] [PubMed] [Google Scholar]
- 38.Yuan CX, Zhou ZW, Yang YX, et al. Danusertib, a potent pan-Aurora kinase and ABL kinase inhibitor, induces cell cycle arrest and programmed cell death and inhibits epithelial to mesenchymal transition involving the PI3K/Akt/mTOR-mediated signaling pathway in human gastric cancer AGSAGS and NCI -N78 cells. Drug Des Dev Ther. 2015;9(default):1293–318. doi: 10.2147/DDDT.S74964. [DOI] [PMC free article] [PubMed] [Google Scholar]